Abstract
Background:
Mediastinitis after a median sternotomy can be life-threatening. The advent of pedicle flap–based treatment has resulted in an improvement in both morbidity and mortality. However, significant morbidities can still occur following the use of flaps for sternal closure, particularly in patients with comorbidities. To minimize an extensive surgical dissection, we modified our approach to reconstruction using a modified subpectoral approach, leaving the overlying skin attached. This technique focuses primarily on controlling wound tension rather than on maximal muscle coverage. This study is a retrospective review of 58 consecutive patients treated with this approach, by a single surgeon.
Methods:
Fifty-eight consecutive patients treated between 2008 and 2019 were included. All patients received the same procedure regardless of the degree of illness, the extent of tissue loss, and the size of sternal defect. Treatment included thorough debridement, with total sternectomy (if required); limited dissection of the pectoralis major muscle off the chest wall to the level of the pectoralis minor without skin and subcutaneous undermining; no release of the insertion of the pectoralis or use of the rectus abdominis; and midline closure over drains connected to wall suction to obliterate dead space.
Results:
Reoperations were required in 7 patients (12%). Of these, only 4 (6.9%) were related to continued sternal osteomyelitis. The other reoperations were for hematoma evacuation, breast fat necrosis, and skin necrosis. There were no operative mortalities.
Conclusion:
Chest closure using minimal dissection and tension release is safe, efficient, and associated with a complication rate equivalent to more extensive procedures reported in the literature despite significant comorbidities.
INTRODUCTION
Median sternotomy wound breakdown after a cardiac surgery has a reported incidence of 0.3%–5%.1 Devascularization of the sternum using the internal mammary artery partially explains this phenomenon,2 along with a high rate of comorbidities, including obesity, diabetes, malnutrition, and macromastia. Mortality rate due to this devascularization can be as high as 25%.3 Management of this condition has evolved over the last 4 decades, with subsequent improvement of outcomes and decrease in postoperative mortality.1,4,5 Replacing the extensively debrided sternal bone and soft tissue with well-vascularized muscle flaps, especially the pectoralis major, is the basis of mediastinal wound dehiscence treatment.1,3 However, to reduce postoperative morbidity and complication rates, we have adopted a technique of minimizing dissection to achieve a minimal tension closure instead of maximal muscle coverage. This procedure allows for shorter operative times and reduced blood loss in these already compromised patients. Since 2008, the senior author (E.C.) has treated all chest closure patients with the same procedure: bilateral pectoralis major muscle dissected only in the plane submuscular to the medial border of the pectoralis minor and drains on continuous suction to control dead space. The pectoralis muscle is not released from its insertion or from the overlying skin. The rectus muscle is not used for the lower sternum.
METHODS
Following Institutional Review Board Approval, a retrospective data review was done for all patients with sternal dehiscence following a median sternotomy between 2008 and 2019. Collected demographics included age, body mass index (BMI), smoking status, and comorbidities (diabetes, renal failure, and coronary artery disease). Patient data encompassed indications for original median sternotomy, time from initial surgery to mediastinal breakdown, level of debridement (soft-tissue debridement, partial sternectomy, hemi-sternectomy, or total sternectomy), blood loss, operative time, time from wound breakdown repair, and subsequent complications. Data analysis was performed using R statistical program language (R Core Team, 2019; Vienna, Austria).
All patients, regardless of their comorbidities or size of defect, were treated in the same manner. Extensive debridement of ischemic, necrotic, or infected bone or soft tissue in the wound was performed initially and was repeated until only healthy tissue remained. Occasionally, a wound vacuum was used, but this was not part of our standard algorithm. When a suitable granulation bed was noted, the patients’ tissue edges were freshened and muscle flaps were developed on each side. Dissection started at the inferior border at the plane above the rectus fascia and was carried cephalad to the pectoralis major. The pectoral origins were detached from the sternum and medial ribs up to the sternal notch. The dissection was carried laterally until the medial border of the pectoralis minor can be seen. The subglandular (breast) plane was not breached, and the skin remained attached to the muscle. Polyglactin sutures (2-0) approximated the bilateral pectoralis fascia. Regardless of the size of the defect, the humeral insertion remained undisturbed. Scarpa’s fascia alone was used to close the soft tissue caudally. No attempt of muscle coverage was done at the lower half of the wound. The skin was closed with polypropylene 2-0 sutures and staples. Three 19-French Jackson-Pratt drains were placed into the wound: 1 under each pectoralis muscle and 1 in the midline over the sternal gap. All 3 drains were connected by Y connectors to a single-wall suction to collapse the dead space (Fig. 1). The wound was dressed with gauze and an antimicrobial adhesive drape (Ioban; 3M, St. Paul, Minn.) that stretched across the chest nipple to nipple. The adhesive drape also tended to reduce tension on the wound in large-breasted patients. A surgical bra was also used in female patients. Continuous wall suction was kept on the drains until the fifth postoperative day. Then the drains were placed individually on bulb suction. Suction was maintained until drainage was minimal.
