INTRODUCTION:
Despite the negative impact of covert hepatic encephalopathy on the outcome of patients with liver cirrhosis, data regarding the ability of different testing strategies to predict overt hepatic encephalopathy (OHE) development and mortality are limited. This study aimed to compare the ability of Psychometric Hepatic Encephalopathy Score (PHES), critical flicker frequency (CFF), simplified animal naming test (S-ANT1), and clinical covert hepatic encephalopathy (CCHE) score to predict OHE development and mortality.
METHODS:
A total of 224 patients with liver cirrhosis were tested with different testing strategies and prospectively followed up regarding clinically relevant outcomes (OHE or death/liver transplantation).
RESULTS:
Prevalence of pathological results varied among the testing strategies: PHES 33.9%, CFF 17.9%, S-ANT1 41.5%, and CCHE score 33.9%. All testing strategies were independent predictors of OHE development after adjusting for model of end-stage liver disease (MELD) score and history of OHE. The predictive performances of PHES (area under the receiver operating characteristic curve, 0.742) and CCHE (area under the receiver operating characteristic curve, 0.785) regarding OHE development during the next 180 days were significantly better than those of CFF and S-ANT1. In multivariable analysis, pathological results in PHES, S-ANT1, and CCHE score were independently associated with higher mortality. CFF did not correlate with mortality in the whole cohort. In the subgroup of patients with a MELD score <15, pathological results in PHES, CFF, or CCHE score were independent predictors of higher mortality.
DISCUSSION:
PHES and CCHE score predict OHE development and mortality in patients with liver cirrhosis. In particular, in patients with low MELD score, both testing strategies could help to identify patients who might benefit from liver transplantation.
INTRODUCTION
Globally, liver cirrhosis is a common cause for morbidity and mortality contributing to more than 1 million deaths in 2010 (1). Among its most relevant complications is the development of hepatic encephalopathy (HE), a brain dysfunction mainly caused by liver insufficiency and portosystemic shunting (2,3). It manifests as a wide spectrum of neurological and psychiatric abnormalities, and depending on severity, HE might roughly be divided into 2 groups: In overt hepatic encephalopathy (OHE), clinically detectable symptoms are present episodically or persistently, whereas covert hepatic encephalopathy (CHE) (which comprises minimal hepatic encephalopathy [MHE] and HE grade 1 [HE1]) is defined as neurocognitive impairment below an unequivocally reliable clinical detection level. However, even CHE leads to impaired quality of life, and each episode of OHE might lead to further cognitive impairment (4,5). Taken together, HE is a marker of poor prognosis, predicts progression of cirrhosis, and is associated with high mortality (6,7).
The diagnostic gold standard for the detection of MHE is the Psychometric Hepatic Encephalopathy Score (PHES) consisting of the number connection test-A, the number connection test-B, the digit symbol test, the serial dotting test, and the line tracing test (2,8). Another established tool is the assessment of the critical flicker frequency (CFF) with a specialized device (HEPAtonorm-Analyzer 2.0) (9). In recent years, several studies have shown that both tests are associated with OHE development and mortality (10–13). However, despite the availability of the different testing strategies, screening for CHE is often overseen or even neglected in clinical practice. Main reasons might be the time-consuming and/or expensive testing process or the unavailability of required devices. A fast and inexpensive tool to evaluate cognitive performance was recently introduced by Campagna et al. In their study, they demonstrated that the simplified animal naming test (S-ANT1) might be a valid tool to detect CHE in clinical practice (14). In addition, our group developed the clinical covert hepatic encephalopathy (CCHE) score, a composite score containing the variables—history of OHE, ascites at clinical examination, albumin, S-ANT1, and the activity subdomain of the chronic liver disease questionnaire-, which can be rapidly conducted in routine clinical practice without additional costs (15). Although the diagnosis of CHE is important in clinical practice because of the higher risk of OHE development, most cutoffs of the respective tests are derived relative to healthy controls. Moreover, apart from diagnosis, data regarding the ability of the different CHE testing strategies to predict clinically relevant outcomes are still limited, in particular in a head-to-head comparison. Therefore, we aimed to compare the ability of PHES, CFF, S-ANT1, and CCHE score to predict OHE development and mortality (death/need for liver transplantation) in patients with liver cirrhosis.
