Abstract
Background
Postoperative pain in children has always been inadequately evaluated. This study aims to evaluate the postoperative pain response using an additional dose of brachial plexus block (BPB) for younger children receiving elbow surgeries under general anesthesia (GA).
Methods
This retrospective case-control study included pediatric patients (3–10 years) who underwent surgeries for elbow injuries between January 2015 and January 2019. Patients with previous history of surgeries around the elbow, neurological impairment of injured limb, polytrauma, undergoing pain management for different causes, and open or old fractures were excluded. Patients were dichotomized into the GA group and the GA + BPB group as per the presence or absence of BPB.
Results
In all, 150 patients (102/48, male/female) in the GA and 150 patients (104/46, male/female) in the GA + BPB group were included. There existed no significant differences between the two groups in age, sex, fracture side, and types of elbow procedures. As for the pain response after lateral condyle fracture of the humerus (LCFH), the FLACC pain scale was significantly higher for those in the GA group (6.2 ± 0.8) when compared to the GA + BPB group (1.6 ± 0.5) (P < 0.001). As for the pain response after medial epicondyle fracture of the humerus (MCFH), the FLACC pain scale was significantly higher for those in the GA group (6.0 ± 0.8) when compared to the GA + BPB group (1.5 ± 0.5) (P < 0.001). As for the pain response after supracondylar fracture of the humerus (SCFH), the FLACC pain scale was significantly higher for those in the GA group (6.0 ± 0.8) when compared to the GA + BPB group (1.6 ± 0.5) (P < 0.001). As for the pain response after cubitus varus correction, the FLACC pain scale was significantly higher for those in the GA group (6.7 ± 0.7) when compared to the GA + BPB group (2.1 ± 0.7) (P < 0.001).
Conclusion
An additional shot of BPB for patients undergoing surgeries for elbow surgeries resulted in better postoperative pain response in younger children without significant BPB-related complications.
Keywords: Brachial plexus block, General anesthesia, Postoperative pain, Children
Background
Most of the orthopedic surgeries in the pediatric population, especially in younger children, are performed under general anesthesia (GA) [1]. Age less than 10 years is considered as a young child at our institute, and usually, GA is chosen as the anesthetic modality for such patients. However, postoperative pain in children has always been inadequately evaluated [2, 3]. Healthcare workers (HCWs) in the postanesthesia care unit (PACU) and residents on night shift always complain about the difficulties tackling with crying and screaming kids in pain [4, 5]. However, pain management is influenced by many factors, such as cultural values, religions, parental beliefs, and anxiety [6, 7]. Besides, the description of pain provided by the children is usually inconclusive [8]. Several non-pharmacological methods, including position adjustment, reassurance [9], and music, have been proposed to alleviate the pain [10–12].
In order to cope with postoperative pain, additional ultrasonography (US)-guided brachial plexus block (BPB) was implemented for pediatric patients under GA since 2017 at our institute. This study aims to compare the postoperative pain response under GA with or without BPB retrospectively.
Methods
From January 2015 to January 2019, all patients who underwent elbow surgeries under GA were retrospectively reviewed. Since 2017, additional BPB was performed in younger children at our institute, and it was consented by the parents. The results are summarized according to the type of fractures that the patient had: lateral condylar fracture of the humerus (LCFH), medial epicondyle fracture of the humerus (MCFH), supracondylar fracture of the humerus (SCFH), and cubitus varus deformity.
Inclusion criteria: (1) pediatric patients aged between 3 and 10 years who underwent surgeries for LCFH, MCFH, SCFH, and corrective osteotomy and fixation for cubitus varus deformity; (2) no previous history of surgeries around the same elbow; and (3) patients without neurological impairment. Exclusion criteria: (1) patients with polytrauma or open fractures, (2) patients with the underlying disease requiring regular pain management, (3) patients not having clear and complete medical records, and (4) delayed presentation of elbow fractures.
