Abstract
Background
Sarcoidosis, one of the most common interstitial lung diseases, has significant health disparities. Approximately 50% of individuals affected with sarcoidosis will undergo spontaneous remission, but those who do not undergo remission often require long-term or lifelong treatment to prevent disease progression. We sought to assess the association between medication adherence and clinical outcomes in sarcoidosis.
Methods
Adult patients in the Johns Hopkins Sarcoidosis Clinic diagnosed with pulmonary sarcoidosis on treatment were eligible for enrollment. Questionnaires were administered to assess medication adherence, health-related quality of life (HRQoL), health-care utilization, and sociodemographic information. Clinical information was abstracted from medical charts including lung function, disease duration, comorbidities, and sarcoidosis organ involvement.
Results
A total of 117 participants were enrolled (57% women; 55% black; median age, 57 years). Within the cohort, 66% of individuals reported at least one nonadherent behavior. Higher medication adherence was associated with better HRQoL (P < .05). There was no association between medication adherence and the odds of health-care utilization, FVC % predicted, FEV1 % predicted, or diffusion capacity of the lungs for carbon monoxide % predicted. Black participants reported lower medication adherence than white participants (P < .05).
Conclusions
This is the first observational study of medication adherence in sarcoidosis. We found that higher medication adherence was associated with better HRQoL, with blacks more likely to report nonadherence. Medication adherence may be an important target to improve patient-reported outcomes and health disparities in sarcoidosis.
Key Words: health-related quality of life, medication adherence, sarcoidosis
Abbreviations: CCI, Charlson Comorbidity Index; Dlco, diffusion capacity of the lungs for carbon monoxide; HRQoL, health-related quality of life; KSQ, King’s Sarcoidosis Health Questionnaire; SGRQ, St. George’s Respiratory Questionnaire
FOR EDITORIAL COMMENT, SEE PAGE 17
Sarcoidosis is one of the most common interstitial lung diseases in the United States. Sarcoidosis is prevalent in all racial and ethnic groups; however, black individuals are estimated to have a three to five times increased incidence.1 Significant health disparities exist in sarcoidosis, with blacks having higher sarcoidosis-related mortality than non-Hispanic whites.2,3 Approximately 50% of individuals affected with sarcoidosis will undergo spontaneous remission.1 Individuals who do not undergo remission often require long-term or lifelong treatment to prevent disease progression.
Glucocorticosteroids are considered first-line therapy in most patients with sarcoidosis who have an indication for treatment.4 Gibson et al5 showed in a randomized controlled trial of 58 patients with unremitting disease that treatment with long-term daily corticosteroids was associated with higher pulmonary function over time compared with the group treated with intermittent, symptom-guided corticosteroids. Results suggest that corticosteroids could modify lung function in sarcoidosis and continuous treatment with corticosteroids is superior to intermittent treatment for individuals with pulmonary sarcoidosis with unremitting disease.
Previous research has shown that approximately 50% of patients with a chronic illness have suboptimal adherence to medications.6 In sarcoidosis, medication nonadherence may resemble intermittent, symptom-based treatment instead of continuous treatment. The findings from the Gibson et al5 trial suggest that individuals with sarcoidosis who have an indication for treatment, but use an intermittent treatment course, may have worse clinical outcomes than those who have continuous treatment. Therefore, the patient’s ability to adhere to a continuous treatment course is critical in modifying disease outcomes in sarcoidosis. In other chronic respiratory diseases, nonadherence has been associated with increased morbidity and mortality.7, 8, 9 To our knowledge, no research to date has evaluated medication adherence in sarcoidosis.
Several patient-level determinants of adherence have been described in the literature including age, sex, race, income, and education.10,11 Patient-level determinants of adherence vary among chronic diseases.10 Understanding determinants of adherence for a specific disease such as sarcoidosis is necessary to start the process of targeting interventions to patients most at risk for nonadherence.
