INTRODUCTION:
To examine the association between phosphodiesterase-5 (PDE-5) inhibitor use and incidence of colorectal cancer among patients with erectile dysfunction treated in the Veterans Affairs (VA) Healthcare System.
METHODS:
A retrospective cohort study using the Veterans Affairs Informatics and Computing Infrastructure was conducted, with data spanning January 2001–December 2016. Patients were followed up from index until (i) the first diagnosis of colorectal cancer, (ii) death, or (iii) the end of study period. Statistical analyses evaluated demographics and baseline characteristics between cohorts (PDE-5 exposed or not) and the effect of additional dosages of each specific PDE-5 inhibitor using adjusted multivariate Cox proportional hazards models.
RESULTS:
A total of 221,538 patients met the study inclusion criteria, 192,691 patients in the PDE-5 cohort and 29,227 patients in the never use PDE-5 cohort. The multivariate Cox proportional hazards model results revealed that the those who had any exposure to a PDE-5 inhibitor have an 18% lower hazard of colorectal cancer (adjusted hazard ratio [HR] = 0.816, 95% confidence interval [CI] = 0.754–0.882). For each additional 100-mg dosage of sildenafil and 10-mg dosage of tadalafil, the hazard of colorectal cancer is reduced by 2.4% (adjusted HR = 0.976, 95% CI = 0.973–0.979) and 1.7% (adjusted HR = 0.983, 95% CI = 0.972–0.996), respectively.
DISCUSSION:
PDE-5 inhibitor usage in patients with erectile dysfunction is associated with a lower hazard of colorectal cancer compared with patients not exposed to PDE-5 inhibitors.
INTRODUCTION
Colorectal cancer is one of the most common and deadly cancers (1). Research suggests that phosphodiesterases (PDEs) are involved in the development and progression of colorectal cancer (2,3). One type of PDE isozyme is the PDE-5, which is specific to cyclic guanosine monophosphate. Currently, PDE-5 inhibitors are approved by the US Food and Drug Administration to treat erectile dysfunction (t) (4). However, PDE-5 inhibitors may be a viable chemopreventive agent. Evidence suggests that targeting cyclic guanosine monophosphate with PDE-5 inhibitors can reduce the incidence of intestinal cancer via altering epithelial homeostasis (5). Furthermore, recent preclinical studies including in vivo, in vitro, and mouse studies suggest that PDE-5 inhibitor use may reduce the risk of colorectal cancer (4,6–11).
Given the preclinical evidence, the purpose of this study is to evaluate the association between PDE-5 inhibitor use and incident colorectal cancer diagnosis in a large national cohort.
METHODS
Data
This retrospective cohort study was conducted using data from the Department of Veterans Affairs. The Veterans Affairs Informatics and Computing Infrastructure was used to obtain individual-level information on demographics, administrative claims, and pharmacy dispensation. The completeness, utility, accuracy, validity, and access methods are described on the Veterans Affairs (VA) website, http://www.virec.research.va.gov. The study was conducted in compliance with the Department of Veterans Affairs requirements and received the Institutional Review Board and Research and Development approval. The study used the inpatient and outpatient data (coded with International Classification of Diseases [ICD] revision 9-CM, revision 10-CM, Current Procedure Terminology) and pharmacy, laboratory, and vital sign data, which include height, weight, and blood pressure, over the time period January 2000–December 2018.
Cohort selection
The cohort for this study includes all male veterans aged 18 years and older with a diagnosis of ED. ICD revision 9 and 10 codes including 302.72, 607.84, and N52.9x were used to code ED between January 2001 and December 31, 2016. The study index date for patients was defined as the first diagnosis of ED. The study period, for each patient, was defined as the time between index and study endpoint. The baseline period was defined as 1 year before study index.
We used several exclusionary criteria. Patients were included if they had at least 1 year between their VA enrollment date and study index date. To account for latency bias, patients were excluded if they had a diagnosis of colorectal cancer or died up until 1 year after index (12,13). Patients were excluded if they did not have any values of estimated glomerular filtration rate (eGFR), systolic, diastolic blood pressure, body mass index (BMI), or serum cholesterol during the baseline period to ensure proper medical surveillance. Furthermore, patients who had a PDE-5 inhibitor prescription during baseline were excluded. Patients with prescriptions for nitroglycerin were excluded from the study because PDE-5 inhibitors are contraindicated for patients on nitroglycerin. To ensure whether patients had the opportunity to be diagnosed with colorectal cancer, all patients were required to have at least 1 colonoscopy during the study period. Colonoscopies were extracted from the outpatient claims using Current Procedure Terminology codes: 45378-45398. Patients with pulmonary arterial hypertension were excluded using the ICD-9/10 diagnosis codes (416.0, 416.8, 416.9, I27.2x, I27.81, I27.89, and I27.9) and any patient with a PDE-5 inhibitor prescription for greater than 1 pill per day. Pills per day were calculated using the quantity supplied divided by the days supplied. Patients were followed up from index until
the first diagnosis of colorectal cancer
death
the end of study period December 31, 2018.
