Abstract
Parkinson disease (PD) is the second-most common neurodegenerative disorder. Its main pathological mechanism is the selective degeneration and deletion of dopaminergic neurons in the dense part of the substantia nigra and the damage of dopaminergic neurons caused by the abnormal deposition of a Lewy body, leading to a decreased dopamine level. Positron emission computed tomography (PET)/single photon emission computed tomography (SPECT) is a molecular imaging technology that can directly or indirectly reflect changes in molecular levels by using a specific tracer. With the research and development on the tracers of related enzymes for labeling dopamine transporter and dopamine receptor and for being involved in dopamine formation, this imaging technology has been applied to all aspects of PD research. It not only contributes to clinical work but also provides an important theoretical basis for exploring the pathological mechanism of PD at a molecular level. Therefore, this review discusses the application value of PET/SPECT in PD in terms of early diagnosis, disease severity evaluation, clinical manifestations, differential diagnosis, and pathological mechanism.
Keywords: Parkinson disease, Positron emission computed tomography, Single photon emission computed tomography, Dopamine transporter, 18F-fluorodeoxyglucose
Introduction
Parkinson disease (PD) is the second-most common neurodegenerative disease, with a prevalence of 1700/100,000 in people over the age of 65 years in China.[1] Its clinical manifestations are mainly divided into motor and non-motor symptoms. Motor symptoms include bradykinesia, rest tremor, rigidity, and postural disorders. Non-motor symptoms include rapid eye movement (REM), sleep behavior disorder (RBD), autonomic nervous dysfunction, mental symptoms, and cognitive decline. At present, the pathological mechanism of PD is unclear, and its diagnosis mostly depends on clinical manifestations. Therefore, misdiagnosis is incurred, especially in the early differential diagnosis of Parkinsonism-plus syndrome. For this reason, an objective biomarker should be developed.
Positron emission computed tomography (PET)/single photon emission computed tomography (SPECT) is a kind of molecular imaging technology that involves the use of a specific tracer for localization and quantification to show the occurrence and development of the disease at a molecular level. In the pathological mechanism of PD, the selective degeneration and deletion of dopaminergic neurons in the substantia nigra pars compacta and the abnormal deposition of a Lewy body cause damage to dopaminergic neurons, resulting in a decreased dopamine level received by the striatum. Therefore, with the rapid advancement of imaging technology, various tracers suitable for PD have been developed. These tracers are mainly divided into the following categories: (1) pre-synaptic dopamine transporter (DAT), such as 2β-carbomethoxy-3β-(4-fluorophenyl)-[N-11C-methyl]tropane (11C-CFT) and [99mTc]technetium[2-[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-yl]-methyl](2-mercaptoe-thyl)amino]-ethyl]amino]ethane-thiolato(3-)-N2,N2’,S2,S2’]oxo-[1R-(exo-exo)] (99mTc-TRODAT-1); (2) post-synaptic dopamine receptor (DR), such as 11C-raclopride and (S)-(-)-3-[123I]iodo-2-hydroxy-6-methoxy-N-[(1-ethyl-2-pyrrolidinyl) methyl]benzamide (123I-IBZM); and (3) intra synaptic enzymes, such as L-3,4-dihydroxy-6-[18F]-fluorophenylalanine (18F-DOPA), and (±)-α-[11C]dihydrotetrabenazine (11C-DTBZ). The intakes of the above tracers are determined via PET/SPECT, which can indirectly reflect the changes in dopamine in the substantia nigra striatum system.[2]
An increasing number of studies on PD have applied PET/SPECT molecular imaging. In this review, the use of PET/SPECT molecular imaging in relation to PD is discussed with the following aspect: (1) early diagnosis, (2) evaluation of disease severity, (3) relation of clinical manifestations, (4) differential diagnosis, and (5) pathological studies.
Early Diagnosis of PD by Using PET/SPECT Molecular Imaging
According to the 2015 The Movement Disorder Society Clinical Diagnostic Criteria for PD,[3] the diagnosis of PD mainly depends on its clinical manifestations. However, its clinical manifestations are relatively obvious when the loss of substantia nigra neurons is more than 50%, and the loss of striatum dopamine is more than 80%. As a consequence, these conditions often lead to the late treatment of patients and weaken the prognosis of patients. Therefore, the early diagnosis of PD is important.[4] In addition, the diagnosis of PD mostly depends on the clinical experience of doctors, and biomarkers with high sensitivity and specificity are lacking. With the development of PET/SPECT, it has been increasingly applied to diagnose PD in early stages. Among PET/SPECT technologies, DAT tracer is the most widely used.
Pérez-Lohman et al[5] examined PD via 11C-DTBZ PET and showed that the sensitivity and specificity of PET for PD diagnosis are 92.9% and 92%, respectively. PET has a great application value in PD diagnosis. A [123I] N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane (123I-FP-CIT) SPECT study found that a DAT decrease in PD presents a specific mode, that is, the DAT intakes of the putamen and caudate nucleus mainly decrease[6]; in particular, using PET with 18F-fluorinated-N-3-fluoropropyl-2-β-carboxymethoxy-3-β-(4-iodophenyl) nortropane (18F-FP-CIT), the loss of DAT in the putamen is more serious, and the stepwise decrease from the posterior putamen nucleus to the ventral putamen nucleus is a considerable sign of PD.[7] de Natale et al[8] further confirmed this finding. By SPECT, the 99mTc-TRODAT-1 uptake of the posterior putamen nucleus on the opposite to the onset side of PD decreases by 81%. Hence, the loss of DAT in the posterior putamen nucleus is remarkable. A related meta-analysis has also been conducted. The dopamine levels in the posterior and anterior putamen in the early to mid-term stages in patients with PD decrease by 58% to 77% and 43% to 59%, respectively; in the caudate nucleus, these levels decrease by 25% to 43%. This decrease is consistent with a stepwise decreasing mode.[9] However, no significant differences were observed in the uptake of 11C-raclopride, which is a post-synaptic PET imaging radiotracer, between PD and normal groups.[10,11]
Hence, DAT PET/SPECT molecular imaging has an important application value in the diagnosis of PD. Moreover, DAT in the posterior putamen decreases in the early stage of PD [Figure 1]. Hence, DAT PET/SPECT is helpful for the early diagnosis of PD.
