Abstract
Background
Mucous exudates occluding the lumen of small airways are associated with reduced lung function and mortality in subjects with COPD; however, luminal plugs in large airways have not been widely studied. We aimed to examine the associations of chest CT scan-identified luminal plugging with lung function, health-related quality of life, and COPD phenotypes.
Methods
We randomly selected 100 smokers without COPD and 400 smokers with COPD from the COPDGene Study. Luminal plugging was visually identified on inspiratory CT scans at baseline and 5-year follow-up. The relationships of luminal plugging to FEV1, St. George’s Respiratory Questionnaire (SGRQ) score, emphysema on CT scan (defined as the percentage of low attenuation area < 950 Hounsfield units [%LAA-950]), and chronic bronchitis were assessed using linear and logistic multivariable analyses.
Results
Overall, 111 subjects (22%) had luminal plugging. The prevalence of luminal plugging was higher in subjects with COPD than those without COPD (25% vs 10%, respectively; P = .001). In subjects with COPD, luminal plugging was significantly associated with FEV1 % predicted (estimate, −6.1; SE, 2.1; P = .004) and SGRQ score (estimate, 4.9; SE, 2.4; P = .04) in adjusted models. Although luminal plugging was associated with log %LAA-950 (estimate, 0.43; SE, 0.16; P = .007), its relationship with chronic bronchitis did not reach statistical significance (P = .07). Seventy-three percent of subjects with COPD with luminal plugging at baseline had it 5 years later.
Conclusions
In subjects with COPD, CT-identified luminal plugging is associated with airflow obstruction, worse health-related quality of life, and emphysema phenotype. This imaging feature may supplement the current clinical assessment of chronic mucus hypersecretion in COPD.
Key Words: airway obstruction, COPD, CT scan, luminal, mucus, plugging, smokers
Abbreviations: FEF25%-75%, forced expiratory flow at 25% to 75% of the FVC; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HRQL, health-related quality of life; %LAA-950, percentage of low attenuation area < 950 Hounsfield units; SGRQ, St. George’s Respiratory Questionnaire
FOR EDITORIAL COMMENT, SEE PAGE 7
Mucus dysfunction is a central pathophysiologic feature of COPD that increases mucus production and plug formation and manifests as chronic cough and phlegm.1 In patients with COPD, mucous exudates in small airways histologically identified in resected lung tissue were known to relate to airflow obstruction and increased mortality.2,3
CT scan measures of relatively large airways are well known to reflect histologic measures of small airways (≤ 2 mm in diameter), the primary site of airflow obstruction in COPD.4 A study identified luminal plugging in relatively large airways on CT scan in patients with severe asthma, and demonstrated that it was associated with reduced lung function and sputum eosinophil levels and inflammatory biomarkers.5 Although it is thought that this imaging feature reflects mucus plugging, this remains to be proven. Consequently, we use the term luminal plugging instead of mucus plugging. Understanding the functional and clinical implications of CT scan-identified luminal plugging in large airways may help guide the development of better therapies targeting mucus dysfunction. Nevertheless, CT scan-identified luminal plugging remains to be explored in COPD. Both emphysema and chronic bronchitis are known as clinical phenotypes of COPD.6 It is conceivable that subjects with chronic bronchitis would likely form plugs in the airways, because increased mucus production is a central feature of this disease,7 whereas the relationship between emphysema and mucus dysfunction remains to be elucidated.
We hypothesized that CT scan-identified luminal plugging is associated with decreased lung function, worse health-related quality of life (HRQL), and COPD clinical phenotypes. Additionally, we aimed to examine changes in CT scan-identified luminal plugging at 5 years of follow-up, using data from the COPDGene Study, a well-characterized cohort of smokers with and without COPD.8
Methods
Study Population
We used baseline (phase 1, 2008-2011) and 5-year follow-up (phase 2, 2012-2016) data from the COPDGene study, designed to determine genetic and epidemiologic determinants of COPD.8 Briefly, African American and non-Hispanic white current and former smokers (≥ 10 pack-years of cigarette smoking) 45 to 80 years of age were recruited for this study (e-Appendix 1). Each study visit included questionnaires, spirometry, and chest CT imaging. We randomly selected 500 subjects stratified by spirometric Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage at phase 1 study visit, yielding 100 subjects without COPD and 400 subjects with COPD (100 from each GOLD stage I-IV). All subjects provided written informed consent to participate in the study. The institutional review board at each participating clinical center approved the COPDGene study, and the Partners HealthCare Research Committee (No. 2007P-000554) approved the current study.
