Abstract
Glioblastoma multiforme (GBM) is the most common and malignant brain tumor of the adult central nervous system and is associated with poor prognosis. The present study aimed to identify the hub genes in GBM in order to improve the current understanding of the underlying mechanism of GBM. The RNA-seq data were downloaded from The Cancer Genome Atlas database. The edgeR package in R software was used to identify differentially expressed genes (DEGs) between two groups: Glioblastoma samples and normal brain samples. Gene Ontology (GO) functional enrichment analysis and the Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis were performed using Database for Annotation, Visualization and Integrated Discovery software. Additionally, Cytoscape and Search Tool for the Retrieval of Interacting Genes/Proteins tools were used for the protein-protein interaction network, while the highly connected modules were extracted from this network using the Minimal Common Oncology Data Elements plugin. Next, the prognostic significance of the candidate hub genes was analyzed using UALCAN. In addition, the identified hub genes were verified by reverse transcription-quantitative (RT-q) PCR. In total, 1,483 DEGs were identified between GBM and control samples, including 954 upregulated genes and 529 downregulated genes (P<0.01; fold-change >16) and these genes were involved in different GO terms and signaling pathways. Furthermore, CDK1, BUB1, BUB1B, CENPA and GNG3 were identified as key genes in the GBM samples. The UALCAN tool verified that higher expression level of CENPA was relevant to poorer overall survival rates. In conclusion, CDK1, BUB1, BUB1B, CENPA and GNG3 were found to be potential biomarkers for GBM. Additionally, ‘cell cycle’ and ‘γ-aminobutyric acid signaling’ pathways may serve a significant role in the pathogenesis of GBM.
Keywords: glioblastoma multiforme, differentially expressed genes, functional enrichment analyses, Kyoto Encyclopedia of Genes and Genomes pathway, prognosis
Introduction
According to the guidelines from the World Health Organization, gliomas are categorized into grades I to IV. Glioblastoma multiforme (GBM) is classified as grade IV and it is a highly malignant form (1,2). GBM has been recognized as the most aggressive type of brain tumor with highly infiltrative ability (3), affecting about ~20,000 people every year in the United States (4). Currently, the treatments for GBM include surgical resection, chemotherapy and radiotherapy (5). However, due to the aggressiveness of gliomas and their resistance to chemotherapy and radiation therapy (6,7), the prognosis of GBM is still poor with a median survival time of 12–15 months for patients with GBM (8,9). In recent years, targeted therapies, such as combinatorial chemotherapy targeting molecular subgroups and gene and immune therapies, have made important progress in preclinical models (10–12). However, the potential molecular mechanism in the pathogenesis of GBM needs to be further investigated. In order to improve the prognosis of GBM patients, it is important to identify molecular mechanism underlying GBM pathogenesis.
In recent years, a number of studies have investigated the potential molecular mechanisms of GBM. It has been reported that the upregulation and mutations in EGFR may be responsible for the resistance of glioma cells to the treatments with chemotherapy and radiation (13–15). Additionally, Annexin-A5 over-activation increases Snail expression level via the PI3K/Akt/NF-κB signaling pathway, which is associated with glioblastoma cells migration and invasion (16). In addition, Siebzehnrubl et al (17) indicated that ZEB1 is linked to tumor chemoresistance and invasion of glioblastoma cells by modulating its downstream effectors, including c-MYB, MGMT and ROBO1. Nevertheless, the exact molecular mechanism and gene networks underlying GBM progression remain to be fully elucidated.
The present study aimed to identify the potential key nodes and molecular mechanisms associated with the progression of GBM. Co-expression interactions between the identified DEGs were conducted using a protein-protein interaction (PPI) network and several critical genes were identified. In addition, a network module analysis was performed according to the PPI network. In conclusion, the present study may improve the current understanding of the pathogenesis underlying GBM and identified key genes that may represent novel targets for the development of novel treatments of GBM.
Materials and methods
Data download
RNA-seq data were downloaded from The Cancer Genome Atlas (TCGA) database (portal.gdc.cancer.gov/) (18). The data of 169 GBM samples and 5 normal brain tissue samples were used and analyzed.
Data preprocessing and DEG analysis
Based on the annotation information contained in the Ensembl database (asia.ensembl.org/html) (19), 23,269 protein coding genes were identified. Then, the DEGs between GBM samples and normal samples were screened using edgeR package in R (20,21). A P<0.01 and |log2 (fc)| >4 were selected as the cutoff criteria for the identification of DEGs. In addition, a volcano plot was drawn using the gplots package in R software (22).
Functional and pathway enrichment analysis of DEGs
To reveal the main functional pathways of GBM, the Database for Annotation, Visualization and Integrated Discovery (DAVID; http://david.ncifcrf.gov/) was used to analyze Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways enriched in the identified DEGs (23). DEG count ≥2 and P<0.05 were used as cutoff to identify the significant biological functions and signaling pathways.
PPI network and module analysis of DEGs
The Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (https://string-db.org/) was used to assess the interacting partners of the DEGs (24), and the PPI network was constructed with a combined score >0.7. Visualization of the network was performed with Cytoscape (version 3.6.1) software (25). After constructing the PPI network, topology characteristics of the network were analyzed using the Network Analyzer plug-in of Cytoscape software, and degree distribution, distribution of the shortest path, average clustering coefficient and closeness centrality were examined (26). Subsequently, the top 10 significant hub genes or key nodes were identified according to their connectivity degrees by using the CytoHubba tool, a plug-in for Cytoscape (27). The MCODE plug-in was used to analyze the highly interconnected clusters of this network with default parameters (28).
UALCAN survival analysis
UALCAN (ualcan.path.uab.edu/) is an interactive web-software that can be used to perform analyses of tumor subgroup gene expression and survival (29). In UALCAN, samples were categorized into two groups: High expression and Low/Medium expression patients. High expression patients refer to the highest quarter of all patients. Low and medium expression patients refer to the remaining three quarters of patients. The P-values from the Kaplan-Meier analysis were based on log-rank. The effects of candidate key gene expression levels on overall survival (OS) were analyzed using UALCAN.
