Abstract
Background
The relationship of uric acid (UA) with the thyroid function among healthy individuals remains unclear. We aimed to examine the relationship between UA contents and thyroid hormone levels in healthy Chinese individuals.
Methods
This was a cross-sectional study of 1186 Chinese adults (736 men and 450 women) who underwent a health check-up at the Huadong Hospital Affiliated to Fudan University (Shanghai, China) between January 1, 2010 and July 31, 2018. Clinical and thyroid hormone levels were compared in different UA groups (in male and menopause women groups, MG1: UA < 5 mg/dL; MG2: 5 mg/dL ≤ UA< 7 mg/dL; and MG3: UA ≥ 7 mg/dL; in female groups, FG1 to FG3 represent the UA levels of <4 mg/dL, 4 mg/dL ≤ UA< 6 mg/dL, and ≥6 mg/dL, respectively). In addition, natural cubic spline regression, together with Pearson correlation analysis, was performed in investigating the correlation of UA with thyroid hormones.
Results
After adjusting for confounding factors, low levels of UA (for males, UA < 5.30 mg/dL; for females, UA < 4.05 mg/dL) were negatively correlated with free triiodothyronine (FT3) both in men and women. UA levels between 4.83 and 6.06 mg/dL may act to protect FT3 in women, while UA levels between 6.39 and 7.09 mg/dL may protect FT3 in men. FT3 levels of low-range UA group reduced compared with mid-range UA and the high-range UA groups in both men and women.
Conclusions
Our results provide epidemiologic evidence to support the negative correlation between low UA contents and FT3 in the Chinese Han population, suggesting that the reduced UA contents may serve as the risk factor to predict poor thyroid function in Chinese individuals.
Keywords: Uric acid, Thyroid, Chinese
Introduction
Thyroid hormones (THs) play their parts in multiple events, including development, growth, metabolism, and reproduction. Recently, attentions have been paid to THs again due to the increasing prevalence of metabolic diseases, because THs boost the body energy metabolism. Research on THs mainly examines the roles of TH in glucose oxidation, oxidative phosphorylation acceleration, fat degradation, as well as additional metabolic activities.[1] In the meantime, TH mimetics are proposed in managing diabetes and obesity. Therefore, it is of vital importance to understand the population-based thyroid dysfunction distribution, as this represents a candidate risk factor of cardiovascular disease, hypercholesterolemia, arrhythmia, osteoporosis, as well as neuropsychiatric diseases.[2] Those adverse thyroid dysfunction effects on the metabolic system have been extensively recognized; however, the associations of THs levels with the hyperuricemia risk among euthyroid subjects remain unclear so far.
Uric acid (UA) is the eventual oxidation product during the metabolism of purine within human body, which exerts the pro-oxidant and antioxidant activities.[3] UA makes no known direct contribution to the metabolic syndrome, but the UA contents in serum usually elevate among such patients.[4,5] The UA content in serum is identified as the factor to independently predict the risk of metabolic syndrome.[6,7] Therefore, it was suggested that UA might be related to the thyroid function. Such association is previously examined among overt thyroid dysfunction patients,[8–10] but it is still unknown about the relationship of UA with thyroid function among the normal subjects. As a result, the current cross-sectional study was performed to evaluate the association of UA with thyroid function in healthy individuals.
Methods
Ethical approval
Our study protocol had gained approval from the local Ethics Committee of Huadong Hospital Affiliated to Fudan University (No. 20190037), and performed following the Declaration of Helsinki. As a retrospective study and data analysis was performed anonymously, it was unnecessary to obtain informed consent from the participants.
Study participants
A total sample size of 1186 was larger than what was needed which was determined using the Fischer equation n = z2×p×(1–p)/e2 (n: sample size, z: z-score associated with a level of confidence, p: sample proportion, e: margin of error). Data from a total of 736 men (age of 56.4 ± 9.2 years) and 450 women (age of 56.6 ± 10.1 years) undergoing routine physical examinations in the Affiliated Huadong Hospital of Fudan University (Shanghai, China) from January 1, 2010 to July 31, 2018 were included into the current work for analysis. Each of the patients paid spontaneous visits to the physical examination center to receive physical examinations, including comprehensive screening to detect malignant tumors, thyroid dysfunction, diabetes, as well as additional age-related disorders. Patients undergoing TH replacement therapy and those who were administered medications that could affect thyroid function such as amiodarone, sulfonylurea, tyrosine kinase inhibitors, and immune modulators, in the past 12 months or over 6 months, were not included in the current work. Subjects who had chronic diseases potentially affecting the thyroid function, like thyroid cancer, hypophyseal disease, gestation and mental disease, abnormality in renal or liver function test, were not included as well, so as to prevent any alterations in thyroid function. The minimal inclusion as well as exclusion criteria were adopted for diminishing the possible bias in patient selection.
