Summary:
Painful neuromas result from traumatic injuries of the hand and digits and cause substantial physical disability, psychological distress, and decreased quality of life among affected patients. The regenerative peripheral nerve interface (RPNI) is a novel surgical technique that involves implanting the divided end of a peripheral nerve into a free muscle graft for the purposes of mitigating neuroma formation and facilitating prosthetic limb control. The RPNI is effective in treating and preventing neuroma pain in major extremity amputations. The purpose of this study was to determine if RPNIs can be used to effectively treat neuroma pain following partial hand and digital amputations. We retrospectively reviewed the use of RPNI to treat symptomatic hand and digital neuromas at our institutions. Between November 2014 and July 2019, we performed 30 therapeutic RPNIs on 14 symptomatic neuroma patients. The average patient follow-up was 37 weeks (6–128 weeks); 85% of patients were pain-free or considerably improved at the last office visit. The RPNI can serve as a safe and effective surgical solution to treat symptomatic neuromas after hand trauma.
INTRODUCTION
Up to 30% of patients with hand and digital amputations develop symptomatic neuromas resulting from the disorganized proliferation of transected peripheral nerves.1–3 Symptomatic neuromas contribute to debilitating pain, resulting in a dramatic decrease in quality of life and productivity among afflicted patients.1–3 Nonsurgical management of symptomatic neuromas includes desensitization, anesthetic and/or steroid injections, transcutaneous electrical nerve stimulation, and opioids.1–3 Many surgical techniques have been used to treat neuromas, including capping of the proximal nerve with silicone, epineural grafts, suture ligature, transposition into muscle, bone, vein graft, and traction neurectomy.1–12 The variety and complexity of these proposed treatments reflect the challenges with managing this difficult problem and the necessity for a more straightforward approach.
PATIENTS AND METHODS
This study received approval from the University of Michigan and University of Texas Institutional Review Boards. All patients treated with neurectomy and regenerative peripheral nerve interfaces (RPNIs) for symptomatic hand or digital neuroma at the institutions between November 2, 2014, and July 29, 2019, were included. Indications for operation included end neuroma and/or phantom pain, with demonstration of a positive Tinel’s sign. Patients were offered RPNI surgery with the primary goal of decreasing neuroma pain. Operative and clinic notes were retrospectively reviewed for analysis.
Operative Technique
Outpatient RPNI surgery was performed under regional or general anesthesia. Under tourniquet control, the symptomatic neuroma was exposed and resected (Fig. 1A). A free muscle graft measuring approximately 1.0 × 1.0 × 0.3 cm3 was harvested for each RPNI along the direction of the muscle fibers from an expendable donor muscle through a separate incision. Under loupe or microscopic magnification, fine monofilament suture (6-0 or 8-0 nylon or prolene) was used to secure the epineurium of the proximal nerve end to the central portion of the muscle graft (Fig. 1B, C). The muscle graft was then wrapped around the nerve end, enveloping it entirely and secured with additional sutures. The RPNI was then buried into the surrounding soft tissue, and the wound was closed (Fig. 1D). A soft dressing was placed for 3 days postoperatively, and patients were allowed to use the hand for light activity with gradual resumption of motion.
Fig. 1.
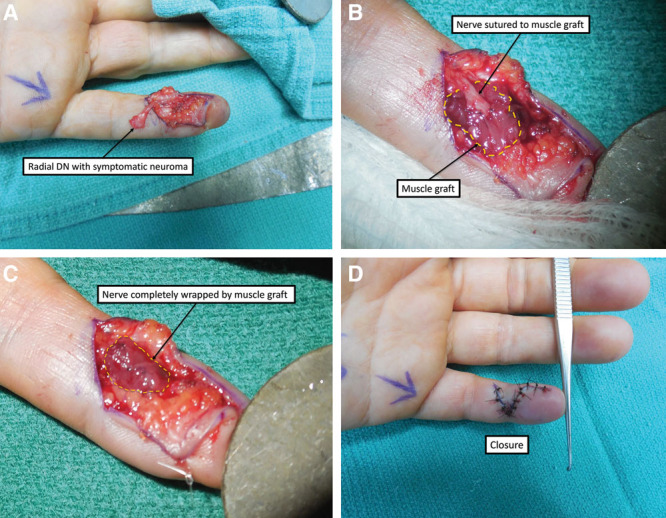
Treatment of a symptomatic left small finger radial digital nerve neuroma with RPNI surgery. A, Radial digital nerve (DN) with symptomatic neuroma. B, Implantation of nerve into muscle graft. C, Nerve completely wrapped in muscle graft. D. Closure.