Figure 1.
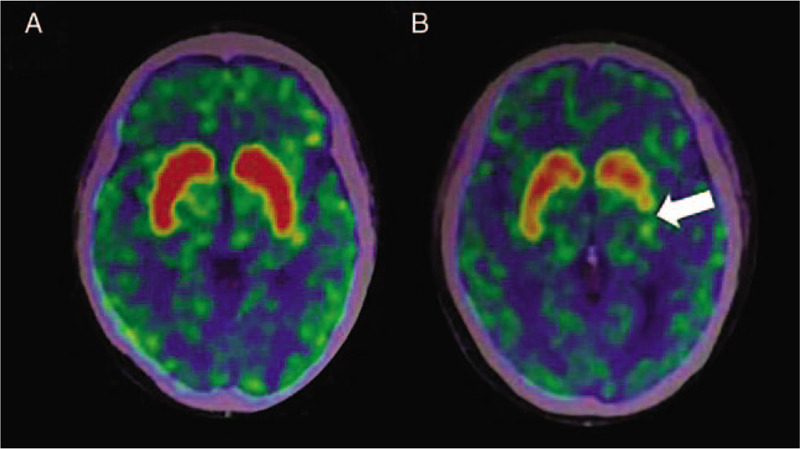
11C-CFT PET images of normal people and patients with PD. (A) No abnormalities were observed in the DAT intake of normal people. (B) DAT intake significantly decreased in the posterior putamen on the opposite to the onset side of PD (white arrow). 11C-CFT: 2β-carbomethoxy-3β-(4-fluorophenyl)-[N-11C-methyl]tropane; DAT: Dopamine transporter; PD: Parkinson disease; PET: Positron emission computed tomography.
Evaluation of the Severity of PD via PET/SPECT Molecular Imaging
The disease severity in patients with PD is often evaluated in accordance with Hoehn-Yahr staging. In this staging, grades 1 to 2.5 indicate early PD, and grades 3 to 5 denote mid-late PD. Patients with PD have mild symptoms and respond well to drugs in early stages, while they experience severe symptoms, poorly respond to drugs, and suffer from complications in mid-late stages. Therefore, if we can quantitatively evaluate the disease severity of patients with PD, we can monitor the disease progression and help choose individualized treatments for patients with PD in different disease stages.
Using SPECT with 99mTc-TRODAT-1, Gupta et al[12] found a remarkably negative correlation between the severity of PD and the increase in the DAT binding rate, which can be used to evaluate the severity of motor symptoms.[13] Furthermore, Tinaz et al[14] emphasized that the 123I-FP-CIT binding rate of the putamen shows a significantly negative correlation with the severity of motor symptoms of patients with PD. However, some think that the correlation of the caudate nucleus is stronger than that of the putamen.[9] In the mid-late stage, patients with PD show a serious loss of dopamine with one or more complications. Ravina et al[15] verified this finding. After undergoing [123I]-(1R)-2-β-carbomethoxy-3-β-(4-iodophenyl)-tropane SPECT, a 22-month follow-up on patients with PD has revealed that patients with the lowest DAT intake are more likely to have cognitive impairment and associated mental disorders. However, for post-synaptic imaging, 11C-raclopride PET showed that DR binding was not significantly correlated with PD dyskinesias.[10]
The severe decrease in the DAT binding rate often indicates a poor prognosis. Therefore, it may be a potential biomarker to evaluate the severity of PD by using DAT PET/SPECT molecular imaging technology.[16]
Some Features in PET/SPECT Molecular Imaging as Reflected by Different Clinical Manifestations of Patients With PD
Motor symptoms
The motor symptoms of PD are mainly manifested as bradykinesia, rest tremor, rigidity, and postural disorders. According to the Unified Parkinson Disease Rating Scale III, PD is divided into tremor dominate type, postural instability/gait disorder type, and mixed type. From a pathophysiological perspective, the clinical manifestations of PD are mainly caused by the lack of dopamine in the striatal system. From an anatomical perspective, the cortex-striatum-pallidum-thalamus-cortex motor circuit is the main loop that directly and indirectly regulates movement flexibility. The direct loop mainly excites the cerebral cortex, thereby making an easier movement. The indirect loop mainly inhibits the cerebral cortex, thereby reducing the movement. The substantia nigra can release the dopaminergic projection nervous tract to regulate the direct and indirect loop systems, including the excitation on the direct loop system and the inhibition on the indirect loop system. For patients with PD, dopaminergic neurons projecting on the striatum from the substantia nigra decrease, thereby inhibiting the direct loop and the excitation of the indirect loop and consequently decreasing movement. In the striatal system, the putamen and internal globus pallidus mainly participate in the motor circuit.[17] Moreover, the pallidum-sub-thalamic loop is the main loop that regulates tremor. The pallidum sends fibers to the sub-thalamic nucleus, and the latter sends fibers back to the pallidum along the original way, causing an inhibitory effect on the pallidum. If the sub-thalamic nucleus on one side is damaged and the pallidum on the same side is released, then the limbs involuntary shake. Therefore, a decrease in dopamine in the specific part of the striatum may be closely related to the different clinical manifestations of PD.
A 99mTc-TRODAT-1 SPECT study found that a decrease in DAT intake is directly related to a specific movement, especially in the putamen.[18] They observed patients with PIGD type PD often experience serious dopamine loss, while tremor has no remarkable correlation with dopamine damage in the striatum.[19] Moreover, Tremblay et al[20] found that the posterior putamen is often the first to be affected in patients with PD.