Outcome
The outcome for this study was the development of colorectal cancer, defined as the first diagnosis of the following ICD-9 and 10 codes: 153.x, 154.0, 154.1, C18.3, C18.4, C18.5, C18.6, C18.7, C18.8, C18.9, C19, and C20 (12).
Study variables
The primary predictor of interest is the use of PDE-5 inhibitors. PDE-5 inhibitors comprised sildenafil, tadalafil, vardenafil, and avanafil. We quantified the exposure to PDE-5 inhibitors in 2 ways. First, patients who had at least 1 prescription for a PDE-5 inhibitor were classified as PDE-5 inhibitor users and those without a prescription for a PDE-5 were classified as never users. Second, we used the cumulative milligrams (mg) prescribed for each PDE-5 inhibitor. Dosages were extracted from the drug name and summed over the study period.
In addition to PDE-5 use, we also included covariates to account for baseline demographic and health differences. Demographic factors included age, race, and index year. Baseline health conditions included the Charlson comorbidity index, diabetes, tobacco usage, BMI, systolic and diastolic blood pressure, eGFR (chronic kidney disease-epi formula), total serum cholesterol, and family history of gastrointestinal malignant neoplasm and inflammatory bowel disease (IBD). Baseline laboratory values including eGFR and serum cholesterol were extracted using logical observation identifiers names and codes (see Appendix, Supplementary Digital Content 1, http://links.lww.com/CTG/A281). Diagnoses of the family history of gastrointestinal malignant neoplasm, diabetes, tobacco usage were coded based on the ICD-9 and ICD-10 diagnoses codes (see Appendix, Supplementary Digital Content 1, http://links.lww.com/CTG/A281).
Statistical analysis
The statistical analysis occurred in 2 steps. First, we compared the baseline characteristics between those exposed to PDE5 inhibitors and the never exposed patients. Second, we displayed the Kaplan–Meier survival curves for the PDE-5 exposed and unexposed cohorts to display the unadjusted survival curves. We used the multivariate Cox proportional hazards models to estimate the hazard of developing colorectal cancer. Our initial model compares the hazard of those exposed to any PDE-5 inhibitor with that of those never exposed. Next, we estimated the effect of additional dosages of each specific PDE-5 inhibitor; however, avanafil was excluded from drug-specific models because of low sample counts. A subanalysis was conducted on patients who had monotherapy (i.e., exposed to only 1 PDE-5 inhibitor) compared with those who were never exposed to sildenafil or vardenafil (due to sample sizes). In addition to estimating the hazard ratios per additional dosages, we also estimated the hazard ratios for the total number of PDE-5 prescriptions. Prescription counts were categorized into 5 groups (1–4, 5–10, 11–15, 16–20, and 21+).
RESULTS
In this study, we found 1.7 million male veterans with a diagnosis for ED over the years 2001–2016. We excluded 406,385 patients who had a PDE-5 inhibitor prescribed before study index. In total, 295,359 patients were excluded because they did not have at least 1 year between VA enrollment and study index; 2,081 patients who had colorectal cancer up until 1 year after index were excluded; 13,392 patients were excluded because they died within 1 year after index; 55,549 patients were excluded because they were missing baseline laboratory test results; 155,668 patients with a prescription for nitroglycerine were removed from the study; 573,987 patients were excluded because they did not have a colonoscopy during the study period; and 8,353 patients were excluded for pulmonary arterial hypertension. After all exclusionary criteria, 221,538 patients remained in the study cohort (Figure 1).
Figure 1.
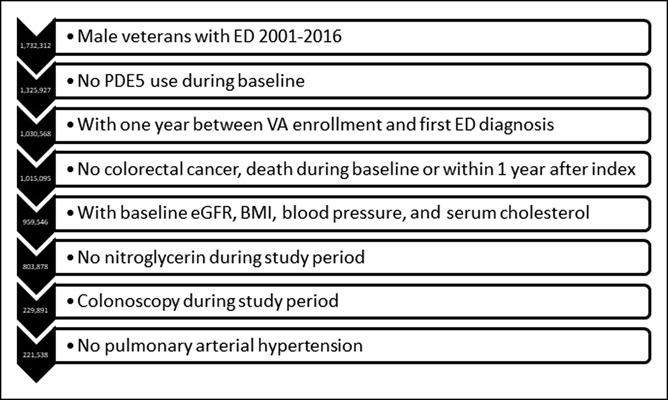
Study attrition. BMI, body mass index; ED, erectile dysfunction; eGFR, estimated glomerular filtration rate; PDE, phosphodiesterase; VA, Veterans Affairs.