Fig. 1.
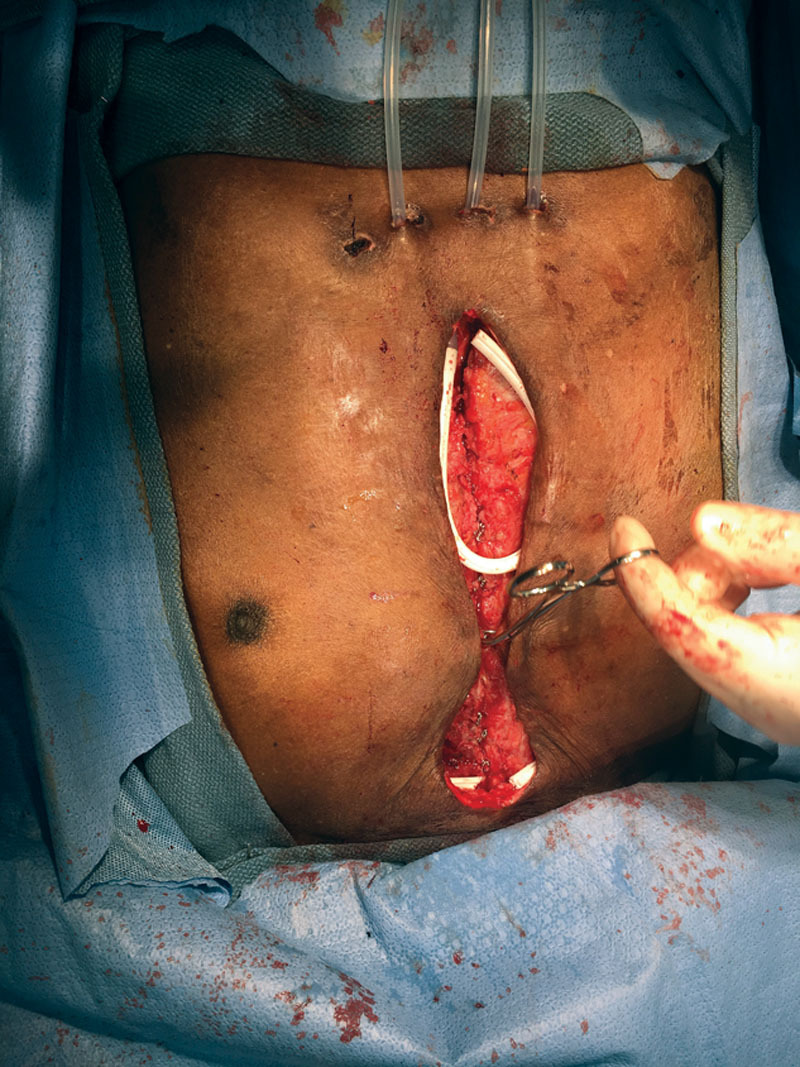
The chest wall is closed after tension release. The instrument marks the inferior border of the pectoralis muscle closure.
RESULTS
Fifty-eight consecutive patients with a median sternotomy with subsequent sternal wound breakdown met the inclusion criteria. Patients’ age ranged from 36 to 87 years, with a median age of 64 years. BMI ranged from 18 to 50 kg/m2, with a median of 32.59 kg/m2. Forty-one patients had insulin-dependent diabetes mellitus (70.7%), 27 patients were active smokers (46.5%), and 23 patients had end-stage renal disease (39.6%).