Despite the known importance of medication adherence in other chronic pulmonary diseases, medication adherence has never been assessed or targeted as a possible way to improve outcomes in sarcoidosis. We sought to assess the association between medication adherence and clinical outcomes in a cross-sectional study of patients with pulmonary sarcoidosis on treatment in the Johns Hopkins Sarcoidosis Clinic. We hypothesized that participants with higher medication adherence would have better clinical outcomes such as better health-related quality of life (HRQoL), higher lung function, and less health-care utilization. We also hypothesized that younger participants, women, blacks, low-income participants, and participants prescribed prednisone would have lower medication adherence.
Materials and Methods
Procedures
The study was reviewed and all recruitment procedures and study protocols were approved by the Johns Hopkins University institutional review board (No. IRB-X 00182289). This was a cross-sectional study conducted from August 2018 to February 2019. Adults ≥ 18 years of age from the Johns Hopkins Sarcoidosis Clinic were eligible for enrollment if they had a diagnosis of pulmonary sarcoidosis and were on treatment for sarcoidosis. Treatment included corticosteroids and steroid sparing agents (eg, methotrexate, azathioprine). Letters describing the study were mailed to each eligible patient prior to their clinic visit, and potential participants were given information on how to decline to be contacted by the study team. If patients did not decline to be contacted, they were approached during their clinic visit for possible enrollment. Oral informed consent was obtained from all study participants. After enrollment, the research team administered questionnaires. The medical charts were also abstracted to assess organ involvement, comorbidities, date of diagnosis, and pulmonary function test results.
Measures
The five-item Medication Adherence Report Scale is a brief, validated self-report instrument of medication adherence12 that has been widely used in other pulmonary diseases.13,14 The measure includes five statements of nonadherence (eg, I forget to take my medications). Participants answer agreement to each statement with a range of answers from 1 (always) to 5 (never). The total possible score on the measure is 25, which indicates the highest level of adherence. Nonadherence is coded if any nonadherent behavior is reported (Medication Adherence Report Scale score < 25).15
HRQoL was assessed using both the St. George’s Respiratory Questionnaire (SGRQ) and the King’s Sarcoidosis Health Questionnaire (KSQ). The SGRQ is an instrument containing 50 items that assesses symptoms, activity, and disease impact. Each response has an empirically derived weight, and the total score is calculated from responses to all 50 items.16, 17, 18 The SGRQ has been used in multiple pulmonary sarcoidosis clinical trials.19,20 A lower SGRQ score indicates a better HRQoL. The KSQ is a 29-item validated measure of sarcoidosis health status.21,22 We used the 16-item lung and general health status subscales. The KSQ has been used as an outcome measure in a sarcoidosis clinical trial23 and has been found to have moderate correlation to lung function in sarcoidosis.21 A higher score on the KSQ indicates a better HRQoL.
Results from lung function testing conducted nearest to the date of the survey completion and < 1 year from survey completion were abstracted from the participants’ medical record. FEV1, FEV1 % predicted, FVC, FVC % predicted, FEV1/FVC, diffusion capacity of the lungs for carbon monoxide (Dlco), and Dlco % predicted were all abstracted, if available. Complete lung function tests within the last year were available for 58% of participants (n = 65).
Health-care utilization was obtained through a self-report questionnaire in which participants reported whether they were hospitalized in the 12 months prior to enrollment. Additionally, participants reported whether they had an ED visit in the 6 months prior to enrollment.
Sociodemographic information was collected by questionnaire. When evaluating race/ethnicity, three participants identified as Asian, one participant identified as Panamanian, one participant identified as Puerto Rican, and one participant identified as Native American. We limited our analysis to participants of white and black races because of the small numbers in the other race categories.