CT Scan Assessment
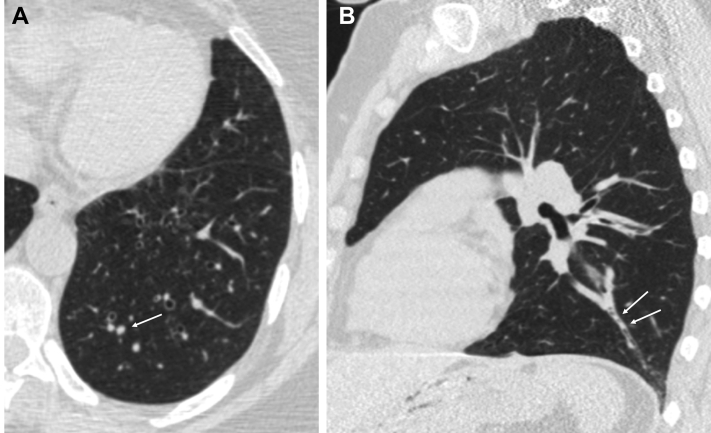
The COPDGene imaging protocols have been published elsewhere.8 Pertinent to this study is the overlapping image reconstruction of the volumetric CT scans with a submillimeter slice thickness, allowing a detailed assessment of the bronchial tree. Phase 1 and phase 2 inspiratory CT scans were independently evaluated with a window width of 1,400 and a level of −500 Hounsfield units, with readers blinded to clinical information.9 We used a sequential reading system described in e-Appendix 1.10 Briefly, a first reader identified and scored luminal plugging for each CT scan. All positive and 20% of negative CT scans were then rated by a second reader. CT scans with discrepant luminal plugging scores were sent to a third reader. We used a scoring system proposed by Dunican et al5 with a modification to comply with Netter’s bronchial anatomy nomenclature.11 This latter nomenclature has 18 bronchopulmonary segments instead of 20 previously used. A luminal plug was defined as an opacity that completely occludes the lumen of an airway, regardless of the airway size or generation (Fig 1). The lung zone within 2 cm from the costal or diaphragmatic pleura was excluded because the airways in that zone are too small to ascertain a complete occlusion by luminal plugs.5 A luminal plug score was generated for each CT scan as an aggregation of the number of bronchopulmonary segments with luminal plugging, ranging from 1 to 18. In those without luminal plugging, the score is 0. If a CT scan required more than one reading, the final luminal plug score used for analysis was an average of the scores from two or three readers. Three readings were required for discrepant CT scans. The intra- and interreader agreement for the luminal plugging score was assessed as described in e-Appendix 1. The reader also visually assessed bronchiectasis12 as detailed in e-Appendix 1. Quantitative CT measurements of lung volume, emphysema, and airways were performed with Thirona software (Thirona) (e-Appendix 1).
Figure 1.
A-B, CT scan findings of luminal plugging. (A) An axial image shows a luminal plug as a round opacity occluding the lumen of a branch of the left posterior basal bronchopulmonary segment (arrow). (B) The sagittal image shows luminal plugs as tubular opacities within the same airway (arrows).
Spirometry
Spirometric measures of lung function were performed before and after the administration of albuterol following the American Thoracic Society13 recommendations. Postbronchodilator FEV1 and FVC are expressed as % predicted values.14 COPD was defined as FEV1/FVC < 0.7, and its severity was classified based on spirometric GOLD stages I through IV.15 We used forced expiratory flow at 25% to 75% of the FVC (FEF25%-75%) (L/s) as a functional measure reflecting small airway disease. Bronchodilator reversibility was defined as at least 12% and 200-mL increase in FEV1 or FVC, or both.16
Clinical Assessment
Demographic and clinical data were collected with standardized questionnaires (available at www.COPDGene.org).8 HRQL was assessed using the St. George’s Respiratory Questionnaire (SGRQ) total score, ranging from 0 to 100; a higher score indicates worse HRQL.17 A subject was considered to have a cough and phlegm if he/she had coughed and brought up phlegm almost every day or most days a week over the last 4 weeks, respectively, based on the SGRQ. A subject was considered to have dyspnea if he/she had a modified Medical Research Council score of ≥ 1.18 Chronic bronchitis was considered present if a subject had chronic cough and phlegm production for ≥ 3 months per year for at least 2 consecutive years.19 A subject was considered to have asthma if he/she reported a current physician diagnosis of asthma.
Statistical Analysis
Differences between subjects with and without luminal plugging at phase 1 visit were compared using the Fisher exact test and the Student t test as appropriate. Because most subjects with luminal plugging had it in one or two bronchopulmonary segments, we report the primary analyses using luminal plugging as a binary variable (presence/absence). Multivariate analyses were performed using linear and logistic regression models for binary and continuous variables, where appropriate. Model building was performed a priori. The main models for FEV1 % predicted, FEF25%-75% SGRQ, chronic bronchitis, and respiratory symptoms included age, sex, race, BMI, current smoking status, pack-years of smoking history, current asthma, bronchiectasis, and CT scan measures of emphysema and airway wall thickness. The models for emphysema on CT scan (ie, as continuous percentage of low attenuation area < −950 Hounsfield units [%LAA-950] and binary variable defined as %LAA-950 ≥ 5%),20 were adjusted for the same above variables but %LAA-950. Secondary models for all those outcomes but spirometric measures of lung function included FEV1 as a covariate. FEF25%-75% and %LAA-950 were log-transformed because they were not normally distributed. P < .05 was considered statistically significant. Analyses were conducted with SAS 9.4 software (SAS Institute) and STATA 14 (StataCorp LLC).