Indications for median sternotomy were coronary artery bypass graft in 46 patients (79.3%), 2 of which were revision coronary artery bypass graft. Valve replacement occurred in 6 patients (10.3%), placement of a left ventricular assist device in 3 patients (5.2%), and repair of type A aortic dissection in 3 patients (5.2%). Preoperative demographics are summarized in Table 1.
Table 1.
Preoperative Demographics
| Category | Median (Range) | No. Patients | % |
|---|---|---|---|
| Age | 64 (36–87) | ||
| BMI | 32.5 (18–50) | ||
| Comorbidity | |||
| Active smoker | 27 | 46.5 | |
| IDDM | 41 | 70.7 | |
| ESRD | 23 | 39.6 | |
| Indication for median sternotomy | |||
| CABG | 46 | 79.3 | |
| Valve replacement | 6 | 10.3 | |
| LVAD | 3 | 5.2 | |
| Type A aortic dissection | 3 | 5.2 | |
CABG, coronary artery bypass graft; ESRD, end-stage renal disease; IDDM, insulin-dependent diabetes mellitus; LVAD, left ventricular assist device.
Wound dehiscence occurred at 1–6 weeks from the index surgery, with an average of 19.6 days. At the time of reclosure, patients had different degrees of debridement. Ten patients had a total sternectomy (17.3%); 13 patients required a hemi-sternectomy, usually on the left (22.4%); 22 patients had a partial sternectomy (37.9%); and 13 patients required only soft-tissue debridement (22.4%). Because of the wide areas of exposed bone surface, re-culture of the entire bone surface was not feasible. Therefore, bone excision was performed until a healthy bleeding bone was observed. The average number of debridement performed by the plastic surgery services was 0.91 (range 0–2). The reason for the lower number of debridements was that the cardiothoracic service may have adequately debrided the wound before referral to the plastic surgery service. The average total number of debridement for both services was 2.16. Between debridements, a wound vacuum was placed in the wound. Wounds were generally closed during the last debridement except for those patients with renal failure. In the latter case, final closure was postponed until after the final debridement. The average length of time between the initial cardiac procedure and the first debridement was 35.89 days (range 7–135 days). The time of the initial debridement probably approximates the time that the infection was first realized by the cardiothoracic surgeons. Seventeen patients had only one debridement, and then the wound was closed.
Average operative time was 81 minutes, and average blood loss was 138 ml. Complications occurred in 15 patients (25.8%). Seven patients required reoperation (12%), and no postoperative mortality was reported during the study. Four patients had a failure of flap closure secondary to continued osteomyelitis (6.8%). One of these patients had mixed gram-negative organisms requiring massive re-debridement and an omental flap, as the defect was too wide to close by re-advancing the pectoralis muscles. The other 3 grew various species of Staphylococcus aureus and were treated with bone re-debridement and reclosure with the pectoralis flaps. The remaining 3 patients had additional surgery for hematoma evacuation, resection of breast fat necrosis, and revision of skin necrosis, respectively. Average follow-up time was 22.7 months, with a range of 2–101 months. Additional complications included 2 seromas, 2 hematomas evacuated at bedside after correction of coagulopathies, and 4 superficial wound breakdown treated conservatively with dressing changes. One patient required a wound vacuum. None of these patients required a return to the operating room. Postoperative data are listed in Table 2. A Firth’s logistic regression model was applied to the data in an effort to determine whether smoking, diabetes, age, BMI, renal failure, number of debridements, or time period from the initial cardiac procedure to the first debridement could be specifically linked to the failure of the procedure in terms of either complications or the need to return to the operating room following the flap surgery. Although the sample size was small, we could find no statistically significant correlation between these comorbid conditions or primary operations and the failure of the initial pectoralis flap closure (Table 3).
Table 2.
Operative Data and Complications
| No. Patients | % | |
|---|---|---|
| Degree of sternal debridement | ||
| Total sternectomy | 10 | 17.3 |
| Hemi-sternectomy | 13 | 22.4 |
| Partial sternectomy | 22 | 37.9 |
| Soft-tissue debridement | 13 | 22.4 |
| Postoperative complications | ||
| Seroma | 2 | 3.4 |
| Hematoma | 3 | 5.2 |
| Superficial wound breakdown | 6 | 10.3 |
| Continued osteomyelitis | 4 | 6.9 |
Table 3.