Therefore, PET/SPECT molecular imaging likely helps us explore the pathological mechanism of PD motor symptoms at a molecular level.
Non-motor symptoms
In addition to motor symptoms, non-motor symptoms, such as RBD, autonomic nervous dysfunction, depression, cognitive decline, and olfactory decline, manifest in patients with PD. More and more studies have explored non-motor symptoms.
RBD
This abnormal sleep type occurs in REM sleep. It is characterized by the achalasia of skeletal muscles, nightmares, and different levels of dream interpretation, often causing self-injury or injury to bedfellows. RBD is often secondary to neurodegenerative diseases, including PD and Dementia with Lewy bodies (DLB). Li et al[21] found that the loss of dopamine is obvious in patients with PD accompanied with RBD and revealed that their condition may be serious. A comparison of patients suffering from RBD and a healthy control group has shown that the former has a decreasing 99mTc-TRODAT-1, but it is not serious compared with that of the PD group. Hence, RBD may further develop to PD, which is consistent with some non-motor symptoms before PD symptoms appear. Iranzo et al[22] subjected 87 patients with RBD to SPECT inspection and found that 123I-FP-CIT uptake in the striatum decreased in 58.6% of the patients. However, after a follow-up of 3 to 5 years, the short-term risk of RBD in patients with abnormal DAT imaging to develop into synuclein disease (including PD and DLB) significantly increased. Hence, RBD may be a precursor of synuclein disease, and DAT SPECT molecular imaging may be used to track the relevant biochemical changes at a molecular level. Moreover, the post-mortem examination of a patient with RBD and abnormal DAT but without PD clinical manifestations has shown that the loss of dopaminergic neurons in the substantia nigra of the patient is 20% to 30%. Considering that this value is less than 50%, it may not show the motor symptoms of PD. Hence, RBD may be a specific manifestation of PD before motor symptoms.
Autonomic dysfunction
This condition mainly includes urinary dysfunction, orthostatic hypotension, and intestinal symptoms. Constipation is more common in clinical settings. The antidromic propagation of misfolded α-synuclein along the efferent fibers of the vagus nerve of the enteric nervous system may form the pathogenesis of PD.[23] Pagano et al[24] found that dopaminergic denervation in the substantia nigra striatum is related to REM, sleep behavior disorder, and depression but is not associated with constipation. By contrast, Hinkle et al[23] observed that constipation is related to a decrease in 123I-FP-CIT intake in the striatum in the early stage of PD. Moreover, the relationship with the DAT binding rate in the caudate nucleus is stronger than that in the putamen nucleus. However, no consistent conclusion has been made on the correlation between constipation and DAT. Hence, further studies are needed.
Depression
Patients with PD often experience depression. This condition may be related to the comprehensive effect of psychosocial factors and the dysfunction of the cortex-limbus-striatum loop involved in motor disorders. A pathological study on PD has shown that degeneration occurs in the limbic and brainstem areas,[25] and this condition is related to emotional disorders and changes in dopamine, norepinephrine, and 5-serotonin metabolism. Ceravolo et al[26] found changes in the 123I-FP-CIT binding in patients with PD accompanied with depression. However, its increase or decrease remains controversial. Some studies have suggested that monoaminergic neurotransmitters decrease in patients with depression, so DAT shows an increasing trend to compensate for this decrease. However, other studies have indicated that damage to dopaminergic neurons in the cortex and limbic system of patients with PD results in a DAT decrease. This phenomenon may be involved in the pathogenesis of depression. Through SPECT, Frosini et al[27] confirmed that 123I-FP-CIT intake in the cingulate cortex of patients with PD accompanied with depression decreases, and this condition is related to the severity of depression. Hence, the DAT of patients with PD accompanied with depression definitely changes, that is, DAT more likely decreases. Furthermore, Di Giuda et al[28] found that 123I-FP-CIT intake in the caudate nucleus decreases, and a low 123I-FP-CIT binding in the caudate nucleus is related to high anxiety and depression levels.[29] Moreover, Yoo et al[30] found that 18F-FP-CIT intake, which involves the caudate nucleus, also decreases in the ventral striatum. The uptake in the striatum shows pre-post and post-post-gradient changes. The loss of DAT in the caudate nucleus and ventral striatum may be involved in the pathophysiological process of depression in PD.
Cognition
Cognitive impairment is a common non-motor symptom in patients with PD, and it is mainly related to executive function, attention, memory, visual construction, and learning. Caspell-Garcia et al[31] examined patients with PD through SPECT and conducted a follow-up for 3 years. They showed that 38% of patients with PD experience cognitive impairment, and a decrease in 123I-FP-CIT intake in the caudate nucleus and putamen nucleus is a predictor of the development of cognitive impairment in these patients. Yoo et al[30] also revealed that the caudate nucleus is closely related to cognition, although it is involved in the pathogenesis of depression. Siepel et al[32] found that the loss of 123I-FP-CIT binding in the caudate nucleus is related to not only the executive function of the frontal lobe but also speech and visual memory. Nobili et al[33] suggested that the functional scoring of the visual space of patients with PD is related to a decrease in 123I-FP-CIT intake in the bilateral putamen. Moreover, 18F-DOPA intake decreases in the anterior cingulate cortex of patients with PD with a low capacity of calculation in the early stage.[34] The cognitive dysfunction of patients with PD is closely related to a decrease in DAT in the striatum.