Biochemical assays
The blood was sampled in the early morning following fasting for 12 h, which were then sent to laboratory for analysis. As the renal function indicator, the estimated glomerular filtration rate (eGFR; mL/min per 1.73 m2) was determined according to Chronic Kidney Disease Epidemiology Collaboration formula. In addition, the contents of UA, free triiodothyronine (FT3), serum creatinine (SCr), free thyroxine (FT4), thyroid-stimulating hormone (TSH), creatinine, triiodothyronine (T3), thyroxine (T4), alkaline phosphatase (ALP), triglycerides (TGs), total cholesterol, low/high-density lipoprotein, serum fasting blood glucose (FBG), and hemoglobin A1c were measured. The patient height and body weight were determined in the absence of shoes. At the same time, the body mass index (BMI) was determined through dividing weight (kg) by the square of height (m2).
Statistical analysis
The categorical and continuous clinical parameters are reported in the manner of percentages and mean ± standard deviation, separately. Analysis of variance was performed to compare the clinical variables, thyroid functions in subjects of each UA group (in male and menopause women groups, MG1–MG3: UA < 5 mg/dL, 5 mg/dL < UA < 7 mg/dL, and ≥7 mg/dL, respectively; in female groups, FG1–FG3: UA < 4 mg/dL, 4 mg/dL < UA < mg/dL, and ≥6 mg/dL, respectively).[11,12] The least significance difference test was applied to examine the homogeneity of variance, whereas the Tamhane T2 test was adopted to examine the inhomogeneity of variation. Factors related to TH levels were identified by Pearson correlation analysis. Thereafter, the relationships between UA contents in serum and TH levels were determined by natural cubic spline regression analysis, following potential confounder adjustment. A difference of P < 0.05 indicated statistical significance. SPSS 18.0 software (IBM Inc, New York, NY, USA). for windows 10.0 was employed for statistical analyses.
Results
Clinical and TH levels in different UA groups
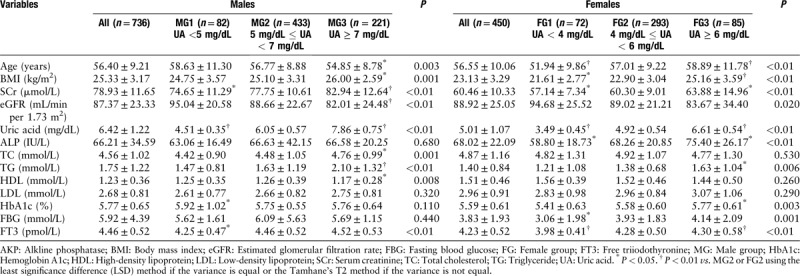
The main clinical variables for the participants are summarized in Table 1. To rule out the effect of UA on THs, we first studied the effect of UA on clinical parameters. The participants were divided into three UA groups. UA, FT3, age, SCr, eGFR, BMI, total cholesterol, TG, and HDL were significantly different in the three UA groups in men (P < 0.05). UA, FT3, age, SCr, eGFR, BMI, TG, ALP, FBG, and hemoglobin A1c were significantly different in the three UA groups in women (P < 0.05).
Table 1.
Clinical characteristic of the study participants and comparison of characteristics according to serum UA groups.
Relationship between serum UA levels and THs
According to Pearson correlation analysis results, while UA showed positive correlation with TG (r = 0.23, P < 0.01), and BMI (r = 0.19, P < 0.01), but it was negatively correlated with eGFR (r = –0.15, P < 0.01) as well as age (r = –0.14, P < 0.01) in men. Those above parameters were identified to be the confounding variables regarding the effect of UA on THs. A natural cubic spline regression model in men was carried out for determining UA effect on FT3, after adjusting for potential confounders. In men, UA had a negative effect on FT3 when it was <5.30 mg/dL and this negative effect increased with decrease in serum UA levels. Meanwhile, UA had a positive effect on FT3 in men in the range of 6.39 to 7.09 mg/dL. These results were statistically significant. When UA > 7.09 mg/dL, UA effect on FT3 was not statistically significant [Figure 1A].