Firth Logistic Regression Model
| OR (95% CI) | P | |
|---|---|---|
| Return to OR as the outcome | ||
| For the outcome variable, Y = 1 when return to OR 2 = 1 | ||
| BMI | 1.033 (0.913–1.169) | 0.587 |
| Renal failure, Yes | 0.306 (0.019–2.478) | 0.276 |
| Diabetes, Yes | 4.335 (0.375–595.699) | 0.272 |
| Age at the time of surgery | 0.960 (0.861–1.067) | 0.429 |
| Current former smoker, Yes | 0.643 (0.040–5.611) | 0.699 |
| No. debridements prior to closure | 1.269 (0.510–3.384) | 0.600 |
| Time between cardiac surgery and initial debridement | 1.006 (0.975–1.031) | 0.667 |
| Success (no complications) as outcome | ||
| For the outcome variable, Y = 1 when success 2 = 1 | ||
| BMI | 0.995 (0.919–1.080) | 0.902 |
| Renal failure, Yes | 1.031 (0.254–4.417) | 0.966 |
| Diabetes, Yes | 1.710 (0.389–7.841) | 0.473 |
| Age at time of surgery | 1.020 (0.957–1.087) | 0.528 |
| Current former smoker, Yes | 1.070 (0.290–4.192) | 0.919 |
| No. debridements before closure | 0.707 (0.383–1.277) | 0.248 |
| Time between cardiac surgery and initial debridement | 0.989 (0.970–1.009) | 0.290 |
CI, confidence interval; OR, operating room.
DISCUSSION
Median sternotomy complications after major cardiac and aortic surgery are uncommon, but significant events. Mediastinitis occurs in 0.25%–5% of patients undergoing median sternotomy, with mortality rates as high as 10%–25% in some reports.1,6–8 Multiple solutions have been advocated for reconstruction: pectoralis flaps, rectus abdominis flaps, latissimus flaps, and omentum. These procedures have improved outcomes and significantly reduced mortality for these patients. Jurkiewicz et al1 in their classic paper reported no deaths in the group treated with muscle flap closure compared to significant mortality in the group treated with closed mediastinal catheter irrigation Regardless of the flap used, a common goal was to remove necrotic tissue and fill tissue gaps.9,10
A review of the literature of the management of deep sternal wound infections reveals 4 variables that may influence the outcome in the patient population. Comorbidities such as significant heart disease, diabetes mellitus, end-stage renal disease, obesity, chronic obstructive pulmonary disease, reoperation for bleeding, and nicotine use are all documented to contribute to complications and poorer outcomes.10–21 Some of these preoperative factors can be optimized. However, the need for cardiovascular intervention often does not allow for patient optimization. The second factor to impact outcome is the timing between recognition of a deep sternal infection and surgical intervention.16,19,22–24 Lo et al25 calculated that each day of delay from diagnosis to flap coverage increased the risk of chronic wound infection by a factor of 1.2/day. The third factor is the adequacy of debridement of all infected tissue and contaminated foreign bodies.14,24–29 Finally, the reconstructive method itself can influence outcomes. Operations that require extensive dissection and inhibition of muscle function can further inhibit healing and rehabilitation. Piwnica-Worms et al14 have shown that the use of vertical rectus abdominis musculocutaneous flap in a series of 119 patients was predictive of increased mortality. Other investigators have demonstrated loss of shoulder function with disinsertion of the pectoralis muscle either as a turnover flap or as an advancement flap.22,30–32 On the other hand, Kamel et al23 showed that patients who had pectoralis advancement flaps had less tissue necrosis than those who had turnover flaps. Furthermore, the pectoralis turnover flap relies on blood supply from the internal mammary artery. If this vessel is used for coronary perfusion, the flap on that side is not available.
The pectoralis major turnover flap and the division of the humeral insertion in the pectoralis advancement flap are examples of the functional sacrifice undertaken in some chest closure techniques.1,3,5,10,11 Advancement flaps without insertion division do not compromise the function of the muscle as much and are not dependent on the internal mammary artery.25,26,33 Suturing the muscle to the contralateral muscle may well preserve most of the origin function of the muscle, although this was not specifically tested.