RESULTS
Thirty RPNIs were performed by 6 surgeons in 14 patients with symptomatic neuromas following traumatic amputation, crush, grease gun injection, or iatrogenic nerve injuries (Table 1). Two patients had further RPNI surgeries for additional neuromas following the initial operation. Symptomatic neuromas were commonly identified along transected proper digital nerves. Brachioradialis and vastus lateralis muscle served as donors in this study. Vastus lateralis is commonly used for lower extremity RPNIs; however, given the small amount of required muscle for hand and digital RPNIs, brachioradialis was used preferentially due to its proximity to the surgical site.
Table 1.
Patient Characteristics and Outcomes
| Patient | Sex | Age | Injury | Area | No. RPNI | Infection | Donor Muscle | Postoperative Tinel’s Sign | Pain Improved after RPNI Surgery? | Follow-Up (wk) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 71 | Neuroma in continuity | Palm | 1 | No | BR | NA | Yes | 128 |
| 2 | Female | 39 | SF crush injury | L SF | 1 | No | BR | NA | Yes | 6 |
| 3 | Male | 63 | L RF amputation | L RF | 2 | No | BR | Negative | Yes | 6 |
| 4 | Male | 56 | Grease gun injury | Dorsal hand | 2 | No | BR | N/A | No | 56 |
| Grease gun injury | L SF | 2 | No | BR | NA | 24 | ||||
| 5 | Female | 41 | Table saw injury | L SF | 1 | No | BR | Negative | Yes | 40 |
| 6 | Male | 55 | Crush injury, L RF/SF amp | L RF/MF | 4 | Yes | FDS | Negative | Yes | 16 |
| 7 | Female | 23 | Crush injury with multiple amp at PIP level | L MF | 2 | No | VL | NA | Yes | 8 |
| Crush injury with multiple amp at PIP level | L MF/RF | 3 | No | VL | NA | 24 | ||||
| Crush injury with multiple amp at PIP level | L MF/RF | 2 | No | VL | NA | 104 | ||||
| 8 | Male | 24 | Table saw injury | 1 digit | 1 | No | BR | Negative | Yes | 8 |
| 9 | Male | 55 | Crush injury, dog bite | Dorsal hand | 1 | No | BR | Negative | Yes | 13 |
| 10 | Male | 33 | Iatrogenic injury | Dorsal wrist | 1 | No | BR | Negative | Yes | 11 |
| 11 | Female | 56 | R thumb crush | Thumb | 2 | No | BR | Negative | Yes | 38 |
| 12 | Male | 32 | MVC, traumatic amputation | L IF | 2 | Yes | BR | Negative | No | 59 |
| 13 | Male | 47 | Traumatic laceration, glass | Volar wrist | 2 | No | BR | Negative | Yes | 47 |
| 14 | Female | 46 | Crush injury | Dorsal wrist | 1 | No | BR | Negative | Yes | 63 |
BR, brachioradialis; FDS, flexor digitorum superficilias; MF, middle finger; MVC, motor vehicle collision; NA, not available, not documented; PIP, proximal interphalangeal; RF, ring finger; SF, small finger; VL, vastus lateralis.
The average follow-up was 37 weeks (range 6–128 weeks). Eighty-five percent of patients reported being completely pain-free or considerably improved at the last clinic visit. Seventy-one percent of patients had a documented negative postoperative Tinel’s sign. Two patients elected not to follow-up beyond 6 weeks because the neuroma pain was completely resolved. Electronic medical records for these patients are available for review, and there have been no encounters related to recurrent digit pain for over 2 years following RPNI surgery. Patient 4 underwent 2 separate RPNI procedures: one to treat neuromas of the dorsal sensory ulnar branches and another to treat symptomatic neuromas of the radial and ulnar digital nerves of the small finger. Patient 7 underwent 3 separate RPNI procedures after sequentially reporting symptomatic neuromas involving different proper digital nerves of several amputated digits following a crush injury. Patient 12 developed a surgical site infection, resulting in resection of the RPNI and implantation of the nerve end into bone. Patient 6 was treated for cellulitis, which resolved with oral antibiotics. It is difficult to ascertain if RPNI surgery directly contributed to these infections because traumatic hand injuries are often contaminated cases and can frequently lead to superficial or deep-space infections. Beyond the 2 patients discussed above, there were no instances of delayed wound healing at the RPNI in the volar aspect of the digit or muscle graft donor site. There were no instances of flexion contractures or difficulty with tendon gliding following RPNI surgery.
