Abstract
Purpose: To estimate the outcomes of definitive concurrent chemoradiotherapy (CCRT) for bulky or advanced-stage cervical squamous cell carcinoma (SCC) and adenocarcinoma (ADC). Patients and methods: We enrolled patients who had been diagnosed as having cervical SCC or ADC and received definitive CCRT. A Cox regression analysis was performed to determine the hazard ratio (HR) and 95% confidence intervals (95% CI); independent predictors were stratified or controlled for in the analysis, and the endpoint was all-cause mortality among patients with cervical SCC and ADC who received CCRT. Propensity score matching was performed to create well-balanced groups. Results: we enrolled 3258 patients who had International Federation of Gynecology and Obstetrics (FIGO) stage IB2-IVA cervical cancer without distant metastasis. Among them, 2927 patients with cervical SCC and 331 patients with cervical ADC received definitive CCRT. The results of multivariate Cox regression analysis indicated that ADC, advanced FIGO stage, no intracavitary brachytherapy, old age, earlier year of diagnosis, and higher comorbidity scores were significant independent poor prognostic factors of all-cause mortality in patients with cervical cancer who received definitive CCRT. Patients with cervical ADC who received definitive CCRT had higher all-cause mortality, locoregional recurrence (LRR), and distant metastasis (DM) (adjusted HR [95% CI]: 2.10 [1.79-2.46], 1.79 [1.35-2.37], and 1.97 [1.54-2.53] for all-cause mortality, LRR, and DM, respectively) compared with patients with cervical SCC who received CCRT. Conclusion: Definitive CCRT in patients with cervical ADC resulted in lower overall survival, higher LRR, and higher DM rate compared with patients with cervical SCC.
Keywords: Cervical cancer, squamous cell carcinoma, adenocarcinoma, concurrent chemoradiotherapy, survival
Introduction
Cervical adenocarcinoma (ADC) and squamous cell carcinoma (SCC) account for 25% and 75% of invasive cervical cancers (CCs), respectively [1]. In Taiwan, approximately 18% of all invasive CCs are cervical ADC [2]. Treatment for CC is mostly based on data from randomized trials in which the majority of subjects were patients with SCC; ADC comprises, on average, 10% of cases [3-5]. No study has reported separate outcomes for ADC, and no prospective study has focused on the treatment of ADC as the sole histology. Consequently, in most institutions, treatment for ADC follows the principles established for cervical SCC [3]. Furthermore, no studies have reported on the effects of different management approaches for cervical ADC and SCC. Initial concurrent chemoradiotherapy (CCRT) rather than surgery is still recommended by the National Comprehensive Cancer Network (NCCN) guidelines in Taiwan for certain subsets of women with invasive cervical ADC, such as patients who display locoregionally advanced-stage IIB to IVA disease, bulky early-stage disease stage IB2-IIA, or evidence of lymph node involvement during imaging or clinical exams [3].
Whether histologic type is an independent prognostic factor in CC remains controversial [6-13]. After adjustment for the stage, some series have supported the prognostic equivalence of cervical ADC and cervical SCC [6-9], whereas others have reported that ADC has a less favorable prognosis [10-13]. One of the largest studies on CC from the Surveillance, Epidemiology, and End Results database, that comprised 77% and 17% patients with SCC and ADC, respectively [13], reported that women with early-stage CC (stage IB1 to IIA) and late-stage disease (stage IIB to IVA) ADC had a higher mortality risk than women with SCC. However, no further details were available regarding the outcomes of stages I-IV, locoregional recurrence (LRR), distant metastasis (DM), or the effects of various treatments for cervical ADC and SCC [13]. A few articles with small sample sizes (ADC < 100 patients) reported that patients with early-stage IB cervical ADC, identified according to the International Federation of Gynecology and Obstetrics (FIGO), who received surgery and adjuvant radiotherapy (RT) exhibited worse outcomes and higher distant metastasis rates [14,15]. An intergroup trial in the United States compared CCRT and RT in 243 patients (50 patients with ADC) with resected stage IA2, IB, or IIA CC and high-risk features [16]. The subgroup analysis revealed that patients with ADC had a less favorable prognosis than patients with SCC when treated with RT alone [16]; however, we did not identify a difference between the patients with ADC and SCC who were concurrently treated with chemotherapy (CT) and RT. Therefore, treatments that combine CT and RT, such as CCRT, may be more effective for treating cervical ADC than RT alone [16].
Our literature review suggested that no large study has estimated the outcomes of definitive CCRT for bulky or advanced stages of cervical SCC and ADC in terms of overall survival, LRR, or DM. Prognostic factors have not been reported either. Therefore, we evaluated the prognostic factors, overall survival (OS), LRR, and DM of definitive CCRT at different stages of cervical SCC and ADC, and we evaluated whether the current definitive CCRT with the platinum-based regimen is also effective for cervical ADC.
Patients and methods
A cohort was established using data from the Taiwan Cancer Registry database. Patients who were diagnosed as having cervical SCC or ADC and received definitive CCRT between January 1, 2007, and December 31, 2015, were enrolled. The follow-up duration was from the index date to December 31, 2014. The Cancer Registry database of the Collaboration Center of Health Information Application contains detailed cancer-related information for each patient, including the clinical stage, treatment modalities, pathology, radiation doses (dose of external beam radiotherapy [EBRT] and intracavitary brachytherapy), and CT regimens [17,18]. The protocols were reviewed and approved by the Institutional Review Board of Taipei Medical University. The diagnoses of the enrolled patients were confirmed using their pathological data, and the patients who were newly diagnosed as having cervical SCC or ADC were confirmed to have no other cancer. Patients were included if they had been diagnosed as having cervical SCC or ADC, they were aged ≥ 20 years, and if their datafile contained a FIGO staging system classification (clinical cancer stage IB-IVA were included). Patients with a history of cancer before cervical SCC or ADC, with DM, missing sex data, undergoing hypofractionation or stereotactic body RT, treated with non-platinum-based chemotherapy, with adenosquamous or small cell carcinoma, or with unclear staging data were excluded. Furthermore, patients with cervical SCC or ADC were excluded if they did not receive definitive CCRT, CT alone, RT alone, or surgical tumor resection.
Patients with CC were enrolled and categorized into two groups according to differences in pathology to compare their definitive CCRT outcomes. The median total dose and fraction size of RT were 52 and 2 Gy per fraction in SCC and ADC groups (Table 1). Comorbidities were scored using the Charlson comorbidity index (CCI) [19,20]. Comorbidities noted within 6 months before the index date were included. Comorbidities were identified on the basis of two separate diagnoses during visits to outpatient clinics and categorized according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
Table 1.