PATIENTS AND METHODS
Patients
In total, 367 patients with liver cirrhosis were screened for this prospective study between March 2017 and December 2018 at the Cirrhosis Center Mainz of the University Medical Center of the Johannes Gutenberg-University, Mainz, and the Diakonie Klinikum, Jung-Stilling, Siegen, Germany. The leading etiology of underlying liver disease was determined according to clinical, serological, and histological findings. Diagnosis of liver cirrhosis was established by histology, conclusive appearance in ultrasound or radiological imaging, endoscopic features of portal hypertension, and medical history. Blood biochemistry (bilirubin, albumin, international normalized ratio, sodium, potassium, creatinine, c-reactive protein, white blood cell count, hemoglobin, and thrombocytes) was assessed in all patients. Model of end-stage liver disease (MELD) and Child–Pugh (CP) score were calculated to determine the severity of underlying liver disease. Patients were excluded if they fulfilled 1 or more of the following criteria: previous episode of OHE during the past 6 weeks, chronic alcohol consumption during the past 3 months, any intake of psychotropic drugs or opioids, the presence of preterminal comorbidities (heart disease with NYHA III–IV, chronic obstructive pulmonary disease with GOLD C and D, and renal failure with creatinine >1.5 mg/dL), the presence of hepatocellular carcinoma or other active malignancies, history of transjugular intrahepatic portosystemic shunt, neurological comorbidities (i.e., dementia or history of stroke), history of recent head trauma, and electrolyte disorders (serum potassium <3.5 mg/dL or >5 mg/dL; serum sodium <130 mg/dL or >150 mg/dL). Patients with a previous episode of OHE, which took place longer than 6 weeks ago, were allowed to participate if they were on consequent therapy with lactulose and/or rifaximin, if clinically indicated. Reasons for hospitalization were to perform liver biopsy, esophagogastroduodenoscopy with potential band ligation, measurement of hepatic venous pressure gradient, preplanned ascites puncture, or evaluation of liver transplantation. No patient included into this study had an active hepatitis C virus infection. Patients with chronic hepatitis B virus infection were on antiviral treatment with nucleoside/nucleotide analogs and had undetectable HBV DNA.
Diagnosis of HE
First, every patient was examined by an experienced hepatologist to rule out OHE. Afterward, portosystemic encephalopathy syndrome test that produces the PHES was performed in all included patients. Interpretation of PHES was done as previously described with German norms (8). A score < −4 was considered as pathological (8). After testing with PHES, the CFF was measured using the validated HEPAtonorm-Analyzer 2.0 (nevoLAB GmbH, Maierhoefen, Germany). Results <39 Hz were considered as pathological (9). After instruction, the patient had a training phase with at least 4 measurements to adapt to the procedure. Finally, 8 measurements were conducted, and a mean was calculated.
In addition, every patient was tested with the S-ANT1 (14). Patients are asked to name as many animals as possible in 1 minute. Repeats and errors were excluded from the calculation. The number of named animals after 1 minute was the definitive score. To compensate for the influence of age and education on the results in ANT1, we calculated the S-ANT1 that has been described recently. In patients with an educational level less than 8 years, 3 animals were added, and in patients with low education (less than 8 years) and high age (more than 80 years), 6 animals were added. In this study, we investigated the predictive ability of 2 cutoffs (<15 animals and <20 animals), which were described elsewhere (14,16). Finally, patients were investigated using the recently developed CCHE score (15). There are 2 cutoff values for the CCHE score to define 3 patient groups with low, intermediate, or high risk of the presence of CHE: (i) a low risk group with CCHE <53.5 points, (ii) an intermediate risk group with 53.5 points ≤ CCHE ≤57.5 points, and (iii) a high risk group with CCHE >57.5 points. In this study, we defined measures above the high-risk cutoff (>57.5 points) as pathological. All tests were performed on the same day with the respective patient. Patients were never tested with either test at the same day of any other intervention to rule out confounding factors.
Follow-up evaluation
All patients were followed up during study visits every 6 months in the outpatient clinic of the Cirrhosis Center Mainz or at the Diakonie Klinikum, Jung-Stilling Hospital, Siegen. At every visit or during unplanned hospitalizations, every patient was examined by an experienced hepatologist to rule in or rule out OHE. Presence of OHE was diagnosed after detailed neurological examination according to the West Haven criteria. Two endpoints were evaluated during the follow-up: (i) the occurrence of an OHE episode requiring hospitalization or an OHE episode during hospitalization for other complications and (ii) the composite of death or need for liver transplantation. Given that all patients who had received a liver transplantation had done so because of final hepatic failure, they were treated as complete cases (=death). Patients who did not participate personally in the follow-up examination were contacted by telephone to assess unplanned hospitalizations in other hospitals or death. In addition, the respective family doctors and hospitals of the patients were contacted in these cases.
Ethics
The study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). The study protocol was approved by the ethics committee of the Landesärztekammer Rheinland-Pfalz (Nr. 837.232.17 [11066]) and the Ärztekammer Westfalen-Lippe (Nr. 2018-250-b-S). A written informed consent was obtained from all participants.
Statistical analysis
Quantitative data are expressed as medians with interquartile ranges. Categorical variables are given as frequencies and percentages, respectively. To analyze the association of the 4 testing strategies with the risk of OHE development, we compared the cumulative OHE incidences of the 2 patient subgroups resulting from each testing strategy. Thereby, we used competing risk methodology where death and liver transplantation were considered as competing events for OHE. In addition, cause-specific OHE hazard ratios (HRs), adjusted for the 2 well-documented predictors of OHE MELD and previous OHE, were obtained for every testing strategy by using multivariable Cox regression analyses.
To investigate the performance of the 4 testing strategies to identify patients developing OHE within the next 180 days, we calculated the area under the receiver operating characteristic curve (AUROC) and its respective 95% confidence interval (CI). Comparisons between the AUROCs were performed using the method of Hanley and McNeil (17).