Health literacy was measured using the Rapid Estimate of Adult Literacy in Medicine,24 a 7-item measure assessing health literacy that has been validated in an age, sex, education, and ethnically diverse sample and has good test-rest reliability.24 Low health literacy (less than seventh or eighth grade reading level) is defined as a score of < 4.24
Sarcoidosis organ involvement, comorbidities, medication regimen including corticosteroid dose when applicable, and disease duration were also collected. The sarcoidosis organ assessment tool that was used in the National Heart, Lung, and Blood Institute–supported Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (GRADS) study25 was used to assess organ involvement. Organ involvement was categorized into three groups as one, two to five, and more than five organs with probable, highly probable, or definite involvement included. The Charlson Comorbidity Index (CCI)26 was used to assess comorbidities. The medication regimen was abstracted and confirmed with participants. Disease duration was calculated by subtracting the date of diagnosis from the date of enrollment.
Statistical Analysis
Descriptive statistics were conducted to evaluate distribution of all data. To examine the association between medication adherence and quality of life, we performed multiple linear regressions to assess the association between SGRQ, KSQ, and lung function % predicted and medication adherence. All models included the following a priori identified covariates: race, age, sex, education, income, organ involvement, CCI, medication regimen, and disease duration. We performed multiple logistic regressions to assess the association between health-care utilization (ED visits and hospitalization both coded as presence or absence) and medication adherence. To examine determinants of adherence, we conducted multiple linear regression to assess the association between medication adherence and potential patient-level determinants of adherence including medication regimen, sex, race, organ involvement, income, education, and disease duration. All analyses were performed using Stata Version 15 (StataCorp LLC).
Results
A total of 257 individuals met the inclusion criteria. Of the 257 eligible individuals, 180 were approached. Of those, 128 individuals were enrolled. The most common reason for not being approached was that the individual missed his or her clinic appointment (n = 41). The most common reasons for not joining the study were individuals being too busy or not interested. Four participants were excluded because of incomplete data and seven were excluded because of limited numbers as previously described. A total of 117 participants were included in the analysis, which represents 46% of all eligible participants (Fig 1). We found no statistical difference in race or sex between those who enrolled vs those who did not (P = .09 and P = .62, respectively). However, there was a significant difference in age (P = .01) with those not enrolled having a mean age of 54 ± 11.15 years vs those enrolled with a mean age of 57 ± 9.87 years.
Figure 1.
Enrollment.
Demographic characteristics are presented in Table 1. The study population consisted of 57% women and 55% blacks with a median age of 57 years. Most participants (47%) were prescribed both corticosteroids and steroid sparing agents compared with 34% prescribed steroids only and 19% prescribed steroid sparing agents only. Most participants (70%) had multiple organ involvement. Only 4% of the cohort had low health literacy. Within the cohort, 66% of individuals reported at least one nonadherent behavior (Medication Adherence Report Scale score < 25) and the median Medication Adherence Report Scale score was 23.
Table 1.
Participant Characteristics (N = 117)
| Characteristic | Value |
|---|---|
| Age, y | 57 (51-65) |
| Sex, female | 57 (67) |
| Race | |
| White | 45 (53) |
| Black | 55 (64) |
| Education | |
| High school diploma or less | 26 (30) |
| Some college or college degree | 45 (53) |
| Graduate degree | 29 (34) |
| Income | |
| < $50,000 | 32 (38) |
| $50,000-$124,999 | 31 (36) |
| > $125,000 | 37 (43) |
| Medication regimen | |
| Corticosteroid only | 34 (40) |
| Corticosteroid + steroid sparing agent | 47 (55) |
| Steroid sparing agent only | 19 (22) |
| Prednisone equivalent corticosteroid dose | 10 (5-15) |
| Organ involvement | |
| 1 organ | 30 (35) |
| 2-4 organs | 62 (72) |
| > 5 organs | 8 (10) |
| Charlson Comorbidity Index | 3 (2-5) |
| Disease duration, y | 7 (3-21) |
| Low health literacya | 4 (5) |
| Five-item Medication Adherence Report Scale | 24 (23-25) |
| St. George’s Respiratory Questionnaire | 33 (16-52) |
| King’s Sarcoidosis Health Questionnaire | 62 (56-70) |
| FVC % predicted (n = 68) | 84 (71-97) |
| FEV1 % predicted (n = 68) | 77 (64-92) |
| Dlco % predicted (n = 65) | 73 (55-85) |
| ED visits in previous 6 mo | 37 (43) |
| Hospitalization in previous 12 mo | 30 (35) |
Values are % (No.) or median (interquartile range). Dlco = diffusion capacity of the lungs for carbon monoxide.