Characteristics of patients with cervical adenocarcinoma or squamous cell carcinoma who received definitive concurrent chemoradiotherapy
| Total (N = 3258) | SCC (N = 2927) | Adenocarcinoma (N = 331) | P value | ||
|---|---|---|---|---|---|
|
|
|
|
|||
| n (%) | n (%) | n (%) | |||
| Age, years | Mean (SD) | 57.6 (12.0) | 57.9 (12.0) | 55.3 (11.6) | .0001 |
| Median (min, max) | 57.0 (25, 91) | 57.0 (25, 91) | 54.0 (28, 88) | ||
| Age group | 20-49 | 821 (25.2) | 711 (24.3) | 110 (33.2) | .0013 |
| 50-59 | 1125 (34.5) | 1013 (34.6) | 112 (33.8) | ||
| 60-69 | 685 (21.0) | 622 (21.3) | 63 (19.0) | ||
| ≥ 70 | 627 (19.2) | 581 (19.8) | 46 (13.9) | ||
| Year of diagnosis | 2007-2009 | 1048 (32.2) | 944 (32.3) | 104 (31.4) | .1124 |
| 2010-2012 | 1058 (32.5) | 964 (32.9) | 94 (28.4) | ||
| 2013-2015 | 1152 (35.4) | 1019 (34.8) | 133 (40.2) | ||
| FIGO stage | IB2 | 506 (15.5) | 438 (15.0) | 68 (20.5) | .0154 |
| II | 1575 (48.3) | 1426 (48.7) | 149 (45.0) | ||
| III | 764 (23.4) | 699 (23.9) | 65 (19.6) | ||
| IVA | 413 (12.7) | 364 (12.4) | 49 (14.8) | ||
| EBRT cumulative dose, Gy | Mean (SD) | 51.8 (18.7) | 51.9 (18.8) | 50.8 (17.0) | .6814 |
| Median (Q1, Q3) | 52 (38, 60) | 52 (38, 60) | 52 (37, 60) | ||
| EBRT cumulative dose | < 50 Gy | 1404 (43.1) | 1262 (43.1) | 142 (42.9) | .9402 |
| ≥ 50 Gy | 1854 (56.9) | 1665 (56.9) | 189 (57.1) | ||
| Platinum cumulative dose, mg | Mean (SD) | 558.3 (480.1) | 555.3 (467.6) | 584.2 (578.8) | .8719 |
| Median (IQR, Q1, Q3) | 500 (300, 600) | 490 (300, 600) | 500 (300, 600) | ||
| Platinum cumulative dose | < 500 mg | 1628 (50.0) | 1470 (50.2) | 158 (47.7) | .3909 |
| ≥ 500 mg | 1630 (50.0) | 1457 (49.8) | 173 (52.3) | ||
| IC Brachytherapy | No | 554 (17.0) | 485 (16.6) | 69 (20.8) | .0497 |
| Yes | 2704 (83.0) | 2442 (83.4) | 262 (79.2) | ||
| IC Brachytherapy dose, cGy | Mean (SD) | 2390.6 (675.8) | 2405.0 (672.5) | 2362.9 (692.9) | .1009 |
| Median (IQR, Q1, Q3) | 2500 (2000, 3000) | 2500 (2000, 3000) | 2500 (2000, 3000) | ||
| IC Brachytherapy dose | No IC Brachytherapy | 554 (17.0) | 485 (16.6) | 69 (20.8) | .1124 |
| < 2500 cGy | 1119 (34.3) | 1005 (34.3) | 114 (34.4) | ||
| ≥ 2500 cGy | 1585 (48.6) | 1437 (49.1) | 148 (44.7) | ||
| CCI Scores | Mean (SD) | 0.4 (0.9) | 0.4 (0.9) | 0.4 (0.9) | .4343 |
| 0 | 2464 (75.6) | 2220 (75.8) | 244 (73.7) | .6608 | |
| 1 | 481 (14.8) | 427 (14.6) | 54 (16.3) | ||
| ≥ 2 | 313 (9.6) | 280 (9.6) | 33 (10.0) | ||
| Income | < 18,000 NTD | 931 (28.6) | 847 (28.9) | 84 (25.4) | .0275 |
| 18,000-22,500 NTD | 1067 (32.8) | 972 (33.2) | 95 (28.7) | ||
| 22,500-30,000 NTD | 465 (14.3) | 414 (14.1) | 51 (15.4) | ||
| ≥ 30,000 NTD | 795 (24.4) | 694 (23.7) | 101 (30.5) | ||
| Hospital type | Medical center | 2391 (73.4) | 2142 (73.2) | 249 (75.2) | .4247 |
| others | 867 (26.6) | 785 (26.8) | 82 (24.8) | ||
| Hospital location | North | 1629 (50.0) | 1476 (50.4) | 153 (46.2) | .0792 |
| Middle | 710 (21.8) | 622 (21.3) | 88 (26.6) | ||
| South/East | 919 (28.2) | 829 (28.3) | 90 (27.2) | ||
| Mean of follow-up time, months (SD) | 54.6 (35.5) | 56.2 (35.4) | 41.1 (33.6) | ||
| Death | 1264 (38.8) | 1081 (36.9) | 183 (55.3) | < .0001 | |
| Local recurrence | 422 (13.0) | 364 (12.4) | 58 (17.5) | < .0001 | |
| Distant metastasis | 503 (15.4) | 428 (14.6) | 75 (22.7) | < .0001 | |
Gy, gray; cGy, centigray; SCC, squamous cell carcinoma; ADC, adenocarcinoma; FIGO, International Federation of Gynecology and Obstetrics; EBRT, external beam radiotherapy; CCI, Charlson comorbidity index; mg, milligrams; SD, standard deviation; IQR, interquartile range; NTD, New Taiwan dollar; IC, intracavitary.
Significant independent predictors, such as age, CCI scores, FIGO clinical stage, year of diagnosis, RT cumulative dose, platinum cumulative dose, intracavitary brachytherapy dose, CCI Scores, income, hospital types, and hospital locations, were determined through multivariate Cox regression analysis to determine the hazard ratio (HR). Independent predictors were stratified or controlled for in the analysis, and the endpoint was all-cause mortality among patients with cervical SCC and ADC who received CCRT. The cumulative incidence of all-cause mortality was estimated using the Kaplan-Meier method, and differences between cervical SCC and ADC were determined according to a log-rank test. The Cox proportional hazards method was adjusted for confounders and used to model the time from the index date until all-cause mortality among patients with cervical SCC or ADC who received CCRT. In the multivariate analysis, HRs were adjusted for age, CCI score, FIGO clinical stage, year of diagnosis, RT cumulative dose, platinum cumulative dose, intracavitary brachytherapy dose, CCI score, income, hospital type, and hospital location. Stratified analyses were performed to evaluate the risk of mortality, LRR, and DM associated with cervical SCC or ADC and FIGO clinical stages. All analyses were performed using SAS statistical software (version 9.3; SAS, Cary, NC, USA). A 2-tailed P value < .05 was considered statistically significant.