Regarding the composite of death/need for liver transplantation (mortality), survival curves for each testing strategy were analyzed using Kaplan–Meier curves and log-rank test. In addition, univariable Cox regression analyses were conducted for different variables. Variables with a P value <0.05 in univariable analyses were included into a multivariable Cox regression model. In all Cox regression analyses containing CCHE score, the variables history of OHE or albumin were excluded because of collinearity.
Our complete data analysis is exploratory. Hence, no adjustments for multiple testing were performed. For all tests, we used a 0.05 level to define statistically relevant deviations from the respective null hypothesis. However, because of the large number of tests, P values should be interpreted with caution and in connection with effect estimates. Data were analyzed using IBM SPSS Statistic Version 23.0 (IBM, Armonk, NY) and R Version 3.4.2 (R Core Team, 2017, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
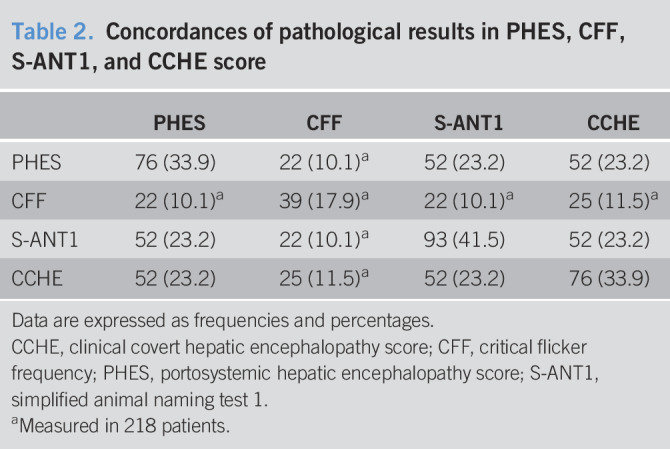
A total of 252 inpatients and outpatients with liver cirrhosis were prospectively enrolled in Mainz (n = 218) and Siegen (n = 34) between March 2017 and December 2018. Follow-up data were available for 224 patients with a median follow-up time of 364 days (interquartile range 202, 508). Twenty-eight patients were lost to follow-up. Most of the patients were men (56.7%) with a median MELD score of 10 (7, 14). The most common etiology of underlying liver disease was chronic alcohol consumption (32.6%), followed by chronic viral hepatitis (20.1%). Frequencies of pathological results in PHES, CFF, S-ANT1, and CCHE are tabulated in Table 1. Concordances of pathological results in PHES, CFF, S-ANT1, and CCHE are displayed in Table 2. Six patients were unable to perform CFF because they did not understand the task.
Table 1.
Demographics and clinical characteristics of the entire cohort at the time of study inclusion
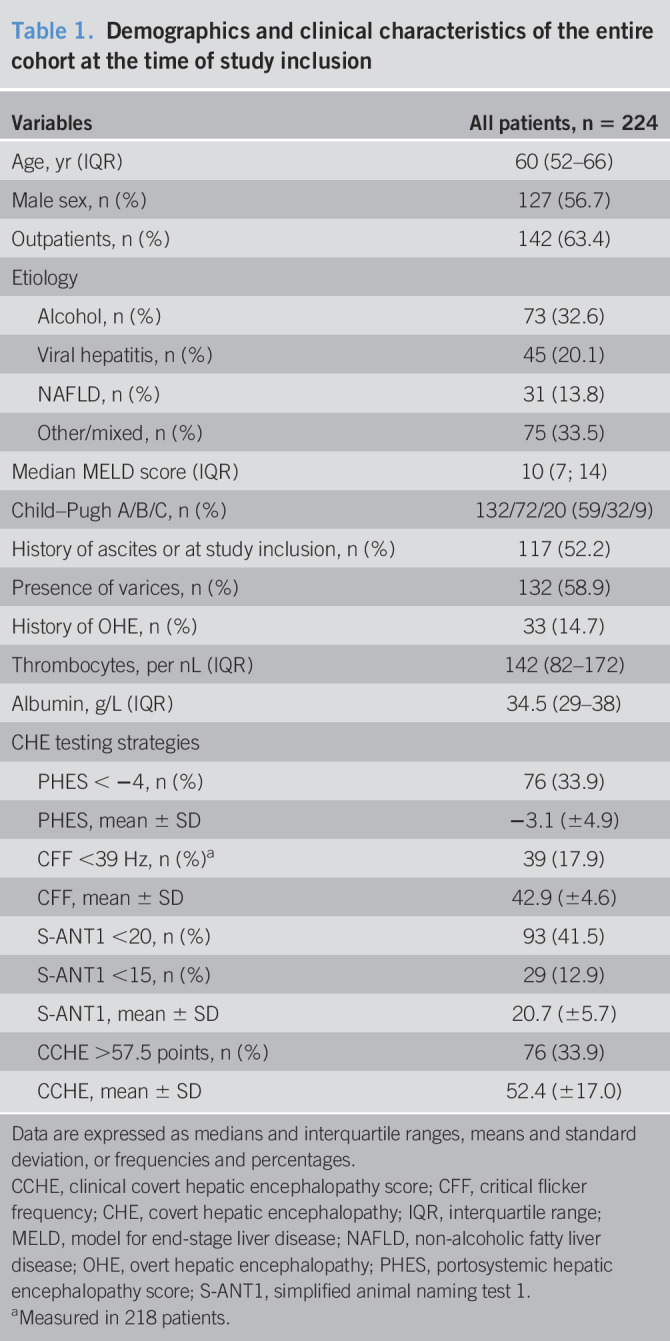
Table 2.