Sample collection and ethics statement
To determine the expression of hub genes in GBM tissues, samples from 20 patients (age, 26–77 years), including nine males and eleven females, who underwent tumor resection surgery were collected at the Shanghai Ninth People's Hospital between September 2010 and March 2018. All patients provided written informed consent, and the study conducted in accordance with the Declaration of Helsinki. In addition, 20 normal brain tissues (used as the control group) were collected from patients who suffered from traumatic brain injury and required internal decompression surgery. After collection, all tissues were frozen in liquid nitrogen and stored at −80°C. All participants in the present study signed informed consents and the study was approved by the Ethics Review Committee of Shanghai Ninth People's Hospital.
RNA extraction and reverse transcription-quantitative (RT-q) PCR
TRIzol (cat. no. 9109; Takara Bio, Inc.) was used to isolate total RNA from each tissue sample according to the manufacturer's instructions. Briefly, 2 ml TRIzol was added to brain tissue (100 mg) to prepare homogenate. After obtaining RNA, TE buffer was used to dilute the RNA. Nanodrop software (ND2000C; Gene Company, Ltd.) was used to determine the OD value. A value of OD260/OD280 within 1.7–2.1 indicated that the extracted RNA was relatively pure. Then, total RNA was reverse transcribed into cDNAs using the PrimeScript RT Master Mix (cat. no. RR036A; Takara Bio, Inc.). RT-qPCR was performed to measure the levels of cDNAs using a SYBR GREEN kit (cat. no. RR420 A; Takara Bio, Inc.). qPCR amplification procedure was performed as follows: Pre-denaturation at 95°C for 30 sec for 1 cycle followed by 40 cycles of 95°C for 5 sec and 60°C for 20 sec. The relative expression level of the five key genes was calculated following comparative CT method, as previously described (30). GAPDH was used to normalize the mRNA expression level. The primer sequences are presented in Table I.
Table I.
Primer sequences for PCR.
| cDNA | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| CDK1 | CACAAAACTACAGGTCAAGTGG | GAGAAATTTCCCGAATTGCAGT |
| BUB1 | GAAAGCATGAGCAATGGGTAAA | CCACCTGATGCAACTTCTTATG |
| BUB1B | ATGGGTCCTTCTGGAAACTTAG | GGAATGTAGTGTCAAAAACCCC |
| CENPA | AAGAGCACACACCTCTTGATAA | CATGTAAGGTGAGGAGATAGGC |
| GNG3 | CGGTGAACAGCACTATGAGTAT | TCACAGTAAGTCATCAGGTCTG |
| GAPDH | GTGGACCTGACCTGCCGTCTAG | GAGTGGGTGTCGCTGTTGAAGTC |
Statistical analysis
Experimental data are presented as the mean ± standard error of the mean. All statistical analyses were performed using SPSS 20.0 (IBM, Corp.) and visualized using GraphPad Prism 6.0 (GraphPad Software Inc.). Differences in expression levels between groups were analyzed by unpaired Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Identification of DEGs
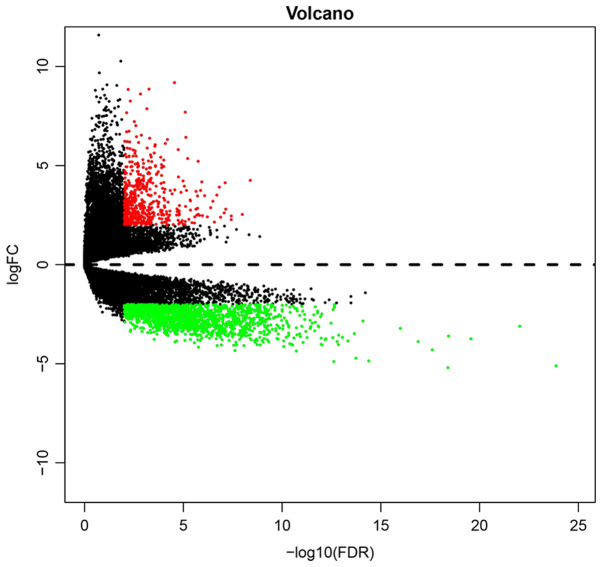
Using P<0.01 as a cutoff, a total of 1,483 DEGs (954 upregulated genes and 529 downregulated) were identified between GBM and control samples (Table S1). The volcano plot of DEGs is presented in Fig. 1. Each individual dot represents a DEG.
Figure 1.
Volcano plot of differentially expressed genes in glioblastoma multiform. Black, non-differentially expressed genes; red, significantly upregulated genes; green, significantly downregulated genes (based on |log 2 FC|>4 and P<0.01). FC, fold-change. FDR, false discovery rate.