The pain response at PACU after extubation was evaluated by the anesthetic nurse using Face, Legs, Activity, Cry, and Consolability (FLACC) pain scale [13]. Pain response in the ward on the first night after surgery was reported by the patient using the Faces Pain Scale-Revised (FPS-R) [14], by the caregiver using the numeric rating scale (NRS) [15], and by the on-call nurse using the FLACC pain scale. Baseline information, including sex, age, operative side, procedures, and application of a tourniquet, was recorded and reviewed.
SPSS statistical package program (SPSS 19.0 version; SPSS Inc., Chicago, Illinois, USA) was used for statistical analysis. The categorical data were analyzed using the chi-square (χ2) test, and the continuous data were analyzed using Student’s t test. Fisher exact test was used under those circumstances with fewer subjects in groups of interest. Data were presented as mean ± SD (range), median (range), or n (%). A P value of less than 0.05 was considered significantly different.
Result
As shown in Table 1, 150 patients (102/48, male/female) in the GA and 150 patients (104/46, male/female) in the GA + BPB group were included in our study. There existed no significant difference between the two groups regarding age, sex, fracture side, and types of procedures.
Table 1.
Demographic and clinical parameters of children with elbow injuries
| Parameters | GA (n = 150) | GA + BPB (n = 150) | P value |
|---|---|---|---|
| Age, years | 7.2 ± 1.9 | 7.5 ± 1.7 | 0.152 |
| Sex, male/female | 102/48 | 104/46 | 0.803 |
| Fracture side, L/R | 95/55 | 92/58 | 0.721 |
| LC | 44 (29.3%) | 45 (30.0%) | 0.998 |
| ME | 23 (15.3%) | 23 (15.3%) | |
| SC | 60 (40.0%) | 60 (40.0%) | |
| CV | 23 (15.3%) | 22 (14.7%) |
GA general anesthesia, BPB brachial plexus block, LC lateral condyle fracture, ME medial epicondyle fracture, SC supracondylar fracture, CV cubitus varus deformity
Data shown as mean ± SD or n (%)
As for the pain response after LCFH (Table 2), there were 44 patients (34/10, male/female) in the GA group and 45 patients (35/10, male/female) in the GA + BPB group. There existed no significant difference between the two groups concerning age, sex, fracture side, duration of surgery, and application of a tourniquet. The FLACC pain scale was significantly higher for those in the GA group (6.2 ± 0.8) when compared to the GA + BPB group (1.6 ± 0.5) (P < 0.001), and all patients in GA group were given additional analgesics in PACU. The pain response from the patient, caregiver, and HCW was significantly better in the GA + BPB group. The frequency of waking up from the sleep, calling nurse/doctor, and utilization of oral ibuprofen was significantly higher in the GA group than the GA + BPB group. Thirty-nine percent (17/44) of patients in the GA group required additional analgesics during the night shift, whereas only 8.9% (4/45) in the GA + BPB group required additional analgesics.
Table 2.