To reduce the effects of potential confounding factors in the comparison of CCRT outcomes between the two groups, the groups were propensity score matched (PSM). Propensity score matching was performed using a multivariable logistic regression model in which the SCC and ADC groups were the dependent variables and potential confounders were covariates. The following confounders were included in the PSM: age, FIGO stage, year of diagnosis, RT cumulative dose, platinum cumulative dose, intracavitary brachytherapy dose, CCI score, income, hospital type, and hospital location. All patients with cervical ADC were matched with patients with cervical SCC at a ratio of 1:2. Independent predictors were controlled in the analysis, and the endpoint was the mortality rate among patients with cervical SCC and ADC.
Results
We enrolled 3258 patients who had FIGO stage IB-IVA CC without DM (Table 1). Among them, 2927 patients with cervical SCC and 331 patients with cervical ADC received definitive CCRT. The mean follow-up duration after the index date was 54.6 months (standard deviation = 35.5 months). The patients with cervical ADC were significantly younger, had a higher income, and had an earlier disease stage. Furthermore, fewer patients with ADC received intracavitary brachytherapy than did patients with cervical SCC (Table 1). The rates of LRR, DM, and mortality after definitive CCRT were higher in patients with cervical ADC than in patients with cervical SCC (LRR: 17.5% and 12.4%, DM: 22.7% and 14.6%, and mortality: 55.3% and 36.9% in patients with cervical ADC and SCC, respectively). The years of diagnosis, EBRT cumulative dose, intracavitary brachytherapy dose, platinum cumulative dose, CCI score, hospital type, and hospital location were not significantly different between the two groups. We further investigated Elixhauser Comorbidities between cervical SCC and ADC, and we did not identify statistically significant differences between the two groups receiving definitive CCRT (Supplemental Table 1) [21,22].
The results of multivariate Cox regression analysis indicated that ADC pathology, advanced FIGO stage, no intracavitary brachytherapy, old age, early year of diagnosis, and high CCI score were significant independent poor prognostic factors of all-cause mortality in patients with CC receiving definitive CCRT (Table 2). Cervical ADC (adjusted HR [aHR], 2.10; 95% confidence interval [CI], 1.79-2.46) was a significant independent prognostic factor for OS (P < .0001) (Table 2). The aHR of intracavitary brachytherapy at ≥ 2500 cGy and < 2500 cGy compared with no intracavitary brachytherapy was 2.27 (95% CI, 1.85-2.79) and 3.85 (95% CI, 3.09-4.80), respectively.
Table 2.
Cox proportional hazards regression analysis of the risk of all-cause mortality among patients with cervical adenocarcinomas or squamous cell carcinoma and received definitive concurrent chemoradiotherapy
| Crude HR (95% CI) | Adjusted HR* (95% CI) | P value | ||
|---|---|---|---|---|
| Pathologic type | SCC | 1 | 1 | < .0001 |
| adenocarcinoma | 1.93 (1.65-2.26) | 2.10 (1.79-2.46) | ||
| FIGO stage | I | 1 | 1 | < .0001 |
| II | 1.33 (1.09-1.61) | 1.39 (1.14-1.69) | ||
| III | 2.46 (2.01-3.01) | 2.27 (1.85-2.79) | ||
| IV | 4.86 (3.93-6.00) | 3.85 (3.09-4.80) | ||
| EBRT cumulative dose | < 50 Gy | 1 | 1 | .5315 |
| ≥ 50 Gy | 1.20 (1.07-1.34) | 0.96 (0.85-1.09) | ||
| Platinum cumulative dose | < 500 mg | 1 | 1 | .2706 |
| ≥ 500 mg | 0.94 (0.84-1.04) | 0.94 (0.84-1.05) | ||
| IC Brachytherapy dose | No IC Brachytherapy | 1 | 1 | < .0001 |
| < 2500 cGy | 0.35 (0.30-0.40) | 0.45 (0.39-0.53) | ||
| ≥ 2500 cGy | 0.29 (0.25-0.33) | 0.42 (0.36-0.49) | ||
| Age, years | ≥ 70 | 1 | 1 | < .0001 |
| 60-69 | 0.93 (0.80-1.07) | 0.85 (0.74-0.99) | ||
| 50-59 | 0.85 (0.72-1.01) | 0.73 (0.61-0.87) | ||
| 20-49 | 1.35 (1.15-1.58) | 1.10 (0.93-1.30) | ||
| Year of diagnosis | 2007-2009 | 1 | 1 | .0199 |
| 2010-2012 | 0.95 (0.83-1.08) | 0.94 (0.83-1.08) | ||
| 2013-2015 | 0.84 (0.72-0.97) | 0.81 (0.70-0.94) | ||
| CCI score | 0 | 1 | 1 | < .0001 |
| 1 | 1.33 (1.15-1.55) | 1.34 (1.15-1.56) | ||
| ≥ 2 | 1.71 (1.45-2.02) | 1.51 (1.27-1.80) | ||
| Income | < 18,000 NTD | 1 | 1 | .0686 |
| 18,000-22,500 NTD | 0.86 (0.75-0.98) | 0.93 (0.81-1.07) | ||
| 22,500-30,000 NTD | 0.81 (0.67-0.97) | 0.89 (0.74-1.07) | ||
| ≥ 30,000 NTD | 0.75 (0.65-0.88) | 0.81 (0.70-0.95) | ||
| Hospital type | Medical center | 1 | 1 | .7094 |
| others | 1.14 (1.01-1.29) | 0.98 (0.86-1.11) | ||
| Area | North | 1 | 1 | .0657 |
| Middle | 0.97 (0.84-1.12) | 1.08 (0.93-1.25) | ||
| South/East | 1.18 (1.04-1.35) | 1.17 (1.03-1.33) |
All variables presented in Table 2 were used in the multivariate analysis.
Gy, gray; SCC, squamous cell carcinoma; ADC, adenocarcinoma; FIGO, International Federation of Gynecology and Obstetrics; EBRT, external beam radiotherapy; CCI, Charlson comorbidity index; mg, milligrams; HR, hazard ratio; CI, confidence interval; NTD, New Taiwan dollar; IC, intracavitary.