Results
The intra- and interreader agreement results and a Bland-Altman plot (e-Fig 1) are shown in e-Appendix 1.
Luminal Plugging at Phase 1
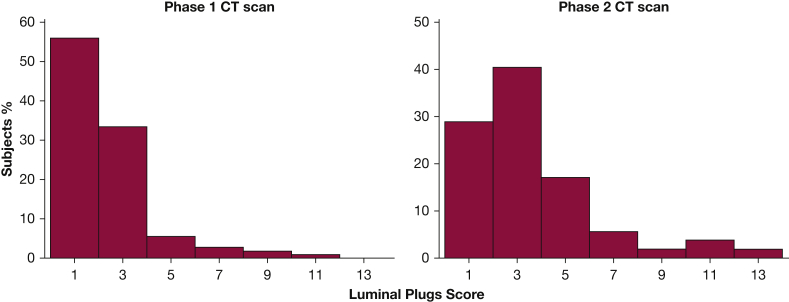
Out of the 500 subjects, 111 (22%) had luminal plugging. Subjects with COPD were more likely to have luminal plugging than subjects without COPD (25% vs 10%, respectively; P = .001), and the prevalence of subjects with luminal plugging increased with GOLD stage (Fig 2). Luminal plugs were mostly found in the lower lobes among subjects with COPD (78%) (e-Fig 2). Overall, the median luminal plug score was 1.5 (range, 1-11). The median luminal plug score across spirometric GOLD stages I through IV was 1 (range, 1-3), 1 (range, 1-11), 2 (range, 1-9), and 2 (range, 1-8), respectively. The distribution of the luminal plug score among all subjects is shown in Figure 3.
Figure 2.
The percentage of subjects with luminal plugging at phase 1 of the COPDGene Study. The bars represent the percentage of subjects with luminal plugging in those without COPD and those with COPD by spirometric GOLD stage. Note that the percentage of subjects with luminal plugging increased with the spirometric GOLD stage. The percentage for each bar was calculated using 100 study subjects for the non-COPD group and each GOLD stage except for overall subjects with COPD, where it was calculated with 400. GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Figure 3.
Distribution of CT scan-identified luminal plug scores at phases 1 (n = 111) and 2 (n = 52) of the COPDGene Study. Subjects without luminal plugging were not included.
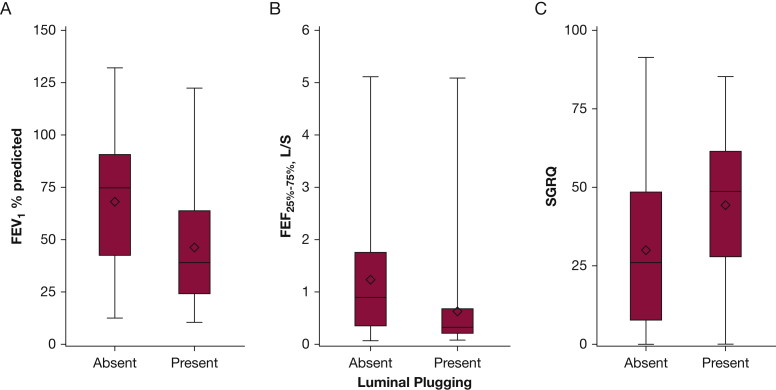
Overall, compared with subjects without luminal plugging, subjects with luminal plugging were older and more likely to have phlegm and dyspnea (Table 1). These subjects with luminal plugging have higher SGRQ total scores (worse HRQL); lower FEV1 % predicted, FVC % predicted, FEV1/FVC ratio, and FEF25%-75%; and greater CT scan measures of emphysema, air trapping, and airway wall thickness than those without plugging (Fig 4, Table 1).
Table 1.