DISCUSSION
Symptomatic neuromas of the hand or digits decreases an individual’s quality of life due to both neuropathic pain and to the fear of pain elicited with attempted use of the affected extremity.1–3,13–16 These patients rely on opioids, neuropathic, and/or antidepressant medications for symptomatic relief. However, such treatments are sedating, associated with dependence and overdose, and often ineffective.1–5,8,14 RPNI surgery has been shown to effectively treat and prevent neuroma pain in upper and lower limb amputation patients and to facilitate myoprosthetic limb control.4,9–12 In this study, 85% of patients experienced clinically significant pain relief. While an encouraging negative Tinel’s sign was documented in 71% of patients postoperatively, this percentage may be even higher with longer follow-up.
The RPNI is a novel, straightforward surgical technique that involves implantation of the proximal cut end of a peripheral nerve into a free muscle graft. The muscle graft undergoes regeneration and reinnervation by the implanted peripheral nerve.4,9 This lends a physiologic endpoint to the outgrowth of the nerve end, thereby assisting in successful pain elimination. Our previous work demonstrated formation of functional neuromuscular junctions within the RPNI, but it remains unclear how regenerating sensory axons interact with a free skeletal muscle graft in a manner that reduces neuroma pain.9 Previous studies investigating the benefits of “sensory protection” procedures, whereby sensory axons “babysit” a denervated muscle to limit atrophy and preserve functional capacity, may help explain how RPNIs prevent neuroma formation.17–19 This phenomenon has been observed clinically in patients with facial paralysis and lower extremity injury.20,21Additionally, some authors have reported that regenerating sensory axons can reinnervate sensory organs within denervated muscle, such as golgi tendon organs and spindle cells.22 Therefore, we hypothesize that because regenerating sensory axons within an RPNI are involved in various physiologic processes, substantially fewer axons are available to form a symptomatic neuroma.
RPNI surgery leverages the reinnervation of denervated muscle to reduce neuroma formation. This mechanism of action is also used by targeted muscle reinnervation (TMR). In contrast to RPNI surgery, TMR involves the sacrifice of motor branches in nearby muscles followed by nerve transfer procedures to redirect regenerating axons to reinnervate the denervated muscle tissue. While TMR may be performed for hand and digital neuromas, there are potential limitations that might restrict its usefulness in these cases. There is a paucity of expendable muscles in the hand; intentional denervation of important intrinsic muscles should be avoided, particularly after any injury that has already compromised hand function. Furthermore, motor branches in the hand (eg, branches to interosseous muscles) are quite small, and therefore coaptation with relatively larger digital nerves will inexorably involve a size mismatch; this will lead to axonal escape, a known risk factor for formation of a neuroma-in-continuity.
This study was limited by its retrospective nature and reliance on data collected from electronic medical records. Despite an average follow-up period of 37 weeks, a handful of our study patients demonstrated follow-up periods of only 6 or 8 weeks. These patients were included to provide a complete account of our experience with RPNI surgery for hand and digital neuromas. Interestingly, half of these patients still displayed a negative Tinel’s sign at the site of neuroma excision (no traction neurectomy was performed) even after a short follow-up interval, and all of them reported improved pain after RPNI surgery. In a subsequent prospective study, we will plan to examine patient outcomes for a longer period of time and also include patient-reported outcomes to better elucidate the efficacy of RPNI surgery. Furthermore, prospective investigation will allow for more granular reporting of other questions, such as whether placement of a small free skeletal muscle graft within a digit during RPNI surgery would lead to diminished joint function. Although our current study did not detect major impairments to joint function after RPNI surgery, a prospectively executed study will include goniometry measurements on study subjects.
We recommend performing RPNI surgery in conjunction with neurectomy (to healthy nerve fascicles) for any patient with symptomatic hand or digital nerve neuromas following traumatic or iatrogenic injury when nerve repair or reconstruction is not possible. In some cases, RPNI surgery may be prophylactically performed at the time of revision amputation to prevent the formation of symptomatic neuromas during rehabilitation. RPNI surgery has minimal morbidity and is easily reproducible as demonstrated by multiple surgeons performing the surgery with consistent results. Patients should be counseled that they are trading pain for numbness with this procedure and that normal sensation in the affected area cannot be expected. RPNI surgery has resulted in dramatic improvement in the neuroma pain experienced by our hand trauma patients and demonstrates promise as both a therapeutic and preventative tool to reduce the formation of symptomatic neuromas.