The patient’s FIGO clinical stage was identified as a crucial, independent predictor. Furthermore, aHRs increased with advancement from FIGO stages II through IV (aHR: 1.39, 2.27, and 3.85 for stages II, III, and IV, respectively) (Table 2). Stratified analyses were performed to evaluate the risk of mortality among patients with cervical ADC and SCC receiving CCRT at different FIGO clinical stages, and a stratified Cox proportional hazard model was used to analyze the risk of mortality at different FIGO stages among patients with CC (Table 3). The aHRs after definitive CCRT for CC were calculated after adjusting for pathologic type, RT cumulative dose, platinum cumulative dose, intracavitary brachytherapy dose, age, year of diagnosis, CCI score, income, hospital type, and hospital location. Compared with the aHRs (95% CIs) of cervical SCC, those of cervical ADC for overall mortality in FIGO clinical stages I, II, and III-IV were 2.21 (95% CI, 1.43-3.42; P = .0004), 2.26 (95% CI, 1.75-2.92; P < .0001), and 2.05 (95% CI, 1.26-2.59; P < .0001), respectively (Table 3). Patients with CC who received intracavitary brachytherapy exhibited better OS compared with patients who did not receive intracavitary brachytherapy, regardless of FIGO stage. Old age and high CCI scores (1 or ≥ 2 compared with 0) in patients with CC receiving definitive CCRT were significant poor prognostic factors in stages II and III-IV FIGO (Table 3).
Table 3.
Cox proportional hazards regression analysis of the risk of all-cause mortality, stratified by disease stage
| Stage I | Stage II | Stage III-IV | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| aHR* (95% CI) | P value | aHR* (95% CI) | P value | aHR* (95% CI) | P value | ||
| Pathologic type | SCC | 1 | .0004 | 1 | < .0001 | 1 | < .0001 |
| adenocarcinoma | 2.21 (1.43-3.42) | 2.26 (1.75-2.92) | 2.05 (1.62-2.59) | ||||
| RT cumulative dose | < 50 Gy | 1 | .1652 | 1 | .3748 | 1 | .8335 |
| ≥ 50 Gy | 1.32 (0.89-1.96) | 0.91 (0.75-1.12) | 0.98 (0.82-1.17) | ||||
| Platinum cumulative dose | < 500 mg | 1 | .2098 | 1 | .8533 | 1 | .5603 |
| ≥ 500 mg | 0.80 (0.56-1.14) | 1.02 (0.85-1.22) | 0.95 (0.81-1.12) | ||||
| IC Brachytherapy dose | No IC Brachytherapy | 1 | .0009 | 1 | < .0001 | 1 | < .0001 |
| < 2500 cGy | 0.42 (0.23-0.74) | 0.34 (0.25-0.44) | 0.45 (0.37-0.55) | ||||
| ≥ 2500 cGy | 0.35 (0.20-0.60) | 0.29 (0.23-0.38) | 0.46 (0.38-0.56) | ||||
| Age, years | ≥ 70 | 1 | .3747 | 1 | < .0001 | 1 | .0352 |
| 60-69 | 0.98 (0.67-1.68) | 0.98 (0.76-1.25) | 0.78 (0.63-0.96) | ||||
| 50-59 | 0.96 (0.55-1.75) | 0.72 (0.54-0.97) | 0.72 (0.56-0.92) | ||||
| 20-49 | 1.49 (0.89-2.49) | 1.43 (1.10-1.87) | 0.85 (0.66-1.09) | ||||
| Year of diagnosis | 2007-2009 | 1 | .3969 | 1 | .0854 | 1 | .0966 |
| 2010-2012 | 0.73 (0.46-1.16) | 0.86 (0.69-1.07) | 1.04 (0.86-1.25) | ||||
| 2013-2015 | 0.93 (0.57-1.49) | 0.76 (0.59-0.97) | 0.84 (0.68-1.03) | ||||
| CCI score | 0 | 1 | .1463 | 1 | < .0001 | 1 | .0034 |
| 1 | 1.59 (0.95-2.65) | 1.18 (0.92-1.50) | 1.43 (1.15-1.78) | ||||
| ≥ 2 | 1.43 (0.82-2.50) | 1.98 (1.51 2.60) | 1.27 (0.99-1.63) | ||||
| Income | < 18,000 NTD | 1 | .3768 | 1 | .7504 | 1 | .0566 |
| 18,000-22,500 NTD | 1.17 (0.75-1.82) | 0.95 (0.76-1.19) | 0.85 (0.70-1.03) | ||||
| 22,500-30,000 NTD | 0.68 (0.36-1.31) | 0.97 (0.71-1.32) | 0.87 (0.68-1.12) | ||||
| ≥ 30,000 NTD | 0.92 (0.55-1.54) | 0.87 (0.67-1.12) | 0.75 (0.60-0.93) | ||||
| Hospital type | Medical center | 1 | .4386 | 1 | .4019 | 1 | .9634 |
| others | 0.84 (0.54-1.31) | 0.91 (0.74-1.13) | 1.00 (0.84-1.20) | ||||
| Area | North | 1 | .1374 | 1 | .7420 | 1 | .1478 |
| Middle | 0.86 (0.53-1.38) | 1.08 (0.85-1.36) | 1.08 (0.87-1.34) | ||||
| South/East | 1.40 (0.91-2.14) | 1.07 (0.87-1.33) | 1.26 (0.95-1.51) | ||||
All variables presented in Table 2 were used in the multivariate analysis.
Gy, gray; SCC, squamous cell carcinoma; ADC, adenocarcinoma; FIGO, International Federation of Gynecology and Obstetrics; EBRT, external beam radiotherapy; CCI, Charlson comorbidity index; mg, milligrams; HR, hazard ratio; CI, confidence interval; NTD, New Taiwan dollar; IC, intracavitary; aHR, adjusted hazard ratio.
The results of using a stratified Cox proportional hazard model to determine the risk of all-cause mortality, LRR, and DM among patients with cervical ADC or SCC who received definitive CCRT are presented in Table 4. Patients with cervical ADC had higher all-cause mortality, LRR, and DM (aHR: 2.10, 1.79, and 1.97, respectively) compared with patients with cervical SCC when the model was not stratified by age (Table 4). Patients with cervical ADC exhibited higher all-cause mortality compared with patients with cervical SCC; the results of multivariate analysis revealed that the aHRs were 2.21, 2.26, and 2.05 in FIGO stage I, II, and III-IV, respectively. Patients with cervical ADC exhibited higher LRR in stages I-II (aHR: 2.58 and 1.84 for stage I, and II, respectively) but no significant difference in FIGO stages III-IV compared with patients with cervical SCC. Compared with patients with cervical SCC, patients with cervical ADC had higher DM in FIGO stages II and III-IV (aHR: 2.31 and 1.90 for stages II and III-IV, respectively) but no significant difference in FIGO stage I.