Hyposmia
Braak et al[25] found that lesions affect the olfactory anterior nucleus and olfactory bulb before the motor symptoms of PD manifest, thus leading to olfactory disorders. Investigations on DAT involving 18F-FP-CIT PET in patients with PD revealed that the decline in DAT binding in the caudate nucleus may be linked to olfactory disorders in patients with PD.[35] This finding is consistent with that of a 123I-FP-CIT SPECT study,[36] and they found that mice lacking DAT are unable to distinguish between habitual smell and new odors, and their inability to do so may be related to an increase in tyrosine hydroxylase and a decrease in DR in the olfactory bulb. In mice with an excised olfactory bulb, tyrosine hydroxylase decreases in the caudate and putamen nuclei, and DR increases in the nucleus accumbens, indicating a strong relationship between olfactory function and dopamine metabolism in the striatum.
Therefore, PET/SPECT molecular imaging is helpful for exploring the correlation between non-motor symptoms and dopamine.
Significance of PET/SPECT Molecular Imaging in the Differential Diagnosis of PD
PD and Parkinsonism-plus syndrome have similar clinical manifestations because Parkinsonism-plus syndrome is often accompanied with damage to the dopamine delivery system. These two diseases are difficult to distinguish when a specific symptom is absent in the early stage of the disease. However, no effective therapeutic regimen has been developed for Parkinsonism-plus syndrome, and the prognosis of patients with this disease is worse than that of patients with PD. With the development of molecular imaging, Inconsistent conclusions on the differential value of post-synaptic imaging in PD and Parkinsonism-plus syndrome have been presented. A meta-analysis on DR SPECT showed that DR binding could not be applied to effectively distinguish the two diseases,[37] but other studies suggested that 18F-desmethoxyfallypride PET may be a potential tool to identify the two diseases.[38] However, the combination of DAT PET/SPECT and 18F-fluorodeoxyglucose (18F-FDG) PET can improve the differential rate of PD and Parkinsonism-plus syndrome to 90%.[39] PET/SPECT has become increasingly important in the differential diagnosis of PD.
Progressive supranuclear palsy (PSP)
PSP is a common atypical Parkinson syndrome.[40] The most common clinical phenotype is progressive supranuclear palsy Richardson syndrome (PSP-RS) clinically characterized by postural instability, vertical supranuclear gaze palsy, pseudobulbar palsy, extrapyramidal symptoms, and mild dementia. The core pathological changes are neurofibrillary tangles and nerve felt filaments, which are mainly located in the brainstem nucleus, basal ganglia, and frontal cortex. It is easily misdiagnosed as PD because of the same involvement of the basal ganglia.
Oh et al[7] confirmed a decrease in 18F-FP-CIT in the basal ganglia, mainly in the ventral putamen and caudate nucleus, of patients with PSP. A decrease in DAT in the caudate nucleus of patients with PSP is more obvious than that of patients with PD. This difference is consistent with rapid PSP progress and disease severity. The DAT intake ratio of the anterior caudate nucleus and ventral striatum has a high diagnostic value in distinguishing PSP and PD. The sensitivity and specificity are 94% and 92%, respectively.[7] Moreover, the latest PSP diagnostic standard shows that 18F-FDG PET can be used as a diagnostic marker. It is commonly manifested as the hypometabolism of glucose in the frontal lobe, caudate nucleus, mid-brain, and thalamus.[41] However, for a typical glucose metabolism pattern associated with PD, metabolism does not decline in the motor and premotor areas, sensory cortex, anterior cingulate zone, frontal cortex, pallidum, and putamen.[42] In addition, SPECT involving 123I-IBZM suggested that the DR binding of PSP-RS decreased, but the small difference failed to discriminate between PD and PSP-RS.[43]
Multiple system atrophy (MSA)
MSA is a sporadic neurodegenerative disease of unknown etiology. It is mainly divided into MSA with pre-dominant Parkinsonism (MSA-P) type, which mainly shows PD-like symptoms (bradykinesia, increased muscle tension, tremor, and postural instability) and MSA with cerebellar features (MSA-C) type, which mainly presents cerebellar symptoms (gait ataxia and microcephaly dysarthria).[44] Its pathological feature is the extensive degeneration of the cerebellum, brain stem, basal ganglia, and spinal cord.[45]
18F-FP-CIT also decreases in the basal ganglia of patients with MSA. Here, MSA-P type decreases more remarkably than MSA-C type. This finding is consistent with the characteristics of MSA-P type, that is, it is mainly reflected as a clinical manifestation of PD. In comparison with PD, MSA-P type is mainly associated with the loss of the putamen and ventral putamen, while the caudate nucleus is relatively reserved[7,46] [Figure 2]. Moreover, the 18F-FDG PET of MSA is often manifested as a decrease in the metabolism in the putamen and cerebellum. Combined DAT PET imaging has a certain specificity, and it has been included as an additional condition for the diagnosis of this disease.[47] Investigations on DR binding involving 11C-raclopride PET in PD and MSA demonstrated that the DR binding of MSA decreased and showed a positive correlation with 11C-CFT. Although the DR binding did not significantly decline in PD, the difference in DR binding might be unable to indicate the distinction between the two groups at early stages. Nevertheless, the difference in DR binding might gradually increase with time.[48]
Figure 2.
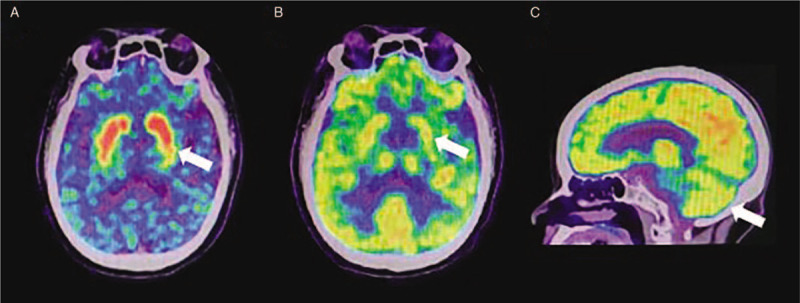
11C-CFT PET and 18F-FDG PET imaging of patients with MSA. (A) DAT intake in the posterior putamen of patients with MSA-P decreases, while the caudate nucleus is relatively reserved (white arrow). (B) FDG metabolism decreases in the posterior putamen (white arrow). (C) FDG metabolism decreases in the cerebellum of patients with MSA-C (white arrow). 11C-CFT: 2β-carbomethoxy-3β-(4-fluorophenyl)-[N-11C-methyl]tropane; DAT: Dopamine transporter; 18F-FDG: 18F-fluorodeoxyglucose; MSA: Multiple system atrophy; MSA-C: MSA with cerebellar features; MSA-P: MSA with predominant Parkinsonism; PET: Positron emission computed tomography.