A number of authors advocate for separation of the muscle from the overlying skin and subcutaneous tissue to allow greater freedom to mobilize the muscle medially.22,27,31,33,34 In 1994 and 1995, Ascherman’s group35,36 published a series of cases where the pectoralis muscle was used as a myocutaneous flap by not separating the muscle from the overlying skin and subcutaneous tissue. The outcome from this series of patients was improved upon by the same group a decade later37 by modifying the procedure by leaving drains longer (reducing the seroma rate), using larger sutures with bigger bites (to decrease dehiscence), and minimally dissecting the pectoralis muscle laterally until the wound could be closed with minimal tension. Prior reports had also stressed the need to fill the presumed dead space between the soft tissue and the myocardium following sternal debridement.22,26,28,33,38 The Ascherman group showed that this was not necessary.35,37–39 In fact, just as in our study, postoperative CT scans obtained for reasons unrelated to the flap surgery have shown early obliteration of the mediastinal space with just the advancement flaps37 (Fig. 2). We have used the Ashcerman technique with several modifications, including using wall suction for 5 days and Ioban to help relieve tension on the closure (particularly in large-breasted women), and we do not mobilize the upper rectus sheath in continuity with the muscle to cover the lower portion of the sternum.37 Our hypothesis is that dead space is obliterated by constant low steady wound tension and scar contracture, possibly facilitated by early wall suction. However, further review of postoperative CT scans would be required to confirm this concept.
Fig. 2.
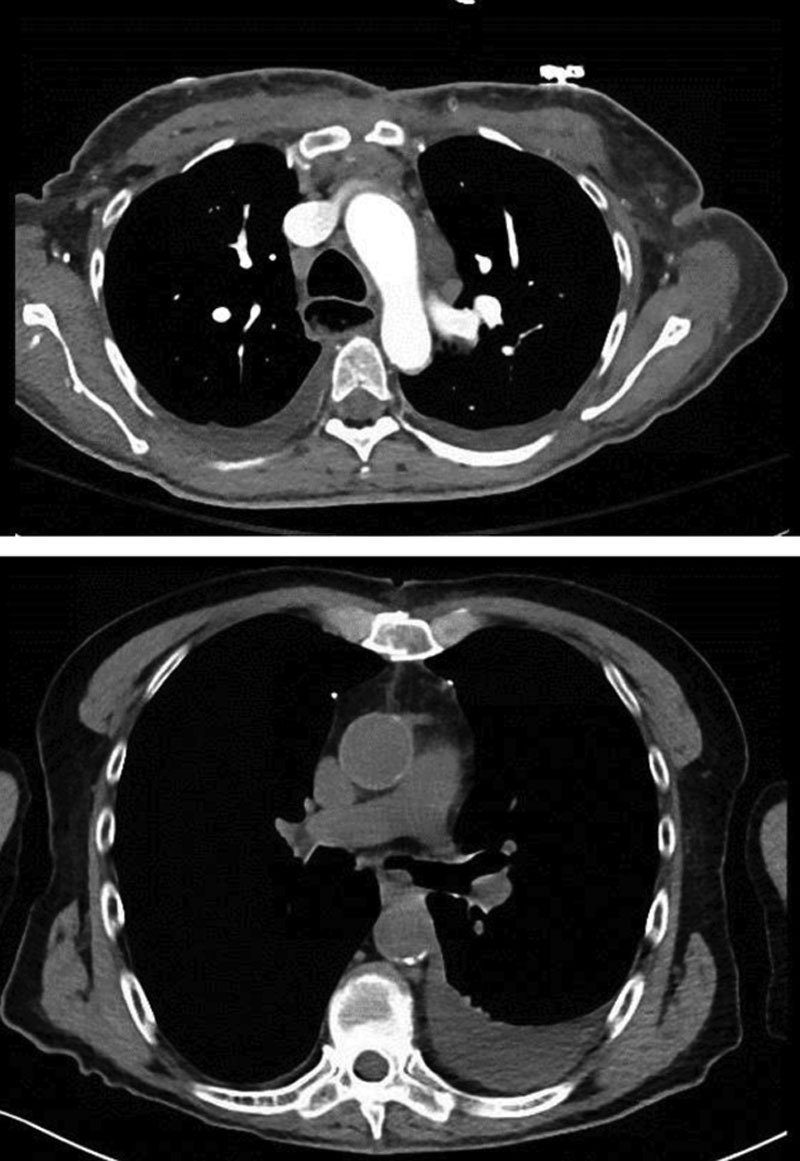
Two different patients’ incidental postoperative computed tomography scan. Sternectomy dead space has narrowed or obliterated on its own.