Table 4.
Stratified Cox proportional hazard model for the risk of all-cause mortality, locoregional recurrence, and distant metastasis among patients with cervical adenocarcinoma or squamous cell carcinoma who received definitive concurrent chemoradiotherapy
| FIGO stage | Event | Pathologic type | Event no (%) | Adjusted HR* (95% CI) | P value |
|---|---|---|---|---|---|
| All patients | All-cause mortality | SCC | 1081 (36.93) | ref. | < .0001 |
| adenocarcinoma | 183 (55.29) | 2.10 (1.79-2.46) | |||
| Locoregional recurrence | SCC | 364 (12.44) | ref. | < .0001 | |
| adenocarcinoma | 58 (17.52) | 1.79 (1.35-2.37) | |||
| Distant metastasis | SCC | 428 (14.62) | ref. | < .0001 | |
| adenocarcinoma | 75 (22.66) | 1.97 (1.54-2.53) | |||
| Stage I | All-cause mortality | SCC | 100 (22.83) | ref. | .0004 |
| adenocarcinoma | 27 (39.71) | 2.21 (1.43-3.42) | |||
| Locoregional recurrence | SCC | 44 (10.05) | ref. | .0047 | |
| adenocarcinoma | 14 (20.59) | 2.58 (1.37-4.86) | |||
| Distant metastasis | SCC | 53 (12.10) | ref. | .2351 | |
| adenocarcinoma | 11 (16.18) | 1.50 (0.77-2.93) | |||
| Stage II | All-cause mortality | SCC | 420 (29.45) | ref. | < .0001 |
| adenocarcinoma | 71 (47.65) | 2.26 (1.75-2.92) | |||
| Locoregional recurrence | SCC | 178 (12.48) | ref. | .0026 | |
| adenocarcinoma | 29 (19.46) | 1.84 (1.24-2.74) | |||
| Distant metastasis | SCC | 175 (12.27) | ref. | < .0001 | |
| adenocarcinoma | 36 (24.16) | 2.31 (1.61-3.32) | |||
| Stage III-IV | All-cause mortality | SCC | 561 (52.78) | ref. | < .0001 |
| adenocarcinoma | 85 (74.56) | 2.05 (1.62-2.59) | |||
| Locoregional recurrence | SCC | 142 (13.36) | ref. | .1672 | |
| adenocarcinoma | 15 (13.16) | 1.47 (0.85-2.52) | |||
| Distant metastasis | SCC | 200 (18.81) | ref. | .0020 | |
| adenocarcinoma | 28 (24.56) | 1.90 (1.27-2.86) |
All variables presented in Table 2 were used in the multivariate analysis.
SCC, squamous cell carcinoma; ADC, adenocarcinoma; HR, hazard ratio; CI, confidence interval; aHR, adjusted hazard ratio.
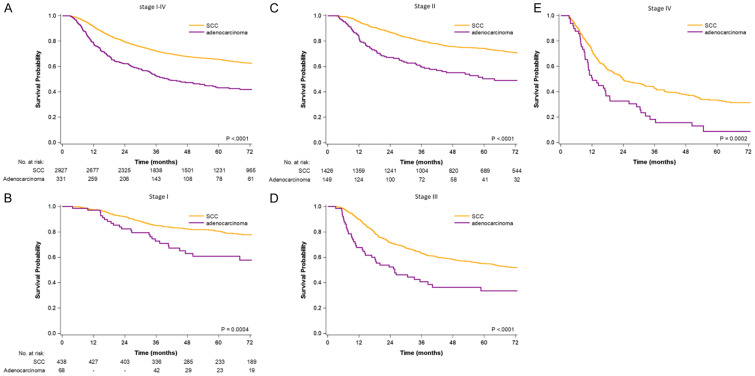
The Kaplan-Meier OS curves for patients with ADC or SCC in stages I, II, III, and IV are provided in Figure 1A-E. The OS rate was higher in patients with cervical SCC who received CCRT than in patients with cervical ADC who received CCRT (log-rank test: P < .0001, P < .0001, P = .0004, P < .0001, and P = .0002 in all stages, stage I, II, III, and IV, respectively). The 3-year OS rates in patients with cervical SCC and ADC who received CCRT were 85% and 73%, 80% and 59%, 63% and 41%, and 43% and 18% in FIGO stage I, II, III, and IV, respectively (Supplemental Table 2). The 5-year OS rates in patients with cervical SCC and ADC who received CCRT were 80% and 61%, 74% and 50%, 55% and 33%, and 33% and 9% in FIGO stage I, II, III, and IV, respectively.
Figure 1.
Survival curves for all-cause mortality determined using the Kaplan-Meier method (A) stage I-IV (B) stage I (C) stage II (D) stage III (E) stage IV.
The matching process yielded a final cohort of 985 patients (655 and 330 patients in the cervical SCC and cervical ADC groups, respectively) who were eligible for further analysis. Patient characteristics for PSM are listed in Supplemental Table 3, and all confounding factors were well matched. Cox proportional hazards regression analysis for the PSM cohort revealed differences in all-cause mortality, LRR, and DM between patients with cervical SCC and ADC who received definitive CCRT (Supplemental Table 4). The trends in all-cause mortality, LRR, and DM after PSM were similar to the trends in the non-PSM cohort (Table 4). Patients with cervical ADC who received definitive CCRT had higher all-cause mortality, LRR, and DM compared with the cervical SCC cohort in all FIGO stages. All-cause mortality was higher in patients with cervical ADC who received CCRT in stages I, II, and III-IV. Patients with cervical ADC had higher LRR in stages I and II compared with patients with cervical ADC, but no significant difference was noted in stage III-IV. Patients with cervical ADC displayed higher DM compared with patients with cervical SCC in stages II and III-IV, but no significant difference was noted in stage I. Supplemental Figure 1 presents the survival curves for all-cause mortality in different stages using the Kaplan-Meier method for the PSM cohort. After propensity score matching, patients with cervical SCC who received CCRT continued to exhibit a higher OS than did patients with cervical ADC who received CCRT in all stages (stage I, II, III, and IV).
Discussion
The present study is the first to report the prognostic factors and outcomes of patients with cervical ADC or SCC who received definitive CCRT. We demonstrated that patients with ADC had a younger age, earlier disease stage, and higher income than did patients with SCC (Table 1). These results accord with those of studies that have demonstrated that ADC incidence is higher in women who are younger [13] and have higher incomes [23-25]. However, a larger number of patients with cervical ADC received definitive CCRT without intracavitary brachytherapy compared with SCC. This may be because patients with cervical ADC had poorer response rates from definitive CCRT and achieved response more slowly than did patients with SCC [8]. Therefore, performing intracavitary brachytherapy is more difficult in patients with cervical ADC than patients with SCC because the response to CCRT in patients with ADC is poorer [8].
