Abstract
To estimate the outcome patterns and predictors of curative surgery for cervical squamous cell carcinoma (SCC) and adenocarcinoma (ADC) for overall survival (OS), locoregional recurrence (LRR), and distant metastasis (DM), we enrolled 4628 patients who had received a diagnosis of cervical SCC or ADC and received curative surgery. Cox regression analysis was employed to calculate hazard ratios and confidence intervals (CIs); independent predictors were controlled for or stratified in the analysis, and the endpoint was all-cause death. Propensity score matching was conducted to create well-balanced groups. Multivariate Cox regression analysis indicated that the pathologic type of ADC, age ≥ 70 years, advanced pathologic stage, positive margin, poorly differentiated cancer, undifferentiated cancer, adjuvant sequential chemotherapy and radiotherapy, earlier year of diagnosis, Charlson comorbidity index (CCI) = 1, CCI ≥ 2, low income levels, and treatment at a nonmedical center were significant independent poor prognostic factors for all-cause mortality in cervical cancer treated with curative surgery. Adjusted hazard ratios (95% CIs) for patients with cervical ADC who received curative surgery were 2.34 (1.96-2.79), 1.15 (0.89-1.49), and 2.16 (1.75-2.66) compared with cervical SCC for all-cause mortality, LRR, and DM, respectively. This study indicated that curative surgery for cervical ADC was associated with poorer OS and higher DM rates relative to cervical SCC, but no significant differences were identified in LRR.
Keywords: Surgery, cervical squamous cell carcinoma, cervical adenocarcinoma, survival, locoregional recurrence, metastasis
Introduction
Adenocarcinoma (ADC) and squamous cell carcinoma (SCC) of the cervix share many similarities, and they are treated with the same approach at most institutions following National Comprehensive Cancer Network (NCCN) guidelines [1]. However, several differences have been identified in epidemiology, prognostic factors, and patterns of failure after primary treatment and possibly in response to specific treatments [2]. Despite these differences, specific treatment strategies tailored to ADC have not yet emerged. Either curative surgery (usually radical hysterectomy, bilateral salpingo-oophorectomy, or pelvic lymph node dissection) or radiotherapy (RT), which is typically administered with concurrent chemoradiotherapy (CCRT), can cure stage IB and IIA cervical cancer (CC). The preference for surgery in cervical ADC is based on data from a prospective trial, in which 343 women with stage IB and IIA CC (14% ADC) were randomly assigned to primary surgery or RT alone [3,4]. At a median follow-up of 87 months, the 5-year overall and disease-free survival rates were the same for both treatment groups (83% and 74% for each group, respectively) [3,4]. However, multivariate analysis demonstrated a survival advantage for patients with ADC who underwent primary surgery [3,4]. Whether this result was due to the increased effectiveness of surgery or the absence of a benefit from RT (possibly due to the lack of concurrent chemotherapy [CT]) in women with ADC [5] remains to be determined. As noted, some data raise the possibility that ADC has poorer outcomes with RT alone than do SCCs, but this relative radioresistance may be overcome through the use of concurrent CT [5]. Thus, when RT is administered for ADC, patients are usually administered concurrent cisplatin-based CT. Presently, curative surgery appears to have preferable outcomes in cervical ADC based on retrospective studies with a small sample size [3,4].
For women with locoregionally advanced International Federation of Gynecology and Obstetrics (FIGO) clinical stage IIB-IVA cervical SCC, primary CCRT has been the treatment of choice at most institutions, although the treatment of choice varies across institutions. In this setting, NCCN guidelines recommend either radical hysterectomy or initial CCRT [1]. However, these patients are initially treated with CCRT because evidence level category 2B is provided for surgery for advanced CC in NCCN guidelines [1]. One of the main arguments against a primary surgical approach to advanced stages is the high potential for multimodal therapy, given that the majority of women will have a high- or intermediate-risk disease, for which adjuvant CCRT is recommended [5]. However, some studies have demonstrated radioresistance in cervical ADC; thus, curative surgery might improve the survival of patients advanced ADC [5,6]. Thus, some patients with advanced stage CC continue to receive curative surgery in Taiwan [6], although adjuvant treatments usually cannot be avoided [1,5].
Patterns of overall survival (OS), locoregional recurrence (LRR), and distant metastasis (DM) in cervical SCC and ADC following curative surgery with or without adjuvant treatments may differ, but the difference remains unclear because no large-scale head-to-head study has estimated outcome patterns of curative surgery for cervical pathologic types of SCC and ADC. In this study, we aimed to determine outcome patterns and predictors of curative surgery for cervical SCC and ADC at pathologic stages I-IIA (early stages) and IIB-IVA (advanced stages) for OS, LRR, and DM.
Patients and methods
We established a cohort by using data from the Taiwan Cancer Registry Database. We enrolled patients who had received a diagnosis of resectable cervical SCC or ADC and underwent curative surgery (radical hysterectomy, bilateral salpingo-oophorectomy, or pelvic lymph node dissection) between January 1, 2007, and December 31, 2015. The index date was the date of the surgery. The follow-up duration was from the index date to December 31, 2014. The Taiwan Cancer Registry Database of the Collaboration Center of Health Information Application contains the detailed cancer-related information of patients, including clinical and pathologic stages, treatment modalities, pathologic characteristics, surgical procedures, RT doses including dose of external beam radiotherapy (EBRT) and intracavitary brachytherapy, and the CT regimens used [7-14]. Our protocols were reviewed and approved by the Institutional Review Board of Taipei Medical University. The diagnoses of the enrolled patients were confirmed using their pathological data, and the patients who received a new diagnosis of resectable cervical SCC or ADC were confirmed to have no other cancer. Patients with a diagnosis of resectable cervical SCC or ADC, aged ≥ 20 years, and clinical cancer stage I-IVA as per the FIGO staging system were included. Pathologic staging according to the American Joint Committee on Cancer (AJCC) staging system, 7th edition, after curative surgery was also used. Patients with a history of cancer before cervical SCC or ADC, distant metastasis, missing sex data, an age of <20 years, nonstandard surgical procedures, nonplatinum-based adjuvant CT or CCRT, hypofraction RT dose of adjuvant RT, adenosquamous cell carcinoma, small cell carcinoma, or unclear staging were excluded. In addition, we excluded patients with cervical SCC or ADC who did not receive surgery within 3 months after the diagnosis date of CC, received CT alone, received neoadjuvant CT, received RT alone, received definitive CCRT, or underwent neoadjuvant CCRT followed by surgery. Finally, we enrolled patients with CC who received curative surgery and categorized them into 2 groups according to the pathologic type and pathologic stage to compare their outcomes. The median total dose and fraction size of adjuvant RT were 50 and 2 Gy per fraction, respectively, in the SCC and ADC groups (Table 1). Comorbidities were scored using the Charlson comorbidity index (CCI) [15,16]. Only comorbidities observed within 6 months before the index date were included; comorbidities were identified and included according to International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) main diagnosis codes for a first admission or 3 or more repeat codes main diagnosis codes for outpatient visits.
Table 1.