Functional and pathway enrichment analysis of the DEGs
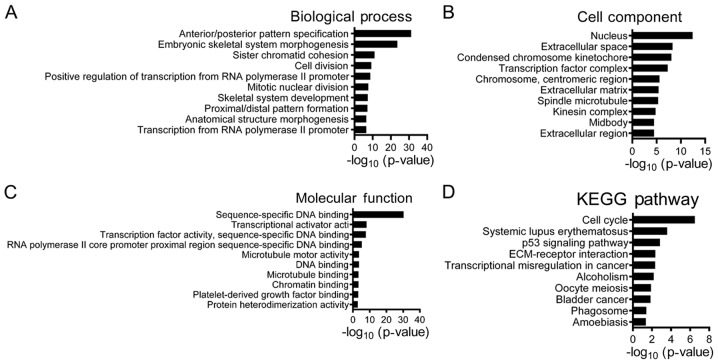
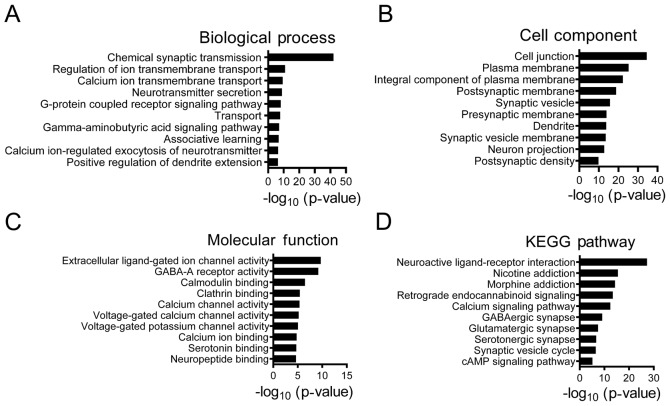
The GO enrichment analysis results revealed that 954 upregulated DEGs were primarily involved in biological process that included ‘anterior/posterior pattern specification’, ‘embryonic skeletal system morphogenesis’, ‘sister chromatid cohesion’ and ‘cell division’ (Fig. 2). According to the ‘cellular components’ analysis, DEGs were found to be primarily localized in the nucleus. In addition, the most significantly enriched molecular functions were related to transcriptional activator activity. In addition, as presented in Fig. 3, 529 downregulated DEGs were significantly associated with ‘chemical synaptic transmission’, ‘regulation of ion transmembrane transport’ and ‘γ-aminobutyric acid signaling pathway’. The top five main biological processes enriched in the upregulated and downregulated DEGs are presented in Tables II and III, respectively. The associated signaling pathways significantly enriched in the identified DEGs are presented in Table IV. The KEGG pathway analysis indicated that upregulated DEGs were mostly involved in pathways such as ‘cell cycle’ and ‘p53 signaling’ pathways, whereas downregulated genes were associated with ‘retrograde endocannabinoid signaling’, ‘GABAergic synapse’ and ‘glutamatergic synapse’.
Figure 2.
Top 10 Gene Ontology analysis of 954 upregulated differentially expressed genes associated with glioblastoma multiforme. (A) ‘Biological Processes’, (B) ‘Cellular Components’ and (C) ‘Molecular Function’, and (D) KEGG pathway analysis. KEGG, Kyoto Encyclopedia of Genes and Genomes.
Figure 3.
Top 10 Gene Ontology analysis of 529 downregulated differentially expressed genes associated with glioblastoma multiforme. (A) ‘Biological Processes’, (B) ‘Cellular Components’ and (C) ‘Molecular Function’, and (D) KEGG pathway analysis. KEGG, Kyoto Encyclopedia of Genes and Genomes.
Table II.
GO analysis of upregulated genes associated with glioblastoma multiforme.
| Category | Term | Count | P-value |
|---|---|---|---|
| GOTERM_BP_DIRECT | GO:0007062~sister chromatid cohesion | 17 | 8.70E-12 |
| GOTERM_BP_DIRECT | GO:0051301~cell division | 26 | 5.28E-10 |
| GOTERM_BP_DIRECT | GO:0007067~mitotic nuclear division | 20 | 2.20E-08 |
| GOTERM_BP_DIRECT | GO:0007051~spindle organization | 3 | 0.026829 |
| GOTERM_BP_DIRECT | GO:0007094~mitoticspindle assembly checkpoint | 3 | 0.04073 |
| GOTERM_CC_DIRECT | GO:0005634~nucleus | 142 | 3.58E-13 |
| GOTERM_CC_DIRECT | GO:0005615~extracellular space | 51 | 5.11E-09 |
| GOTERM_CC_DIRECT | GO:0000777~condensed chromosome kinetochore | 13 | 9.06E-09 |
| GOTERM_MF_DIRECT | GO:0043565~sequence-specific DNA binding | 57 | 5.42E-31 |
| GOTERM_MF_DIRECT | GO:0003677~DNA binding | 46 | 2.76E-04 |
| GOTERM_MF_DIRECT | GO:0003682~chromatin binding | 17 | 2.76E-04 |
GO, Gene Ontology; BP, ‘Biological Process’; CC, ‘Cellular Component’; MF, ‘Molecular Function’.
Table III.
GO analysis of downregulated genes associated with glioblastoma multiforme.
| Category | Term | Count | P-value |
|---|---|---|---|
| GOTERM_BP_DIRECT | GO:0007268~chemical synaptic transmission | 54 | 1.09E-42 |
| GOTERM_BP_DIRECT | GO:0034765~regulation of ion transmembrane transport | 18 | 1.37E-11 |
| GOTERM_BP_DIRECT | GO:0007269~neurotransmitter secretion | 12 | 1.33E-09 |
| GOTERM_BP_DIRECT | GO:0007187~G-protein coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger | 11 | 7.02E-09 |
| GOTERM_BP_DIRECT | GO:0007214~gamma-aminobutyric acid signaling pathway | 8 | 7.68E-08 |
| GOTERM_CC_DIRECT | GO:0005886~plasma membrane | 162 | 7.00E-26 |
| GOTERM_CC_DIRECT | GO:0005887~integral component of plasma membrane | 85 | 4.55E-23 |
| GOTERM_CC_DIRECT | GO:0045211~postsynaptic membrane | 32 | 1.74E-19 |
| GOTERM_MF_DIRECT | GO:0005230~extracellular ligand-gated ion channel activity | 11 | 1.99E-10 |
| GOTERM_MF_DIRECT | GO:0004890~GABA-A receptor activity | 9 | 6.42E-10 |
| GOTERM_MF_DIRECT | GO:0005516~calmodulin binding | 17 | 3.29E-07 |
GO, Gene Ontology; BP, biological process; CC, cellular component; MF, molecular function.
Table IV.