All Subjects’ Baseline Characteristics by CT Scan-Identified Luminal Plugging Status
| Characteristics | Subjects Without Luminal Plugging (n = 389) | Subjects With Luminal Plugging (n = 111) | P Value |
|---|---|---|---|
| Age, y | 62 ± 9 | 65 ± 8 | .0002 |
| Female | 57 | 54 | .59 |
| African American race | 29 | 22 | .12 |
| BMI, kg/m2 | 28 ± 6 | 26 ± 6 | .0007 |
| Current smoking status, yes | 45 | 41 | .39 |
| Pack-years smoked | 46 ± 23 | 55 ± 35 | .002 |
| Cough | 47 | 58 | .053 |
| Phlegm | 39 | 55 | .002 |
| Dyspnea | 57 | 81 | < .0001 |
| Asthma | 11 | 20 | .03 |
| Chronic bronchitis | 22 | 35 | .006 |
| Bronchiectasis on CT scan | 28 | 50 | < .0001 |
| SGRQ total score | 30 ± 24 | 44 ± 23 | < .0001 |
| FEV1 % predicteda | 68 ± 29 | 46 ± 26 | < .0001 |
| FVC % predicteda | 87 ± 21 | 74 ± 19 | < .0001 |
| FEV1/FVC, %a | 58 ± 17 | 45 ± 17 | < .0001 |
| FEF25%-75%, L/seca | 1.2 ± 1.1 | 0.6 ± 0.8 | < .0001 |
| TLCCT % predicted | 99 ± 17 | 106 ± 15 | .0002 |
| %LAA-950 | 9 ± 12 | 17 ± 14 | < .0001 |
| %LAA-856 | 28.7 ± 21 | 46 ± 21 | < .0001 |
| Pi10, mm2 | 2.5 ± 0.6 | 2.8 ± 0.6 | < .0001 |
Values are mean ± SD, %, or as otherwise indicated. Missing data: TLCCT, %LAA-950, and Pi10, 24 each; %LAA-856, 83. FEF25%-75% = forced expiratory flow at 25% to 75% of the FVC; %LAA-856 = percentage of low attenuation areas < −856 Housfield units; %LAA-950 = percentage of low attenuation area < −950 Hounsfield units; Pi10 = the wall area of a theoretical airway of 10 mm luminal perimeter; SGRQ = St. George’s Respiratory Questionnaire. TLCCT = CT measure of total lung capacity.
Postbronchodilator pulmonary function measurements are presented.
Figure 4.
(A) FEV1 % predicted, (B) FEF25%-75%, and (C) SGRQ total score among subjects with COPD by luminal plug status at phase 1. The box plots show the median (horizontal line in the middle of the box), mean (diamond), and 25th and 75th percentiles (bottom and top lines of the box) of FEV1 % predicted, FEF25%-75%, and SGRQ total score. The whiskers represent the upper and lower values (1.5 times above the 75th percentile and below the 25th percentile, respectively). P < .0001 for the difference in FEV1 % predicted, FEF25%-75%, and SGRQ between those with and without luminal plugging in univariate analysis. See text for details in adjusted models for these outcomes. FEF25%-75% = forced expiratory flow at 25% to 75% of the FVC; SGRQ = St. George’s Respiratory Questionnaire.
Characteristics of the subjects with and without COPD and by luminal plugging status and by GOLD stage are shown in e-Tables 1 and 2. The prevalence of luminal plugging among subjects with COPD with asthma, chronic bronchitis, or bronchiectasis was higher than those without these conditions (37% vs 23%, P = .04; 34% vs 22%, P = .02; 36% vs 20%, P = .001, respectively). When asthma is excluded, the prevalence of luminal plugging among subjects with COPD (n = 340) was 23%. The prevalence of luminal plugging was higher in subjects with bronchodilator reversibility (n = 133) than those without bronchodilator reversibility (32% vs 22%, respectively; P = .012). Those having one or more COPD exacerbations in the previous year had a nonsignificant trend of a higher prevalence of luminal plugging than those who reported no exacerbation (30% vs 23%, respectively; P = .09). There were no differences in luminal plugging prevalence between current and former smokers with COPD (P = .64).
Associations of Luminal Plugging With Lung Function and SGRQ in Subjects With COPD
CT scan-identified luminal plugging was significantly associated with FEV1 % predicted (estimate, −6.1%; SE, 2.1%; P = .004) in adjusted models. The association between CT scan-identified luminal plugging and FEF25%-75% (log-transformed estimate, −0.11; SE, 0.06 L/s, P = .08) did not reach statistical significance. The robust association between luminal plugging and FEV1 suggests that luminal plugging is in the causal line of lung function impairment. Therefore, in subsequent main models for SGRQ, COPD phenotypes, and respiratory symptoms, we did not use FEV1 as a covariate to allow assessing the potential relationship of a modifiable feature (ie, luminal plugging) and those outcomes, regardless of disease severity. For completeness, secondary models with FEV1 as covariate are also shown. After adjustment for the same covariates used for the FEV1 model, luminal plugging was associated with SGRQ (estimate, 4.9; SE, 2.4; P = .04) (Table 2). The association between luminal plugging and SGRQ was not statistically significant when FEV1 was added to the model (P = .26).
Table 2.
Multivariable Analyses for the Associations Between CT Scan-Identified Luminal Plugging at Phase 1 and Spirometric Measures of Lung Function and SGRQ in Subjects With COPD
| Luminal Plugging | FEV1 % Predicted | Log FEF25%-75% (L/s) | SGRQ |
|---|---|---|---|
| Absent | Ref | Ref | Ref |
| Present | −6.1 (2.1), .004 | −0.11 (0.06), .08 | 4.9 (2.4), .04 |
Values are estimate (SE), P value. All models were adjusted for age, sex, race, BMI, current smoking status, smoking pack-years history, asthma, CT scan-identified bronchiectasis, and CT measures of emphysema and airway wall thickness. All analyses were the results from multivariable linear regression models. Ref = reference. See Table 1 legend for expansion of other abbreviations.