Concordances of pathological results in PHES, CFF, S-ANT1, and CCHE score
Prediction of OHE
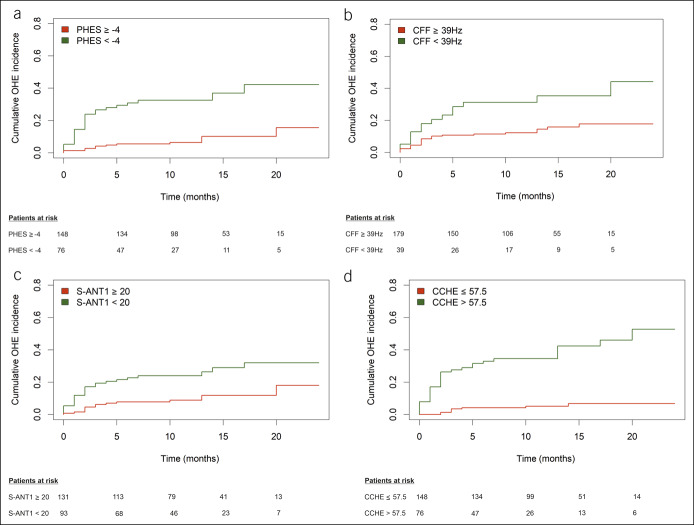
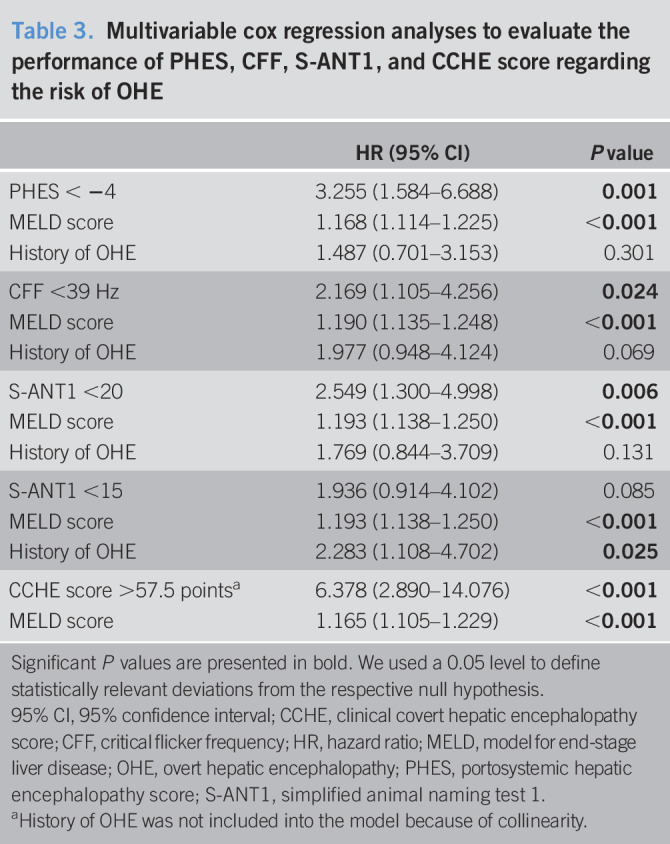
During follow-up, 39 patients (17.0%) developed an episode of OHE. Fourteen (35.9%) episodes were caused by infections, 6 (15.4%) by upper gastrointestinal bleeding, 3 (7.7%) by constipation, 1 (2.6%) by electrolyte disorders, and 6 (15.4%) by multiple factors, and in 9 (23.1%) episodes, no precipitating factor could be identified. Seven patients had more than 1 OHE episode during follow-up. Of these, 1 patient had 4 episodes, 1 patient 3 episodes, and the remaining 5 had 2 episodes each. Twenty-seven patients had an episode of OHE before they died or were transplanted. Seventeen patients died or required liver transplantation before reaching the primary endpoint and were handled as competing events. In total, 53 patients were at least once hospitalized during follow-up because of liver-related events other than OHE. Cumulative OHE incidences for the subgroups defined by all 4 testing strategies are shown in Figure 1. In multivariable Cox regression analyses, each adjusted for the well-known risk factors MELD score and history of OHE, all 4 testing strategies were independent predictors of OHE development during follow-up (Table 3). Within the testing strategies, pathological results in CCHE score had numerically the highest HR (6.378, 95% CI 2.890–14.076, P < 0.001).
Figure 1.