Characteristics of patients with cervical adenocarcinoma or squamous cell carcinoma who received curative surgery
| AJCC pathologic stages I-IIA | AJCC pathologic stages IIB-IVA | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| SCC (N = 2790) | Adenocarcinoma (N = 788) | P-value | SCC (N = 798) | Adenocarcinoma (N = 252) | P-value | ||
|
|
|
||||||
| n (%) | n (%) | n (%) | n (%) | ||||
| Age, years | Mean (SD) | 51.3 (12.3) | 48.6 (10.2) | <.0001 | 55.1 (11.8) | 51.5 (10.4) | <.0001 |
| Median (IQR: Q1, Q3) | 51 (20, 90) | 48 (25, 83) | 54 (24, 88) | 52 (27, 83) | |||
| Age group | 20-39 | 508 (18.2) | 138 (17.5) | <.0001 | 73 (9.1) | 31 (12.3) | .0015 |
| 40-49 | 772 (27.7) | 304 (38.6) | 167 (20.9) | 70 (27.8) | |||
| 50-59 | 811 (29.1) | 235 (29.8) | 296 (37.1) | 91 (36.1) | |||
| 60-69 | 475 (17.0) | 86 (10.9) | 164 (20.6) | 49 (19.4) | |||
| 70+ | 224 (8.0) | 25 (3.2) | 98 (12.3) | 11 (4.4) | |||
| Year of diagnosis | 2007-2009 | 1106 (39.6) | 265 (33.6) | .0085 | 306 (38.3) | 71 (28.2) | .0129 |
| 2010-2012 | 945 (33.9) | 298 (37.8) | 279 (35.0) | 105 (41.7) | |||
| 2013-2015 | 739 (26.5) | 225 (28.6) | 213 (26.7) | 76 (30.2) | |||
| FIGO stages | I-IIA | 2764 (99.1) | 780 (99.0) | .8314 | 630 (78.9) | 185 (73.4) | .0661 |
| IIB-IVA | 26 (0.9) | 8 (1.0) | 168 (21.1) | 67 (26.6) | |||
| AJCC pathologic stage | I | 2594 (93.0) | 744 (94.4) | .1532 | |||
| IIA | 196 (7.0) | 44 (5.6) | |||||
| IIIB | 199 (24.9) | 28 (11.1) | <.0001 | ||||
| IVA | 515 (64.5) | 167 (66.3) | |||||
| 4 | 84 (10.5) | 57 (22.6) | |||||
| Grade | I (well differentiated) | 149 (5.3) | 237 (30.1) | <.0001 | 21 (2.6) | 40 (15.9) | <.0001 |
| II (moderately differentiated) | 1287 (46.1) | 332 (42.1) | 512 (64.2) | 133 (52.8) | |||
| III (poorly differentiated) | 449 (16.1) | 98 (12.4) | 174 (21.8) | 56 (22.2) | |||
| IV (undifferentiated) | 9 (0.3) | 6 (0.8) | 3 (0.4) | 5 (2.0) | |||
| Missing | 896 (32.1) | 115 (14.6) | 88 (11.0) | 18 (7.1) | |||
| Surgical margin | No residual | 2457 (88.1) | 725 (92.0) | <.0001 | 590 (73.9) | 185 (73.4) | .3999 |
| Residual | 179 (6.4) | 19 (2.4) | 137 (17.2) | 50 (19.8) | |||
| Unknown | 154 (5.5) | 44 (5.6) | 71 (8.9) | 17 (6.7) | |||
| Adjuvant treatment | Adjuvant CCRT | 179 (6.4) | 71 (9.0) | .0080 | 408 (51.1) | 120 (47.6) | .0126 |
| Adjuvant sequential CT and RT | 24 (0.9) | 14 (1.8) | 53 (6.6) | 33 (13.1) | |||
| Adjuvant RT | 339 (12.2) | 97 (12.3) | 164 (20.6) | 51 (20.2) | |||
| No adjuvant | 2248 (80.6) | 606 (76.9) | 173 (21.7) | 48 (19.0) | |||
| RT cumulative dose, Gy | Mean (SD) | 45.8 (11.6) | 45.9 (11.7) | .9971 | 48.8 (14.9) | 48.8 (15.4) | .4020 |
| Median (IQR: Q1, Q3) | 50 (37, 56) | 50 (36, 56) | 50 (37, 56) | 50 (37, 56) | |||
| EBRT cumulative dose | No EBRT | 2272 (81.4) | 620 (78.7) | .2146 | 226 (28.3) | 81 (32.1) | .4015 |
| <50 Gy | 250 (9.0) | 83 (10.5) | 247 (31.0) | 79 (31.3) | |||
| 50+ Gy | 268 (9.6) | 85 (10.8) | 325 (40.7) | 92 (36.5) | |||
| Platinum cumulative dose, MG | Mean (SD) | 446.0 (224.3) | 533.2 (428.8) | .5141 | 488.4 (317.0) | 628.3 (614.7) | .1517 |
| Median (IQR: Q1, Q3) | 420 (300, 600) | 420 (300, 600) | 450 (300, 600) | 500 (300, 600) | |||
| Platinum cumulative dose | No CT | 2587 (92.7) | 703 (89.2) | .0058 | 337 (42.2) | 99 (39.3) | .5886 |
| <500 MG | 115 (4.1) | 47 (6.0) | 239 (29.9) | 75 (29.8) | |||
| 500+ MG | 88 (3.2) | 38 (4.8) | 222 (27.8) | 78 (31.0) | |||
| Intracavitary brachytherapy dose, cGy | Mean (SD) | 2275.2 (943.9) | 2213.7 (794.8) | .3015 | 2043.2 (812.6) | 2267.2 (753.3) | .8284 |
| Median (IQR: Q1, Q3) | 2500 (1500, 3000) | 2000 (1500, 2500) | 2000 (1500, 3000) | 2000 (1500, 2500) | |||
| Intracavitary brachytherapy dose | No intracavitary brachytherapy | 2167 (77.7) | 614 (77.9) | .3963 | 241 (30.2) | 89 (35.3) | .3123 |
| <2500 cGy | 490 (17.6) | 145 (18.4) | 454 (56.9) | 133 (52.8) | |||
| 2500+ cGy | 133 (4.8) | 29 (3.7) | 103 (12.9) | 30 (11.9) | |||
| CCI Score | Mean (SD) | 0.3 (0.7) | 0.3 (0.7) | .1054 | 0.3 (0.7) | 0.4 (0.8) | .2343 |
| 0 | 2216 (79.4) | 647 (82.1) | .2362 | 637 (79.8) | 192 (76.2) | .4216 | |
| 1 | 396 (14.2) | 95 (12.1) | 105 (13.2) | 41 (16.3) | |||
| 2+ | 178 (6.4) | 46 (5.8) | 56 (7.0) | 19 (7.5) | |||
| Income | <NTD 18,000 | 695 (24.9) | 148 (18.8) | .0002 | 218 (27.3) | 62 (24.6) | .5471 |
| NTD 18,000-22,500 | 995 (35.7) | 283 (35.9) | 266 (33.3) | 82 (32.5) | |||
| NTD 22,500-30,000 | 398 (14.3) | 108 (13.7) | 118 (14.8) | 35 (13.9) | |||
| NTD 30,000+ | 702 (25.2) | 249 (31.6) | 196 (24.6) | 73 (29.0) | |||
| Hospital level | Medical center | 1950 (69.9) | 579 (73.5) | .0509 | 509 (63.8) | 186 (73.8) | .0034 |
| Other | 840 (30.1) | 209 (26.5) | 289 (36.2) | 66 (26.2) | |||
| Hospital area | North | 1388 (49.7) | 435 (55.2) | .0238 | 332 (41.6) | 130 (51.6) | .0003 |
| Middle | 601 (21.5) | 147 (18.7) | 141 (17.7) | 55 (21.8) | |||
| South/East | 801 (28.7) | 206 (26.1) | 325 (40.7) | 67 (26.6) | |||
| Mean follow-up time, months (SD) | 79.5 (31.8) | 75.0 (31.1) | 66.2 (35.4) | 46.0 (31.0) | |||
| Death | 206 (7.38) | 79 (10.03) | 245 (30.70) | 143 (56.75) | |||
| Local recurrence | 207 (7.42) | 52 (6.60) | 94 (11.78) | 38 (15.08) | |||
| Distant metastasis | 103 (3.69) | 68 (8.63) | 180 (22.56) | 91 (36.11) | |||
CCRT, concurrent chemoradiotherapy; cGy, centigray; Gy, gray; SCC, squamous cell carcinoma; FIGO, International Federation of Gynecology and Obstetrics; CT, chemotherapy; RT, radiotherapy; EBRT, external beam radiotherapy; CCI, Charlson comorbidity index; MG, milligrams; SD, standard deviation; IQR, interquartile range; NTD, New Taiwan dollar, AJCC, American Joint Committee on Cancer.