Figure 1.
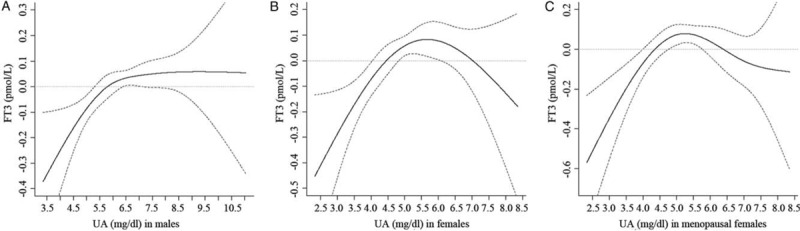
Natural cubic spline regression analysis for the effect of serum UA (independent variable) on serum FT3 (dependent variables) in different models. (A) The effect of serum UA on serum FT3 in males. (B) The effect of serum UA on serum FT3 in females. (C) The effect of serum UA on serum FT3 in menopausal females. Solid line refers to the effect of UA on FT3; the 95% CI are depicted in dotted line. The 95% CI containing 0 implies the effect is not statistically significant. UA: Uric acid; FT3: Free triiodothyronine; CI: Confidence interval.
In women, UA was positively associated with age (r = 0.21, P < 0.01), BMI (r = 0.38, P < 0.01), FBG (r = 0.18, P < 0.01), together with TG (r = 0.16, P = 0.001). UA was inversely related to eGFR (r = –0.18, P < 0.01). The above parameters were identified to be the confounding parameters regarding the effect of UA on THs. A natural cubic spline regression model in women was carried out for determining UA effect on FT3, when the possible confounders were adjusted. In females, UA negatively affected FT3 at <4.05 mg/dL, and this negative effect increased with decrease in serum UA levels. Meanwhile, UA had a positive effect on FT3 in women within 4.83 to 6.06 mg/dL range. The results were statistically significant. When UA > 6.06 mg/dL, the effect of UA on FT3 was not statistically significant [Figure 1B].
In menopausal women over 50 years old, UA was positively associated with BMI (r = 0.34, P < 0.01), FBG (r = 0.23, P < 0.01), along with TG (r = 0.16, P = 0.003). UA was inversely related to eGFR (r = –0.18, P < 0.01). The above parameters were identified to be the confounding parameters regarding the effect of UA on THs. In menopausal women, UA had a negative effect on FT3 at <4.02 mg/dL, and this negative effect increased as the UC levels in serum decreased. Meanwhile, UA had a positive effect on FT3 within 4.75 to 5.76 mg/dL range. The results were statistically significant. When UA > 5.76 mg/dL, the effect of UA on FT3 was not statistically significant [Figure 1C].
TH levels in different UA groups
Based on analysis of variance results, men within low-range UA group displayed markedly lower FT3 [Figure 2A] than those in the high- and mid-range UA groups (P < 0.01 and P = 0.001).
Figure 2.
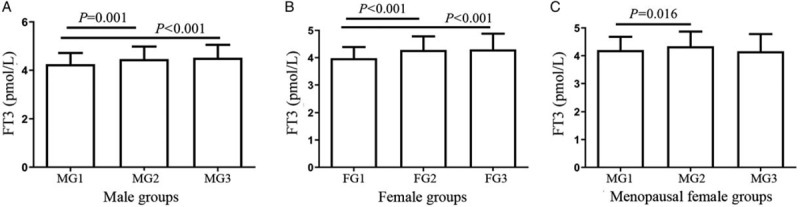
Serum FT3 levels according to serum UA groups in males, females and menopause women. (A) FT3 in men was compared among three groups of serum UA levels using one-way ANOVA. MG1: UA < 5 mg/dL, MG2: 5 mg/dL ≤ UA < 7 mg/dL, MG3: UA ≥ 7 mg/dL. (B) FT3 in females was compared among three groups of serum UA levels using one-way ANOVA. FG1: UA < 4 mg/dL, FG2: 4 mg/dL ≤ UA < 6 mg/dL, FG3: UA ≥ 6 mg/dL. (C) FT3 in menopause women compared among three groups of serum UA levels using one-way ANOVA. MG1: UA < 5 mg/dL, MG2: 5 mg/dL ≤ UA < 7 mg/dL, MG3: UA ≥ 7 mg/dL. FT3: Free triiodothyronine; UA: Uric acid; ANOVA: Analysis of variance.