Corticobasal degeneration (CBD)
CBD is diagnosed on the basis of pathological changes. The main pathological manifestation is the extensively hyperphosphorylated Tau protein deposits in the neurons and glial cells of the cortex, basal ganglia, and brain stem. As a consequence, neuronal loss occurs in the cortex and substantia nigra. The characteristic pathological sign of CBD is the star cell spot on the prefrontal lobe and premotor area. Its core clinical symptoms include progressive asymmetrical myotonia and disuse.
In a 123I-FP-CIT SPECT study, CBD is also characterized by a decrease in DAT. It is mainly manifested as a decrease in the DAT in the asymmetric cortex and basal ganglia. CBD and PD show a decrease in DAT in the putamen and caudate nuclei, but CBD presents a more obvious asymmetry than PD does. 18F-FDG PET imaging further reveals that glucose metabolism decreases in the asymmetric cerebral hemisphere, striatum, and thalamus [Figure 3]. Therefore, CBD can be distinguished from PD and other Parkinsonism-plus syndromes.[6,39] In another 18F-FDG PET imaging, the metabolism and perfusion of glucose in the asymmetric frontotemporal lobe and basal ganglia also decrease.[49]
Figure 3.
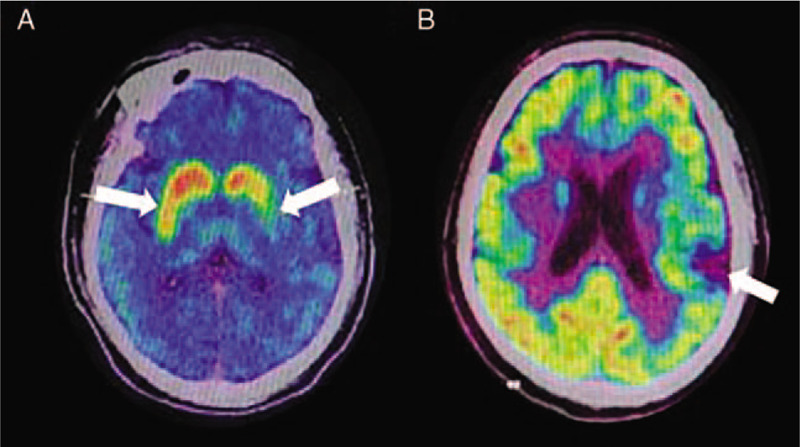
11C-CFT PET and 18F-FDG PET imaging of patients with CBD. (A) patients with CBD have an obvious asymmetric decrease in DAT intake (white arrows); (B) the metabolism of FDG in the left cerebral hemisphere of patients with CBD decreases (white arrow), but no obvious abnormality is observed on the right side, which is asymmetric. 11C-CFT: 2β-carbomethoxy-3β-(4-fluorophenyl)-[N-11C-methyl]tropane; CBD: Corticobasal degeneration; DAT: Dopamine transporter; 18F-FDG: 18F-fluorodeoxyglucose; PET: Positron emission computed tomography.
DLB
DLB is the second-most common neurodegenerative dementia. The main clinical manifestations of DLB include fluctuating cognitive dysfunction, visual hallucination, and PD-like motor symptoms. Its main pathological manifestation is that DLB is widely distributed in the cerebral cortex and brainstem. Patients with DLB often show motor symptoms similar to those of PD, suggesting a possible striatal involvement in this disease.
Joling et al[50] found that the 123I-FP-CIT intake in the bilateral caudate nucleus of patients with PD and DLB is lower than that of healthy people. Moreover, striatal dopamine loss is observed in patients with DLB. However, the two diseases cannot be distinguished on the basis of the differences in DAT imaging. Therefore, Caminiti et al[42] applied 18F-FDG PET to examine patients with DLB and found that the main symptoms of these patients include the hypometabolism of the temporal parietal lobe and occipital lobe. This condition is significantly different from those of patients with PD. DLB can be distinguished from PD with an accuracy of above 90%. Cousins et al[51] focused on a decrease in glucose metabolism in the occipital cortex of patients with DLB. Except the occipital lobe, the island sign of the cingulate gyrus detected by 18F-FDG PET has been identified as an imaging feature with an obvious significance. The island sign of the cingulate gyrus is regarded as a supportive marker in DLB's diagnostic consensus guidelines.[52]
Therefore, the combination of DAT PET/SPECT with 18F- FDG PET has an important value in the differential diagnosis of PD.
Significance of PET/SPECT Molecular Imaging in Pathological Studies on PD
α-Synuclein is a neuron protein composed of 140 residues. Under physiological conditions, most of them exist in presynaptic terminals. The pathological aggregation of synuclein is one of the characteristics of PD and other neurodegenerative diseases.