Footnotes
Published online 4 June 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Vlot MA, Wilkens SC, Chen NC, et al. Symptomatic neuroma following initial amputation for traumatic digital amputation. J Hand Surg Am. 2018;43:86.e1–86.e8. [DOI] [PubMed] [Google Scholar]
- 2.van der Avoort DJ, Hovius SE, Selles RW, et al. The incidence of symptomatic neuroma in amputation and neurorrhaphy patients. J Plast Reconstr Aesthet Surg. 2013;66:1330–1334. [DOI] [PubMed] [Google Scholar]
- 3.Wolvetang NHA, Lans J, Verhiel SHWL, et al. Surgery for symptomatic neuroma: anatomic distribution and predictors of secondary surgery. Plast Reconstr Surg. 2019;143:1762–1771. [DOI] [PubMed] [Google Scholar]
- 4.Woo SL, Kung TA, Brown DL, et al. Regenerative peripheral nerve interfaces for the treatment of postampuation neuroma pain: a pilot study. Plas Reconstr Surg Glob Open. 2016;4:e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar N, Stevenson JH. Intractable digital neuroma pain; the ultimate solution? Br J Plast Surg. 1990;43:122–123. [DOI] [PubMed] [Google Scholar]
- 6.Yüksel F, Kişlaoğlu E, Durak N, et al. Prevention of painful neuromas by epineural ligatures, flaps and grafts. Br J Plast Surg. 1997;50:182–185. [DOI] [PubMed] [Google Scholar]
- 7.Dellon AL, Mackinnon SE. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg. 1986;77:427–438. [DOI] [PubMed] [Google Scholar]
- 8.Lewin-Kowalik J, Marcol W, Kotulska K, et al. Prevention and management of painful neuroma. Neurol Med Chir (Tokyo). 2006;46:62–67; discussion 67. [DOI] [PubMed] [Google Scholar]
- 9.Kung TA, Langhals NB, Martin DC, et al. Regenerative perihperal nerve interface viability and signal transduction with an implanted electrode. Plast Recontr Surg. 2014;133:1380–1394. [DOI] [PubMed] [Google Scholar]
- 10.Frost CM, Ursu DC, Flattery SM, et al. Regenerative peripheral nerve interfaces for real-time, proportional control of a neuroprosthetic hand. J Neuroeng Rehabil. 2018;15:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubiak CA, Kemp SWP, Cederna PS. Regenerative peripheral nerve interface for management of postamputation neuroma. JAMA Surg. 2018;153:681–682. [DOI] [PubMed] [Google Scholar]
- 12.Kubiak CA, Kung TA, Brown DL, et al. State-of-the-art techniques in treating peripheral nerve injury. Plast Reconstr Surg. 2018;141:702–710. [DOI] [PubMed] [Google Scholar]
- 13.Watson J, Gonzalez M, Romero A, et al. Neuromas of the hand and upper extremity. J Hand Surg Am. 2010;35:499–510. [DOI] [PubMed] [Google Scholar]
- 14.Yao C, Zhou X, Zhao B, et al. Treatments of traumatic neuropathic pain: a systematic review. Oncotarget. 2017;8:57670–57679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dellon AL. Surgical treatment of upper extremity pain. Hand Clin. 2016;32:71–80. [DOI] [PubMed] [Google Scholar]
- 16.Arnold DMJ, Wilkens SC, Coert JH, et al. Diagnostic criteria for symptomatic neuroma. Ann Plast Surg. 2019;82:420–427. [DOI] [PubMed] [Google Scholar]
- 17.Li QT, Zhang PX, Yin XF, et al. Functional recovery of denervated skeletal muscle with sensory or mixed nerve protection: a pilot study. PLoS One. 2013;8:e79746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozusko SD, Kaminsky AJ, Boyd LC, et al. Sensory neurotization of muscle: past, present, and future considerations. J Plast Hand Surg. 2018;53:31–36. [DOI] [PubMed] [Google Scholar]
- 19.Hynes NM, Bain JR, Thoma A, et al. Preservation of denervated muscle by sensory protection in rats. J Reconstr Microsurg. 1997;13:337–343. [DOI] [PubMed] [Google Scholar]
- 20.Bain JR, Veltri KL, Chamberlain D, et al. Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience. 2001;103:503–510. [DOI] [PubMed] [Google Scholar]
- 21.Placheta E, Wood MD, Lafontaine C, et al. Enhancement of facial nerve motor neuron regeneration through cross-face nerve grafts by adding end-to-side sensory axons. Plast Recontr Surg. 2015;135:460–468. [DOI] [PubMed] [Google Scholar]
- 22.Kuiken TA, Barlow AK, Hargrove L, et al. Targeted muscle reinnervation for the upper and lower extremity. Tech Orthop. 2017;32:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]