Results of KEGG pathway analysis.
| A, Upregulated genes | ||
|---|---|---|
| Term | Count | P-value |
| hsa04110: Cell cycle | 12 | 3.26E-07 |
| hsa05322: Systemic lupus erythematosus | 9 | 2.49E-04 |
| hsa04115: p53 signaling pathway | 6 | 0.001428 |
| hsa04512: ECM-receptor interaction | 6 | 0.004496 |
| hsa05202: Transcriptional misregulation in cancer | 8 | 0.004710 |
| B, Downregulated genes | ||
| Term | Count | P-value |
| hsa04080: Neuroactive ligand-receptor interaction | 44 | 5.35E-28 |
| hsa04723: Retrograde endocannabinoid signaling | 20 | 4.27E-14 |
| hsa04020: Calcium signaling pathway | 24 | 3.72E-13 |
| hsa04727: GABAergic synapse | 15 | 7.40E-10 |
| hsa04724: Glutamatergic synapse | 15 | 3.76E-08 |
KEGG, Kyoto Encyclopedia of Genes and Genomes.
PPI network construction of DEGs
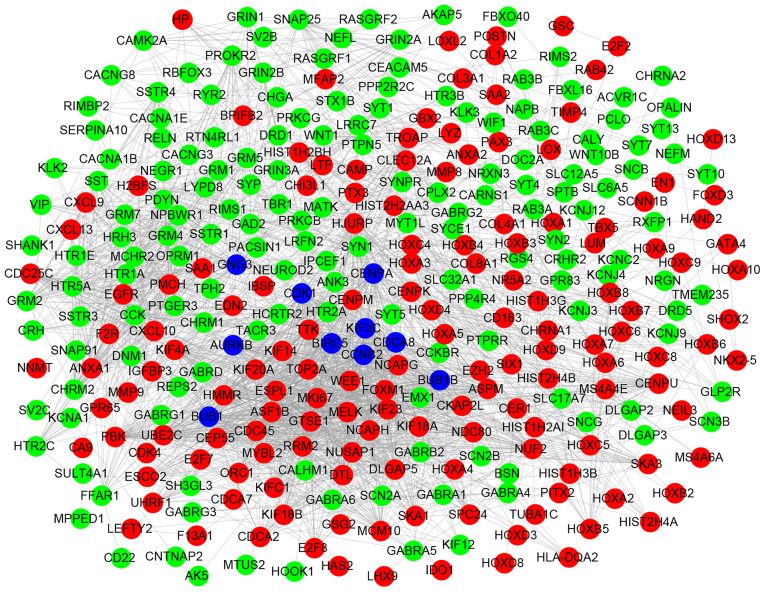
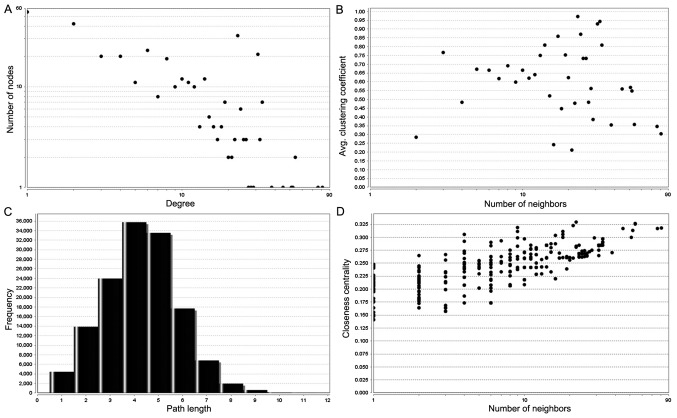
Based on the STRING database and Cytoscape software, PPI networks were constructed with combined scores >0.7, including 645 nodes and 1,779 edges (Fig. 4). Subsequently, the node degree distribution of this network was analyzed using a pattern of power-law according to the topology property (Fig. 5). In this network, a degree value >40 was used as the cutoff criterion for the CytoHubba tool. Finally, 10 DEGs were identified as the hub genes with the highest connectivity degree: cyclin dependent kinase 1 (CDK1), centromere protein A (CENPA), G protein subunit γ 3 (GNG3), BUB1 mitotic checkpoint serine/threonine kinase (BUB1), cyclin B2 (CCNB2), kinesin family member 2C (KIF2C), aurora kinase B (AURKB), baculoviral IAP repeat containing 5 (BIRC5), cell division cycle associated 8 (CDCA8) and BUB1 mitotic checkpoint serine/threonine kinase B (BUB1B).
Figure 4.
The giant protein-protein interaction network. Key nodes in the network are presented in different colors: Blue stands for the hub genes, red corresponds to the upregulated genes and green corresponds to the downregulated genes in glioblastoma multiforme.
Figure 5.
The topology properties. (A) Distribution of degrees, (B) Average clustering coefficient, (C) Shortest path distribution and (D) Closeness centrality.
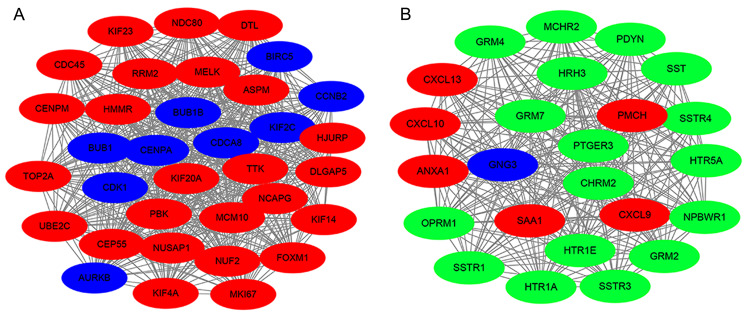
Module analysis of the PPI network
Generally, biological networks contain several functional modules and they may interact in various biological process (31). In the present study, the top two clusters were identified from the PPI network using MCODE analysis. The most significant cluster involved 35 nodes and 572 edges, and CDK1, BUB1, BUB1B, CENPA were highly enriched (Fig. 6A). Cluster 2 is presented in Fig. 6B, consisting of 24 nodes and 276 edges. Specifically, the hub gene GNG3 was enriched in cluster 2, which indicated that it may be involved in the pathogenesis of GBM by cooperating with other genes.
Figure 6.
Top 2 modules were extracted from the protein-protein interaction network. Blue stands for the hub genes, red corresponds to the upregulated genes and green corresponds to the downregulated genes in glioblastoma multiforme. (A) Cluster 1 and (B) cluster 2.