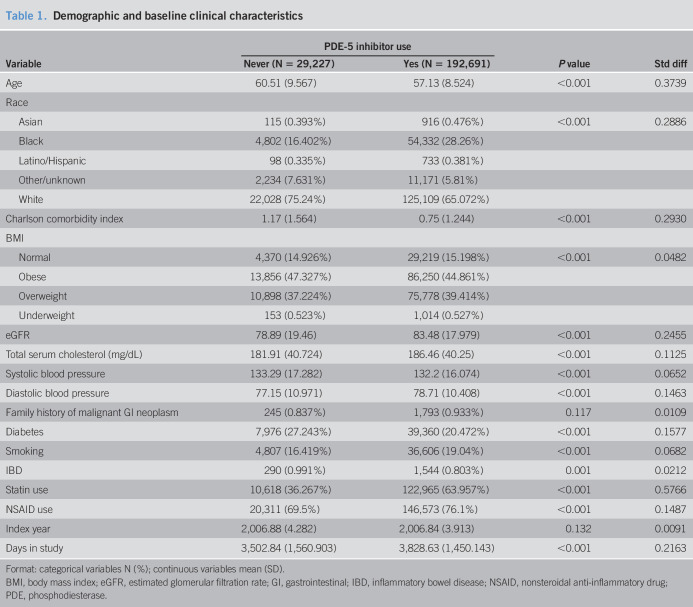
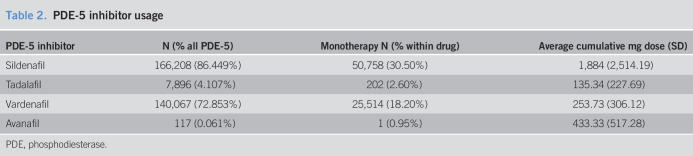
Table 1 displays the demographic and baseline clinical characteristics for patients who never used a PDE-5 inhibitor (n = 29,227) and those who had at least 1 PDE-5 inhibitor prescription (n = 192,691). On average, those who never used a PDE-5 inhibitor are older (60.51 vs 57.13 years, respectively), have a higher Charlson comorbidity index (1.17 vs 0.75, respectively), have a lower eGFR (78.89 vs 83.48), have lower total serum cholesterol (181.91 vs 186.46), and have a higher prevalence of diabetes (27.24% vs 20.47%). Patients who took a PDE-5 inhibitor have a higher prevalence of statin usage (63.95% vs 36.27%) and are more likely to be smokers (19.04% vs 16.42%). The breakdown of PDE-5 use by drug is shown in Table 2. Of the PDE-5 inhibitor users, 86% has at least 1 prescription for sildenafil, 72% had a prescription for vardenafil, 4% for tadalafil, and 0.9% for avanafil. Among those with a sildenafil prescription, 30% had no other PDE-5 inhibitors. Among those with a vardenafil prescription, 18% had no other PDE-5 inhibitors. Among those with tadalafil exposure, 2.6% were exposed only to tadalafil. Among those exposed to avanafil, only 1 patient was exposed only to avanafil. Figure 2 presents the unadjusted Kaplan–Meier survival curves for those exposed and unexposed to a PDE-5 inhibitor. The Kaplan–Meier curves showed a lower risk of colorectal cancer throughout the study time. In Table 3, we present the results of the Cox proportional hazard model, examining the association between any PDE-5 inhibitor use and colorectal cancer. Those who had any exposure to a PDE-5 inhibitor have an 18% lower hazard of colorectal cancer (hazard ratio [HR] = 0.816, 95% confidence interval [CI] = 0.754–0.882). Black patients had a higher hazard of colorectal cancer compared with white patients (HR = 1.168, 95% CI = 1.089–1.252). Other risk factors for colorectal cancer included age (HR = 1.056, 95% CI = 1.052–1.060), Charlson comorbidity (HR = 1.067, 95% CI = 1.042–1.093), total serum cholesterol (HR = 1.005, 95% CI = 1.003–1.007), diastolic blood pressure (HR = 1.002, 95% CI = 1.001–1.003), diabetes (HR = 1.196, 95% CI = 1.105–1.295), smoking (HR = 1.164, 95% CI = 1.075–1.259), and IBD (HR = 1.411, 95% CI = 1.061–1.876). Statins, consistent with Poynter et al. (14), are associated with a decrease in the hazard of colorectal cancer (HR = 0.613, 95% CI = 0.576–0.652).
Table 1.
Demographic and baseline clinical characteristics
Table 2.
PDE-5 inhibitor usage
Figure 2.