Pain response in children with LC
| Parameters | GA (n = 44) | GA + BPB (n = 45) | P value | |
|---|---|---|---|---|
| Age, years | 7.2 ± 1.8 | 7.2 ± 1.8 | 0.964 | |
| Sex, male/female | 34/10 | 35/10 | 0.834 | |
| Fracture side, L/R | 34/10 | 32/13 | 0.498 | |
| Duration of surgery, min | 49.6 ± 6.7 | 48.9 ± 7.0 | 0.633 | |
| Tourniquet | 0 | 0 | > 0.999 | |
| FLACC in PACU | 6.2 ± 0.8 | 1.6 ± 0.5 | < 0.001* | |
| Analgesic in PACU | 44 (100%) | 0 | < 0.001* | |
| FPS-R, patient | 5.1 ± 1.0 | 3.4 ± 1.0 | < 0.001* | |
| NRS, caregiver | 3.8 ± 0.8 | 2.3 ± 0.5 | < 0.001* | |
| FLACC in ward | 3.1 ± 0.9 | 1.9 ± 0.5 | < 0.001* | |
| Wake from sleeping, times | 0 | 0 | 27 (60.0%) | < 0.001* |
| 1 | 0 | 7 (15.6%) | ||
| 2 | 10 (22.7%) | 11 (24.4%) | ||
| ≥ 3 | 34 (87.3%) | 0 | ||
| Call nurse/doctor, times | 0 | 9 (20.5%) | 24 (53.3%) | < 0.001* |
| 1 | 6 (13.6%) | 14 (31.1%) | ||
| 2 | 16 (36.4%) | 7 (15.6%) | ||
| ≥ 3 | 13 (29.5%) | 0 | ||
| Oral ibuprofen, times | 0 | 0 | 45 (100%) | < 0.001* |
| 1 | 0 | 0 | ||
| 2 | 19 (43.2%) | 0 | ||
| ≥ 3 | 25 (56.8%) | 0 | ||
| Additional analgesic | 17 (38.6%) | 4 (8.9%) | < 0.001* | |
PACU postanesthesia care unit; FLACC Face, Leg, Activity, Cry, and Consolability; FPS-R Faces Pain Scale-Revised; NRS numeric rate scale
Data shown as mean ± SD or n (%)
* < 0.05
As for the pain response after MCFH (Table 3), there were 23 patients (18/5, male/female) in the GA group and 23 patients (18/5, male/female) in the GA + BPB group. There existed no significant difference between the two groups concerning age, sex, fracture side, duration of surgery, and application of a tourniquet. The FLACC pain scale was significantly higher for those in the GA group (6.0 ± 0.8) when compared to the GA + BPB group (1.5 ± 0.5) (P < 0.001), and all patients in the GA group required additional analgesics in PACU. The pain response from the patient, caregiver, and HCW was significantly better in the GA + BPB group. The frequency of waking up from the sleep, calling nurse/doctor, and utilization of oral ibuprofen was significantly higher in the GA than the GA + BPB. Fifty-two percent (12/23) of patients in the GA group required additional analgesics during the night shift, whereas only 13% (3/23) in the GA + BPB group required additional analgesics.
Table 3.
Pain response in children with medial epicondyle fractures
| Parameters | GA (n = 23) | GA + BPB (n = 23) | P value | |
|---|---|---|---|---|
| Age, years | 7.9 ± 1.2 | 8.5 ± 1.0 | 0.073 | |
| Sex, male/female | 18/5 | 18/5 | > 0.999 | |
| Fracture side, L/R | 10/13 | 9/14 | 0.925 | |
| Duration of surgery, min | 45.4 ± 8.3 | 43.9 ± 8.2 | 0.533 | |
| Tourniquet | 0 | 0 | > 0.99 | |
| FLACC in PACU | 6.0 ± 0.8 | 1.5 ± 0.5 | < 0.001* | |
| Analgesic in PACU | 23 (100%) | 0 | < 0.001* | |
| FPS-R, patient | 5.0 ± 1.0 | 3.1 ± 1.0 | < 0.001* | |
| NRS, caregiver | 4.0 ± 0.8 | 2.5 ± 0.5 | < 0.001* | |