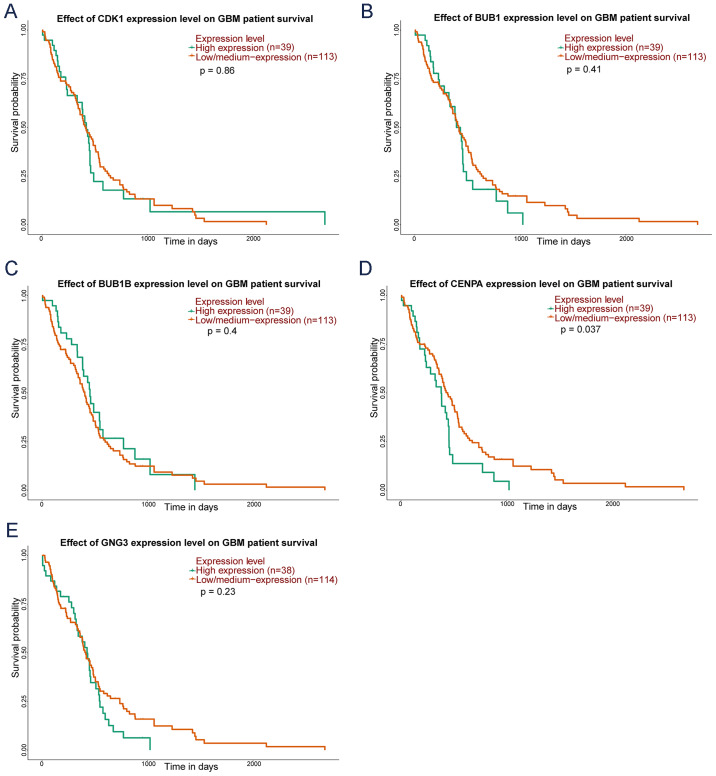
UALCAN survival analysis
UALCAN was used to analyze the OS of 169 patients with GBM based on TCGA data. Briefly, survival analysis indicated that the higher expression of CENPA gene was significantly correlated with shorter OS time in GBM patients, whereas the expression of CDK1, BUB1, BUB1B and GNG3 was not correlated with OS (Fig. 7).
Figure 7.
Kaplan-Meier curve of five key genes in patients with GBM. The red lines represent patients with low gene expression, and green lines represent patients with high gene expression. (A) CDK1, (B) BUB1, (C) BUB1B, (D) CENPA and (E) GNG3. GBM, glioblastoma multiforme. CDK1, cyclin dependent kinase 1; BUB1, BUB1 mitotic checkpoint serine/threonine kinase; BUB1B, BUB1 mitotic checkpoint serine/threonine kinase B; CENPA, centromere protein A; GNG3, G protein subunit γ 3.
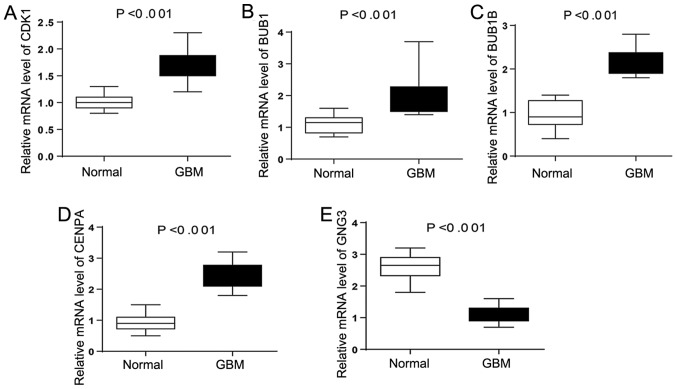
Expression of hub genes in GBM
To further verify the expression level of hub genes in GBM samples, RT-qPCR was performed to calculate the mRNA levels of the five hub genes identified in the present study (CDK1, BUB1, BUB1B, CENPA and GNG3) in GBM samples. As illustrated in Fig. 8, the expression of CDK1, BUB1, BUB1B and CENPA were significantly upregulated in GBM samples compared with normal control tissues. By contrast, the expression level of GNG3 was higher in the control tissues compared with tumor tissues. The present RT-qPCR results were in line with the aforementioned bioinformatics analysis, suggesting that these key genes may be linked to the molecular mechanism underlying GBM.
Figure 8.
The mRNA expression of five hub genes in tissues of GBM patients. (A) CDK1, (B) BUB1, (C) BUB1B, (D) CENPA and (E) GNG3. Expression of these genes was normalized against GAPDH expression. The statistical significance of differences was analyzed by t-test. GBM, glioblastoma multiforme. CDK1, cyclin dependent kinase 1; BUB1, BUB1 mitotic checkpoint serine/threonine kinase; BUB1B, BUB1 mitotic checkpoint serine/threonine kinase B; CENPA, centromere protein A; GNG3, G protein subunit γ 3.
Discussion
Glioblastoma multiforme (GBM) is the most common aggressive brain tumor, and is associated with a poor patient survival rate (32). However, the most important challenge in treating GBM is the presence of significant intra-tumor heterogeneity (13,33,34). Although previous studies have reported numerous potential biomarkers associated with the progression of GBM, the potential molecular mechanism underlying its pathogenesis has not been comprehensively investigated (35–37). In the present study, a total of 1,483 DEGs were identified, containing 954 upregulated genes and 529 downregulated genes. The present results suggested that these DEGs were primarily enriched in cell division, mitotic nuclear division and chemical synaptic transmission. In addition, the upregulated and downregulated genes were related to cell cycle, p53 signaling pathway and synapse. A PPI network was constructed and the highly connected module were identified. After module analysis, several hub genes with higher degree of connectivity were identified, including CDK1, BUB1, BUB1B, CENPA and GNG3.
In the TCGA database, there were only 5 control samples. Although it is of a smaller size when compared with the 169 GBM samples, it should not have great influence on the results of the present study, as there was relatively little individual difference in the control simples from different patients, whereas the difference was much more evident in tumor samples due to intratumoral heterogeneity between different patients.