Cumulative incidence of overt hepatic encephalopathy for patients with liver cirrhosis tested with (a) PHES, (b) CFF, (c) S-ANT1 (cutoff <20 animals), and (d) CCHE score. (a) P < 0.001, (b) P = 0.003, (c) P = 0.001, (d) P < 0.001. CCHE, clinical covert hepatic encephalopathy score; CFF, critical flicker frequency; PHES, Psychometric Hepatic Encephalopathy Score.
Table 3.
Multivariable cox regression analyses to evaluate the performance of PHES, CFF, S-ANT1, and CCHE score regarding the risk of OHE
To analyze the diagnostic performance of the 4 testing strategies regarding the prediction of first-time OHE during follow-up, we excluded the 33 patients with a history of OHE. In Cox regression analyses adjusted for MELD, only PHES (HR 3.900, 95% CI 1.780–8.544, P = 0.001), CCHE score (HR 5.866, 95% CI 2.559–13.447, P < 0.001), and S-ANT1 with a cutoff <20 animals (HR 2.740, 95% CI 1.290–5.822, P = 0.009) remained independent predictors of first-time OHE development. CFF did not predict first-time OHE (HR 1.910, 95% CI 0.840–4.345, P = 0.123).
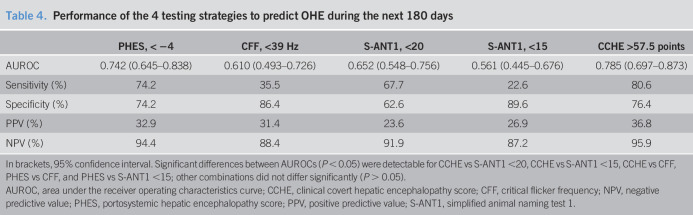
To analyze the diagnostic performance of the 4 testing strategies to predict the development of an OHE episode during the next 180 days of follow-up receiver operating characteristic (ROC) curves were conducted. A total of 31 OHE episodes occurred in the 218 patients with at least 6 months of follow-up. Ten patients were excluded in this analysis because of competing events (liver transplantation, n = 5; died, n = 5) before reaching the primary endpoint (OHE development). When comparing the AUROCs for each testing strategy (Table 4), CCHE score and PHES outperform CFF and S-ANT1 regarding the prediction of OHE development during the next 180 days (all P < 0.05). Although AUROC for CCHE score was numerically higher compared with that of PHES, there was no statistically significant difference (CCHE score vs PHES, P = 0.280). CCHE score was the most sensitive (80.6%) testing strategy with a specificity of 76.4%, a positive predictive value of 36.8%, and a negative predictive value of 95.9% (Table 4).
Table 4.
Performance of the 4 testing strategies to predict OHE during the next 180 days
To analyze the diagnostic performance of the 4 testing strategies to predict the development of an OHE episode requiring hospitalization during the next 180 days of follow-up, additional ROC curves were conducted. In this analysis, an OHE episode during hospitalization because of other causes was not counted as reaching the endpoint. A total of 20 OHE episodes occurred in the 218 patients with at least 6 months of follow-up. As mentioned earlier, 10 patients were excluded in this analysis because of competing events. The respective AUROCs were as follows: PHES 0.677 (95% CI 0.551–0.804), CFF 0.628 (95% CI 0.487–0.770), S-ANT1 0.628 (95% CI 0.501–0.756), and CCHE 0.820 (95% CI 0.734–0.907).
Prediction of the composite of death and need for liver transplantation (mortality)
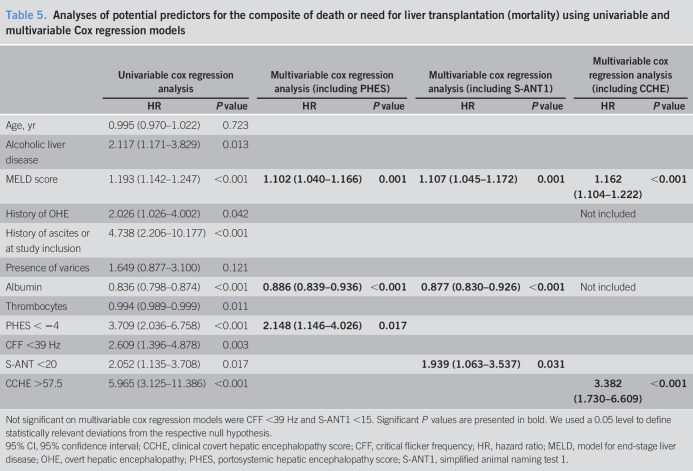
Given that all patients who had received a liver transplantation had done so because of final hepatic failure, they were treated as complete cases. In total, 45 patients died (n = 28) or received a liver transplantation (n = 17) during the follow-up. The number of patients dead or received a transplantation was highest in the group of patients with pathological results in CCHE score (n = 32; log-rank P < 0.001), followed by PHES (n = 27; log-rank P < 0.001), S-ANT1 (cutoff < 20 animals: n = 26; log-rank P = 0.015; cutoff <15 animals: n = 9; log-rank P = 0.080), and CFF (n = 15; log-rank P = 0.002) (see Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A287). Univariable Cox regression analyses identified alcoholic liver disease, higher MELD score, history of OHE, presence of ascites, lower albumin, lower thrombocytes, and the 4 testing strategies as predictors for mortality (Table 5). Using the aforementioned variables, we calculated multivariable Cox regression analyses for every testing strategy. In this study, all testing strategies except for CFF were associated with higher mortality (Table 5).