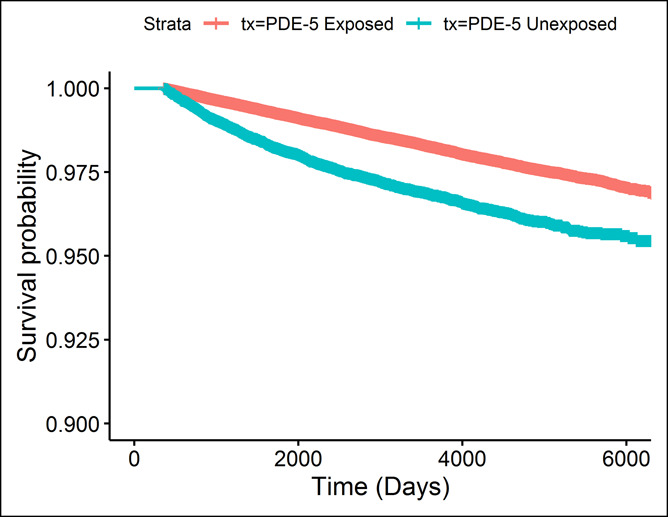
Kaplan–Meier survival curves. PDE, phosphodiesterase.
Table 3.
Multivariate Cox proportional hazards model estimating the hazard of developing colorectal cancer
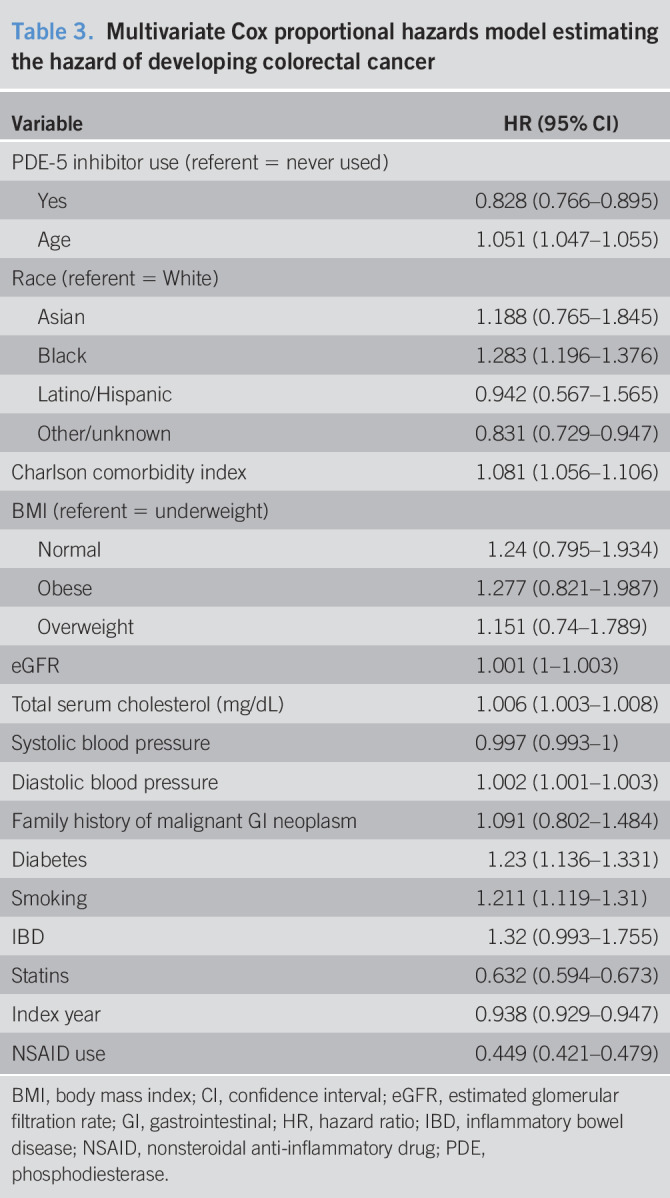
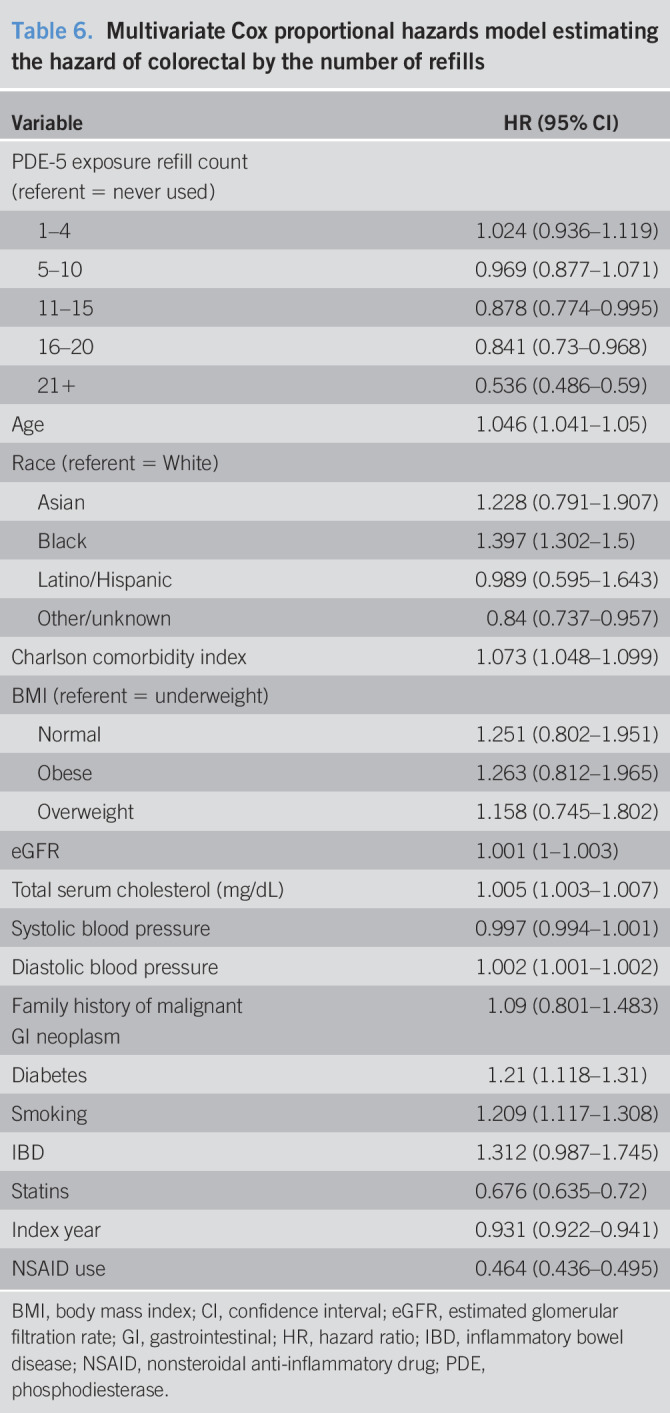
The results from the association between drug dosage and colorectal cancer are shown in Table 4. For each additional 100-mg dosage of sildenafil, the hazard of colorectal cancer is reduced by 2.4% (HR = 0.976, 95% CI = 0.973–0.979). For each additional 10-mg dose of tadalafil, the hazard of colorectal cancer is reduced by 1.7% (HR = 0.983, 95% CI = 0.972–0.996). We find no statistically significant association for each additional 10-mg vardenafil dose (HR = 1.000, 95% CI = 0.999–1.002). Other results are similar to those of the cox proportional hazards model examining the association between any PDE-5 inhibitor use and colorectal cancer. Table 5 displays the subanalysis conducted on those with no PDE-5 inhibitor exposure and those with sildenafil-only treatment, and secondarily those with no PDE-5 inhibitor exposure and vardenafil-only treatment. We find that each additional 100 mg of sildenafil, the hazard of colorectal cancer is reduced by 1% (HR = 0.99, 95% CI = 0.984–0.995). For each additional 10 mg of vardenafil, there is an associated 0.7% higher hazard of colorectal cancer (HR = 1.007, 95% CI = 1.002–1.011). Table 6 displays the results of the Cox model estimating PDE-5 exposure by prescription counts. We found that patients with less than or equal to 10 prescriptions are not statistically significantly different in the risk of colorectal cancer compared with the unexposed (1–4 HR = 1.024, 95% CI = 0.936–1.119; 5–10 HR = 0.969, 95% CI = 0.877–1.071). Patients with greater than 10 prescriptions for a PDE-5 have a statistically significantly lower risk of colorectal cancer (11–15 HR = 0.878, 95% CI = 0.774–0.995; 16–20 HR = 0.841, 95% CI = 0.73–0.968; 21+ HR = 0.536, 95% CI = 0.486–0.59). Furthermore, the estimates suggest that the risk of colorectal cancer decreases for additional prescriptions of a PDE-5, ultimately resulting in a 46% reduction in risk for those with 21 or more prescriptions (21+ HR = 0.536, 95% CI = 0.486–0.59).
Table 4.