Association of Luminal Plugging With Emphysema and Chronic Bronchitis Phenotypes and Respiratory Symptoms in Subjects With COPD
Emphysema, defined as %LAA-950 ≥ 5% on CT scan, was present in 60% of the subjects with COPD. Luminal plugging was more prevalent in those with emphysema than those without emphysema (33% vs 13%, respectively; P < .0001). Based on emphysema and luminal plugging statuses, subjects were grouped into 4 groups and their characteristics are shown in e-Table 3. In a multivariable model, luminal plugging was related to the emphysema phenotype as continuous (estimate, 0.43; SE, 0.16) and binary (OR, 2.06; 95% CI, 1.09-3.90) variable (Table 3). Chronic bronchitis was present in 112 subjects with COPD (28%), and the prevalence of luminal plugging was higher in those with COPD and chronic bronchitis than those with COPD alone (34% vs 22%, respectively; P = .02). In a multivariable model, luminal plugging tended to increase the odds of chronic bronchitis, but the relationship did not reach statistical significance (OR, 1.68; 95% CI, 0.95-2.97; P = .07) (Table 3). Luminal plugging was related to dyspnea but not to phlegm in the main multivariable models (e-Table 4).
Table 3.
Multivariable Analyses for the Associations of CT Scan-Identified Luminal Plugging at Phase 1 With Emphysema and Chronic Bronchitis in Subjects With COPD
| Model | Log Emphysema on CT Scan | Emphysema ≥ 5% on CT Scan (Yes/No) | Chronic Bronchitis | |
|---|---|---|---|---|
| Model 1 | ||||
| Luminal plugging | Absent | Ref | Ref | Ref |
| Present | 0.43 (0.16), .007 | 2.06 (1.09-3.90), .02 | 1.68 (0.95-2.97), .07 | |
| Model 2 | ||||
| Luminal plugging | Absent | Ref | Ref | Ref |
| 0.21 (0.14), .14 | 1.52 (0.76-3.07), .24 | 1.55 (0.87-2.76), .14 | ||
Values are estimate (SE), P value or OR (95% CI), P value. Model 1 for chronic bronchitis was adjusted for age, sex, race, BMI, current smoking status, smoking pack-years history, asthma, CT scan-identified bronchiectasis, and CT scan measures of emphysema and airway wall thickness. The same covariates were used in the model for emphysema on CT scan but log-transformed %LAA-950. Model 2 has all model 1 covariates and FEV1. See Table 1 and 2 legends for expansion of abbreviations.
Luminal Plugging at Phase 2
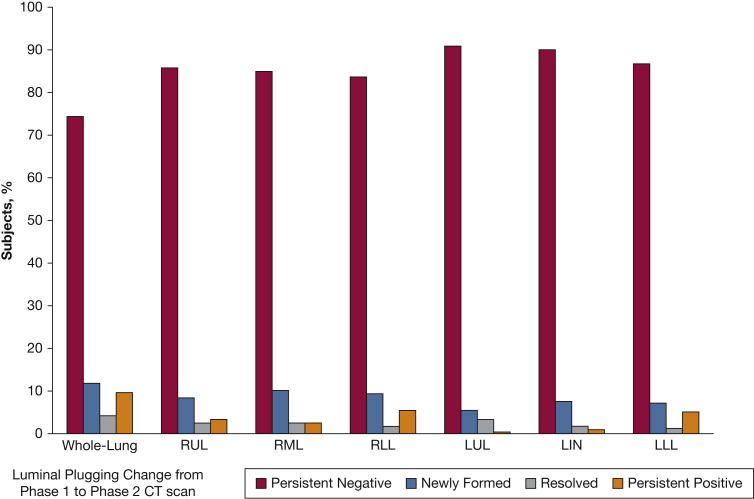
Out of 500 subjects, 287 returned to phase 2 study visit 5 years later, whereas 113 were deceased, and 100 were lost to follow-up. Phase 2 CT scan data were available on 238 subjects, including 178 subjects with COPD and 60 subjects without COPD. Luminal plugging was found in 52 subjects, with a higher prevalence in those with COPD than those without COPD (28% vs 5%, respectively). The distribution of luminal plug scores among all subjects is shown in Figure 3, and the changes in luminal plugging status from phase 1 CT scan to phase 2 CT scan is shown in Figure 5. In subjects with COPD, luminal plugs on phase 2 CT scan were also most frequently (82%) found in the lower lobes (Fig 2). Among subjects with COPD with luminal plugging at phase 1 (n = 30), 22 subjects (73%) had and 8 (27%) did not have luminal plugging at phase 2. Out of 148 subjects with COPD without luminal plugging at phase 1, 18% had luminal plugging at phase 2.
Figure 5.