Significant independent predictors, such as pathologic type, age, AJCC pathologic stage, surgical margin, grade of differentiation, adjuvant treatment, intracavitary brachytherapy, year of diagnosis, CCI score, income level, hospital level, and hospital region, were determined using multivariate Cox regression analysis to determine the hazard ratio (HR); independent predictors were controlled for or stratified in the analysis, and the endpoint was all-cause death among patients with cervical SCC or ADC who received curative surgery. The cumulative incidence of all-cause mortality was estimated using the Kaplan-Meier method, and differences between cervical SCC and ADC were determined using the log-rank test. After adjustment for confounders, the Cox proportional hazard method was used to model the time from the index date to all-cause mortality incidence among patients. In multivariate analysis, HRs were adjusted for pathologic type, age, AJCC pathologic stage, surgical margin, grade of differentiation, adjuvant treatment, intracavitary brachytherapy, year of diagnosis, CCI score, income level, hospital level, and hospital area. Stratified analyses were performed to evaluate the risks of mortality, LRR, and DM associated with resectable cervical SCC or ADC at various AJCC pathologic stages. All analyses were performed using SAS 9.3 software (SAS, Cary, NC, USA). Two-sided P<.05 was considered significant.
To reduce the effects of potential confounders, propensity score matching (PSM) was employed to create well-balanced groups. PSM scores were adjusted using a multivariable logistic regression model, in which the SCC and ADC groups were dependent variables and potential confounders were covariates. The following confounders were included in PSM: pathologic type, age, AJCC pathologic stage, surgical margin, grade of differentiation, adjuvant treatment, intracavitary brachytherapy, RT cumulative dose, platinum cumulative dose, intracavitary brachytherapy dose, year of diagnosis, CCI score, income level, hospital level, and hospital area. All patients with cervical ADC were matched at a ratio of 1:2 to patients with cervical SCC (if ADC cannot be matched at a ratio of 1:2 to SCC, ADC might be matched at a ratio of 1:1 to SCC). The independent predictors were controlled for in the analysis.
Results
We enrolled 4628 patients who had FIGO stage I-IVA CC without distant metastasis (Table 1). Of these, 3588 patients with cervical SCC received curative surgery, and 1040 patients with cervical ADC received curative surgery. Overall, 3578 patients received curative surgery at pathologic stages I-IIA, and 1050 patients received curative surgery at pathologic stages IIB-IVA. The mean follow-up duration after the index date was 79.5 and 75.0 months for pathologic stages I-IIA and IIB-IVA cervical SCC, respectively, and 66.2 and 46.0 months for pathologic stages I-IIA and IIB-IVA cervical ADC, respectively. Relative to patients with cervical SCC, patients with cervical ADC were significantly younger and more frequently received treatment at a medical center; had higher income levels, lower incidence of pathologic stage IIB, and more frequent well-differentiated or undifferentiated cancer; and were more likely to be margin clear in the early pathologic stage, more likely to receive adjuvant treatment, more likely to receive adjuvant sequential CT and RT in early pathologic stages, and more likely to live in northern Taiwan (Table 1). Incidence rates of DM and death for ADC were also higher. No statistically significant differences were identified in the platinum cumulative dose, RT cumulative dose, intracavitary brachytherapy dose, CCI score, or FIGO stage; however, we identified significant stage variation (from FIGO stage to AJCC pathologic stage) and significant differences in FIGO stage IIB (Supplementary Table 1). Clinical and pathologic stages were significantly inconsistent for cervical ADC relative to SCC. In addition, clinical stage IIB for cervical ADC is usually underestimated; up-pathologic stages were identified in 62.2% of patients.
The results of multivariate Cox regression analysis indicated that the pathologic type of ADC, age ≥ 70 years, advanced AJCC pathologic stage, positive margin, poorly differentiated cancer, undifferentiated cancer, adjuvant sequential CT and RT, earlier year of diagnosis, CCI = 1, CCI ≥ 2, low income level, and treatment at a nonmedical center were significant independent poor prognostic factors for all-cause mortality in CC treated with curative surgery (Table 2). Cervical ADC with curative surgery (adjusted HR [aHR], 2.34; 95% confidence interval [CI], 1.96-2.79) was a significant independent prognostic factor for OS (P <.0001) (Table 2). The aHRs (95% CIs) of age ≥ 70 years and AJCC pathologic stages IIA, IIB, III, and IVA were 2.89 (2.10-3.99), 2.05 (1.46-2.87), 2.31 (1.66-3.22), 4.76 (3.79-5.98), and 10.07 (7.54-13.46), respectively. The aHRs (95% CIs) of positive margin, poorly differentiated cancer, undifferentiated cancer, adjuvant sequential CT and RT, 2013-2015 as year of diagnosis, CCI = 1, CCI ≥ 2, higher income (≥ 30,000 NTD), and treatment at a nonmedical center were 1.80 (1.45-2.24), 1.41 (1.01-1.95), 3.21 (1.56-6.60), 1.52 (1.08-2.15), 0.77 (0.61-0.97), 1.24 (1.00-1.53), 1.76 (1.37-2.28), 0.72 (0.58, 0.89), and 1.19 (1.01-1.41), respectively.
Table 2.
Results of Cox proportional hazard regression analysis of the risk of all-cause mortality among patients with cervical adenocarcinoma or squamous cell carcinoma who received curative surgery
| Crude HR (95% CI) | Adjusted HR* (95% CI) | P-value | ||
|---|---|---|---|---|
| Pathologic type | SCC | 1 | 1 | <.0001 |
| Adenocarcinoma | 1.88 (1.60, 2.21) | 2.34 (1.96, 2.79) | ||
| Age | 0-39 | 1 | 1 | <.0001 |
| 40-49 | 1.17 (0.87, 1.57) | 1.01 (0.75, 1.36) | ||
| 50-59 | 1.57 (1.19, 2.07) | 1.07 (0.81, 1.43) | ||
| 60-69 | 2.04 (1.52, 2.74) | 1.25 (0.91, 1.70) | ||
| 70+ | 4.71 (3.50, 6.33) | 2.89 (2.10, 3.99) | ||
| AJCC pathologic stage | I | 1 | 1 | <.0001 |
| IIA | 2.75 (2.00, 3.80) | 2.05 (1.46, 2.87) | ||
| IIB | 3.49 (2.58, 4.73) | 2.31 (1.66, 3.22) | ||
| III | 6.18 (5.17, 7.38) | 4.76 (3.79, 5.98) | ||
| IVA | 16.95 (13.31, 21.59) | 10.07 (7.54, 13.46) | ||
| Surgical margin | No residual | 1 | 1 | <.0001 |
| Residual | 3.33 (2.74, 4.06) | 1.80 (1.45, 2.24) | ||
| Unknown | 1.89 (1.45, 2.48) | 1.38 (1.04, 1.83) | ||
| Grade | I (well differentiated) | 1 | 1 | .0017 |
| II (moderately differentiated) | 1.30 (0.98, 1.72) | 1.19 (0.88, 1.61) | ||
| III (poorly differentiated) | 1.63 (1.20, 2.22) | 1.41 (1.01, 1.95) | ||
| IV (undifferentiated) | 4.69 (2.32, 9.50) | 3.21 (1.56, 6.60) | ||
| Missing | 0.62 (0.45, 0.87) | 0.96 (0.68, 1.37) | ||
| Adjuvant treatment | No adjuvant | 1 | 1 | .0381 |
| Adjuvant CCRT | 3.41 (2.84, 4.09) | 0.96 (0.76, 1.22) | ||
| Adjuvant sequential CT and RT | 5.66 (4.17, 7.70) | 1.52 (1.08, 2.15) | ||
| Adjuvant RT | 2.92 (2.40, 3.56) | 1.11 (0.88, 1.41) | ||
| IC brachytherapy | 2.81 (2.41, 3.27) | 1.14 (0.92, 1.50) | .1307 | |
| Year of diagnosis | 2007-2009 | 1 | 1 | .0553 |
| 2010-2012 | 1.05 (0.88, 1.25) | 0.86 (0.72, 1.02) | ||
| 2013-2015 | 0.96 (0.77, 1.21) | 0.77 (0.61, 0.97) | ||
| CCI score | 0 | 1.00 | 1 | <.0001 |
| 1 | 1.52 (1.24, 1.85) | 1.24 (1.00, 1.53) | ||
| 2+ | 2.27 (1.79, 2.88) | 1.76 (1.37, 2.28) | ||
| Income | <NTD 18,000 | 1 | 1 | .0051 |
| NTD 18,000-22,500 | 0.78 (0.65, 0.94) | 0.74 (0.61, 0.89) | ||
| NTD 22,500-30,000 | 0.73 (0.57, 0.95) | 0.73 (0.62, 0.85) | ||
| NTD 30,000+ | 0.72 (0.58, 0.88) | 0.72 (0.58, 0.89) | ||
| Hospital level | Medical center | 1 | 1 | .0422 |
| Nonmedical center | 1.36 (1.16, 1.59) | 1.19 (1.01, 1.41) | ||
| Region | North | 1 | 1 | .2881 |
| Central | 1.06 (0.86, 1.30) | 1.05 (0.85, 1.30) | ||
| South/East | 1.31 (1.10, 1.55) | 1.16 (0.96, 1.39) |
All the variables included in Table 2 were used in the multivariate analysis.