Table 5.
Analyses of potential predictors for the composite of death or need for liver transplantation (mortality) using univariable and multivariable Cox regression models
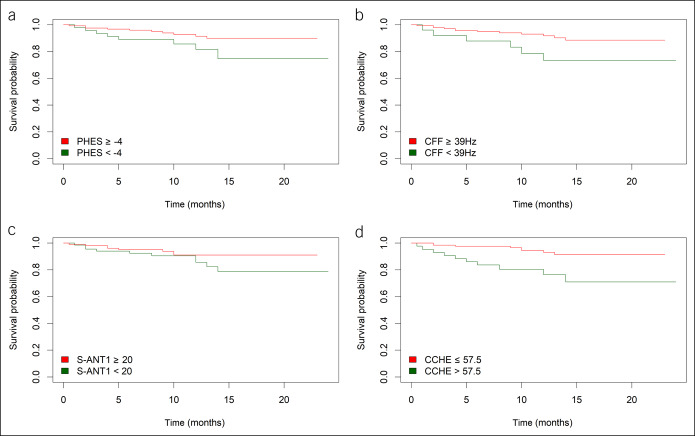
To compare the usefulness of the different testing strategies for the prediction of mortality (death or need for liver transplantation) in low-risk patients (MELD score <15), Kaplan–Meier curves were conducted (Figure 2). In this study, PHES (log-rank, 3.923; P = 0.048), CFF (log-rank, 4.613; P = 0.032), and CCHE score (log-rank, 10.845; P = 0.001) were able to predict survival in this special subset of patients. In contrast, both cutoffs of S-ANT1 were not able to identify patients at risk of death or need for liver transplantation (cutoff <20 animals: log-rank, 2.014; P = 0.156; cutoff <15 animals: log-rank, 0.905; P = 0.342).
Figure 2.
Impact of pathological results of (a) PHES, (b) CFF, (c) S-ANT1, and (d) CCHE score on risk of mortality (death/need for liver transplantation) in patient with low MELD score (<15). (a) P = 0.048, (b) P = 0.032, (c) P = 0.156, and (d) P = 0.001. CCHE, clinical covert hepatic encephalopathy score; CFF, critical flicker frequency; MELD, model of end-stage liver disease; PHES, Psychometric Hepatic Encephalopathy Score.
To analyze the diagnostic performance of the 4 testing strategies to predict mortality (death or need for liver transplantation) during the next 180 days of follow-up, ROC curves were conducted. A total of 27 of 218 patients with at least 6 months of follow-up reached the endpoint. The respective AUROCs were as follows: PHES 0.689 (95% CI 0.580–0.799), CFF 0.617 (95% CI 0.492–0.742), S-ANT1 0.579 (95% CI 0.463–0.695), and CCHE 0.745 (95% CI 0.647–0.843).
DISCUSSION
Still, there is a need to improve quality of care in the management of patients with HE, and potential targets have been recently described (18). In particular, it is of utmost importance to identify patients at high risk to meet clinically relevant endpoints; however, data regarding the ability of the available CHE testing strategies to predict outcome are still limited. In this study, we could demonstrate that pathological results in PHES, CFF, S-ANT1, and CCHE score predicted OHE development, whereas abnormal PHES and CCHE score were also associated with a higher risk of mortality in the whole cohort and in the subgroup of patients with low MELD score (<15). In addition, our data showed that CCHE score and PHES were superior to other tests in predicting OHE development during the next 180 days of follow-up.
Until now, there is a discussion about the best MHE/CHE testing strategy. CHE is a continuum of signs and symptoms, and not every symptom has to be present in every patient at every given time. For that reason, current guidelines propose the use of 2 established tests (e.g., PHES and CFF) for the diagnosis of CHE (2). However, a recent multicenter study across North America by Duarte-Rojo et al. (13) showed that the use of 2 tests does not automatically improve the accuracy of OHE prediction. Moreover, the 2-test strategy does not often seem to be applicable in routine clinical practice because even testing with a single test is often neglected owing to lack of time and reimbursement (19). As a consequence, more rapid and simplified testing strategies such as the S-ANT1 or the CCHE score came to the fore in recent years (14,15). Our results show that all 4 testing strategies might predict a higher risk of OHE development. In particular, the findings regarding PHES and CFF are in line with previous studies (12,20). However, until now, data regarding the ability of S-ANT1 and CCHE score to predict outcomes were limited. Only Campagna et al. assessed the usefulness of S-ANT1 for the prediction of OHE in which the group of patients with less than 15 mentioned animal names had a higher frequency of OHE episodes during follow-up (14). In this study, we could demonstrate that CCHE score and PHES are superior to CFF and S-ANT1 regarding the prediction of OHE development during the next 180 days. However, it has to be mentioned that CCHE score is a composite score containing different clinical and biochemical variables and can, therefore, per definition, never be diagnostic for CHE. However, it is not surprising that CCHE has value in predicting OHE events. CCHE score comprises questions regarding body care, mobility, diet behavior, and capability to eat. Moreover, albumin serum levels are an important part of this composite score. These factors may associate with malnutrition and potential sarcopenia, which is common in patients with cirrhosis and associated with HE (21,22). Moreover, liver function plays an important role in CCHE score as expressed by albumin serum levels, presence of ascites, and history of OHE. Taken together, our hypothesis is that CCHE seems to identify patients with some degree of brain dysfunction (S-ANT1) and a more fragile state of their liver disease and/or body composition. Therefore, our data indicate that CCHE score may be an ideal, a fast, and an inexpensive tool do identify patients at high risk of clinically relevant outcomes in routine practice.