Defined as a score of < 4 on the Rapid Estimate of Adult Literacy in Medicine - Short Form.
Association of Medication Adherence and HRQoL
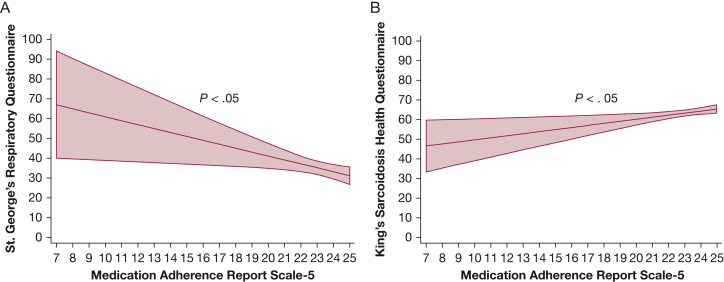
In multiple linear regressions, participants who had higher scores on the Medication Adherence Report Scale, suggesting higher adherence, had significantly better HRQoL as measured by both the SGRQ and KSQ (P < .05) after controlling for race, age, sex, education, income, organ involvement, CCI, medication regimen, and disease duration (Fig 2, Table 2).
Figure 2.
A-B, Higher adherence associated with better health-related quality of life (HRQoL) by the (A) St. George’s Respiratory Questionnaire (SGRQ) and the (B) King’s Sarcoidosis Health Questionnaire (KSQ). Lower scores on the SGRQ indicates better HRQoL. Higher scores on the KSQ indicates better HRQoL.
Table 2.
Association Between Adherence and Clinical Outcomes in Sarcoidosis
| Dependent Variable | B Value | P Value |
|---|---|---|
| Health-related quality of lifea | ||
| St. George’s Respiratory Questionnaire | −1.98 | .02 |
| King’s Sarcoidosis Health Questionnaire | 1.05 | .01 |
| Pulmonary function testsa | ||
| FVC % predicted (n = 68) | 1.45 | .27 |
| FEV1 % predicted (n = 68) | 1.37 | .34 |
| Dlco % predicted (n = 65) | 0.70 | .62 |
| Health-Care Utilizationb | OR | P Value |
|---|---|---|
| ED visits in last 6 mo | 0.91 | .32 |
| Hospitalization in last 12 mo | 1.11 | .40 |
Covariates for all models included the following: age, race, education, income, organ involvement, medication regimen, Charlson Comorbidity Index, and disease duration. Lower scores on the St. George’s Respiratory Questionnaire indicate better heath-related quality of life and higher scores on the King’s Sarcoidosis Health Questionnaire indicate better heath-related quality of life. See Table 1 legend for expansion of abbreviation.
Multiple linear regression.
Multiple logistic regression.
Association of Medication Adherence and Lung Function
In multiple linear regressions, we found no association between medication adherence and lung function including FVC % predicted, FEV1 % predicted, and Dlco % predicted after controlling for covariates as previously mentioned (Table 2).
Association of Medication Adherence and Health-Care Utilization
In multiple logistic regressions, we found no association between medication adherence and ED visits in the last 6 months or hospitalization in the last 12 months after controlling for controlling for covariates as previously mentioned (Table 2).