CCRT, concurrent chemoradiotherapy; Gy, gray; SCC, squamous cell carcinoma; FIGO, International Federation of Gynecology and Obstetrics; EBRT, external beam radiotherapy; CCI, Charlson comorbidity index; MG, milligrams; HR, hazard ratio; CI, confidence intervals; NTD, New Taiwan dollar; IC, intracavitary; AJCC, American Joint Committee on Cancer; CT, chemotherapy; RT, radiotherapy.
Stratified analyses were performed to evaluate the risk of mortality among patients for different AJCC pathologic stages, and a stratified Cox proportional hazard model was used to analyze the risk of mortality associated with different pathologic stages (Table 3). After adjustment for the pathologic type, age, AJCC pathologic stage, surgical margin, grade of differentiation, adjuvant treatment, intracavitary brachytherapy, year of diagnosis, CCI score, income level, hospital level, and hospital area, the aHRs (95% CIs) of pathologic stage I-IIA for overall mortality in ADC, age ≥ 70 years, pathologic stage IIA, poorly differentiated cancer, undifferentiated cancer, adjuvant sequential CT and RT, CCI = 1, and CCI ≥ 2 were 1.93 (1.45-2.57), 3.68 (2.32-5.82), 1.62 (1.14, 2.31), 2.02 (1.23-3.32), 1.96 (1.45-3.46), 2.83 (1.30-6.16), 1.62 (1.20-2.20), and 2.56 (1.82-3.60), respectively (Table 3). In pathologic stages IIB-IVA, the aHRs (95% CIs) of ADC, age ≥ 70 years, pathologic stage III, pathologic stage IVA, positive margin, poorly differentiated cancer, undifferentiated cancer, adjuvant CCRT, 2013-2015 year of diagnosis, and income ≥ 30,000 NTD were 2.59 (2.05-3.28), 2.00 (1.27-3.16), 1.79 (1.30-2.45), 3.79 (2.62-5.47), 2.08 (1.61-2.69), 1.09 (1.01-1.41), 2.77 (1.17-6.52), 0.70 (0.53-0.92), 0.71 (0.53-0.96), and 0.73 (0.55-0.98), respectively (Table 3).
Table 3.
Results of Cox proportional hazard regression analysis of the risk of all-cause mortality, stratified by AJCC pathologic stage
| Stages I-IIA | Stages IIB-IV | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Adjusted HR (95% CI) | P-value | Adjusted HR* (95% CI) | P-value | ||
| Pathologic type | SCC | 1 | <.0001 | 1 | <.0001 |
| Adenocarcinoma | 1.93 (1.45, 2.57) | 2.59 (2.05, 3.28) | |||
| Age | 0-39 | 1 | <.0001 | 1 | .0019 |
| 40-49 | 0.72 (0.46, 1.15) | 1.20 (0.81, 1.79) | |||
| 50-59 | 0.86 (0.55, 1.34) | 1.09 (0.75, 1.60) | |||
| 60-69 | 1.49 (0.94, 2.36) | 0.99 (0.64, 1.52) | |||
| 70+ | 3.68 (2.32, 5.82) | 2.00 (1.27, 3.16) | |||
| AJCC pathologic stage | I | 1 | .0075 | - | <.0001 |
| IIA | 1.62 (1.14, 2.31) | - | |||
| IIB | - | 1 | |||
| III | - | 1.79 (1.30, 2.45) | |||
| IVA | - | 3.79 (2.62, 5.47) | |||
| Surgical margin | No residual | 1 | .5868 | 1 | <.0001 |
| Residual | 1.16 (0.72, 1.88) | 2.08 (1.61, 2.69) | |||
| Unknown | 1.23 (0.79, 1.92) | 1.47 (1.01, 2.12) | |||
| Grade | I (well differentiated) | 1 | .0007 | 1 | .0305 |
| II (moderately differentiated) | 1.46 (0.92, 2.31) | 0.93 (0.62, 1.38) | |||
| III (poorly differentiated) | 2.02 (1.23, 3.32) | 1.09 (1.01, 1.41) | |||
| IV (undifferentiated) | 1.96 (1.45, 3.46) | 2.77 (1.17, 6.52) | |||
| Missing | 0.90 (0.54, 1.52) | 1.07 (0.66, 1.75) | |||
| Adjuvant treatment | No adjuvant | 1 | .0747 | 1 | .0558 |
| Adjuvant CCRT | 1.05 (0.67, 1.63) | 0.70 (0.53, 0.92) | |||
| Adjuvant sequential CT and RT | 2.83 (1.30, 6.16) | 0.95 (0.64, 1.40) | |||
| Adjuvant RT | 1.03 (0.72, 1.47) | 0.84 (0.61, 1.14) | |||
| Intracavitary brachytherapy | 1.93 (0.40, 2.67) | .3301 | 0.87 (0.69, 1.09) | .2309 | |
| Year of diagnosis | 2007-2009 | 1 | .3536 | 1 | .0755 |
| 2010-2012 | 0.82 (0.62, 1.09) | 0.95 (0.75, 1.20) | |||
| 2013-2015 | 0.99 (0.68, 1.43) | 0.71 (0.53, 0.96) | |||
| CCI score | 0 | 1.00 | <.0001 | 1 | .6955 |
| 1 | 1.62 (1.20, 2.20) | 0.97 (0.71, 1.32) | |||
| 2+ | 2.56 (1.82, 3.60) | 1.18 (0.78, 1.79) | |||
| Income | <NTD 18,000 | 1 | .1167 | 1 | .1359 |
| NTD 18,000-22,500 | 0.72 (0.54, 0.97) | 0.84 (0.64, 1.09) | |||
| NTD 22,500-30,000 | 0.67 (0.43, 1.05) | 0.99 (0.71, 1.37) | |||
| NTD 30,000+ | 0.83 (0.60, 1.15) | 0.73 (0.55, 0.98) | |||
| Hospital level | Medical center | 1 | .0717 | 1 | .2800 |
| Other | 1.27 (0.98, 1.64) | 1.13 (0.90, 1.42) | |||
| Area | North | 1 | .3236 | 1 | .6597 |
| Central | 1.04 (0.76, 1.44) | 0.98 (0.73, 1.31) | |||
| South/East | 1.23 (0.93, 1.62) | 1.11 (0.87, 1.41) | |||
All the variables included in Table 2 were used in the multivariate analysis.