CDK1, also named cell division control protein 2, is an important cell cycle regulator functioning as a serine/threonine kinase (38,39). During G2 and early mitosis, active CDK1-cyclin complexes can phosphorylate various downstream proteins, leading to re-organization of the cytoskeleton, nuclear envelope breakdown and chromosome condensation (40,41). Previous studies have reported that downregulation of CDK1 could inhibit proliferative ability of human glioma cells, while overexpression of CDK1 contributed to senescence escape of the cells and promoted oncogenesis of human gliomas (42–44). In the present study, CDK1 was also observed to be significantly upregulated in the module analysis of the PPI network constructed. The present results suggested that CDK1, interacting with other genes identified in the present module analysis, may be associated with the progression of GBM by modulating the cell cycle.
In addition, CDK1, BUB1, BUB1B and CENPA were also identified as key nodes in the current study. BUB1 and BUB1B are spindle assembly checkpoint (SAC) genes that serve as a controller of mitotic checkpoints and chromosome segregation (32,45). BUB1 mRNA was reported to be upregulated in glioma samples and the expression level was positively correlated with glioma grade (46). In addition, BUB1B was identified to be enriched in glioblastoma cells and associated with radio-resistance and recurrence of glioblastoma (47,48). In accordance with these previous studies, the present study identified that the expression levels of both BUB1 and BUB1B in GBM samples were higher compared with the negative controls. Collectively, accumulating evidence suggested that BUB1 and BUB1B may exert a significant influence on the progression of GBM by regulating mitotic spindle assembly checkpoint and sister chromosome segregation during mitosis. It has been previously reported that SAC inhibition promoted the response of glioblastoma cells to antimitotic drugs and enhanced the efficacy of tumor treating fields, which impair mitosis by disturbing the spindle formation (49,50). Therefore, inhibition of BUB1 or BUB1B may be a therapeutic strategy to treat GBM by influencing SAC during the process of cell division. In addition, CENPA, which encodes a centromere-associated protein, plays an important role in cell division by directing the assembly of active kinetochores (51). Failure in this process could lead to dysfunction of chromosome segregation, which has been found to be associated with initiation and progression of cancer (52–54). Notably, the present study identified a higher expression level of CENPA in brain tissue samples from GBM patients and high levels of CENPA were significantly associated with shorter OS. In addition, GO enrichment analyses showed that CENPA was involved in the biological function of sister chromatid cohesion, nucleosome assembly and mitotic cytokinesis. The present results suggested that CENPA gene may regulate the mitosis of glioma cells by interacting with other genes in the cluster 1, thus suggesting an association with pathogenesis and prognosis in GBM. The present study identified a novel potential target molecule in GBM, and inhibition of CENPA may be a novel potential therapeutic strategy for GBM.
The present results showed that GNG3 may be associated with the GABAergic synapse pathway. GNG3 is a gene that encodes the γ subunits of G proteins (55), and the disruption of GNG3 may induce the dysfunction of the GABAB1 receptor signaling pathway (56). Notably, a previous study reported that switching GABA catabolism toward γ-hydroxybutyric acid production could suppress glioblastoma cell tumorigenic properties (57). Therefore, it was hypothesized that low expression of GNG3 may be associated with the pathogenesis of GBM by regulating related signaling pathways (Table SII). However, further studies are required to confirm the role of GNG3 in GBM.
Collectively, the present study has identified that several hub genes (CDK1, BUB1, BUB1B, CENPA and GNG3) were involved in the mechanism of GBM. The present results suggested that CDK1, BUB1, BUB1B and CENPA may play an essential role in the cell cycle pathway, leading to GBM progression. In addition, GNG3 was found to be a potential therapeutic target to treat GBM. The present results may provide novel insights into the molecular mechanisms of GBM. Additional studies investigating the hub genes identified in the present study are required to examine their detailed function and interaction in GBM. However, several classical molecular traits, such as IDH1 mutation status, were not analyzed with these hub genes in our study. The authors believe that the IDH mutation analysis for these hub genes, such as CENPA, need to be investigated in the future. Furthermore, it is also appealing to investigate whether these hub genes are regulated by oncogenic mutations of themselves or other genes in the future.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This research was financially supported by The Shanghai Science & Technology Research Program (grant no. 14411972200) and Shanghai Wu Mengchao Medical Science Foundation (grant no. JJHM-2019009).
Availability of data and materials
The datasets analyzed in the present study are available from The Cancer Genome Atlas database (https://portal.gdc.cancer.gov/).