Determinants of Adherence
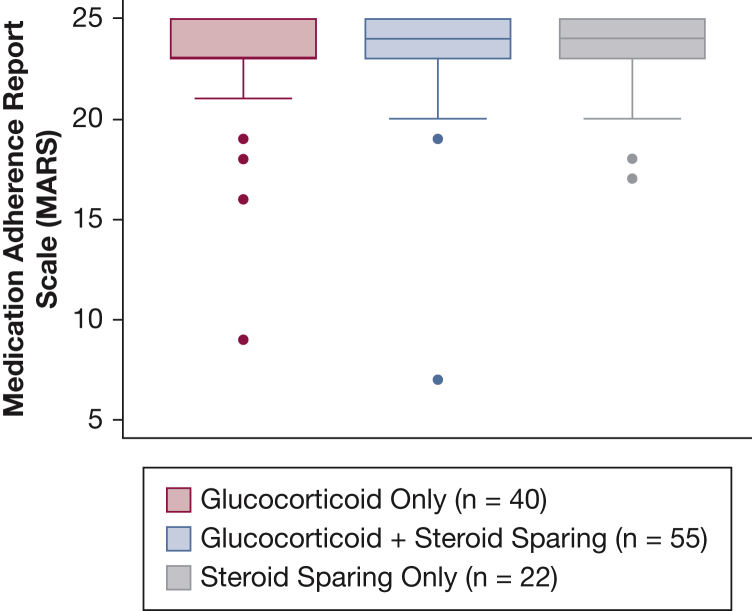
In a multiple linear regression, we found an association between race and medication adherence with black participants reporting lower medication adherence than white participants (P < .05) (Table 3). There was no association between sex, organ involvement, income, education, and disease duration. We found no association between corticosteroid dose and medication adherence (P = .28). Given the low numbers of participants with low health literacy, we did not include health literacy in the final model. Additionally, we found no association between medication regimen including corticosteroid only, corticosteroids and steroid sparing agent, and steroid sparing agent only and medication adherence defined by the Medication Adherence Report Scale (Fig 3, Table 3).
Table 3.
Determinants of Medication Adherence
| Characteristic | Mean Difference | P Value |
|---|---|---|
| Sex | 0.56 | .29 |
| Race | −1.25 | .02 |
| Education | ||
| High school diploma or less | ||
| Some college or college degree | −0.57 | .34 |
| Graduate degree | −0.11 | .99 |
| Income | ||
| < $50,000 | ||
| $50,000-$124,999 | −0.06 | .93 |
| > $125,000 | 0.47 | .46 |
| Medication regimen | ||
| Corticosteroid only | ||
| Corticosteroid + steroid sparing agent | 0.59 | .26 |
| Steroid sparing agent only | 0.47 | .49 |
| Organ involvement | ||
| 1 organ | ||
| 2-4 organs | 0.20 | .70 |
| > 5 organs | −0.20 | .83 |
| B Value | P Value | |
|---|---|---|
| Disease duration, y | 0.02 | .23 |
Figure 3.
Medication adherence similar across medication regimens.
Discussion
In a cross-sectional study of 117 participants with pulmonary sarcoidosis on treatment, we examined the association between medication adherence and clinical outcomes including HRQoL, pulmonary function, and health-care utilization. This is the first study, to our knowledge, to investigate medication adherence in sarcoidosis. Consistent with other diseases in which corticosteroids and steroid sparing agents are prescribed, the cohort had a high level of nonadherence by self-report.27 We found that higher medication adherence was associated with better HRQoL by a sarcoidosis specific measure (KSQ) and the SGRQ. We found no association between medication adherence and pulmonary function or health-care utilization. Additionally, we found that black participants reported lower medication adherence than white participants.
Corticosteroid therapy is the cornerstone of treatment in sarcoidosis, but is associated with adverse effects.28 Corticosteroid dose was not significantly associated with medication adherence in the cohort. However, because some participants were not on corticosteroid therapy, and few were on moderately high to high doses, this preliminary result is likely insufficiently powered to detect clinically significant differences. Future studies are necessary to explore the effect of corticosteroid dose and duration of therapy on adherence because increasing adverse effects from corticosteroids are known to be associated with higher doses; however, the tolerability can differ markedly from person to person. Steroid sparing therapies such as methotrexate, azathioprine, and antitumor necrosis factor therapies are not effective or poorly tolerated in 20% to 40% of patients.29,30 We did not find any difference in medication adherence among medication regimens including corticosteroid only, corticosteroids and steroid sparing agents, and steroid sparing agent only in the cohort. However, given that 66% of participants reported nonadherent behavior in our study, medication adherence may be an important factor to measure in determining real-world effectiveness of medication regimens in sarcoidosis, suggesting the importance of discussing medication adherence with patients.