CCRT, concurrent chemoradiotherapy; Gy, gray; SCC, squamous cell carcinoma; FIGO, International Federation of Gynecology and Obstetrics; EBRT, external beam radiotherapy; CCI, Charlson comorbidity index; MG, milligrams; HR, hazard ratio; CI, confidence intervals; NTD, New Taiwan dollar; IC, intracavitary; AJCC, American Joint Committee on Cancer; CT, chemotherapy; RT, radiotherapy.
Stratified Cox proportional hazard model results for the risks of all-cause mortality, LRR, and DM are presented in Table 4. Without stratification by stage, patients with cervical ADC had higher all-cause mortality and DM; aHRs (95% CIs) were 2.34 (1.96-2.79) and 2.16 (1.75-2.66) for all-cause mortality and DM (Table 4), respectively. After multivariate analysis, patients with pathologic stages I-IIA cervical ADC had higher all-cause mortality, with aHRs (95% CIs) of 1.93 (1.45-2.57) and 2.50 (1.78-3.51) for all-cause mortality and DM, respectively. Patients with pathologic stages IIB-IVA cervical ADC also had higher all-cause mortality, with aHRs (95% CIs) of 2.59 (2.05-3.28) and 1.84 (1.39-2.42) for all-cause mortality and DM, respectively (Table 4).
Table 4.
Results of Cox proportional hazard model for the risk of all-cause mortality, locoregional recurrence, and distant metastasis, stratified by AJCC pathologic stage
| AJCC pathologic stage | Event | Pathologic type | Patient (n) | Event (n) (%) | Adjusted HR* (95% CI) | P-value |
|---|---|---|---|---|---|---|
| All patients | All-cause mortality | SCC | 3588 | 451 (12.57) | 1 | <.0001 |
| Adenocarcinoma | 1040 | 222 (21.35) | 2.34 (1.96, 2.79) | |||
| Locoregional recurrence | SCC | 3588 | 301 (8.39) | 1 | .2905 | |
| Adenocarcinoma | 1040 | 90 (8.65) | 1.15 (0.89, 1.49) | |||
| Distant metastasis | SCC | 3588 | 283 (7.89) | 1 | <.0001 | |
| Adenocarcinoma | 1040 | 159 (15.29) | 2.16 (1.75, 2.66) | |||
| Stages I-IIA | All-cause mortality | SCC | 2790 | 206 (7.38) | 1 | <.0001 |
| Adenocarcinoma | 788 | 79 (10.03) | 1.93 (1.45, 2.57) | |||
| Locoregional recurrence | SCC | 2790 | 207 (7.42) | 1 | .9737 | |
| Adenocarcinoma | 788 | 52 (6.60) | 1.01 (0.72, 1.41) | |||
| Distant metastasis | SCC | 2790 | 103 (3.69) | 1 | <.0001 | |
| Adenocarcinoma | 788 | 68 (8.63) | 2.50 (1.78, 3.51) | |||
| Stages IIB-IVA | All-cause mortality | SCC | 798 | 245 (30.70) | 1 | <.0001 |
| Adenocarcinoma | 252 | 143 (56.75) | 2.59 (2.05, 3.28) | |||
| Locoregional recurrence | SCC | 798 | 94 (11.78) | 1 | .2891 | |
| Adenocarcinoma | 252 | 38 (15.08) | 1.26 (0.82, 1.92) | |||
| Distant metastasis | SCC | 798 | 180 (22.56) | 1 | <.0001 | |
| Adenocarcinoma | 252 | 91 (36.11) | 1.84 (1.39, 2.42) |
All the variables included in Table 2 were used in the multivariate analysis.
SCC, squamous cell carcinoma; HR, hazard ratio; CI, confidence intervals; AJCC, American Joint Committee on Cancer.
Kaplan-Meier OS curves for patients with ADC or SCC at all pathologic stages, stage I-IIA, and IIB-IVA are provided in Figure 1. The OS rate was higher for patients with cervical SCC (log-rank test: P<.0001, P = .0059, and P<0.0001, respectively). The 5-year OS rates of patients with cervical SCC or ADC who received CCRT were 90% and 80%, 95% and 92%, and 74% and 42% for all pathologic stages, stages I-IIA, and stages IIB-IVA, respectively (Supplementary Table 2).
Figure 1.
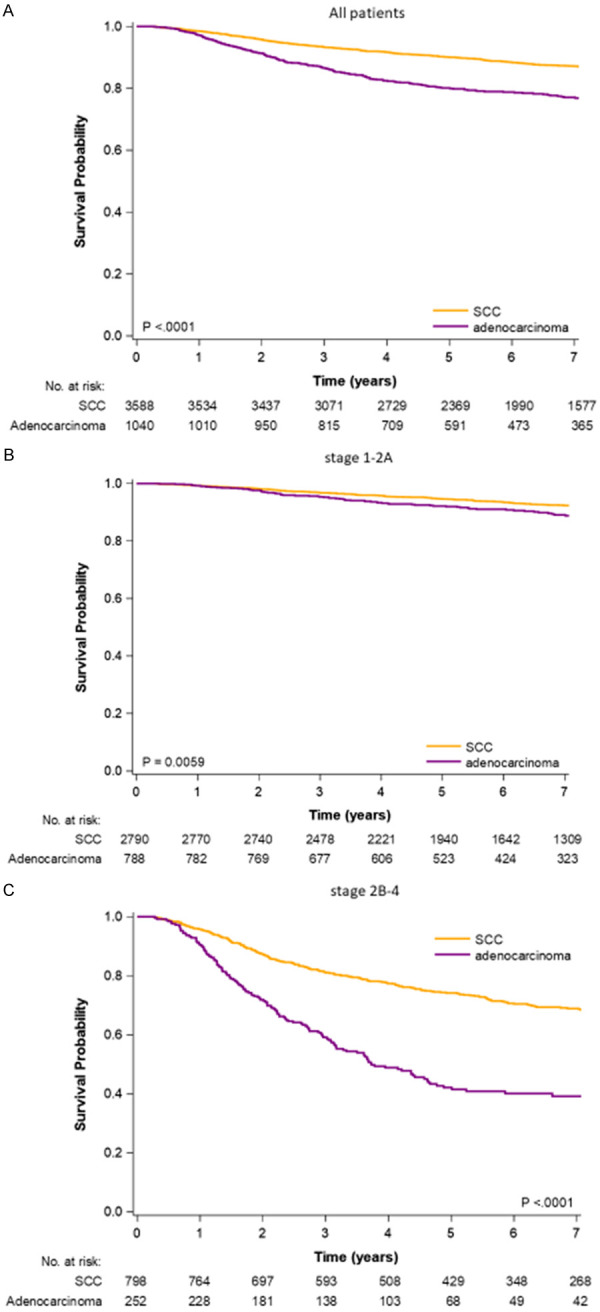
Kaplan-Meier survival curves of all-cause death for patients with cervical adenocarcinoma or squamous cell carcinoma who received curative surgery. A. All study patients. B. AJCC pathologic stages I-IIA. C. AJCC pathologic stages IIB-IV.
The matching process yielded a final cohort of 2479 patients (1218 vs. 667 patients with cervical SCC or cervical ADC at pathologic stages I-IIA and 384 vs. 210 patients with cervical SCC or cervical ADC at pathologic stages IIB-IVA) eligible for further analysis. Patient characteristics for PSM were identified, and all confounders were well matched (Supplementary Table 3). Cox proportional hazard regression analysis of the all-cause mortality, LRR, and DM of our PSM cohorts with cervical SCC or ADC was conducted (Supplementary Table 4). After PSM, the trends for all-cause death, LRR, and DM were similar to those in the non-PSM cohort (Table 4). Patients with cervical ADC who received curative surgery had higher all-cause death and DM than those with cervical SCC at both early and advanced pathologic stages. No significant difference was observed in LRR for either early or advanced pathologic stages. Supplementary Figure 1 presents the survival curves for all-cause death, as calculated using the Kaplan-Meier method, for the PSM cohort in different stages. After PSM, those with cervical SCC still exhibited superior OS for all pathologic stages, stages I-IIA, and stages III-IVA.