Women of low-range UA group had remarkably lower FT3 [Figure 2B] than those in the mid- and high-range UA groups (P < 0.01).
Menopausal women of low-range UA group had evidently lower FT3 [Figure 2C] than those in the mid-range UA group (P = 0.016).
Discussion
As far as we know, the current cross-sectional study has demonstrated a relationship of FT3 with UA contents among the general population with no obvious thyroid dysfunction. In this study involving 1186 Chinese adults, UA showed positive correlation with FT3 levels by Pearson correlation and by natural cubic spline regression after adjusting for confounding factors. Our result suggests that UA is closely correlated with thyroid function.
Our results provide epidemiologic evidence that the UA levels in serum are related to FT3, rather than to FT4 and T4 levels in a Chinese Han population. Currently, several articles investigate the relationship of severe thyroid dysfunction with hyperuricemia. According to the cross-sectional research by Ye et al,[13] UA content was associated with FT4 but not with FT3 or T3 in subjects without overt thyroid dysfunction; Moreover, according to one case-control study, the UA contents in serum markedly increase among cases suffering from overt hyperthyroidism, which are tightly associated with the T3 and T4 levels in serum prior to and in the treatment.[14] On the contrary, based on one article involving 2359 cases suffering from thyroid dysfunction to different degrees, UA was not related to T3 or T4 contents.[9] This result conflicts with our own data. Several factors, such as the heterogeneities in study objects (like general subjects vs. patients), laboratory approaches for defining T3/T4 level and TSH, and sample size, could account for these discrepancies.
THs are related to oxidative stress (OS) as well as the antioxidant status, which is because that they can alter the respiratory rate and promote the basal metabolism within mitochondria.[15] Changes in THs contents may serve as the major physiologic regulators for cellular OS in vivo.[16] The available data concerning OS in thyroid dysfunction are controversial and insufficient. A low amount of free radicals are generated in hypothyroidism, since the decreased THs content led to suppression of metabolism.[17] Additionally, T3 can decrease the superoxide dismutase 1 expression; by contrast, the persistent hypothyroidism results in the increased superoxide dismutase 1 activity within rat brains.[18] Evidence suggests that, as the antioxidant, UA exerts its physiologic benefits based on its ability to scavenge free radicals.[19] As a matter of fact, UA takes up about 1/2 of the antioxidants within the plasma of human beings.[20] As a result, it can be hypothesized that, the moderate elevation in plasma UA content may affect THs due to its potent anti-oxidation. As suggested by a large study, the UA contents in serum is linearly correlated with the quartiles of FT4 contents, and the elevated UA contents are expected within the upper FT4 quartiles.[13] This result is also consistent with our own data, in which low UA content in serum were inversely related to FT3 for both men and women.
BMI has been shown to be associated with thyroid functions. As discovered by investigators, the increased BMI is related to thyroid function; besides, it has been suggested that thyroid function was partly mediated by BMI. Abdi et al[21] evaluated the correlation of changes of thyroid functional tests in euthyroid range with BMI among individuals having normal baseline weight. Their results found that in normal weight euthyroid individuals, changes of the FT4 levels in serum, rather than TSH, possibly led to the changed body weight among euthyroid subjects with normal weight. Schmid et al[22] reported that the thyroid cancer risk was markedly higher in subjects with over-weight (25%) and obesity (55%) than in normal weight patients. An increase of 5 units on the BMI scale is associated with a 30% higher thyroid cancer risk. This study also suggested that low UA content was still inversely related to FT3 after adjusting for BMI. Therefore, it is less likely, at least in our cohort, that BMI has no effect on FT3.