A few studies evaluated the impact of MHE on survival. Ampuero et al. could demonstrate in 2 independent cohorts that presence of MHE defined by CFF has an impact on survival of patients with liver cirrhosis. However, in their study, PHES failed to predict survival (10). Patidar et al. (12) were able to demonstrate that PHES is associated with higher mortality in 170 outpatients with liver cirrhosis. Our data extend the literature by showing that PHES, S-ANT1 (validating the data of Campagna et al. (14)), and CCHE could predict mortality in a large cohort of patients with liver cirrhosis. Surprisingly, CFF was not significantly associated with higher mortality in the multivariable Cox regression analyses. This finding contrasts with the study conducted by Ampuero et al. (10). However, the main difference between the study by Ampuero et al. and our study is the varying follow-up time. Although the aforementioned study investigated the ability of CFF and PHES to predict long-term outcome, we analyzed a cohort with medium-term follow-up. Nevertheless, our findings agree with the study of Ampuero et al. regarding the ability of CFF to predict higher risk of mortality in patients with low MELD score.
In most countries, patients with a MELD score <15 are not evaluated or listed for liver transplantation. However, there is a subset of patients, especially those with OHE, in whom the risk of mortality is not well reflected by MELD score. Ampuero et al. found that patients with a MELD score of 10–15 and MHE defined by a CFF <39 Hz had a poorer prognosis compared with patients without MHE (10). Our data confirm these findings. In addition, we could show that PHES and CCHE score were also able to identify patients at high risk of mortality in this low MELD subgroup. These findings indicate that all 3 testing strategies, but, especially, PHES and CCHE score, are a suitable adjunct for the risk stratification of ostensibly stable patients with compensated liver cirrhosis and might be valuable tools to facilitate decision-making regarding which patients need to be evaluated and listed for liver transplantation.
Our study has some limitations. First, our results are based on an exploratory analysis of a prospective study with a medium-term follow-up. Nevertheless, the frequency of OHE episodes and deaths/liver transplantations in our analysis was clearly sufficient to establish the predictive value of the different testing strategies. Second, we included patients with a history of OHE into our analysis, who are at higher risk of a recurrent OHE episode or death. However, we adjusted for this variable in the respective regression models, and therefore, the potential bias should be negligible. We excluded patients with hepatocellular carcinoma, electrolyte disorders, transjugular intrahepatic portosystemic shunt, or preterminal comorbidities. Therefore, our results might not be generalized to all patients with liver cirrhosis. However, it should be taken into account that advanced comorbidities can lead to cognitive impairment per se, which might be indistinguishable from HE and affect the results of almost all CHE tests such as the PHES (23), in the end justifying our stringent exclusion criteria. In total, 28 patients were lost to follow-up, which represents more than 10% of our cohort. It has to be acknowledged that this loss to follow-up rate might represent a bias. Finally, apart from esophageal varices, our patients were not examined regarding the presence of portosystemic shunts, which might represent another potential bias in our analyses, especially regarding the OHE endpoint.
In conclusion, our study demonstrates that PHES, S-ANT1, and CCHE score might predict OHE development and mortality in patients with liver cirrhosis. In addition, PHES and CCHE score were able to identify patients with low MELD score (<15) at risk of higher mortality. Using 1 of these 2 testing strategies could facilitate risk stratification in clinical practice, ultimately leading to the improvement of care in patients with liver cirrhosis.
CONFLICTS OF INTEREST
Guarantor of the article: Marcus-Alexander Wörns, MD, PhD.
Specific author contributions: Performed research: C.L. and M.-A.W. Contributed to acquisition of data: C.L., J.M.S., M.N., Y.H., J.U.M., J.L., and M.-A.W. Designed the experiments and analyzed the data: C.L., G.T., and M.-A.W. Contributed reagents/materials/analysis tools: C.L., J.L., P.R.G., and M.-A.W. Wrote the paper: C.L. and M.-A.W. Statistical analysis: G.T. and C.L. All authors approved the final version of the manuscript and the authorship list.
Financial support: None to report.
Potential competing interests: None to report.
Previous presentation: Parts of this study were presented as a poster at the meeting of the German Association for the Study of the Liver (GASL); 2020; Mainz, Germany.
Study Highlights.