Discussion
No large-scale, head-to-head study estimating outcome patterns for cervical ADC and SCC treated with curative surgery has been conducted. Our study is the first to describe and compare the failure and survival patterns of patients with different pathologic types of CC who received surgery. Our findings can be confirmed in further research on CC with SCC or ADC types. According to our literature review, this study is the largest cohort study to estimate predictors, patterns of failure, and survival for cervical ADC and SCC treated with surgery. PSM was also used to control for numerous confounders.
The significant characteristics of cervical ADC and SCC treated with curative surgery are compatible with those reported in previous studies, including younger age and higher income level of patients with ADC than those with SCC [17-19]. However, significant clinical characteristics not mentioned in previous reports were also identified, including the gradual increase in the incidence of cervical ADC in recent years, high inconsistency in clinic and pathologic stages of ADC, more advanced pathologic stages of ADC, more well-differentiated and undifferentiated ADC, more adjuvant CCRT for patients with ADC at early pathologic stages, more positive margin with ADC, higher prevalence of ADC in northern Taiwan, and more patients with ADC who underwent surgery at a medical center relative to patients with cervical SCC. A higher proportion of ADC patients with a positive margin and adjuvant CCRT might be associated with more advanced pathologic stages in ADC patients compared with SCC patients (Table 1). That cervical ADC was associated with higher income levels, living in northern Taiwan, and surgery at medical centers may be because northern Taiwanese people are wealthier than southern Taiwanese people [20]. The most notable clinical findings of our study were the stage variation from FIGO stage to AJCC pathologic stage, especially for cervical ADC at FIGO stage IIB (Supplementary Table 1). FIGO stages IIB of cervical ADC were usually underestimated, with 62.2% up-staging. This inconsistency and underestimation in FIGO and pathologic stages in cervical ADC is a novel finding. The clinical FIGO stages of cervical ADC were underestimated, which may partially explain why patients with cervical ADC at early clinic stages receiving RT or CCRT had poorer survival than those with cervical SCC [4,5,21].
We also estimated predictors of all-cause mortality using Cox proportional hazard regression analysis. The risk factors identified were ADC, age ≥ 70 years, advanced pathologic stage (with aHRs increasing gradually from IIA to IVA), positive margin, poorly differentiated cancer, undifferentiated cancer, adjuvant sequential CT and RT, earlier year of diagnosis, high CCI, low income, and nonmedical center treatment. These factors were compatible with those described in previous studies, for example, ADC, old age, advanced stages, positive margin, poorly differentiated cancer, undifferentiated cancer, and earlier year of diagnosis [22-29]. However, age ≥ 70 years, low income level, CCI = 1 or ≥ 2, and treatment at nonmedical centers have been reported for first time. The higher risk of all-cause mortality for treatment at a nonmedical center might be associated with the hospital case volume [30-32]. That adjuvant sequential CT and RT were poor prognostic factors for survival may be because adjuvant sequential CT and RT are insufficient adjuvant treatments for CC patients with high pathologic risk factors when compared with adjuvant CCRT or adjuvant RT [33-35].
Cox proportional hazard regression analysis stratified by AJCC pathologic early and advanced stages was conducted for examining the risk of all-cause mortality (Table 3). The risk factors were similar to non-stage-stratified outcomes. The risk factors for pathologic stages I-IIA were ADC, age ≥ 70 years, CCI = 1, CCI ≥ 2, pathologic stage IIA, poorly differentiated cancer, undifferentiated cancer, adjuvant sequential CT and RT, and low income level. The predictors in pathologic stage I-IIA CC were similar to all stages-CC (Tables 2 and 3). No significant differences in medical versus nonmedical center, year of diagnosis, or positive margin were noted, possibly because they might be associated with adjuvant treatments, which could cover these risk factors at early pathologic stages (Table 3) [5,36]. In advanced pathologic stages, adjuvant CCRT results in better OS of pathologic IIB-IVA CC, which is compatible with the finding of previous studies [5,36]. Our results support that adjuvant CCRT is an effective adjuvant treatment for advanced pathologic stage CC (Table 3). Other nonsignificant factors, including high CCI, high grade of differentiated cancer, and treatment at a nonmedical center might be associated with the relatively small sample size of patients with pathologic stage IIB-IVA CC (798 SCC and 252 ADC cases) compared with early pathologic stage CC (Table 1).
Figure 1 displays Kaplan-Meier survival curves of all-cause death for patients with cervical ADC or SCC who received curative surgery. Although curative surgery is generally preferred over CCRT for cervical ADC [4,21], this surgery resulted in inferior OS compared with SCC at all-stages, pathologic stages I-IIA, and stages IIB-IVA CC (Figure 1 and Supplementary Table 2). The distances between the survival curves of SCC and ADC were larger in advanced pathologic stages than in early pathologic stages (Figure 1B and 1C). Thus, relative to those with SCC, the OS of patients with ADC was poorer at advanced pathologic stages than at early pathologic stages (Figure 1). In early pathologic stages, the 5-year OS of SCC and ADC was similar (95% and 92%, respectively). By contrast, 5-year OS was poor for ADC at pathologic stages IIB-IVA relative to SCC (74% and 42%, respectively). The median OS of cervical ADC treated with curative surgery was only 3.73 years. According to our results, curative surgery with adjuvant treatments for cervical ADC is not optimal in improving OS. Other clinical trials with novel therapy would be necessary for advanced-stage cervical ADC.
Analysis stratified by the AJCC pathologic stage was performed to determine the risks of all-cause mortality, LRR, and DM (Table 4). In previous studies, radioresistance and higher LRR were noted for cervical ADC than for cervical SCC [5,37]. In our study, curative surgery improved LRR for cervical ADC, and no significance differences were observed in LRR between the groups (Table 4) in either early or advanced pathologic stages. However, OS and DM were poorer for ADC. Curative surgery could improve LRR, but not OS or DM rates, for cervical ADC. Finding new therapeutic modalities to reduce the high DM rate in patients with cervical ADC is essential to improving OS. New therapeutic strategies such as induction CT with novel regimens, induction CCRT, or adjuvant CT with nonplatinum-based regimens might decrease DM and improve OS [38-42].
Many risk factors for all-cause death in patients with CC who received curative surgery were identified (Table 2). Therefore, we conducted a PSM cohort study to match the risk factors to estimate the outcome patterns of patients with cervical ADC or SCC with early or advanced pathologic types who received curative surgery (Supplementary Table 3). All covariates were matched well. After PSM, the outcomes (Supplementary Table 4) were similar to those of the non-PSM cohort (Table 4). We created non-PSM and PSM cohorts to compare clinical characteristics, risk factors of survival, and head-to-head outcomes patterns of cervical ADC and SCC (Tables 1, 2 and Supplementary Table 4). An advantage of our study is its view on overall characteristics of cervical SCC and ADC (Tables 1 and 2) and the effects of curative surgery after PSM with all covariates controlled for (Supplementary Table 3). The survival curves of ADC and SCC for the PSM cohort were also similar with those of the non-PSM cohort (Figure 1 and Supplementary Figure 1). The distance of the survival curves was larger in advanced than in early pathologic stages. The finding means curative surgery for cervical ADC at early pathologic stages would be more helpful for OS than curative surgery for cervical ADC at advanced pathologic stages. Although curative surgery was performed for cervical ADC with advanced pathologic stages, OS remained poor (Supplementary Figure 1 and Supplementary Table 2). Hence, new translational medicine studies must be conducted to combine basic and clinic data and uncover novel therapeutic strategies to overcome DM and further improve OS, because most failure patterns are associated with DM rather than LRR after curative surgery (Supplementary Table 4).