Meanwhile, THs are also associated with renal dysfunction. A prospective investigation demonstrated that a greater FT4, rather than FT3 or T3, related to the elevated Chronic Kidney Disease risk as well as rapid decrease in eGFR among the old and middle-aged Chinese.[23] Such finding conformed to this study, suggesting that FT3 and T3 were related to UA when the eGFR was adjusted. Another consideration is that serum T3 levels are known to be affected by age,[24] but we have found that the correlation of UA and FT3 remained in men and women after adjusting for age, suggesting that the effects of age may not have substantial effect on serum FT3 levels.
There are several noteworthy strengths to our study. Most importantly, we analyzed cross-sectional data separated from women and men for the specific investigation on the UA impacts on TH. Second, rigorous exclusion criteria were set according to routine laboratory findings and medical histories, which contributed to carefully adjusting the potential confounders for the appropriate investigation on those physiologic impacts of UA on thyroid function. Additionally, we noticed different effects of low levels of UA and high levels of UA on FT3 in the Chinese cohort.
Nonetheless, certain limitations should be noted in the current work. Firstly, the causal relationship of the UA content in serum with thyroid dysfunction was not determined due to the nature of cross-sectional study. Secondly, subjects visiting the physical examination center were also included as the study population in this study; as a result, the representativeness of general population might be lacking, thus leading to the selection bias. Thirdly, participant's smoking history was not recorded in this study due to its health check-up and cross-sectional nature, which can impact thyroid function test results.
In conclusion, this study demonstrates that low levels of UA were negatively correlated with FT3 both in men and women. UA levels between 4.83 and 6.06 mg/dL may act to protect FT3 in women, while UA levels between 6.39 and 7.09 mg/dL may also protect FT3 in men. FT3 level of low-range UA group reduces compared with mid- and the high-range UA groups both in men and women. UA, the natural antioxidant, can serve as the risk factor of thyroid disease at a low content. For prevention and treatment in hyperuricemia, the most appropriate UA target has to be selected and considered for how long it needs to be maintained for. More investigation is needed to explore the underlying mechanism responsible for the various UA effects on THs.
Funding
This study was supported by a grant of Shanghai Municipal Commission of Health and Family Planning Project Grant (No. 2018LQ003).
Conflicts of interest
None.
Footnotes
How to cite this article: XJ, Qian XW, Zhang X, Han L, Zheng YQ, Wu T, Qin GY, Ye ZB, Xiao J. Association of serum uric acid with thyroid function in health check-up participants. Chin Med J 2020;133:1409–1414. doi: 10.1097/CM9.0000000000000840
References
- 1.Damiano F, Rochira A, Gnoni A, Siculella L. Action of thyroid hormones, T3 and T2, on hepatic fatty acids: differences in metabolic effects and molecular mechanisms. Int J Mol Sci 2017; 18:E744.doi: 10.3390/ijms18040744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87:489–499. doi: 10.1210/jc.87.2.489. [DOI] [PubMed] [Google Scholar]
- 3.So A, Thorens B. Uric acid transport and disease. J Clin Invest 2010; 120:1791–1799. doi: 10.1172/jci42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirillo P, Sato W, Reungjui S, Heinig M, Gersch M, Sautin Y, et al. Uric acid, the metabolic syndrome, and renal disease. J Am Soc Nephrol 2006; 17:S165–S168. doi: 10.1681/ASN.2006080909. [DOI] [PubMed] [Google Scholar]
- 5.Heinig M, Johnson RJ. Uric acid in hypertension, renal disease, and metabolic syndrome. Cleve Clin J Med 2006; 73:1059–1064. doi: 10.3949/ccjm.73.12.1059. [DOI] [PubMed] [Google Scholar]
- 6.Goncalves JP, Oliveira A, Severo M, Santos AC, Lopes C. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine 2012; 41:450–457. doi: 10.1007/s12020-012-9629-8. [DOI] [PubMed] [Google Scholar]