Table 2 displays some of the poor prognostic factors for definitive CCRT in patients with CC, such as ADC pathologic type, advanced FIGO stage, high CCI score, old age, earlier year of diagnosis, and no intracavitary brachytherapy. Old age, advanced FIGO stage, earlier year of diagnosis, no intracavitary brachytherapy, and ADC have previously been reported as poor prognostic factors [13,26-29]. However, high CCI scores had not previously been identified as a poor prognostic factor for patients with CC who have received CCRT. Furthermore, this is the first study to report lower OS in patients with cervical ADC than in patients with SCC following definitive CCRT at all FIGO stages after multivariate analysis (Table 2 and Figure 1). In Table 3, we stratified stages I, II, III-IV, which revealed similar prognostic factors to those presented in Table 2. ADC and no intracavitary brachytherapy were independent poor prognostic factors for patients with stage I CC who received CCRT; ADC, no intracavitary brachytherapy, old age, and high CCI scores were independent poor prognostic factors for patients with stages II or III-IV CC who received CCRT. Old age and high CCI scores were not statistically significant factors for patients with stage I CC who received CCRT, possibly because of the small sample size in stage I (Table 1).
We further used a stratified Cox proportional hazard model to assess the risk of all-cause mortality, LRR, and DM among cervical patients with ADC or SCC who received definitive CCRT (Table 4). This study is the first to evaluate the OS outcomes of definitive CCRT in patients with cervical ADC and SCC, LRR, and DM. All-cause mortality was significantly higher in all stages (stage I, II, III, and IV) in patients with cervical ADC compared with patients with cervical SCC. Studies have reported that a higher DM in patients with cervical ADC who have received CCRT might be associated with poor survival [7,30,31], despite the differences in OS not reaching statistical significance. Furthermore, we demonstrated that the LRR after definitive CCRT was significantly higher in patients with ADC compared with patients with SCC; thus, both the DM rate and LRR were higher in patients with ADC. The higher LRR rate in patients with cervical ADC is compatible with the findings of a study that had a small sample size and inconsistent treatments [31]. Although a study indicated that the addition of CT to RT may reduce the treatment failure rate in patients with cervical ADC [16], our results demonstrated that CCRT could not overcome the high LRR and high DM rate in patients with cervical ADC. Our findings did not indicate a significant difference in LRR between patients with ADC and SCC in stages III-IV (Table 4), but the trend maintained an aHR of 1.47. This may be because the LRRs were high in both cervical SCC and ADC in the advanced stages III-IV [8], and thus a larger sample size might be necessary. Similarly, we did not observe significant differences in DM in stage I between patients with ADC and those with SCC, but the trend was present with an aHR of 1.50. The improved control of DM after CCRT between stage I ADC and SCC may have contributed to these results [16]; thus, a larger sample size in stage I may be necessary to verify differences. Overall, patients with cervical ADC who received definitive CCRT had lower OS, higher DM, and higher LRR compared with patients with cervical SCC who received definitive CCRT (Table 4 and Figure 1). Therefore, improving local control modality and systemic treatments for reducing DM is crucial for cervical ADC. Conventional CCRT with a platinum-based CT regimen was insufficient for patients with cervical ADC; the five-year OS of stage IV patients with cervical ADC who received definitive CCRT was only 9% in our study (Supplemental Table 2).
To balance the confounding factors, we conducted a PSM study, the results of which are displayed in Supplemental Table 3 and demonstrate that the confounding factors were well matched between patients with cervical ADC and SCC who received CCRT. The results of Cox proportional hazards regression analysis for the PSM cohort also demonstrated lower OS, higher DM, and higher LRR in all FIGO stages (Supplemental Table 4). OS was lower in stages I, II, III, and IV in patients with cervical ADC compared with patients with the same stage of cervical SCC (Supplemental Table 4 and Supplemental Figure 1). Patients with cervical ADC who received definitive CCRT had a higher LRR rate in stages I and II compared with patients with cervical SCC who received CCRT. Moreover, in stages II and III-IV, the DM rate was higher in patients with cervical ADC who received CCRT than in patients with cervical SCC who received CCRT. Our results demonstrated that current definitive CCRT was insufficient for patients with cervical ADC and resulted in a low OS, high DM, and high LRR. The 5-year OS of patients with cervical ADC who received CCRT was extremely poor (Supplemental Table 2). Based on the inconsistent outcomes after CCRT in patients with cervical SCC and ADC, we suggest more aggressive treatments for better local control and less distant failure to improve survival. Carbon-ion RT or high dose intracavitary brachytherapy could be considered for patients with cervical ADC because of their greater affordance of local control, and trials for patients with cervical ADC should be performed [32-34]. However, cervical ADC is similarly sensitive to CT, at least for advanced diseases [6,35]. The use of neoadjuvant CT may be beneficial in selected women [36,37]. Novel systemic regimens with paclitaxel, cisplatin, carboplatin, bevacizumab, etoposide, or mitomycin could be considered in future clinical trials because they may reduce high distant failure rates in patients with cervical ADC [38,39].
The main strength of our study is that it is the largest cohort study to estimate the outcomes, including OS, LRR, and DM, of definitive CCRT with platinum-based CT for patients with cervical SCC and ADC. Furthermore, the treatment was highly homogenous because we only used definitive CCRT. PSMs were also performed before and after to eliminate possible confounding factors (Supplemental Table 3), preserve clinical characteristics in patients with ADC and SCC (Table 1), and evaluate the outcomes of definitive CCRT in patients with cervical SCC and ADC (Table 4 and Supplemental Table 4). Our findings demonstrated that patients with cervical ADC had lower OS, higher LRR, and higher DM than did patients with cervical SCC following conventional definitive CCRT. These findings indicate that physicians should consider that standard CCRT following NCCN guidelines is insufficient and results in poor survival outcomes among patients with cervical ADC (Supplemental Table 2, and Figure 1). The study outcomes indicate that increasing local control and reducing DM are crucial for patients with cervical ADC and can be achieved using charged-particle [32] and novel systemic regimens [39], respectively. Definitive CCRT may be suitable for patients with cervical SCC but insufficient for patients with cervical ADC (Supplemental Table 2). These findings could also be considered in future clinical practice and randomized controlled studies.