Five-year changes in luminal plugging presence for the whole lung and by lobe in all study subjects with both phase 1 and phase 2 CT scans available (n = 238). The bars show the percentage of subjects by group as follows: persistent negative (no luminal plugging on phase 1 and 2 CT scans); persistent positive (luminal plugging on phase 1 and 2 CT scans); newly formed (no luminal plugging on phase 1 CT scan and luminal plugging on phase 2 CT scan); and resolved (luminal plugging on phase 1 CT scan and no luminal plugging on phase 2 CT scan). LIN = lingula; LLL = left lower lobe; LUL = left upper lobe; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe.
Discussion
We identified luminal plugging in relatively large intraparenchymal airways on chest CT scans from 500 current and former smokers with and without COPD, and found that the prevalence of luminal plugging was higher in subjects with COPD (vs non-COPD) and increased with spirometric GOLD stage. In COPD, luminal plugging is associated with decreased lung function, worse HRQL, and the emphysema phenotype. Furthermore, 73% of subjects with COPD with luminal plugging at baseline had this abnormality at 5-year follow-up. This study provides novel information on CT scan-identified luminal plugging and its change over time in COPD.
Increased mucus production, impaired mucociliary clearance, and more viscous mucus lead to plug formation in subjects with COPD.21 We found that 25% of subjects with COPD surveyed had luminal plugging, which was less prevalent than that reported in patients with asthma (58%-67%).5,22 The prevalence of subjects with luminal plugging increased with disease severity of COPD. This finding is similar to that reported in a lung tissue study where the extent of mucous exudates in the lumen of small airways increased with spirometric GOLD stage.3
In our study, CT scan-identified luminal plugging was associated with lower FEV1 % predicted, independently of overlapping asthma, bronchiectasis, and CT scan measures of emphysema and airway wall thickness. These findings are consistent with previous studies on patients with asthma, which reported a 25% decrease in FEV1 % predicted in those with high luminal plug score (≥ 4) compared with those without plugging, and that luminal plugging was associated with ventilation heterogeneity on MRI.5,22 Although our study cannot explain the mechanisms underlying these associations, possibilities include the following: (1) complete obstruction of the airways by luminal plugging leading to a regional or more widespread airflow limitation; (2) differing susceptibility to developing luminal plugging among subjects with severe vs mild COPD; and (3) COPD exacerbations, which are associated with luminal plugging, leading to loss of lung function. Further longitudinal studies are warranted to understand our observations better. Most subjects had lower lobe predominant luminal plugging. One explanation for this finding is gravity, which may make more mucus pooling in the lower lobe airways than upper lobe airways.
A novel finding in this study was that luminal plugging was directly associated with the emphysema phenotype of COPD, both when used as a continuous and binary variable. We are not aware of any other studies reporting this association. The association we observed does not prove causality; possible explanations for this relationship are as follows. First, subjects with COPD with luminal plugging have an increased inflammatory burden that may independently contribute to or facilitate lung parenchyma damage. In severe asthma, higher IL-5 and IL-13 concentrations in sputum cells were associated with high mucus plugs on CT scan, and IL-5 blood levels are higher in subjects with COPD with emphysema vs without emphysema.23 Second, subjects with COPD with emphysema might be prone to develop luminal plugging because of loss of airway tethering. Airways without tethering tend to collapse, and their lumen narrows.24 For a given amount of mucus, a collapsible airway may tend to form plugs more easily than a noncollapsible airway. In our main models for emphysema, we did not use FEV1 as a covariate based on the robust relationship between luminal plugging and airflow; and our goal was to examine this imaging feature, which might be amenable for therapeutic intervention as in other diseases, such as cystic fibrosis, regardless of the severity of airflow limitation.25
In contrast to the luminal plugging-emphysema relationship observed, we found luminal plugging was not related to chronic bronchitis or cough and phlegm. This finding is in agreement with a study on patients with COPD reporting a lack of relationship between luminal plugs in small airways and symptoms of cough and phlegm.3 Another study also failed to demonstrate an association between luminal plugging on CT scan and cough and phlegm in severe asthma.5 A possibility to explain this lack of association is that the airways where plugs form may have fewer cough receptors.5 We also found that 30% of subjects with COPD with CT scan-identified luminal plugging reported neither cough nor phlegm, suggesting that the identification of chronic mucus hypersecretion should not rely only on symptoms and that CT scan may provide a unique phenotype for mucus dysfunction.
Another interesting finding was that subjects with CT scan-identified luminal plugging (vs those without luminal plugging) had a thicker airway wall as measured by the wall area of a theoretical airway of 10 mm luminal perimeter. This wall thickening may reflect the remodeling that occurs in subjects susceptible to forming intraluminal plugs in large airways in a similar manner to what was described in small airways. Hogg et al2 demonstrated that in addition to mucous exudates occluding the lumen of small airways, the walls were also thickened. We also found that the prevalence of luminal plugging was higher in subjects with bronchodilator reversibility than those who did not have that response. Subjects with asthma and COPD (vs COPD alone) had a higher prevalence of luminal plugging as well. Because bronchodilator reversibility is used to diagnose asthma, these findings are consistent with the high prevalence of mucus plugging reported in asthma studies. Further studies are needed to understand the potential clinical impact of luminal plugging in subjects with asthma-COPD overlap.