- 7.Yang T, Chu CH, Bai CH, You SL, Chou YC, Chou WY, et al. Uric acid level as a risk marker for metabolic syndrome: a Chinese cohort study. Atherosclerosis 2012; 220:525–531. doi: 10.1016/j.atherosclerosis.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Giordano N, Santacroce C, Mattii G, Geraci S, Amendola A, Gennari C. Hyperuricemia and gout in thyroid endocrine disorders. Clin Exp Rheumatol 2001; 19:661–665. doi: 10.1002/1529-0131(200111)44:11<2706::AID-ART454>3.0.CO;2-X. [PubMed] [Google Scholar]
- 9.Raber W, Vukovich T, Vierhapper H. Serum uric acid concentration and thyroid-stimulating-hormone (TSH): results of screening for hyperuricaemia in 2359 consecutive patients with various degrees of thyroid dysfunction. Wien Klin Wochenschr 1999; 111:326–328. [PubMed] [Google Scholar]
- 10.Saini V, Yadav A, Arora MK, Arora S, Singh R, Bhattacharjee J. Correlation of creatinine with TSH levels in overt hypothyroidism - a requirement for monitoring of renal function in hypothyroid patients? Clin Biochem 2012; 45:212–214. doi: 10.1016/jclinbiochem.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Bellomo G, Selvi A. Uric acid: the lower the better? Contrib Nephrol 2018; 192:69–76. doi: 10.1159/000484280. [DOI] [PubMed] [Google Scholar]
- 12.Schumacher HR. Crystal-induced arthritis: an overview. Am J Med 1996; 100:46S–52S. doi: 10.1016/s0002-9343(97)89546-0. [DOI] [PubMed] [Google Scholar]
- 13.Ye Y, Gai X, Xie H, Jiao L, Zhang S. Association between serum free thyroxine (FT4) and uric acid levels in populations without overt thyroid dysfunction. Ann Clin Lab Sci 2015; 45:49–53. [PubMed] [Google Scholar]
- 14.Sato A, Shirota T, Shinoda T, Komiya I, Aizawa T, Takemura Y, et al. Hyperuricemia in patients with hyperthyroidism due to graves’ disease. Metabolism 1995; 44:207–211. doi: 10.1016/0026-0495(95)90266-X. [DOI] [PubMed] [Google Scholar]
- 15.Pereira B, Rosa LF, Safi DA, Bechara EJ, Curi R. Control of superoxide dismutase, catalase and glutathione peroxidase activities in rat lymphoid organs by thyroid hormones. J Endocrinol 1994; 140:73–77. doi: 10.1677/joe.0.1400073. [DOI] [PubMed] [Google Scholar]
- 16.Guerrero A, Pamplona R, Portero-Otin M, Barja G, Lopez Torres M. Effect of thyroid status on lipid composition and peroxidation in the mouse liver. Free Rad Biol Med 1999; 26:73–80. doi: 10.1016/S0891-58499800173-7. [DOI] [PubMed] [Google Scholar]
- 17.Erdamar H, Demirci H, Yaman H, Erbil MK, Yakar T, Sancak B, et al. The effect of hypothyroidism, hyperthyroidism, and their treatment on parameters of oxidative stress and antioxidant status. Clin Chem Lab Med 2008; 46:1004–1010. doi: 10.1515/CCLM.2008.183. [DOI] [PubMed] [Google Scholar]
- 18.Santos GM, Afonso V, Barra B, Togashi M, Webb P, Neves FA, et al. Negative regulation of superoxide dismutase-1promoter by thyroid hormone. Mol Pharmacol 2006; 70:793–800. doi: 10.1124/mol.106.025627. [DOI] [PubMed] [Google Scholar]
- 19.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleos Nucleot Nucl Acids 2008; 27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masseoud D, Rott K, Liu-Bryan R, Agudelo C. Overview of hyperuricaemia and gout. Curr Pharm Des 2005; 11:4117–4124. doi: 10.2174/138161205774913318. [DOI] [PubMed] [Google Scholar]
- 21.Abdi H, Kazemian E, Gharibzadeh S, Amouzegar A, Mehran L, Tohidi M, et al. Association between thyroid function and body mass index: a 10-year follow-up. Ann Nutr Metab 2017; 70:338–345. doi: 10.1159/000477497. [DOI] [PubMed] [Google Scholar]
- 22.Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev 2015; 16:1042–1054. doi: 10.1111/obr.12321. [DOI] [PubMed] [Google Scholar]
- 23.Huang X, Ding L, Peng K, Lin L, Wang T, Zhao Z, et al. Thyroid hormones associate with risk of incident chronic kidney disease and rapid decline in renal function: a prospective investigation. J Transl Med 2016; 14:336.doi: 10.1186/s12967-016-1081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 2014; 24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]