Despite known health disparities in sarcoidosis, there is still a lack of understanding around why such disparities in sarcoidosis exist. Our finding of lower medication adherence among blacks is consistent with findings in other chronic diseases that have shown lower rates of medication adherence in blacks.11,31 In asthma, we found that health beliefs was a mediator between race and medication adherence, such that blacks were more likely to have concerns about medications, resulting in lower adherence.32 More research is needed to examine this potential mechanism between race and medication adherence in sarcoidosis.
Sarcoidosis is characterized by clinical heterogeneity, and it may be important to understand how medication adherence influences clinical outcomes in this disease and for future studies evaluating novel treatments for long-term management of pulmonary sarcoidosis. We recognize that our study is cross-sectional and therefore is unable to evaluate the directionality of associations. Our findings may be limited by the small sample size of 117; however, this sample size is comparable with other observational sarcoidosis studies.28 We also found that the enrolled sample was slightly older than those that did not enroll; therefore, we have included age as a covariate in our models. Despite a modest sample size, this study will help inform a larger longitudinal study in this area that is desperately needed to extend these findings. In this context, a longitudinal study will be necessary to understand the impact medication adherence may have on specific clinical outcomes. The use of a self-report measure of adherence, which has been shown to be susceptible to social desirability bias33 and has been shown to underestimate adherence in other diseases such as asthma and COPD34,35 is another limitation. Future studies using objective measures of adherence such as electronic medication monitors and pharmacy records will be required. Our study lacks data on all the potential determinants of adherence. Although we collected sociodemographic covariates such as income, we did not have enough individuals with low health literacy to assess the association between medication adherence and health literacy. In diseases such as diabetes, health literacy has been associated with self-management.36 Incomplete lung function data, which limits the interpretation of our finding of no significant association with medication adherence, is an additional limitation. A longitudinal study assessing both lung function and medication adherence together will be important to assess the importance of medication adherence on possible lung function outcomes. Finally, although our inclusion criteria required that participants had pulmonary sarcoidosis, more than one-half of the participants had multiorgan disease and the effect of coinvolvement of these other organ systems remains unclear.
Despite the limitations of our study, this initial study will inform future longitudinal studies using objective measures of adherence with lung function and HRQoL measurements over time. In addition, dissecting the determinants of adherence should include studies investigating beliefs about medications, the impact of side effects of medications including corticosteroid dosage, the number and cost of medications, and health literacy. Understanding the impact of medication adherence among different populations is especially important given the significant health disparities that exist in sarcoidosis and our finding of medication adherence being different among blacks.
Conclusions
This is the first study to investigate medication adherence in sarcoidosis, finding that higher medication adherence was associated with better HRQoL. Our findings suggest that medication adherence may be an important target to improve patient-reported outcomes and health disparities in sarcoidosis. Future studies are needed to understand the impact of medication adherence and its determinants may have on clinical outcomes and health disparities in sarcoidosis.
Acknowledgments
Author contributions: M. N. E. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. M. S., M. N. E., T. B., M. S. C., C. S. R., and D. R. M. contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. R. M. is Chairman and Chief Technical Officer of Sarcoidosis Diagnostic Testing, LLC; has received funding including past salary support under the NHLBI STTR program [Grant R41 HL129728]; was a consultant to Merck, Dicerna, aTYR, and Roivant; serves on the Scientific Advisory Board of the Foundation for Sarcoidosis Research; receives royalties from Hodder Education for editing a book on Sarcoidosis; will receive royalties from Taylor & Francis Group for editing a book on interstitial lung disease; has received research support including salary support from NHLBI [Grant U01HL112708] (GRADS) and from subaward to parent [Grant R01HL136681] (National Jewish Health); has received honoraria for lecturing on sarcoidosis; and serves as an expert legal consultant/witness for legal proceedings concerning sarcoidosis. None declared (M. S., T. B., E. S. C., C. S. R., M. N. E.).