WHAT IS KNOWN
✓ CHE affects roughly 50% of all patients with liver cirrhosis and impairs prognosis.
✓ Data regarding the ability of different CHE testing strategies to predict OHE development and mortality are limited.
WHAT IS NEW HERE
✓ Predictive performance of PHES and CCHE score regarding OHE development was better than that of CFF and S-ANT1.
✓ Pathological results in PHES and CCHE score are associated with higher mortality even in patients with MELD scores <15.
TRANSLATIONAL IMPACT
✓ PHES and CCHE score might be useful tools to predict OHE development and mortality in patients with liver cirrhosis.
✓ Both testing strategies could help to identify patients with low MELD score who might benefit from liver transplantation.
Supplementary Material
ACKNOWLEDGMENTS
We thank J.S. Baron, C. Schilling, and L. Beul for excellent technical assistance. This work was not supported by any grant or funding source.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A287.
References
- 1.Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med 2014;12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–35. [DOI] [PubMed] [Google Scholar]
- 3.Ferenci P. Therapy of acute and chronic hepatic encephalopathy in patients with liver cirrhosis. Z Gastroenterol 1998;36:909–16. [PubMed] [Google Scholar]
- 4.Bajaj JS, Schubert CM, Heuman DM, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology 2010;138:2332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labenz C, Baron JS, Toenges G, et al. Prospective evaluation of the impact of covert hepatic encephalopathy on quality of life and sleep in cirrhotic patients. Aliment Pharmacol Ther 2018;48:313–21. [DOI] [PubMed] [Google Scholar]
- 6.Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: A Danish population-based cohort study. Hepatology 2010;51:1675–82. [DOI] [PubMed] [Google Scholar]
- 7.Ampuero J, Montoliu C, Simon-Talero M, et al. Minimal hepatic encephalopathy identifies patients at risk of faster cirrhosis progression. J Gastroenterol Hepatol 2018;33:718–25. [DOI] [PubMed] [Google Scholar]
- 8.Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol 2001;34:768–73. [DOI] [PubMed] [Google Scholar]
- 9.Kircheis G, Wettstein M, Timmermann L, et al. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology 2002;35:357–66. [DOI] [PubMed] [Google Scholar]
- 10.Ampuero J, Simon M, Montoliu C, et al. Minimal hepatic encephalopathy and critical flicker frequency are associated with survival of patients with cirrhosis. Gastroenterology 2015;149:1483–9. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen KL, Macnaughtan J, Tritto G, et al. Clinical and pathophysiological characteristics of cirrhotic patients with grade 1 and minimal hepatic encephalopathy. PLoS One 2016;11:e0146076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patidar KR, Thacker LR, Wade JB, et al. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. Am J Gastroenterol 2014;109:1757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte-Rojo A, Allampati S, Thacker LR, et al. Diagnosis of covert hepatic encephalopathy: A multi-center study testing the utility of single versus combined testing. Metab Brain Dis 2019;34:289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campagna F, Montagnese S, Ridola L, et al. The animal naming test: An easy tool for the assessment of hepatic encephalopathy. Hepatology 2017;66:198–208. [DOI] [PubMed] [Google Scholar]
- 15.Labenz C, Toenges G, Huber Y, et al. Development and validation of a prognostic score to predict covert hepatic encephalopathy in patients with cirrhosis. Am J Gastroenterol 2019;114:764–70. [DOI] [PubMed] [Google Scholar]
- 16.Labenz C, Beul L, Toenges G, et al. Validation of the simplified Animal Naming Test as primary screening tool for the diagnosis of covert hepatic encephalopathy. Eur J Intern Med 2019;60:96–100. [DOI] [PubMed] [Google Scholar]
- 17.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839–43. [DOI] [PubMed] [Google Scholar]
- 18.Bajaj JS, O'Leary JG, Tandon P, et al. Targets to improve quality of care for patients with hepatic encephalopathy: Data from a multi-centre cohort. Aliment Pharmacol Ther 2019;49:1518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajaj JS, Etemadian A, Hafeezullah M, et al. Testing for minimal hepatic encephalopathy in the United States: An AASLD survey. Hepatology 2007;45:833–4. [DOI] [PubMed] [Google Scholar]
- 20.Barone M, Shahini E, Iannone A, et al. Critical flicker frequency test predicts overt hepatic encephalopathy and survival in patients with liver cirrhosis. Dig Liver Dis 2018;50:496–500. [DOI] [PubMed] [Google Scholar]
- 21.Nardelli S, Lattanzi B, Merli M, et al. Muscle alterations are associated with minimal and overt hepatic encephalopathy in patients with liver cirrhosis. Hepatology 2019;70:1704–13. [DOI] [PubMed] [Google Scholar]
- 22.Nardelli S, Lattanzi B, Torrisi S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol 2017;15:934–6. [DOI] [PubMed] [Google Scholar]
- 23.Lauridsen MM, Poulsen L, Rasmussen CK, et al. Effects of common chronic medical conditions on psychometric tests used to diagnose minimal hepatic encephalopathy. Metab Brain Dis 2016;31:267–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.