Accordingly, Bellucci et al[53] found that α-synuclein aggregation may be responsible for the synaptic dysfunction of dopaminergic neurons in the substantia nigra striatum system of PD. Moreover, α-synuclein causes an increase in dopamine in the free cytoplasm by regulating the DAT expression, thus leading to the production of toxic dopamine metabolites and reactive oxygen species, such as superoxide and hydroxyl radicals, during automatic oxidation and enzyme metabolism, resulting in neuronal death. This condition is associated with the selective damage to dopaminergic neurons in PD.[54] Subsequently, Bellucci et al[55] proposed a retrograde pattern of degeneration in the substantia nigra and striatum of PD, that is, the progression from the end of a caudate nucleus and a putamen nucleus to the cells in the substantia nigra. The mechanism may depend on the aggregation of α-synuclein at synaptic sites. [18F](E)-N-(3-iodoprop-2-enyl)-2β-carbofluoroethoxy-3β-(4’-methyl-phenyl)nortropane PET study has shown that DAT decreases to a greater extent at the end of the axon than in the cell body and axon levels of dopaminergic neurons in the early symptomatic phase of the disease.[56] This condition is consistent with the action site of α-synuclein. Gao et al[57] collected the labial gland tissues of patients with PD to detect α-synuclein and demonstrated the improvement of 11C-CFT PET. The α-Synuclein aggregation in the labial gland is consistent with the change in 11C-CFT PET imaging. Moreover, the combination of labial gland biopsy and DAT PET is helpful to improve early diagnosis. Therefore, PET/SPECT has an important value in the pathology of PD.
In conclusion, PET/SPECT has a unique molecular imaging function and has been applied to all aspects of PD research. In the future, more in-depth research on PET/SPECT can provide clinical help and explore the pathophysiological mechanism of PD at a molecular level.
Funding
This work was supported by grants from the National Key Laboratory of Infectious Disease Prevention and Control Independent Research Open Project (No. 2018SKLID307); Science and Technology Fund Project of Guizhou Health and Health Commission (No. gzwjkj2019-1-065); Science and Technology Plan Project of Guiyang (No. [2017]30-30), and the Department of Science and Technology of Guizhou Province (No. [2017]5718).
Conflicts of interest
None.
Footnotes
How to cite this article: Yao NT, Zheng Q, Xu QZ, Yin JH, Lu LG, Zuo Q, Yang S, Zhang CL, Jiao L. Positron emission computed tomography/single photon emission computed tomography in Parkinson disease. Chin Med J 2020;133:1448–1455. doi: 10.1097/CM9.0000000000000836
Nian-Ting Yao and Qian Zheng contributed equally to this work.
References
- 1.Zhang ZX, Roman GC, Hong Z, Wu CB, Qu QM, Huang JB, et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet 2005; 365:595–597. doi: 10.1016/S0140-6736(05)17909-4. [DOI] [PubMed] [Google Scholar]
- 2.Abbasi Gharibkandi N, Hosseinimehr SJ. Radiotracers for imaging of Parkinson's disease. Eur J Med Chem 2019; 166:75–89. doi: 10.1016/j.ejmech.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015; 30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 4.Bhat S, Acharya UR, Hagiwara Y, Dadmehr N, Adeli H. Parkinson's disease: Cause factors, measurable indicators, and early diagnosis. Comput Biol Med 2018; 102:234–241. doi: 10.1016/j.compbiomed.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Lohman C, Kerik NE, Díaz-Meneses IE, Cervantes-Arriaga A, Rodríguez-Violante M. Diagnostic utility of [11C]DTBZ positron emission tomography in clinically uncertain parkinsonism: experience of a single tertiary center. Rev Invest Clin 2018; 70:285–290. doi: 10.24875/RIC.18002644. [DOI] [PubMed] [Google Scholar]
- 6.Meyer PT, Hellwig S. Update on SPECT and PET in Parkinsonism - part 1: imaging for differential diagnosis. Curr Opin Neurol 2014; 27:390–397. doi: 10.1097/WCO.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 7.Oh M, Kim JS, Kim JY, Shin KH, Park SH, Kim HO, et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med 2012; 53:399–406. doi: 10.2967/jnumed.111.095224. [DOI] [PubMed] [Google Scholar]
- 8.de Natale ER, Niccolini F, Wilson H, Politis M. Molecular imaging of the dopaminergic system in idiopathic Parkinson's disease. Int Rev Neurobiol 2018; 141:131–172. doi: 10.1016/bs.irn.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Kaasinen V, Vahlberg T. Striatal dopamine in Parkinson disease: a meta-analysis of imaging studies. Ann Neurol 2017; 82:873–882. doi: 10.1002/ana.25103. [DOI] [PubMed] [Google Scholar]
- 10.Niccolini F, Su P, Politis M. Dopamine receptor mapping with PET imaging in Parkinson's disease. J Neurol 2014; 261:2251–2263. doi: 10.1007/s00415-014-7302-2. [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Xu B, Guo Z, Chen T, Zhang J, Chen Y, et al. Suite PET/CT neuroimaging for the diagnosis of Parkinson's disease: statistical parametric mapping analysis. Nucl Med Commun 2017; 38:164–169. doi: 10.1097/MNM.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 12.Gupta V, Ranjan R, Verma R, Belho ES, Malik D, Mahajan H. Correlation of 99mTc-TRODAT-1 SPECT imaging findings and clinical staging of Parkinson disease. Clin Nucl Med 2019; 44:347–350. doi: 10.1097/RLU.0000000000002529. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Lao-Kaim NP, Roussakis AA, Martín-Bastida A, Valle-Guzman N, Paul G, et al. 11C-PE2I and 18 F-Dopa PET for assessing progression rate in Parkinson's: a longitudinal study. Mov Disord 2018; 33:117–127. doi: 10.1002/mds.27183. [DOI] [PubMed] [Google Scholar]