In this study, we also found that subjects with luminal plugging on CT scan had worse HRQL than those without plug formation. The difference in SGRQ total score was not only statistically significant but clinically meaningful (> 4 points). This finding expands prior studies in COPD linking CT scan measures of emphysema and airway wall thickness with HRQL,25 an outcome used in ongoing clinical trials in smokers.26
The assessment of 5-year follow-up CT images provides novel insight into temporal changes in CT scan-identified luminal plugging in COPD. Seventy-three percent of subjects with COPD with luminal plugging at phase 1 had this imaging feature in phase 2, suggesting that a subgroup of subjects tends to be persistent producers of mucus plugs. Although our study does not address the underlying mechanism of mucus plug formation, it has been suggested to be a result of elevated type 2 inflammation as seen in severe asthma.5
This study has several limitations. First, we used a cohort of heavy smokers with and without COPD, and in our study design, severe and very severe COPD is overrepresented. Although this might not represent the full spectrum of smokers, our sampling did include the full spectrum of COPD stages. Second, although our findings lack histologic validations, our main results were consistent with those from the previous lung tissue studies in COPD.2,3 Third, we used luminal plugging as a binary variable, not the score, for our primary analyses. Moreover, although our score slightly differed from that used in asthma studies making the scores not directly comparable, we found similar findings to the previous asthma study that used a three-category luminal plug score.5 We think the validation and clinical utility of a luminal plug score in subjects with COPD deserves further study. Finally, although the number of subjects with phase 2 CT scans available was relatively low, limiting our ability to assess the clinical impact of luminal plugging over time, we did provide novel data on this imaging feature in COPD at two points in time. Further large longitudinal studies may be warranted.
In summary, CT scan-identified luminal plugging is frequent in subjects with COPD and related to disease severity, worse HRQL, and emphysema phenotype of COPD. CT imaging might be a useful tool to assess luminal plugging and potentially to evaluate novel therapies targeting mucus dysfunction.
Acknowledgments
Author contributions: All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Y. O. and A. A. D contributed to study concept/study design. Y. O., P. N., D. A. L., G. R. W., R. S. J. E, and A. A. D. contributed to data acquisition and analysis. All authors contributed to interpretation for the work. Y. O., C. E. C., and A. A. D. contributed to drafting the work. All authors contributed to critical revision for relevant intellectual content and final approval of this manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: C. E. C. reports a grant from the NIH/NHLBI during the conduct of the study. A. Y. receives salary support from NIH-funded research. N. T. is site PI for a UAB subcontract on a grant for a study on Subclinical Interstitial Lung in MESA and FAR-ILD. D. A. L. has received grant support from the NHLBI for COPDGene. B. J. M. reports the following: Astra Zeneca: international PI for multicenter clinical trial, medical advisory board, disease-state presentation, and grant funds provided to and controlled by the National Jewish Health; Spiration: reviewed clinical trial data, and data and safety monitoring board; Glaxo Smith Kline: advisory board member, disease-state presentation, and multicenter trial funds provided to and controlled by National Jewish Health; Sunovian Medical Board: member, and grant funds provided to and controlled by National Jewish Health; Mt. Sinai Medical Center: CME activity; WebMD: CME activity; National Jewish Health: CME activity; Novartis: CME activity; NHLBI: grant funds provided to and controlled by National Jewish Health; American College of Chest Physicians: CME activity; Projects in Knowledge: CME activity; Hybrid Communications: CME activity; Pearl Research: funds provided to and controlled by the National Jewish Health; Medscape: CME activity; Verona: medical advisory board; Boehringer Ingelheim: medical advisory board; Thervance: medical advisory board; Ultimate Medical Academy: CME activity; Circassia: medical advisory board; Third Pole: consultant for proposed trial; Shire: data and safety monitoring board; Phillips: medical advisory board; Eastern Pulmonary Society: CME activity; Catamount Medical: CME activity; Science 24/7: medical advisory board; Eastern VA Medical Center: CME activity; and Academy Continued Health Care Learning: CME activity. E. K. S. has received grant and travel support from GlaxoSmithKline. G. R. W. reports grants from the NIH, Boehringer Ingelheim, BTG Interventional Medicine, and Janssen Pharmaceuticals; reports consultancies/advisory board participation for Boehringer Ingelheim, Janssen Pharmaceuticals, GlaxoSmithKline, PulmonX, Novartis, Philips, and Vertex; is a cofounder and equity share holder of Quantitative Imaging Solutions; and his wife works for Biogen. R. S. J. E. reports grants from the NIH, during the conduct of the study; grants from the NHLBI; personal fees from Toshiba, Boehringer Ingelheim, Eolo Medical, and Leuko Labs, outside the submitted work; and is also a founder and co-owner of Quantitative Imaging Solutions, which is a company that provides image-based consulting and develops software to enable data sharing. A. A. D. has received funding from the NHLBI [Grant R01-HL133137] and through the Brigham and Women’s Hospital Minority Faculty Career Development Award. None declared (Y. O., P. N., S. K. S., H. P. N., S. A. G., A. A., S. K., K. J.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The National Heart, Lung, and Blood Institute (NHLBI) supports the COPDGene Study [Grants U01 HL089897, U01 HL089856]. The COPD Foundation also supports the COPDGene Study [Grant NCT00608764] through contributions made to an Industry Advisory Committee composed of AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Sunovion. Dr Diaz is supported by the NHLBI [Grant R01-HL133137] and the Brigham and Women’s Hospital Minority Faculty Career Development Award.