Multivariate Cox proportional hazards model estimating the hazard of colorectal cancer by dose
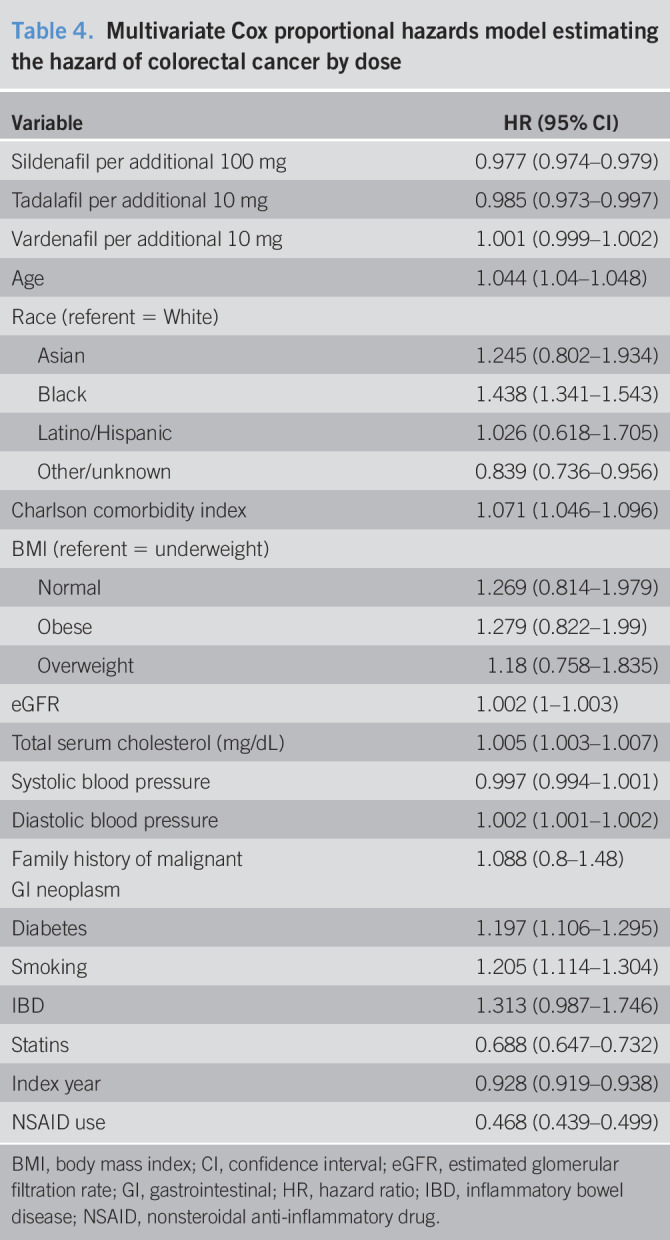
Table 5.
Multivariate Cox proportional hazards model estimating the hazard of colorectal cancer for those on monotherapy
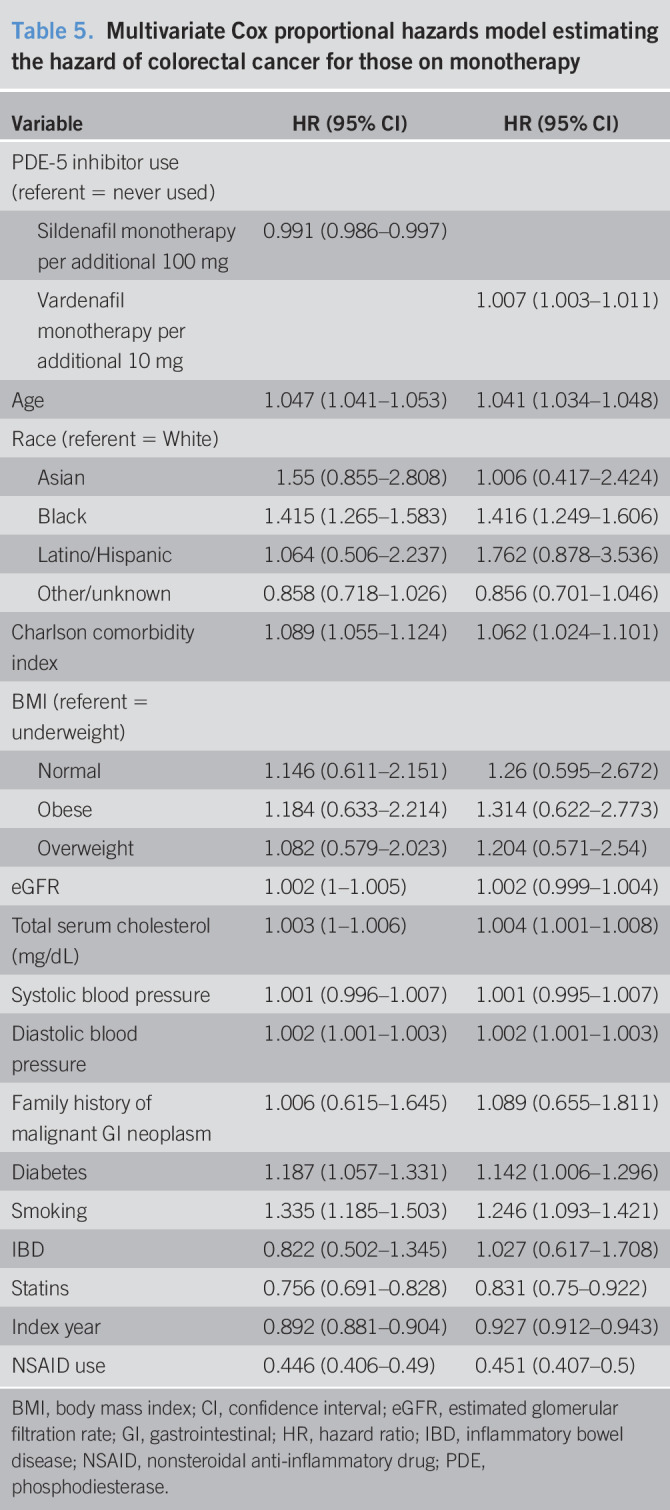
Table 6.