- 14.Tinaz S, Chow C, Kuo PH, Krupinski EA, Blumenfeld H, Louis ED, et al. Semiquantitative analysis of dopamine transporter scans in patients with Parkinson disease. Clin Nucl Med 2018; 43:e1–e7. doi: 10.1097/RLU.0000000000001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravina B, Marek K, Eberly S, Oakes D, Kurlan R, Ascherio A, et al. Dopamine transporter imaging is associated with long-term outcomes in Parkinson's disease. Mov Disord 2012; 27:1392–1397. doi: 10.1002/mds.25157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi H, Watanabe Y, Tanaka H, Mochizuki H, Kato H, Hatazawa J, et al. Quantifying the severity of Parkinson disease by use of dopaminergic neuroimaging. AJR Am J Roentgenol 2019; 213:1–6. doi: 10.2214/AJR.18.20655. [DOI] [PubMed] [Google Scholar]
- 17.Poston KL, Eidelberg D. Functional brain networks and abnormal connectivity in the movement disorders. Neuroimage 2012; 62:2261–2270. doi: 10.1016/j.neuroimage.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corrêa PS, Pagnussat AS, Cabeleira M, Schifino GP, Rieder C, da Silva Junior N, et al. Is the dopaminergic loss associated with gait and postural impairments in subjects with Parkinson's disease at different motor stages. Eur J Neurosci 2019; 50:3889–3895. doi: 10.1111/ejn.14522. [DOI] [PubMed] [Google Scholar]
- 19.Liu FT, Ge JJ, Wu JJ, Wu P, Ma Y, Zuo CT, et al. Clinical, dopaminergic, and metabolic correlations in Parkinson disease: a dual-tracer PET study. Clin Nucl Med 2018; 43:562–571. doi: 10.1097/RLU.0000000000002148. [DOI] [PubMed] [Google Scholar]
- 20.Tremblay L, Worbe Y, Thobois S, Sgambato-Faure V, Féger J. Selective dysfunction of basal ganglia subterritories: from movement to behavioral disorders. Mov Disord 2015; 30:1155–1170. doi: 10.1002/mds.26199. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Kang W, Yang Q, Zhang L, Zhang L, Dong F, et al. Predictive markers for early conversion of iRBD to neurodegenerative synucleinopathy diseases. Neurology 2017; 88:1493–1500. doi: 10.1212/WNL.0000000000003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iranzo A, Santamaría J, Valldeoriola F, Serradell M, Salamero M, Gaig C, et al. Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 2017; 82:419–428. doi: 10.1002/ana.25026. [DOI] [PubMed] [Google Scholar]
- 23.Hinkle JT, Perepezko K, Mills KA, Mari Z, Butala A, Dawson TM, et al. Dopamine transporter availability reflects gastrointestinal dysautonomia in early Parkinson disease. Parkinsonism Relat Disord 2018; 55:8–14. doi: 10.1016/j.parkreldis.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagano G, Yousaf T, Wilson H, Niccolini F, Polychronis S, Chaudhuri KR, et al. Constipation is not associated with dopamine transporter pathology in early drug-naïve patients with Parkinson's disease. Eur J Neurol 2018; 25:307–312. doi: 10.1111/ene.13503. [DOI] [PubMed] [Google Scholar]
- 25.Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol 2000; 247: Suppl 2: II3–II10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- 26.Ceravolo R, Frosini D, Poletti M, Kiferle L, Pagni C, Mazzucchi S, et al. Mild affective symptoms in de novo Parkinson's disease patients: relationship with dopaminergic dysfunction. Eur J Neurol 2013; 20:480–485. doi: 10.1111/j.1468-1331.2012.03878.x. [DOI] [PubMed] [Google Scholar]
- 27.Frosini D, Unti E, Guidoccio F, Del Gamba C, Puccini G, Volterrani D, et al. Mesolimbic dopaminergic dysfunction in Parkinson's disease depression: evidence from a 123I-FP-CIT SPECT investigation. J Neural Transm (Vienna) 2015; 122:1143–1147. doi: 10.1007/s00702-015-1370-z. [DOI] [PubMed] [Google Scholar]
- 28.Di Giuda D, Camardese G, Bentivoglio AR, Cocciolillo F, Guidubaldi A, Pucci L, et al. Dopaminergic dysfunction and psychiatric symptoms in movement disorders: a 123I-FP-CIT SPECT study. Eur J Nucl Med Mol Imaging 2012; 39:1937–1948. doi: 10.1007/s00259-012-2232-7. [DOI] [PubMed] [Google Scholar]
- 29.Picillo M, Santangelo G, Erro R, Cozzolino A, Amboni M, Vitale C, et al. Association between dopaminergic dysfunction and anxiety in de novo Parkinson's disease. Parkinsonism Relat Disord 2017; 37:106–110. doi: 10.1016/j.parkreldis.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Yoo SW, Oh YS, Hwang EJ, Ryu DW, Lee KS, Lyoo CH, et al. Depressed” caudate and ventral striatum dopamine transporter availability in de novo Depressed Parkinson's disease. Neurobiol Dis 2019; 132:104563.doi: 10.1016/j.nbd.2019.104563. [DOI] [PubMed] [Google Scholar]
- 31.Caspell-Garcia C, Simuni T, Tosun-Turgut D, Wu IW, Zhang Y, Nalls M, et al. Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS One 2017; 12:e0175674.doi: 10.1371/journal.pone.0175674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siepel FJ, Brønnick KS, Booij J, Ravina BM, Lebedev AV, Pereira JB, et al. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson's disease. Mov Disord 2014; 29:1802–1808. doi: 10.1002/mds.26051. [DOI] [PubMed] [Google Scholar]
- 33.Nobili F, Campus C, Arnaldi D, De Carli F, Cabassi G, Brugnolo A, et al. Cognitive-nigrostriatal relationships in de novo, drug-naïve Parkinson's disease patients: a [I-123]FP-CIT SPECT study. Mov Disord 2010; 25:35–43. doi: 10.1002/mds.22899. [DOI] [PubMed] [Google Scholar]