Supplementary Data
References
- 1.Burgel P.R., Martin C. Mucus hypersecretion in COPD: Should we only rely on symptoms? Eur Respir Rev. 2010;19(116):94–96. doi: 10.1183/09059180.00004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg J.C., Chu F., Utokaparch S. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 3.Hogg J.C., Chu F.S., Tan W.C. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med. 2007;176(5):454–459. doi: 10.1164/rccm.200612-1772OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakano Y., Wong J.C., de Jong P.A. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171(2):142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 5.Dunican E.M., Elicker B.M., Gierada D.S. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128(3):997–1009. doi: 10.1172/JCI95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim V., Criner G.J. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(3):228–237. doi: 10.1164/rccm.201210-1843CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kesimer M., Ford A.A., Ceppe A. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377(10):911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan E.A., Hokanson J.E., Murphy J.R. Genetic epidemiology of COPD (COPDGene) study design. Copd. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bankier A.A., Fleischmann D., Mallek R. Bronchial wall thickness: appropriate window settings for thin-section CT and radiologic-anatomic correlation. Radiology. 1996;199(3):831–836. doi: 10.1148/radiology.199.3.8638013. [DOI] [PubMed] [Google Scholar]
- 10.Washko G.R., Lynch D.A., Matsuoka S. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. 2010;17(1):48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netter F. Medical Education and Publishing, Ciba-Geigy Corporation; Summit, NJ: 1989. Atlas of Human Anatomy. [Google Scholar]
- 12.Diaz A.A., Young T.P., Maselli D.J. Quantitative CT measures of bronchiectasis in smokers. Chest. 2017;151(6):1255–1262. doi: 10.1016/j.chest.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 15.Vestbo J., Hurd S.S., Agusti A.G. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 16.Pellegrino R., Viegi G., Brusasco V. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 17.Diaz A.A., Petersen H., Meek P., Sood A., Celli B., Tesfaigzi Y. Differences in health-related quality of life between New Mexican Hispanic and non-Hispanic white smokers. Chest. 2016;150(4):869–876. doi: 10.1016/j.chest.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz A.A., Valim C., Yamashiro T. Airway count and emphysema assessed by chest CT imaging predicts clinical outcome in smokers. Chest. 2010;138(4):880–887. doi: 10.1378/chest.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim V., Crapo J., Zhao H. Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc. 2015;12(3):332–339. doi: 10.1513/AnnalsATS.201411-518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han M.K., Tayob N., Murray S. Association between emphysema and chronic obstructive pulmonary disease outcomes in the COPDGene and SPIROMICS cohorts: a post hoc analysis of two clinical trials. Am J Respir Crit Care Med. 2018;198(2):265–267. doi: 10.1164/rccm.201801-0051LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim V., Evans C.M., Dickey B.F. Dawn of a new era in the diagnosis and treatment of airway mucus dysfunction. Am J Respir Crit Care Med. 2019;199(2):133–134. doi: 10.1164/rccm.201808-1444ED. [DOI] [PubMed] [Google Scholar]
- 22.Svenningsen S., Haider E., Boylan C. CT and functional MRI to evaluate airway mucus in severe asthma. Chest. 2019;155(6):1178–1189. doi: 10.1016/j.chest.2019.02.403. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M., Nakamura H., Minematsu N. Plasma cytokine profiles related to smoking-sensitivity and phenotypes of chronic obstructive pulmonary disease. Biomarkers. 2014;19(5):368–377. doi: 10.3109/1354750X.2014.915342. [DOI] [PubMed] [Google Scholar]
- 24.Diaz A.A., Han M.K., Come C.E. Effect of emphysema on CT scan measures of airway dimensions in smokers. Chest. 2013;143(3):687–693. doi: 10.1378/chest.12-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez C.H., Chen Y.H., Westgate P.M. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67(5):399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.University of Michigan and National Heart, Lung, and Blood Institute. RETHINC: REdefining THerapy In Early COPD for the Pulmonary Trials Cooperative. NCT02867761. ClinicalTrials.gov. Bethesda, MD:National Institutes of Health; 2007. https://clinicaltrials.gov/ct2/show/NCT02867761. Updated July 26, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.