Multivariate Cox proportional hazards model estimating the hazard of colorectal by the number of refills
DISCUSSION
In this study, among patients receiving care in the VA system with ED, we found that those who had any exposure to a PDE-5 inhibitor had a lower hazard of colorectal cancer. Furthermore, we estimated the effect of additional dosages of each specific PDE-5 inhibitor (excluding avanafil). We found for each additional 100-mg dosage of sildenafil and each additional 10-mg dose of tadalafil, the hazard of colorectal cancer is reduced. In the subanalysis, patients who had monotherapy with sildenafil only had a decreased hazard of colorectal cancer for each additional 100 mg of treatment. However, for each additional 10 mg of vardenafil, there is a higher hazard of colorectal cancer for those on vardenafil only.
More in vivo studies have demonstrated the effectiveness of PDE-5 inhibitors effect on the colon compared with in vitro studies. Some in vivo studies showed that the sildenafil suppressed the polyp multiplicity in the colon, even by 50% by administering varying dosages of the inhibitor (6,7,10). Growth inhibition of colon tumor cells occurred with the use of PDE-5 inhibitors in vitro (and in vivo) while enhancing anticancer and other drugs (8,11). Mei et al. (4) demonstrated the effect of sildenafil on proliferation and apoptosis in vitro and in vivo by implanting nude mice with SW480 or HCT116 human cancer cells by administering sildenafil orally. Similar results were shown in vitro and in vivo with sildenafil and tadalafil inhibiting tumor growth in immune-competent mice by 50%–70% (9).
Although there is a lack of observational studies evaluating the association of the use of PDE-5 inhibitors and colorectal cancer, some results from our study is consistent with other studies. A UK-matched cohort study found a decreased risk for colorectal cancer for those who were PDE-5 inhibitor users (HR = 0.91, 95% CI = 0.85–0.98, P = 0.01); however, they did not limit their analyses to men with ED (15). In a Swedish study of men diagnosed with benign colorectal neoplasm had a reduced risk of colorectal cancer (HR = 0.65, 95% CI = 0.49–0.85) while using PDE-5 and an increased cumulative dosage of PDE-5 (16). PDE-5 is effective enough to selectively stimulate apoptosis of colon tumor cells while having minimal effects on normal colon cells (17,18).
Some demographic and risk factors that we adjusted for in our models are included in other literature concerning colorectal cancer. Differences in colorectal cancer are seen in racial disparities, as blacks are associated with having a higher incidence (19,20). Clinical studies of IBD report that patients have a higher risk of colorectal cancer as well (7,21). Statin users had a decreased risk of colorectal cancer compared with nonstatin users (13). Patients with diabetes, consistent with Overbeek et al. (22), have an increased risk of developing colorectal cancer; however, the study did not account for smoking status nor BMI in estimating models as we did. Unlike Mamtani et al., we found an increased total serum cholesterol to be associated with an increased hazard of colorectal cancer similar to another study (13,23). A meta-analysis demonstrated that cigarette smoking is associated with an increased incidence and mortality of colorectal cancer among a sample of 106 studies (24).
To our knowledge, this study is the first population-based study to examine the function of PDE-5 inhibitors along with the risk of colorectal cancer among patients with ED. Our study is unique because it (i) evaluated a national cohort of patients with ED, (ii) evaluated patients who were prescribed PDE-5 inhibitors (sildenafil, tadalafil, vardenafil, and avanafil), and (iii) evaluated PDE-5 usage 2 ways (binary and dosage). However, like many observational studies, there are some factors that may have influenced the outcomes which were not accounted for within this study. First, the population is US male veterans with ED; therefore, our findings may not be generalizable to other patients. We were unable to exclude unmeasured potential confounding factors that may have influenced our outcomes because patients were not randomized to different treatments. Although we attempted to control for select variables by using adjusted models, residual confounding may remain. Finally, medication usage was measured from filled prescriptions, and we cannot confirm that patients adhered to their prescriptions; however, studies have advocated that pharmacy refill rates and prescription claims data are a good depiction for actual medication adherence (25). Despite the limitations of this study, we believe that the data are robust for our large sample within the US Veteran population and will add to the current literature on the incidence of colorectal cancer and the use of PDE-5 inhibitors.
Our study evaluated the association of colorectal cancer and PDE-5 inhibitors among a national cohort of patients with ED within the VA system. Those who were prescribed PDE-5 inhibitors had a reduced hazard of colorectal cancer compared with those who were never prescribed PDE-5 inhibitors. For each additional 100-mg dosage of sildenafil and each additional 10-mg dosage of tadalafil, the hazard of colorectal cancer was reduced as well. According to our research, clinicians should monitor and understand the risks and benefits of PDE-5 inhibitors with the occurrence of colorectal cancer among patients with ED. Further research is needed to confirm the observed association.
CONFLICTS OF INTEREST
Guarantor of the article: Joseph Magagnoli, MS.
Specific author contributions: Study concept and design: S.S.S. and J.M. Acquisition of data: S.S.S. and J.M. Analysis and interpretation of data: J.M. Drafting of the manuscript: all authors. Analysis and interpretation: all authors.