Although multiple debridements were done in our patient population until the wound bed was presumed clean by the presence of widespread granulation tissue, in most cases only 1–2 debridements were required. Then a relatively minimal procedure was undertaken to close the wound, concentrating on tension release rather than on full-muscle coverage of the wound and dead space. Pectoralis undermining is used for mobilization of the overlying skin, not for full coverage of the mediastinum. Dead spaces were collapsed using Jackson-Pratt drains on wall suction.
The total complication rate was 25.8%, including minor and major (requiring reoperation) complications. Although significant, it should be noted that only 4 patients (6.8%) required additional surgery. This is a fairly low percentage of reoperations given the high rate of comorbidities in this patient population. These numbers are comparable to other, more extensive, methods of closure5,11,37,39 (Table 4). Hematomas occurred in 3 patients. These were detected by persistent sanguineous drain output. For 2 patients, this was treated by correcting coagulopathy; the other required operative wound exploration. Flap necrosis or complete wound dehiscence was not encountered. Four cases of continued osteomyelitis, despite widespread sternal debridement, were the only form of deep wound complications. This problem manifested as persistent purulent drainage from the area of the wound with nonhealing or partial dehiscence. Three patients were treated successfully with re-debridement and simple reclosure. Only one required secondary closure with an omental flap.
Table 4.
Comparison with Other Techniques
| Author | Technique | No. Patients | Morbidity, % | Mortality, % |
|---|---|---|---|---|
| Nahai et al5 | PMF, OF, RAF | 211 | 64 | 5.3 |
| Pairolero et al11 | PMF, OF, RAF, OEF, LDF | 100 | 56 | 2 |
| Ascherman et al37 | PMF + rectus fascia | 114 | 16.7 | 0.9 |
| Zahiri et al21 | PMF, OF, LDF, RAF | 106 | 26 | 2 |
| Preminger et al39 | Modified PMF | 25 | 24 | 0 |
| Spindler et al28 | LDF | 69 | 35 | 20 |
| Barbera et al 201940 | PMF | 73 | 9.6 | 2.7 |
| Piwnica-Worms et al14 | PMF, OF, VRAM | 119 | 65 | 15.1 |
| This Study | Refined PMF | 59 | 25 | 0 |
EOF, external oblique flap; LDF, latissmus dorsi flap; OF, omental flap; PMF, pectoralis major flap; RAF, rectus abdominis flap; VRAM, vertical rectus abdominis flap.
In summary, this technique has allowed the authors to achieve comparable results but with a lower operative time, limited dissection, and probably less functional muscle morbidity than seen with division of the muscle insertion.
Our experience emphasized the importance of sternal debridement and infection control before chest closure. The major complications were noted early in the series, due to an underappreciation of suboptimal bone debridement and osteomyelitis control. In these 4 cases, the same bacteria were cultured before flap closure and before final debridement and reclosure, indicating a probably inadequate initial debridement.
Thus, based on the findings of this series of patients, we believe that limited pectoralis dissection—combined with adequate debridement and wall suction drainage, immediately postoperatively—is sufficient to treat the majority of sternal wound infections and breakdown after an open-heart surgery.