This study had some limitations. First, because all patients with cervical ADC were enrolled from an Asian population, the corresponding ethnic susceptibility remains unclear; therefore, caution should be exercised when extrapolating these results to non-Asian populations. However, differences in outcomes of definitive CCRT for CC between Asian and non-Asian populations have not been reported. Second, the diagnoses of all comorbid conditions were based on ICD-9-CM codes. However, the Taiwan Cancer Registry Administration randomly reviews charts and interviews patients to verify the accuracy of diagnoses, and hospitals with outlier chargers or practices may be audited and subsequently heavily penalized if malpractice or discrepancies are identified. Third, to prevent the creation of several subgroups, various adjuvant treatments after curative definitive CCRT were not categorized separately during the analyses. Subsequently, the effects of different adjuvant treatments after CCRT remain unclear. Therefore, a large-scale randomized trial comparing carefully selected patients undergoing suitable treatments is essential to obtain crucial information regarding population specificity and disease occurrence. Finally, the Taiwan Cancer Registry database does not contain information regarding dietary habits, socioeconomic status, or body mass index, all of which may be risk factors for mortality. However, considering the magnitude and statistical significance of the observed effects in this study, these limitations are unlikely to affect the conclusions.
Conclusions
Definitive CCRT resulted in lower OS, higher LRR, and higher DM rates in patients with cervical ADC than in patients with cervical SCC. Improving local control and DM are crucial for the treatment of patients with cervical ADC, and thus standard CCRT might be insufficient for patients with cervical ADC.
Acknowledgements
Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, supports Szu-Yuan Wu’s work (Funding Number: 10908 and 10909). Lei Qin’s work is supported by University of International Business and Economics Huiyuan outstanding young scholars research funding (17YQ15), “the Fundamental Research Funds for the central Universities” in UIBE (CXTD10-10).
Disclosure of conflict of interest
None.
Abbreviations
- AHR
adjusted hazard ratio
- CCRT
concurrent chemoradiotherapy
- Gy
gray
- US
United States
- SCC
squamous cell carcinoma
- ADC
adenocarcinoma
- HR
hazard ratio
- CI
confidence intervals
- FIGO
the International Federation of Gynecology and Obstetrics
- LRR
locoreional recurrence
- DM
distant metastasis
- NCCN
National Comprehensive Cancer Network
- CC
cervical cancers
- SEER
Surveillance, Epidemiology and End Results
- CT
chemotherapy
- RT
radiotherapy
- OS
overall survival
- EBRT
external beam radiotherapy
- CCI
Charlson comorbidity index
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- PSM
Propensity scores matched
Supporting Information
References
- 1.Alfsen GC, Thoresen SO, Kristensen GB, Skovlund E, Abeler VM. Histopathologic subtyping of cervical adenocarcinoma reveals increasing incidence rates of endometrioid tumors in all age groups: a population based study with review of all nonsquamous cervical carcinomas in Norway from 1966 to 1970, 1976 to 1980, and 1986 to 1990. Cancer. 2000;89:1291–1299. [PubMed] [Google Scholar]
- 2.National Health Insurance Administration, Ministry of Health and Welfare, Taiwan, R.O.C. (2015) 2017 [Google Scholar]
- 3.NCCN Clinical practice guidelines in oncology. National Comprehensive Cancer Network (NCCN) Version 3.2019. 2019. January 29, 2020:Version 3.2019. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp, 2020.
- 4.Rotman M, Sedlis A, Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, Zaino RJ. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65:169–176. doi: 10.1016/j.ijrobp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Wu SY, Huang EY, Lin H. Optimal treatments for cervical adenocarcinoma. Am J Cancer Res. 2019;9:1224–1234. [PMC free article] [PubMed] [Google Scholar]
- 6.Kastritis E, Bamias A, Efstathiou E, Gika D, Bozas G, Zorzou P, Sarris K, Papadimitriou C, Dimopoulos MA. The outcome of advanced or recurrent non-squamous carcinoma of the uterine cervix after platinum-based combination chemotherapy. Gynecol Oncol. 2005;99:376–382. doi: 10.1016/j.ygyno.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Lee KB, Lee JM, Park CY, Lee KB, Cho HY, Ha SY. What is the difference between squamous cell carcinoma and adenocarcinoma of the cervix? A matched case-control study. Int J Gynecol Cancer. 2006;16:1569–1573. doi: 10.1111/j.1525-1438.2006.00628.x. [DOI] [PubMed] [Google Scholar]
- 8.Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol. 2012;125:292–296. doi: 10.1016/j.ygyno.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald OK, Chen J, Dodson M, Lee CM, Gaffney DK. Prognostic significance of histology and positive lymph node involvement following radical hysterectomy in carcinoma of the cervix. Am J Clin Oncol. 2009;32:411–416. doi: 10.1097/COC.0b013e31819142dc. [DOI] [PubMed] [Google Scholar]
- 10.Davy ML, Dodd TJ, Luke CG, Roder DM. Cervical cancer: effect of glandular cell type on prognosis, treatment, and survival. Obstet Gynecol. 2003;101:38–45. doi: 10.1016/s0029-7844(02)02275-5. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi T, Ishikawa H, Suzuki Y, Inoue T, Nakamura S, Kuzuya K. A comparison of prognoses of pathologic stage Ib adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2000;79:289–293. doi: 10.1006/gyno.2000.5935. [DOI] [PubMed] [Google Scholar]
- 12.Lee YY, Choi CH, Kim TJ, Lee JW, Kim BG, Lee JH, Bae DS. A comparison of pure adenocarcinoma and squamous cell carcinoma of the cervix after radical hysterectomy in stage IB-IIA. Gynecol Oncol. 2011;120:439–443. doi: 10.1016/j.ygyno.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Galic V, Herzog TJ, Lewin SN, Neugut AI, Burke WM, Lu YS, Hershman DL, Wright JD. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012;125:287–291. doi: 10.1016/j.ygyno.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Schorge JO, Lee KR, Lee SJ, Flynn CE, Goodman A, Sheets EE. Early cervical adenocarcinoma: selection criteria for radical surgery. Obstet Gynecol. 1999;94:386–390. doi: 10.1016/s0029-7844(99)00312-9. [DOI] [PubMed] [Google Scholar]