The strength of our study is the size of the cohorts, which allowed estimation of outcomes patterns of curative surgery for cervical SCC and ADC, including OS, LRR, and DM. The treatment was homogenous in our study, as curative surgical procedures were consistent. PSM was also conducted to eliminate possible confounders (Supplementary Table 3), preserve clinical characteristics (Table 1), and further evaluate the outcomes patterns (Table 4 and Supplementary Table 4). The outcomes patterns for cervical ADC differed markedly from those of cervical SCC. Curative surgery might be suitable for patients with cervical SCC but might be insufficient for those with cervical ADC (Supplementary Table 2). Cervical ADC is unique regarding both OS and DM, and treatment should be modified accordingly. The patterns of failure in cervical ADC are related to DM; no significant differences were observed in LRR (Table 4 and Supplementary Table 4). Novel therapeutic modalities such as novel CT regimens or other effective systemic treatments in a neoadjuvant or adjuvant setting to decrease DM would be necessary for patients with cervical ADC who received curative surgery [38-43]. These findings could be considered in future clinical practice and randomized controlled studies.
This study has limitations. First, because all the patients were enrolled from an East Asian population, corresponding ethnic susceptibility remains unclear; our results should be cautiously extrapolated to other populations. No evidence indicates differences between populations in outcomes following curative surgery for CC. Second, the diagnoses of all comorbid conditions were based on ICD-9-CM codes. Nevertheless, the Taiwan Cancer Registry Administration randomly reviews charts and interviews patients to verify the accuracy of the diagnoses, and hospitals with outlier charges or practices may be audited and subsequently heavily penalized if malpractice or discrepancies are identified. Third, toxicity induced by curative surgeries and adjuvant treatments for advanced stages of CC could not be determined; therefore, treatment-related mortality estimates may have been biased. However, we conducted a PSM cohort study with well-matched stages and adjuvant treatments, and the outcomes were compatible to those of our non-PSM cohort. Accordingly, to obtain crucial information on population specificity and disease occurrence, a large-scale randomized trial comparing carefully selected patients undergoing suitable treatments is essential. Finally, the Taiwan Cancer Registry database does not contain information regarding dietary habits, socioeconomic status, or body mass index, all of which may be risk factors for mortality. However, considering the magnitude and statistical significance of the observed effects in this study, these limitations are unlikely to affect the conclusions.
Conclusion
Curative surgery for patients with cervical ADC was associated with poorer OS and higher DM rates than those with cervical SCC, but no significant differences were observed in LRR at early or advanced pathologic stages. Novel therapeutic strategies are necessary for reducing DM in patients with cervical ADC who receive curative surgery.
Acknowledgements
Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, supports Szu-Yuan Wu’s work (Funding Number: 10908 and 10909).
Disclosure of conflict of interest
None.
Abbreviations
- aHR
adjusted hazard ratio
- CCRT
concurrent chemoradiotherapy
- Gy
gray
- US
United States
- SCC
squamous cell carcinoma
- ADC
adenocarcinoma
- HR
hazard ratio
- CI
confidence intervals
- FIGO
the International Federation of Gynecology and Obstetrics
- LRR
locoregional recurrence
- DM
distant metastasis
- NCCN
National Comprehensive Cancer Network
- CC
cervical cancers
- SEER
Surveillance, Epidemiology and End Results
- CT
chemotherapy
- RT
radiotherapy
- OS
overall survival
- EBRT
external beam radiotherapy
- CCI
Charlson comorbidity index
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- PSM
Propensity scores matched
- AJCC
The American Joint Committee on Cancer
Supporting Information
References
- 1.NCCN Clinical practice guidelines in oncology. 2019. January 29, 2020:Version 3.2019. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 2.Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma: a unique cervical cancer. Gynecol Oncol. 2010;116:140–146. doi: 10.1016/j.ygyno.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, Favini G, Ferri L, Mangioni C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 4.Baalbergen A, Veenstra Y, Stalpers LL, Ansink AC. Primary surgery versus primary radiation therapy with or without chemotherapy for early adenocarcinoma of the uterine cervix. Cochrane Database Syst Rev. 2010:CD006248. doi: 10.1002/14651858.CD006248.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr, Alberts DS. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 6.Wu SY, Huang EY, Lin H. Optimal treatments for cervical adenocarcinoma. Am J Cancer Res. 2019;9:1224–1234. [PMC free article] [PubMed] [Google Scholar]
- 7.Lin WC, Ding YF, Hsu HL, Chang JH, Yuan KS, Wu ATH, Chow JM, Chang CL, Chen SU, Wu SY. Value and application of trimodality therapy or definitive concurrent chemoradiotherapy in thoracic esophageal squamous cell carcinoma. Cancer. 2017;123:3904–3915. doi: 10.1002/cncr.30823. [DOI] [PubMed] [Google Scholar]
- 8.Yen YC, Chang JH, Lin WC, Chiou JF, Chang YC, Chang CL, Hsu HL, Chow JM, Yuan KS, Wu ATH, Wu SY. Effectiveness of esophagectomy in patients with thoracic esophageal squamous cell carcinoma receiving definitive radiotherapy or concurrent chemoradiotherapy through intensity-modulated radiation therapy techniques. Cancer. 2017;123:2043–2053. doi: 10.1002/cncr.30565. [DOI] [PubMed] [Google Scholar]
- 9.Chang CL, Tsai HC, Lin WC, Chang JH, Hsu HL, Chow JM, Yuan KS, Wu ATH, Wu SY. Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother Oncol. 2017;125:73–79. doi: 10.1016/j.radonc.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Chang WW, Hsiao PK, Qin L, Chang CL, Chow JM, Wu SY. Treatment outcomes for unresectable intrahepatic cholangiocarcinoma: nationwide, population-based, cohort study based on propensity score matching with the Mahalanobis metric. Radiother Oncol. 2018;129:284–292. doi: 10.1016/j.radonc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Chen TM, Lin KC, Yuan KS, Chang CL, Chow JM, Wu SY. Treatment of advanced nasopharyngeal cancer using low- or high-dose concurrent chemoradiotherapy with intensity-modulated radiotherapy: a propensity score-matched, nationwide, population-based cohort study. Radiother Oncol. 2017;129:23–29. doi: 10.1016/j.radonc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Lin YK, Hsieh MC, Chang CL, Chow JM, Yuan KS, Wu ATH, Wu SY. Intensity-modulated radiotherapy with systemic chemotherapy improves survival in patients with nonmetastatic unresectable pancreatic adenocarcinoma: a propensity score-matched, nationwide, population-based cohort study. Radiother Oncol. 2018;129:326–332. doi: 10.1016/j.radonc.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Lin YK, Hsieh MC, Wang WW, Lin YC, Chang WW, Chang CL, Cheng YF, Wu SY. Outcomes of adjuvant treatments for resectable intrahepatic cholangiocarcinoma: chemotherapy alone, sequential chemoradiotherapy, or concurrent chemoradiotherapy. Radiother Oncol. 2018;128:575–583. doi: 10.1016/j.radonc.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Yen YC, Hsu HL, Chang JH, Lin WC, Chang YC, Chang CL, Chow JM, Yuan KS, Wu ATH, Wu SY. Efficacy of thoracic radiotherapy in patients with stage IIIB-IV epidermal growth factor receptor-mutant lung adenocarcinomas who received and responded to tyrosine kinase inhibitor treatment. Radiother Oncol. 2018;129:52–60. doi: 10.1016/j.radonc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 16.Chen JH, Yen YC, Yang HC, Liu SH, Yuan SP, Wu LL, Lee FP, Lin KC, Lai MT, Wu CC, Chen TM, Chang CL, Chow JM, Ding YF, Wu SY. Curative-intent aggressive treatment improves survival in elderly patients with locally advanced head and neck squamous cell carcinoma and high comorbidity index. Medicine (Baltimore) 2016;95:e3268. doi: 10.1097/MD.0000000000003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States--a 24-year population-based study. Gynecol Oncol. 2000;78:97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 18.Bray F, Carstensen B, Moller H, Zappa M, Zakelj MP, Lawrence G, Hakama M, Weiderpass E. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev. 2005;14:2191–2199. doi: 10.1158/1055-9965.EPI-05-0231. [DOI] [PubMed] [Google Scholar]