Role of sponsors: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
FUNDING/SUPPORT: Research reported in this study was supported by an American Thoracic Society Fellowship in Health Equality Pearl M Stetler Fellowship Award and by the National Heart, Lung, and Blood Institute of the National Institutes of Health [Award T32HL007534].
References
- 1.Chen E.S., Moller D.R. Sarcoidosis--scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7(8):457–467. doi: 10.1038/nrrheum.2011.93. [DOI] [PubMed] [Google Scholar]
- 2.Coultas D.B., Zumwalt R.E., Black W.C., Sobonya R.E. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150(4):967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 3.Gerke A.K., Judson M.A., Cozier Y.C., Culver D.A., Koth L.L. Disease burden and variability in sarcoidosis. Ann Am Thorac Soc. 2017;14(suppl 6):S421–S428. doi: 10.1513/AnnalsATS.201707-564OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley B., Branley H.M., Egan J.J. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1–v58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 5.Gibson G.J., Prescott R.J., Muers M.F. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51(3):238–247. doi: 10.1136/thx.51.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabate E. World Health Organization; Geneva, Switzerland: 2003. Adherence to Long-Term Therapies: Evidence for Action. [Google Scholar]
- 7.Suissa S., Ernst P., Benayoun S., Baltzan M., Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343(5):332–336. doi: 10.1056/NEJM200008033430504. [DOI] [PubMed] [Google Scholar]
- 8.Vestbo J., Anderson J.A., Calverley P.M. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–943. doi: 10.1136/thx.2009.113662. [DOI] [PubMed] [Google Scholar]
- 9.Eakin M.N., Bilderback A., Boyle M.P., Mogayzel P.J., Riekert K.A. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cyst Fibros. 2011;10(4):258–264. doi: 10.1016/j.jcf.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kardas P., Lewek P., Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4(91) doi: 10.3389/fphar.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber B.S., Cho Y.I., Arozullah A.M., Lee S.-Y.D. Racial differences in medication adherence: a cross-sectional study of Medicare enrollees. Am J Geriatr Pharmacother. 2010;8(2):136–145. doi: 10.1016/j.amjopharm.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen J.L., Mann D.M., Wisnivesky J.P. Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: the Medication Adherence Report Scale for Asthma. Ann Allergy Asthma Immunol. 2009;103(4):325–331. doi: 10.1016/s1081-1206(10)60532-7. [DOI] [PubMed] [Google Scholar]
- 13.Khdour M.R., Hawwa A.F., Kidney J.C., Smyth B.M., McElnay J.C. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD) Eur J Clin Pharmacol. 2012;68(10):1365–1373. doi: 10.1007/s00228-012-1279-5. [DOI] [PubMed] [Google Scholar]
- 14.Krauskopf K., Federman A.D., Kale M.S. Chronic obstructive pulmonary disease illness and medication beliefs are associated with medication adherence. Copd. 2015;12(2):151–164. doi: 10.3109/15412555.2014.922067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAdam-Marx C., Bellows B.K., Unni S. Impact of adherence and weight loss on glycemic control in patients with type 2 diabetes: cohort analyses of integrated medical record, pharmacy claims, and patient-reported data. J Manag Care Spec Pharm. 2014;20(7):691–700. doi: 10.18553/jmcp.2014.20.7.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barr J.T., Schumacher G.E., Freeman S., LeMoine M., Bakst A.W., Jones P.W. American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin Ther. 2000;22(9):1121–1145. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt G.H., Townsend M., Keller J., Singer J., Nogradi S. Measuring functional status in chronic lung disease: conclusions from a randomized control trial. Respir Med. 1989;83(4):293–297. doi: 10.1016/s0954-6111(89)80199-4. [DOI] [PubMed] [Google Scholar]