- 15.Baalbergen A, Veenstra Y, Stalpers LL, Ansink AC. Primary surgery versus primary radiation therapy with or without chemotherapy for early adenocarcinoma of the uterine cervix. Cochrane Database Syst Rev. 2010:CD006248. doi: 10.1002/14651858.CD006248.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr, Alberts DS. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 17.Yen YC, Chang JH, Lin WC, Chiou JF, Chang YC, Chang CL, Hsu HL, Chow JM, Yuan KS, Wu ATH, Wu SY. Effectiveness of esophagectomy in patients with thoracic esophageal squamous cell carcinoma receiving definitive radiotherapy or concurrent chemoradiotherapy through intensity-modulated radiation therapy techniques. Cancer. 2017;123:2043–2053. doi: 10.1002/cncr.30565. [DOI] [PubMed] [Google Scholar]
- 18.Chen TM, Lin KC, Yuan KS, Chang CL, Chow JM, Wu SY. Treatment of advanced nasopharyngeal cancer using low- or high-dose concurrent chemoradiotherapy with intensity-modulated radiotherapy: a propensity score-matched, nationwide, population-based cohort study. Radiother Oncol. 2018;129:23–29. doi: 10.1016/j.radonc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 20.Chen JH, Yen YC, Yang HC, Liu SH, Yuan SP, Wu LL, Lee FP, Lin KC, Lai MT, Wu CC, Chen TM, Chang CL, Chow JM, Ding YF, Wu SY. Curative-intent aggressive treatment improves survival in elderly patients with locally advanced head and neck squamous cell carcinoma and high comorbidity index. Medicine (Baltimore) 2016;95:e3268. doi: 10.1097/MD.0000000000003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieffers JR, Baracos VE, Winget M, Fassbender K. A comparison of Charlson and Elixhauser comorbidity measures to predict colorectal cancer survival using administrative health data. Cancer. 2011;117:1957–1965. doi: 10.1002/cncr.25653. [DOI] [PubMed] [Google Scholar]
- 22.Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score Work. Med Care. 2015;53:e65–72. doi: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States--a 24-year population-based study. Gynecol Oncol. 2000;78:97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 24.Bray F, Carstensen B, Moller H, Zappa M, Zakelj MP, Lawrence G, Hakama M, Weiderpass E. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev. 2005;14:2191–2199. doi: 10.1158/1055-9965.EPI-05-0231. [DOI] [PubMed] [Google Scholar]
- 25.van der Horst J, Siebers AG, Bulten J, Massuger LF, de Kok IM. Increasing incidence of invasive and in situ cervical adenocarcinoma in the Netherlands during 2004-2013. Cancer Med. 2017;6:416–423. doi: 10.1002/cam4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu SY, Huang EY, Chanchien CC, Lin H, Wang CJ, Sun LM, Chen HC, Fang FM, Hsu HC, Huang YJ. Prognostic factors associated with radiotherapy for cervical cancer with computed tomography-detected para-aortic lymph node metastasis. J Radiat Res. 2014;55:129–138. doi: 10.1093/jrr/rrt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn BA, Deng X, Colton A, Bandyopadhyay D, Carter JS, Fields EC. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy. 2019;18:29–37. doi: 10.1016/j.brachy.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturdza A, Potter R, Fokdal LU, Haie-Meder C, Tan LT, Mazeron R, Petric P, Segedin B, Jurgenliemk-Schulz IM, Nomden C, Gillham C, McArdle O, Van Limbergen E, Janssen H, Hoskin P, Lowe G, Tharavichitkul E, Villafranca E, Mahantshetty U, Georg P, Kirchheiner K, Kirisits C, Tanderup K, Lindegaard JC. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120:428–433. doi: 10.1016/j.radonc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Fokdal L, Sturdza A, Mazeron R, Haie-Meder C, Tan LT, Gillham C, Segedin B, Jurgenliemk-Schultz I, Kirisits C, Hoskin P, Potter R, Lindegaard JC, Tanderup K. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: analysis from the retroEMBRACE study. Radiother Oncol. 2016;120:434–440. doi: 10.1016/j.radonc.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Eifel PJ, Morris M, Oswald MJ, Wharton JT, Delclos L. Adenocarcinoma of the uterine cervix. Prognosis and patterns of failure in 367 cases. Cancer. 1990;65:2507–2514. doi: 10.1002/1097-0142(19900601)65:11<2507::aid-cncr2820651120>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Huang YT, Wang CC, Tsai CS, Lai CH, Chang TC, Chou HH, Lee SP, Hong JH. Clinical behaviors and outcomes for adenocarcinoma or adenosquamous carcinoma of cervix treated by radical hysterectomy and adjuvant radiotherapy or chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;84:420–427. doi: 10.1016/j.ijrobp.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Shiba S, Wakatsuki M, Kato S, Ohno T, Okonogi N, Karasawa K, Kiyohara H, Tsujii H, Nakano T, Kamada T, Shozu MM The Working Group of the Gynecological Tumor. Carbon-ion radiotherapy for locally advanced cervical cancer with bladder invasion. J Radiat Res. 2016;57:684–690. doi: 10.1093/jrr/rrw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho O, Chun M. Management for locally advanced cervical cancer: new trends and controversial issues. Radiat Oncol J. 2018;36:254–264. doi: 10.3857/roj.2018.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assenholt MS, Vestergaard A, Kallehauge JF, Mohamed S, Nielsen SK, Petersen JB, Fokdal L, Lindegaard JC, Tanderup K. Proof of principle: applicator-guided stereotactic IMRT boost in combination with 3D MRI-based brachytherapy in locally advanced cervical cancer. Brachytherapy. 2014;13:361–368. doi: 10.1016/j.brachy.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Lissoni A, Gabriele A, Gorga G, Tumolo S, Landoni F, Mangioni C, Sessa C. Cisplatin-, epirubicin- and paclitaxel-containing chemotherapy in uterine adenocarcinoma. Ann Oncol. 1997;8:969–972. doi: 10.1023/a:1008221310453. [DOI] [PubMed] [Google Scholar]
- 36.Zanetta G, Lissoni A, Gabriele A, Landoni F, Colombo A, Perego P, Mangioni C. Intense neoadjuvant chemotherapy with cisplatin and epirubicin for advanced or bulky cervical and vaginal adenocarcinoma. Gynecol Oncol. 1997;64:431–435. doi: 10.1006/gyno.1996.4561. [DOI] [PubMed] [Google Scholar]
- 37.Benedetti-Panici P, Greggi S, Scambia G, Salerno MG, Amoroso M, Maneschi F, Cutillo G, Caruso A, Capelli A, Mancuso S. Locally advanced cervical adenocarcinoma: is there a place for chemo-surgical treatment? Gynecol Oncol. 1996;61:44–49. doi: 10.1006/gyno.1996.0094. [DOI] [PubMed] [Google Scholar]
- 38.Iwasaka T, Fukuda K, Hara K, Yokoyama M, Nakao Y, Uchiyama M, Sugimori H. Neoadjuvant chemotherapy with mitomycin C, etoposide, and cisplatin for adenocarcinoma of the cervix. Gynecol Oncol. 1998;70:236–240. doi: 10.1006/gyno.1998.5079. [DOI] [PubMed] [Google Scholar]
- 39.Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, Monk BJ. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.