- 34.Jokinen P, Karrasch M, Brück A, Johansson J, Bergman J, Rinne JO. Cognitive slowing in Parkinson's disease is related to frontostriatal dopaminergic dysfunction. J Neurol Sci 2013; 329:23–28. doi: 10.1016/j.jns.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Oh YS, Kim JS, Hwang EJ, Lyoo CH. Striatal dopamine uptake and olfactory dysfunction in patients with early Parkinson's disease. Parkinsonism Relat Disord 2018; 56:47–51. doi: 10.1016/j.parkreldis.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Pak K, Kim K, Lee MJ, Lee JM, Kim BS, Kim SJ, et al. Correlation between the availability of dopamine transporter and olfactory function in healthy subjects. Eur Radiol 2018; 28:1756–1760. doi: 10.1007/s00330-017-5147-7. [DOI] [PubMed] [Google Scholar]
- 37.Vlaar AM, van Kroonenburgh MJ, Kessels AG, Weber WE. Meta-analysis of the literature on diagnostic accuracy of SPECT in Parkinsonian syndromes. BMC Neurol 2007; 7:27.doi: 10.1186/1471-2377-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segovia F, Górriz JM, Ramírez J, Martínez-Murcia FJ, Levin J, Schuberth M, et al. Multivariate analysis of 18F-DMFP PET data to assist the diagnosis of Parkinsonism. Front Neuroinform 2017; 11:23.doi: 10.3389/fninf.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer PT, Frings L, Rücker G, Hellwig S. 18F-FDG PET in Parkinsonism: differential diagnosis and evaluation of cognitive impairment. J Nucl Med 2017; 58:1888–1898. doi: 10.2967/jnumed.116.186403. [DOI] [PubMed] [Google Scholar]
- 40.Boxer AL, Yu JT, Golbe LI, Litvan I, Lang AE, Höglinger GU. Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol 2017; 16:552–563. doi: 10.1016/S1474-4422(17)30157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zalewski N, Botha H, Whitwell JL, Lowe V, Dickson DW, Josephs KA. FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. J Neurol 2014; 261:710–716. doi: 10.1007/s00415-014-7256-4. [DOI] [PubMed] [Google Scholar]
- 42.Caminiti SP, Sala A, Iaccarino L, Beretta L, Pilotto A, Gianolli L, et al. Brain glucose metabolism in Lewy body dementia: implications for diagnostic criteria. Alzheimers Res Ther 2019; 11:20.doi: 10.1186/s13195-019-0473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen LH, Liao MH, Tseng YC. Recent advances in imaging of dopaminergic neurons for evaluation of neuropsychiatric disorders. J Biomed Biotechnol 2012; 2012:259349.doi: 10.1155/2012/259349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JY, Yang D, Yang HJ, Kim HA, Kim S, Heo D, et al. Quantitative autonomic function test in differentiation of multiple system atrophy from idiopathic Parkinson disease. Chin Med J 2019; 132:1919–1924. doi: 10.1097/CM9.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klockgether T. The art of making a clinical diagnosis of multiple system atrophy. Brain 2019; 142:2555–2557. doi: 10.1093/brain/awz255. [DOI] [PubMed] [Google Scholar]
- 46.Kim HW, Kim JS, Oh M, Oh JS, Lee SJ, Oh SJ, et al. Different loss of dopamine transporter according to subtype of multiple system atrophy. Eur J Nucl Med Mol Imaging 2016; 43:517–525. doi: 10.1007/s00259-015-3191-6. [DOI] [PubMed] [Google Scholar]
- 47.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008; 71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishibashi K, Nishina H, Ishiwata K, Ishii K. Individual time course of pre- and postsynaptic PET imaging may improve differential diagnosis of Parkinson's disease and multiple system atrophy: a case report. BMC Res Notes 2015; 8:496.doi: 10.1186/s13104-015-1522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardini M, Huey ED, Spina S, Kreisl WC, Morbelli S, Wassermann EM, et al. FDG-PET patterns associated with underlying pathology in corticobasal syndrome. Neurology 2019; 92:e1121–e1135. doi: 10.1212/WNL.0000000000007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joling M, Vriend C, Raijmakers P, van der Zande JJ, Lemstra AW, Berendse HW, et al. Striatal DAT and extrastriatal SERT binding in early-stage Parkinson's disease and dementia with Lewy bodies, compared with healthy controls: an 123I-FP-CIT SPECT study. Neuroimage Clin 2019; 22:101755.doi: 10.1016/j.nicl.2019.101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cousins O, Yousaf T, Wilson H, Pagano G, Politis M. Molecular imaging of dementia with Lewy bodies. Int Rev Neurobiol 2019; 144:59–93. doi: 10.1016/bs.irn.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 52.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 2017; 89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bellucci A, Navarria L, Falarti E, Zaltieri M, Bono F, Collo G, et al. Redistribution of DAT/α-synuclein complexes visualized by “in situ” proximity ligation assay in transgenic mice modelling early Parkinson's disease. PLoS One 2011; 6:e27959.doi: 10.1371/journal.pone.0027959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sidhu A, Wersinger C, Vernier P. Alpha-synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease. FEBS Lett 2004; 565:1–5. doi: 10.1016/j.febslet.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 55.Bellucci A, Mercuri NB, Venneri A, Faustini G, Longhena F, Pizzi M, et al. Review: Parkinson's disease: from synaptic loss to connectome dysfunction. Neuropathol Appl Neurobiol 2016; 42:77–94. doi: 10.1111/nan.12297. [DOI] [PubMed] [Google Scholar]
- 56.Fazio P, Svenningsson P, Cselényi Z, Halldin C, Farde L, Varrone A. Nigrostriatal dopamine transporter availability in early Parkinson's disease. Mov Disord 2018; 33:592–599. doi: 10.1002/mds.27316. [DOI] [PubMed] [Google Scholar]
- 57.Gao L, Chen H, Li X, Li F, Ou-Yang Q, Feng T. The diagnostic value of minor salivary gland biopsy in clinically diagnosed patients with Parkinson's disease: comparison with DAT PET scans. Neurol Sci 2015; 36:1575–1580. doi: 10.1007/s10072-015-2190-5. [DOI] [PubMed] [Google Scholar]