Footnotes
Published online 23 June 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Jurkiewicz MJ, Bostwick J, III, Hester TR, et al. Infected median sternotomy wound. Successful treatment by muscle flaps. Ann Surg. 1980;191:738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossi EA, Esposito R, Harris LJ, et al. Sternal wound infections and use of internal mammary artery grafts. J Thorac Cardiovasc Surg. 1991;102:342–346; discussion 346. [PubMed] [Google Scholar]
- 3.Francel TJ, Kouchoukos NT. A rational approach to wound difficulties after sternotomy: reconstruction and long-term results. Ann Thorac Surg. 2001;72:1419–1429. [DOI] [PubMed] [Google Scholar]
- 4.Lee AB, Jr, Schimert G, Shaktin S, et al. Total excision of the sternum and thoracic pedicle transposition of the greater omentum; useful strategems in managing severe mediastinal infection following open heart surgery. Surgery. 1976;80:433–436. [PubMed] [Google Scholar]
- 5.Nahai F, Rand RP, Hester TR, et al. Primary treatment of the infected sternotomy wound with muscle flaps: a review of 211 consecutive cases. Plast Reconstr Surg. 1989;84:434–441. [DOI] [PubMed] [Google Scholar]
- 6.Sarr MG, Gott VL, Townsend TR. Mediastinal infection after cardiac surgery. Ann Thorac Surg. 1984;38:415–423. [DOI] [PubMed] [Google Scholar]
- 7.Loop FD, Lytle BW, Cosgrove DM, et al. J. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg. 1990;49:179–186; discussion 186. [DOI] [PubMed] [Google Scholar]
- 8.Gummert JF, Barten MJ, Hans C, et al. Mediastinitis and cardiac surgery: an updated risk factor analysis in 10,373 consecutive patients. J Thorac Cardiovasc Surg. 2002;50:87. [DOI] [PubMed] [Google Scholar]
- 9.Milano CA, Georgiade G, Muhlbaier LH, et al. Comparison of omental and pectoralis flaps for poststernotomy mediastinitis. Ann Thorac Surg. 1999;67:377–380; discussion 380. [DOI] [PubMed] [Google Scholar]
- 10.Jones G, Jurkiewicz MJ, Bostwick J, et al. Management of the infected median sternotomy wound with muscle flaps. The Emory 20-year experience. Ann Surg. 1997;225:766–776; discussion 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pairolero PC, Arnold PG, Harris JB. Long-term results of pectoralis major muscle transposition for infected sternotomy wounds. Ann Surg. 1991;213:583–589; discussion 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Feo M, Renzulli A, Ismeno G, et al. Variables predicting adverse outcome in patients with deep sternal wound infection. Ann Thorac Surg. 2001;71:324–331. [DOI] [PubMed] [Google Scholar]
- 13.Zahiri HR, Medina D, Kelishadi S, et al. Risk factors for increased morbidity post tissue flap reconstruction of complicated sternal wound. Plast Reconstr Surg. 2011;127:94. [Google Scholar]
- 14.Piwnica-Worms W, Azoury SC, Kozak G, et al. Flap reconstruction for deep sternal wound infections: factors influencing morbidity and mortality. Ann Thorac Surg. 2020;109:1584–1590. [DOI] [PubMed] [Google Scholar]
- 15.Buja A, Zampieron A, Cavalet S, et al. An update review on risk factors and scales for prediction of deep sternal wound infections. Int Wound J. 2012;9:372–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phoon PHY, Hwang N. Deep sternal wound infection: diagnosis, treatment, and prevention. J Cardiothoracic Vasc Anesth. 2020;34:1602–1613. [DOI] [PubMed] [Google Scholar]
- 17.Patel NV, Woznick AR, Welsh KS, et al. Predictors of mortality after muscle flap advancement for deep sternal wound infections. Plast Reconstr Surg. 2009;123:132–138. [DOI] [PubMed] [Google Scholar]
- 18.Toumpoulis IK, Anagnostopoulos CE, Derose JJ, Jr, et al. The impact of deep sternal wound infection on long-term survival after coronary artery bypass grafting. Chest. 2005;127:464–471. [DOI] [PubMed] [Google Scholar]
- 19.Abboud CS, Wey SB, Baltar VT. Risk factor for mediastinitis after cardiac surgery. Ann Thorc Surg. 2004;77:676–683. [DOI] [PubMed] [Google Scholar]
- 20.Omran AS, Karimi A, Ahmadi SH, et al. Superficial and deep sternal wound infection after more than 9000 Coronary Artery Bypass Grafts (CABG): incidence, risk factors and mortality. BMC Infect Dis. 2007;7:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zahiri HR, Lumpkins K, Kelishadi SS, et al. Significant predictors of complications after sternal wound reconstruction: a 21-year experience. Ann Plast Surg. 2012;69:439–441. [DOI] [PubMed] [Google Scholar]