- 18.Jones P.W., Quirk F.H., Baveystock C.M., Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 19.Baughman R.P., Drent M., Kavuru M. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 20.Judson M.A., Baughman R.P., Costabel U. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014;44(5):1296–1307. doi: 10.1183/09031936.00000914. [DOI] [PubMed] [Google Scholar]
- 21.Patel A.S., Siegert R.J., Creamer D. The development and validation of the King's Sarcoidosis Questionnaire for the assessment of health status. Thorax. 2013;68(1):57–65. doi: 10.1136/thoraxjnl-2012-201962. [DOI] [PubMed] [Google Scholar]
- 22.Van Manen M.J., Wapenaar M., Strookappe B. Validation of the King's Sarcoidosis Questionnaire (KSQ) in a Dutch sarcoidosis population. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33(1):75–82. [PubMed] [Google Scholar]
- 23.Baughman R.P., Sweiss N., Keijsers R. Repository corticotropin for chronic pulmonary sarcoidosis. Lung. 2017;195(3):313–322. doi: 10.1007/s00408-017-9994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arozullah A.M., Yarnold P.R., Bennett C.L. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007;45(11):1026–1033. doi: 10.1097/MLR.0b013e3180616c1b. [DOI] [PubMed] [Google Scholar]
- 25.Moller D.R., Koth L.L., Maier L.A. Rationale and Design of the Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (GRADS) study. Sarcoidosis protocol. Ann Am Thorac Soc. 2015;12(10):1561–1571. doi: 10.1513/AnnalsATS.201503-172OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Koneru S., Shishov M., Ware A. Effectively measuring adherence to medications for systemic lupus erythematosus in a clinical setting. Arthritis Rheum. 2007;57(6):1000–1006. doi: 10.1002/art.22898. [DOI] [PubMed] [Google Scholar]
- 28.Judson M.A., Chaudhry H., Louis A., Lee K., Yucel R. The effect of corticosteroids on quality of life in a sarcoidosis clinic: the results of a propensity analysis. Respir Med. 2015;109:526–531. doi: 10.1016/j.rmed.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lower E.E., Baughman R.P. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med. 1994;155(8):846–851. [PubMed] [Google Scholar]
- 30.Vorselaars A.D.M., Wuyts W.A., Vorselaars V.M.M. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144(3):805–812. doi: 10.1378/chest.12-1728. [DOI] [PubMed] [Google Scholar]
- 31.Adams A.S., Trinacty C.M., Zhang F. Medication adherence and racial differences in A1C control. Diabetes Care. 2008;31(5):916–921. doi: 10.2337/dc07-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le T.T., Bilderback A., Bender B. Do asthma medication beliefs mediate the relationship between minority status and adherence to therapy? J Asthma. 2008;45(1):33–37. doi: 10.1080/02770900701815552. [DOI] [PubMed] [Google Scholar]
- 33.Stirratt M.J., Dunbar-Jacob J., Crane H.M. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470–482. doi: 10.1007/s13142-015-0315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel M., Perrin K., Pritchard A. Accuracy of patient self-report as a measure of inhaled asthma medication use. Respirology. 2013;18(3):546–552. doi: 10.1111/resp.12059. [DOI] [PubMed] [Google Scholar]
- 35.Tommelein E., Mehuys E., Van Tongelen I., Brusselle G., Boussery K. Accuracy of the Medication Adherence Report Scale (MARS-5) as a quantitative measure of adherence to inhalation medication in patients with COPD. Ann Pharmacother. 2014;48(5):589–595. doi: 10.1177/1060028014522982. [DOI] [PubMed] [Google Scholar]
- 36.Osborn C.Y., Bains S.S., Egede L.E. Health literacy, diabetes self-care, and glycemic control in adults with type 2 diabetes. Diabetes Technol Therap. 2010;12(11):913–919. doi: 10.1089/dia.2010.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]