Authors' contributions
GB and XS conceived and designed the study. YP downloaded and analyzed the RNA-seq data. XC and MY collected the samples. XC and MY analyzed the data. XC wrote the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Informed consent was obtained from all patients and the present study was approved by the Ethics Review Committee of Shanghai Ninth People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Kloosterhof NK, de Rooi JJ, Kros M, Eilers PH, Sillevis Smitt PA, van den Bent MJ, French PJ. Molecular subtypes of glioma identified by genome-wide methylation profiling. Gene Chromosomes Cancer. 2013;52:665–674. doi: 10.1002/gcc.22062. [DOI] [PubMed] [Google Scholar]
- 4.Rao SS, Lannutti JJ, Viapiano MS, Sarkar A, Winter JO. Toward 3D biomimetic models to understand the behavior of glioblastoma multiforme cells. Tissue Eng Part B Rev. 2014;20:314–327. doi: 10.1089/ten.teb.2013.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alifieris C, Trafalis DT. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82. doi: 10.1016/j.pharmthera.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Yang B, Ma YB, Chu SH. Silencing SATB1 overcomes temozolomide resistance by downregulating MGMT expression and upregulating SLC22A18 expression in human glioblastoma cells. Cancer Gene Ther. 2018;25:309–316. doi: 10.1038/s41417-018-0040-3. [DOI] [PubMed] [Google Scholar]
- 7.Doan NB, Nguyen HS, Al-Gizawiy MM, Mueller WM, Sabbadini RA, Rand SD, Connelly JM, Chitambar CR, Schmainda KM, Mirza SP. Acid ceramidase confers radioresistance to glioblastoma cells. Oncol Rep. 2017;38:1932–1940. doi: 10.3892/or.2017.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez R, Rohde V, Schackert G. Different molecular patterns in glioblastoma multiforme subtypes upon recurrence. J Neurooncol. 2010;96:321–329. doi: 10.1007/s11060-009-9967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witthayanuwat S, Pesee M, Supaadirek C, Supakalin N, Thamronganantasakul K, Krusun S. Survival analysis of glioblastoma multiforme. Asian Pac J Cancer Prev. 2018;19:2613–2617. doi: 10.22034/APJCP.2018.19.9.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrlinger U, Tzaridis T, Mack F, Steinbach JP, Schlegel U, Sabel M, Hau P, Kortmann RD, Krex D, Grauer O, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet. 2019;393:678–688. doi: 10.1016/S0140-6736(18)31791-4. [DOI] [PubMed] [Google Scholar]
- 11.Hashemi M, Hadjighassem M, Zali A, Hashemi J. Tumoricidal effect of human olfactory ensheathing cell mediated suicide gene therapy in human glioblastoma cells. Mol Biol Rep. 2018;45:2263–2273. doi: 10.1007/s11033-018-4388-0. [DOI] [PubMed] [Google Scholar]
- 12.Chao CN, Yang YH, Wu MS, Chou MC, Fang CY, Lin MC, Tai CK, Shen CH, Chen PL, Chang D, Wang M. Gene therapy for human glioblastoma using neurotropic JC virus-like particles as a gene delivery vector. Sci Rep. 2018;8:2213. doi: 10.1038/s41598-018-19825-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathanson DA, Gini B, Mottahedeh J, Visnyei K, Koga T, Gomez G, Eskin A, Hwang K, Wang J, Masui K, et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science. 2014;343:72–76. doi: 10.1126/science.1241328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou K, Yao H, Zhang X, Liu J, Qi Z, Xie X, Xu X, Zhou Y, Yu Z, Wang Z, et al. Next generation sequencing and molecular imaging identify EGFR mutation and amplification in a glioblastoma multiforme patient treated with an EGFR inhibitor: A case report. Oncotarget. 2017;8:50305–50313. doi: 10.18632/oncotarget.18148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12:675–684. doi: 10.1593/neo.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji C, Guo H, Zhang P, Kuang W, Fan Y, Wu L. AnnexinA5 promote glioma cell invasion and migration via the PI3K/Akt/NF-κB signaling pathway. J Neurooncol. 2018;138:469–478. doi: 10.1007/s11060-018-2818-4. [DOI] [PubMed] [Google Scholar]
- 17.Siebzehnrubl FA, Silver DJ, Tugertimur B, Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT, Kupper MD, Neal D, et al. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol Med. 2013;5:1196–1212. doi: 10.1002/emmm.201302827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Liu D, Yang Z, Yang Z. High LAMC1 expression in glioma is associated with poor prognosis. OncoTargets Ther. 2019;12:4253–4260. doi: 10.2147/OTT.S205333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yates AD, Achuthan P, Akanni W, Allen J, Allen J, Alvarez-Jarreta J, Amode MR, Armean IM, Azov AG, Bennett R, et al. Ensembl 2020. Nucleic Acids Res. 2020;48:D682–D688. doi: 10.1093/nar/gkz966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, Robinson MD. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat Protoc. 2013;8:1765–1786. doi: 10.1038/nprot.2013.099. [DOI] [PubMed] [Google Scholar]
- 22.Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A, Moeller S, et al. gplots: Various R programming tools for plotting data. R Package Version 2.4. 2009 [Google Scholar]
- 23.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 24.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz-Montana JJ, Gomez-Vela F, Diaz-Diaz N. GNC-app: A new Cytoscape app to rate gene networks biological coherence using gene-gene indirect relationships. Biosystems. 2018;166:61–65. doi: 10.1016/j.biosystems.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2018;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandettini WP, Kellman P, Mancini C, Booker OJ, Vasu S, Leung SW, Wilson JR, Shanbhag SM, Chen MY, Arai AE. MultiContrast delayed enhancement (MCODE) improves detection of subendocardial myocardial infarction by late gadolinium enhancement cardiovascular magnetic resonance: A clinical validation study. J Cardiovasc Magn Reson. 2012;14:83. doi: 10.1186/1532-429X-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Chen X, Chen Z. Identification of key candidate genes in dairy cow in response to escherichia coli mastitis by bioinformatical analysis. Front Genet. 2019;10:1251. doi: 10.3389/fgene.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watkins S, Sontheimer H. Unique biology of gliomas: Challenges and opportunities. Trends Neurosci. 2012;35:546–556. doi: 10.1016/j.tins.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng J, da Hora CC, Kantar RS, Nakano I, Wakimoto H, Batchelor TT, Chiocca EA, Badr CE, Tannous BA. Dissecting inherent intratumor heterogeneity in patient-derived glioblastoma culture models. Neuro Oncol. 