- 19.van der Horst J, Siebers AG, Bulten J, Massuger LF, de Kok IM. Increasing incidence of invasive and in situ cervical adenocarcinoma in the Netherlands during 2004-2013. Cancer Med. 2017;6:416–423. doi: 10.1002/cam4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CA, Lahiri S, Hsu C. Population aging and regional income inequality in Taiwan: a spatial dimension. Social Indicators Research. 2015;122:757–777. [Google Scholar]
- 21.Schorge JO, Lee KR, Lee SJ, Flynn CE, Goodman A, Sheets EE. Early cervical adenocarcinoma: selection criteria for radical surgery. Obstet Gynecol. 1999;94:386–390. doi: 10.1016/s0029-7844(99)00312-9. [DOI] [PubMed] [Google Scholar]
- 22.Wu SY, Huang EY, Chanchien CC, Lin H, Wang CJ, Sun LM, Chen HC, Fang FM, Hsu HC, Huang YJ. Prognostic factors associated with radiotherapy for cervical cancer with computed tomography-detected para-aortic lymph node metastasis. J Radiat Res. 2014;55:129–138. doi: 10.1093/jrr/rrt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galic V, Herzog TJ, Lewin SN, Neugut AI, Burke WM, Lu YS, Hershman DL, Wright JD. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012;125:287–291. doi: 10.1016/j.ygyno.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Quinn BA, Deng X, Colton A, Bandyopadhyay D, Carter JS, Fields EC. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy. 2019;18:29–37. doi: 10.1016/j.brachy.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturdza A, Potter R, Fokdal LU, Haie-Meder C, Tan LT, Mazeron R, Petric P, Segedin B, Jurgenliemk-Schulz IM, Nomden C, Gillham C, McArdle O, Van Limbergen E, Janssen H, Hoskin P, Lowe G, Tharavichitkul E, Villafranca E, Mahantshetty U, Georg P, Kirchheiner K, Kirisits C, Tanderup K, Lindegaard JC. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120:428–433. doi: 10.1016/j.radonc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Fokdal L, Sturdza A, Mazeron R, Haie-Meder C, Tan LT, Gillham C, Segedin B, Jurgenliemk-Schultz I, Kirisits C, Hoskin P, Potter R, Lindegaard JC, Tanderup K. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: analysis from the retroEMBRACE study. Radiother Oncol. 2016;120:434–440. doi: 10.1016/j.radonc.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Bradbury M, Founta C, Taylor W, Kucukmetin A, Naik R, Ang C. Pathological risk factors and outcomes in women with stage IB2 cervical cancer treated with primary radical surgery versus chemoradiotherapy. Int J Gynecol Cancer. 2015;25:1476–1483. doi: 10.1097/IGC.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 28.McCann GA, Taege SK, Boutsicaris CE, Phillips GS, Eisenhauer EL, Fowler JM, O’Malley DM, Copeland LJ, Cohn DE, Salani R. The impact of close surgical margins after radical hysterectomy for early-stage cervical cancer. Gynecol Oncol. 2013;128:44–48. doi: 10.1016/j.ygyno.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kristensen GB, Abeler VM, Risberg B, Trop C, Bryne M. Tumor size, depth of invasion, and grading of the invasive tumor front are the main prognostic factors in early squamous cell cervical carcinoma. Gynecol Oncol. 1999;74:245–251. doi: 10.1006/gyno.1999.5420. [DOI] [PubMed] [Google Scholar]
- 30.Glance LG, Li Y, Osler TM, Dick A, Mukamel DB. Impact of patient volume on the mortality rate of adult intensive care unit patients. Crit Care Med. 2006;34:1925–1934. doi: 10.1097/01.CCM.0000226415.93237.84. [DOI] [PubMed] [Google Scholar]
- 31.Phibbs CS, Bronstein JM, Buxton E, Phibbs RH. The effects of patient volume and level of care at the hospital of birth on neonatal mortality. JAMA. 1996;276:1054–1059. [PubMed] [Google Scholar]
- 32.Prabhakaran S, Fonarow GC, Smith EE, Liang L, Xian Y, Neely M, Peterson ED, Schwamm LH. Hospital case volume is associated with mortality in patients hospitalized with subarachnoid hemorrhage. Neurosurgery. 2014;75:500–508. doi: 10.1227/NEU.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 33.Shu T, Zhao D, Li B, Wang Y, Liu S, Li P, Zuo J, Bai P, Zhang R, Wu L. Prognostic evaluation of postoperative adjuvant therapy for operable cervical cancer: 10 years’ experience of National Cancer Center in China. Chin J Cancer Res. 2017;29:510–520. doi: 10.21147/j.issn.1000-9604.2017.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, Rotman M, Gershenson D, Mutch DG. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J. Clin. Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 35.Chiara S, Bruzzone M, Merlini L, Bruzzi P, Rosso R, Franzone P, Orsatti M, Vitale V, Foglia G, Odicino F, et al. Randomized study comparing chemotherapy plus radiotherapy versus radiotherapy alone in FIGO stage IIB-III cervical carcinoma. GONO (North-West Oncologic Cooperative Group) Am J Clin Oncol. 1994;17:294–297. doi: 10.1097/00000421-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Monk BJ, Wang J, Im S, Stock RJ, Peters WA 3rd, Liu PY, Barrett RJ 2nd, Berek JS, Souhami L, Grigsby PW, Gordon W Jr, Alberts DS Gynecologic Oncology Group; Southwest Oncology Group; Radiation Therapy Oncology Group. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol Oncol. 2005;96:721–728. doi: 10.1016/j.ygyno.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol. 2012;125:292–296. doi: 10.1016/j.ygyno.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 38.Iwasaka T, Fukuda K, Hara K, Yokoyama M, Nakao Y, Uchiyama M, Sugimori H. Neoadjuvant chemotherapy with mitomycin C, etoposide, and cisplatin for adenocarcinoma of the cervix. Gynecol Oncol. 1998;70:236–240. doi: 10.1006/gyno.1998.5079. [DOI] [PubMed] [Google Scholar]
- 39.Kastritis E, Bamias A, Efstathiou E, Gika D, Bozas G, Zorzou P, Sarris K, Papadimitriou C, Dimopoulos MA. The outcome of advanced or recurrent non-squamous carcinoma of the uterine cervix after platinum-based combination chemotherapy. Gynecol Oncol. 2005;99:376–382. doi: 10.1016/j.ygyno.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Lissoni A, Gabriele A, Gorga G, Tumolo S, Landoni F, Mangioni C, Sessa C. Cisplatin-, epirubicin- and paclitaxel-containing chemotherapy in uterine adenocarcinoma. Ann Oncol. 1997;8:969–972. doi: 10.1023/a:1008221310453. [DOI] [PubMed] [Google Scholar]
- 41.Zanetta G, Lissoni A, Gabriele A, Landoni F, Colombo A, Perego P, Mangioni C. Intense neoadjuvant chemotherapy with cisplatin and epirubicin for advanced or bulky cervical and vaginal adenocarcinoma. Gynecol Oncol. 1997;64:431–435. doi: 10.1006/gyno.1996.4561. [DOI] [PubMed] [Google Scholar]
- 42.Benedetti-Panici P, Greggi S, Scambia G, Salerno MG, Amoroso M, Maneschi F, Cutillo G, Caruso A, Capelli A, Mancuso S. Locally advanced cervical adenocarcinoma: is there a place for chemo-surgical treatment? Gynecol Oncol. 1996;61:44–49. doi: 10.1006/gyno.1996.0094. [DOI] [PubMed] [Google Scholar]
- 43.Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, Monk BJ. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