Financial support: None to report.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Recent preclinical studies including in vivo, in vitro, and mouse studies suggest that PDE-5 inhibitor use may reduce the risk of colorectal cancer.
WHAT IS NEW HERE
✓ We use a large national cohort of patients being treated in the VA and show that PDE-5 inhibitor use is associated with a lower risk of colorectal cancer.
TRANSLATIONAL IMPACT
✓ PDE-5 inhibitors show evidence to support a new drug development or additional indications for PDE-5 inhibitors.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A281
REFERENCES
- 1.Cronin KA, Lake AJ, Scott S, et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2018;124:2785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehrer S, Rheinstein PH, Rosenzweig KE. Mutations of the PDE5A gene confer a survival advantage in patients with colon cancer. Cancer Prev Res 2018;11:439–40. [DOI] [PubMed] [Google Scholar]
- 3.Zhu B, Vemavarapu L, Thompson WJ, et al. Suppression of cyclic GMP-specific phosphodiesterase 5 promotes apoptosis and inhibits growth in HT29 cells. J Cell Biochem 2005;94:336–50. [DOI] [PubMed] [Google Scholar]
- 4.Mei XL, Yang Y, Zhang YJ, et al. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am J Cancer Res 2015;5:3311–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Sharman SK, Islam BN, Hou Y, et al. Cyclic-GMP-elevating agents suppress polyposis in Apc Min mice by targeting the preneoplastic epithelium. Cancer Prev Res (Phila) 2018;11:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islam BN, Sharman SK, Hou Y, et al. Sildenafil suppresses inflammation-driven colorectal cancer in mice. Cancer Prev Res (Phila) 2017;10:377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin S, Wang J, Wang L, et al. Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC. Am J Cancer Res 2017;7:41–52. [PMC free article] [PubMed] [Google Scholar]
- 8.Booth L, Roberts JL, Cruickshanks N, et al. PDE5 inhibitors enhance celecoxib killing in multiple tumor types. J Cell. Physiol 2015;230:1115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006;203:2691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capuano G, Rigamonti N, Grioni M, et al. Modulators of arginine metabolism support cancer immunosurveillance. BMC Immunol 2009;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavallai M, Hamed HA, Roberts JL, et al. Nexavar/stivarga and viagra interact to kill tumor cells. J Cell Physiol 2015;230:2281–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murff HJ, Roumie CL, Greevy RA, et al. Metformin use and incidence cancer risk: Evidence for a selective protective effect against liver cancer. Cancer Causes Control 2018;29:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamtani R, Lewis JD, Scott FI, et al. Disentangling the association between statins, cholesterol, and colorectal cancer: A nested case-control study. PLoS Med 2016;13:e1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poynter J, Gruber SB, Higgins PD, et al. Statins and the risk of colorectal cancer. N Engl J Med 2005;352:2184–92. [DOI] [PubMed] [Google Scholar]
- 15.Matthews A, Langan SM, Douglas IJ, et al. Phosphodiesterase type 5 inhibitors and risk of malignant melanoma: matched cohort study using primary care data from the UK clinical practice research datalink. PLoS Med 2016;13:e1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W, Sundquist J, Sundquist K, et al. Use of phosphodiesterase 5 inhibitors associates with risk of colorectal cancer in men with benign colorectal neoplasms. Gastroenterology 2019;157:672–81.e4. [DOI] [PubMed] [Google Scholar]
- 17.Tinsley HN, Gary BD, Thaiparambil J, et al. Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition. Cancer Prev Res (Phila) 2010;3:1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abadi AH, Gary BD, Tinsley HN, et al. Synthesis, molecular modeling and biological evaluation of novel tadalafil analogues as phosphodiesterase 5 and colon tumor cell growth inhibitors, new stereochemical perspective. Eur J Med Chem 2010;45:1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao SR, Breen N, Graubard BI. Trends in Black-White disparities in breast and colorectal cancer screening rates in a changing screening environment: the Peters-Belson approach using United States National Health Interview Surveys 2000-2010. Med Care 2016;54:133–9. [DOI] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–93. [DOI] [PubMed] [Google Scholar]
- 21.Stidham RW, Higgins PDR. Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal Surg 2018;31:168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overbeek JA, Kuiper JG, van der Heijden AAWA, et al. Sex- and site-specific differences in colorectal cancer risk among people with type 2 diabetes. Int J Colorectal Dis 2019;34:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muka T, Kraja B, Ruiter R, et al. Dietary polyunsaturated fatty acids intake modifies the positive association between serum total cholesterol and colorectal cancer risk: The Rotterdam Study. J Epidemiol Community Health 2016;70:881–7. [DOI] [PubMed] [Google Scholar]
- 24.Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765–78. [DOI] [PubMed] [Google Scholar]
- 25.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. [DOI] [PubMed] [Google Scholar]