2017;19:820–832. doi: 10.1093/neuonc/now253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonavia R, Inda MM, Cavenee WK, Furnari FB. Heterogeneity maintenance in glioblastoma: A social network. Cancer Res. 2011;71:4055–4060. doi: 10.1158/0008-5472.CAN-11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao S, Yang Z, Lv R, Zhao J, Wu M, Liao Y, Liu Q. miR-135b contributes to the radioresistance by targeting GSK3β in human glioblastoma multiforme cells. PLoS One. 2014;9:e108810. doi: 10.1371/journal.pone.0108810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu J, Ye J, Zhang L, Xia L, Hu H, Jiang H, Wan Z, Sheng F, Ma Y, Li W, et al. Differential expression of circular RNAs in glioblastoma multiforme and its correlation with prognosis. Transl Oncol. 2017;10:271–279. doi: 10.1016/j.tranon.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song YC, Lu GX, Zhang HW, Zhong XM, Cong XL, Xue SB, Kong R, Li D, Chang ZY, Wang XF, et al. Proteogenomic characterization and integrative analysis of glioblastoma multiforme. Oncotarget. 2017;8:97304–97312. doi: 10.18632/oncotarget.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fourest-Lieuvin A, Peris L, Gache V, Garcia-Saez I, Juillan-Binard C, Lantez V, Job D. Microtubule regulation in mitosis: Tubulin phosphorylation by the cyclin-dependent kinase Cdk1. Mol Biol Cell. 2006;17:1041–1050. doi: 10.1091/mbc.e05-07-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: A changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 40.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 42.Li W, Liu J, Fu W, Zheng X, Ren L, Liu S, Wang J, Ji T, Du G. 3-O-acetyl-11-keto-β-boswellic acid exerts anti-tumor effects in glioblastoma by arresting cell cycle at G2/M phase. J Exp Clin Cancer Res. 2018;37:132. doi: 10.1186/s13046-018-0805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song Z, Pan Y, Ling G, Wang S, Huang M, Jiang X, Ke Y. Escape of U251 glioma cells from temozolomide-induced senescence was modulated by CDK1/survivin signaling. Am J Transl Res. 2017;9:2163–2180. [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Huang Q, Dong J, Zhai DZ, Wang AD, Lan Q. Overexpression of CDC2/CyclinB1 in gliomas, and CDC2 depletion inhibits proliferation of human glioma cells in vitro and in vivo. BMC Cancer. 2008;8:29. doi: 10.1186/1471-2407-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Voer RM, Geurts van Kessel A, Weren RD, Ligtenberg MJ, Smeets D, Fu L, Vreede L, Kamping EJ, Verwiel ET, Hahn MM, et al. Germline mutations in the spindle assembly checkpoint genes BUB1 and BUB3 are risk factors for colorectal cancer. Gastroenterology. 2013;145:544–547. doi: 10.1053/j.gastro.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Bie L, Zhao G, Cheng P, Rondeau G, Porwollik S, Ju Y, Xia XQ, McClelland M. The accuracy of survival time prediction for patients with glioma is improved by measuring mitotic spindle checkpoint gene expression. PLoS One. 2011;6:e25631. doi: 10.1371/journal.pone.0025631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Q, Liu Y, Shang L, Yu J, Qu Q. The FOXM1/BUB1B signaling pathway is essential for the tumorigenicity and radioresistance of glioblastoma. Oncol Rep. 2017;38:3367–3375. doi: 10.3892/or.2017.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee E, Pain M, Wang H, Herman JA, Toledo CM, DeLuca JG, Yong RL, Paddison P, Zhu J. Sensitivity to BUB1B inhibition defines an alternative classification of glioblastoma. Cancer Res. 2017;77:5518–5529. doi: 10.1158/0008-5472.CAN-17-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tannous BA, Kerami M, Van der Stoop PM, Kwiatkowski N, Wang J, Zhou W, Kessler AF, Lewandrowski G, Hiddingh L, Sol N, et al. Effects of the selective MPS1 inhibitor MPS1-IN-3 on glioblastoma sensitivity to antimitotic drugs. J Natl Cancer Inst. 2013;105:1322–1331. doi: 10.1093/jnci/djt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kessler AF, Frombling GE, Gross F, Hahn M, Dzokou W, Ernestus RI, Löhr M, Hagemann C. Effects of tumor treating fields (TTFields) on glioblastoma cells are augmented by mitotic checkpoint inhibition. Cell Death Discov. 2018;4:12. doi: 10.1038/s41420-018-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Rop V, Padeganeh A, Maddox PS. CENP-A: The key player behind centromere identity, propagation, and kinetochore assembly. Chromosoma. 2012;121:527–538. doi: 10.1007/s00412-012-0386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomonaga T, Matsushita K, Yamaguchi S, Oohashi T, Shimada H, Ochiai T, Yoda K, Nomura F. Overexpression and mistargeting of centromere protein-A in human primary colorectal cancer. Cancer Res. 2003;63:3511–3516. [PubMed] [Google Scholar]
- 53.Rajput AB, Hu N, Varma S, Chen CH, Ding K, Park PC, Chapman JA, Sengupta SK, Madarnas Y, Elliott BE, Feilotter HE. Immunohistochemical assessment of expression of centromere protein-A (CENPA) in Human invasive breast cancer. Cancers. 2011;3:4212–4227. doi: 10.3390/cancers3044212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li YM, Liu XH, Cao XZ, Wang L, Zhu MH. Expression of centromere protein A in hepatocellular carcinoma. Zhonghua Bing Li Xue Za Zhi. 2007;36:175–178. (In Chinese) [PubMed] [Google Scholar]
- 55.Downes GB, Copeland NG, Jenkins NA, Gautam N. Structure and mapping of the G protein gamma3 subunit gene and a divergently transcribed novel gene, gng3lg. Genomics. 1998;53:220–230. doi: 10.1006/geno.1998.5508. [DOI] [PubMed] [Google Scholar]
- 56.Schwindinger WF, Giger KE, Betz KS, Stauffer AM, Sunderlin EM, Sim-Selley LJ, Selley DE, Bronson SK, Robishaw JD. Mice with deficiency of G protein gamma3 are lean and have seizures. Mol Cell Biol. 2004;24:7758–7768. doi: 10.1128/MCB.24.17.7758-7768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El-Habr EA, Dubois LG, Burel-Vandenbos F, Bogeas A, Lipecka J, Turchi L, Lejeune FX, Coehlo PL, Yamaki T, Wittmann BM, et al. A driver role for GABA metabolism in controlling stem and proliferative cell state through GHB production in glioma. Acta Neuropathol. 2017;133:645–660. doi: 10.1007/s00401-016-1659-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in the present study are available from The Cancer Genome Atlas database (https://portal.gdc.cancer.gov/).