- 22.Cabbabe EB, Cabbabe SW. Immediate versus delayed one-stage sternal débridement and pectoralis muscle flap reconstruction of deep sternal wound infections. Plast Reconstr Surg. 2009;123:1490–1494. [DOI] [PubMed] [Google Scholar]
- 23.Kamel GN, Jacobson J, Rizzo AM, et al. Analysis of immediate versus delayed sternal reconstruction with pectoralis major advancement versus turnover muscle flaps. J Reconstr Microsurg. 2019;35:602–608. [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Chung KC, Waljee JF, et al. A national study of the impact of initial debridement timing on outcomes for patients with deep sternal wound infection. Plast Reconstr Surg. 2016:137:414e–423e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo S, Hutson K, Hallam MJ, et al. The importance of early flap coverage in deep sternal wounds. Ann Plast Surg. 2014;73:588–590. [DOI] [PubMed] [Google Scholar]
- 26.Schiraldi L, Jabbour G, Centofanti P, et al. Deep sternal wound infections: evidence for prevention, treatment, and reconstructive surgery. Arch Plast Surg. 2019;46:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Zhang J, Liu Z. Vacuum-assisted closure therapy combined with bi-pectoral muscle flap for the treatment of deep sternal wound infections. Int Wound J. 2020;17:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spindler N, Biereigel C, Pieroh P, et al. Clinical and microbiological analysis of deep sternal wound infections in fifty-two consecutive patients. Surg Infect (Larchmt). 2020;21:370–377. [DOI] [PubMed] [Google Scholar]
- 29.Izaddoost S, Withers EH. Sternal reconstruction with omental and pectoralis flaps: a review of 415 consecutive cases. Ann Plast Surg. 2012;69:296–300. [DOI] [PubMed] [Google Scholar]
- 30.Ringelman PR, Vander Kolk CA, Cameron D, et al. Long-term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg. 1994;93:1208–1214; discussion 1215. [PubMed] [Google Scholar]
- 31.Daigeler A, Falkenstein A, Pennekamp W, et al. Sternal osteomyelitis: long-term results after pectoralis muscle flap reconstruction. Plast Reconstr Surg. 2009;123:910–917. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson J, Huljebrant I, Nettelblad H, et al. Functional impairment after treatment with pectoral muscle flaps because of deep sternal wound infection. Scand Cardiovasc J. 2011;3:174–180. [DOI] [PubMed] [Google Scholar]
- 33.Tomos P, Lachanas E, Michail PO, et al. Alternative bi-pectoral muscle flaps for postoperative sternotomy mediastinitis. Ann Thorac Surg. 2006;81:754–755. [DOI] [PubMed] [Google Scholar]
- 34.Kaul P. Sternal reconstruction after post-sternotomy mediastinitis. J. Cardiothorac Surg. 2017;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ascherman JA, Hugo NE, Sultan MR, et al. Single-stage treatment of sternal wound complications in heart transplant recipients in whom pectoralis major myocutaneous advancement flaps were used. J Thorac Cardiovasc Surg. 1995;1104 Pt 11030–1036. [DOI] [PubMed] [Google Scholar]
- 36.Hugo HE, Sultan MR, Ascherman JA, et al. Single stage management of 74 consecutive sternal wound complications with pectoralis major myocutaneous advancement flaps. Plast Reconst Surg. 1994;93:1433–1441. [DOI] [PubMed] [Google Scholar]
- 37.Ascherman JA, Patel SM, Malhotra SM, et al. Management of sternal wounds with bilateral pectoralis major myocutaneous advancement flaps in 114 consecutively treated patients: refinements in technique and outcomes analysis. Plast Reconstr Surg. 2004;114:676–683. [DOI] [PubMed] [Google Scholar]
- 38.Levy AS, Ascherman JA. Sternal wound reconstruction made simple. Plast Reconstr Surg Glob Open. 2019;7:e2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Preminger BA, Yaghoobzadeh Y, Ascherman JA. Management of sternal wounds by limited debridement and partial bilateral pectoralis major myocutaneous advancement flaps in 25 patients: a less invasive approach. Ann Plast Surg. 2014;72:446–450. [DOI] [PubMed] [Google Scholar]
- 40.Barbera F, Lorenzetti F, Marsili R. The impact of preoperative negative-pressure wound therapy on pectoralis major muscle flap reconstruction for deep sternal wound infections. Ann Plast Surg. 2019;83:195–200. [DOI] [PubMed] [Google Scholar]