| FLACC in ward | 3.2 ± 0.7 | 2.0 ± 0.6 | < 0.001* | |
| Wake from sleeping, times | 0 | 0 | 10 (43.5%) | < 0.001* |
| 1 | 0 | 6 (26.1%) | ||
| 2 | 6 (26.1%) | 7 (30.4%) | ||
| ≥ 3 | 17 (73.9%) | 0 | ||
| Call nurse/doctor, times | 0 | 1 (4.3%) | 10 (43.5%) | 0.002* |
| 1 | 9 (39.1%) | 10 (43.5%) | ||
| 2 | 10 (43.5%) | 3 (13.0%) | ||
| ≥ 3 | 3 (13.0%) | 0 | ||
| Oral ibuprofen, times | 0 | 0 | 23 (100%) | < 0.001* |
| 1 | 0 | 0 | ||
| 2 | 12 (52.2%) | 0 | ||
| ≥ 3 | 11 (47.8%) | 0 | ||
| Additional analgesic | 12 (52.2%) | 3 (13.0%) | 0.005* | |
PACU postanesthesia care unit; FLACC Face, Leg, Activity, Cry, and Consolability; FPS-R Faces Pain Scale-Revised; NRS numeric rate scale
Data shown as mean ± SD or n (%)
* < 0.05
As for the pain response after SCFH (see Fig. 1, Table 4), there were 60 patients (38/22, male/female) in the GA group and 60 patients (36/24, male/female) in the GA + BPB group. There existed no significant difference between the two groups concerning age, sex, fracture side, duration of surgery, and application of a tourniquet. The FLACC pain scale was significantly higher for those in the GA group (6.0 ± 0.8) than the GA + BPB group (1.6 ± 0.5) (P < 0.001), and 75.0% (45/60) patients in GA group required additional analgesics in PACU. The pain response from the patient, caregiver, and HCW was significantly better in the GA + BPB group. The frequency of waking up from the sleep, calling nurse/doctor, and utilization of oral ibuprofen was significantly higher in the GA group than the GA + BPB group. Twenty-five percent (15/60) of patients in the GA group required additional analgesics during the night shift, whereas no patients in the GA + BPB group required additional analgesics.
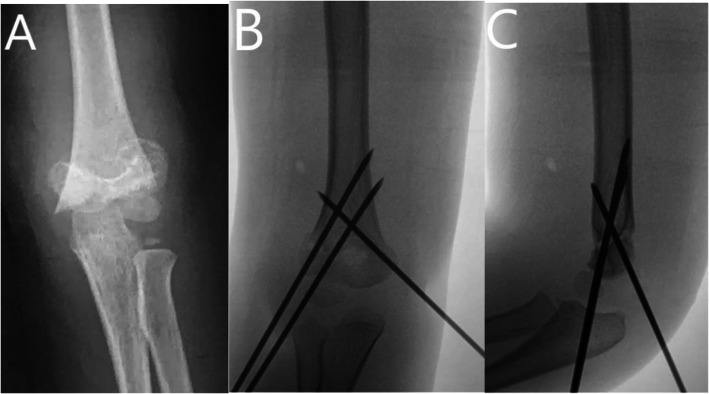
Fig. 1.
A 7-year boy of left supracondylar fracture treated with CRPP. a AP view of the elbow before the surgery. b AP view of the elbow after surgery. c Lateral view of the elbow after surgery
Table 4.
Pain response in children with supracondylar fractures
| Parameters | GA (n = 60) | GA + BPB (n = 60) | P value | |
|---|---|---|---|---|
| Age, years | 6.4 ± 1.9 | 7.5 ± 1.7 | 0.002* | |
| Sex, male/female | 38/22 | 36/24 | 0.658 | |
| Fracture side, L/R | 35/25 | 35/25 | > 0.999 | |
| Duration of surgery, min | 44.0 ± 9.2 | 43.5 ± 7.9 | 0.752 | |
| Tourniquet | 0 | 0 | > 0.999 | |
| FLACC in PACU | 6.0 ± 0.8 | 1.6 ± 0.5 | < 0.001* | |
| Analgesic in PACU | 45 (75.0%) | 0 | < 0.001* | |
| FPS-R, patient | 5.6 ± 1.1 | 3.4 ± 1.0 | < 0.001* | |
| NRS, caregiver | 4.1 ± 0.8 | 2.4 ± 0.5 | < 0.001* | |
| FLACC in ward | 3.1 ± 0.9 | 1.9 ± 0.6 | < 0.001* | |
| Wake from sleeping, times | 0 | 0 | 28 (46.7%) | < 0.001* |
| 1 | 27 (45.0%) | 21 (35.0%) | ||
| 2 | 26 (43.3%) | 11 (18.3%) | ||
| ≥ 3 | 7 (11.7%) | 0 | ||
| Call nurse/doctor, times | 0 | 25 (41.7%) | 36 (60.0%) | 0.067 |
| 1 | 17 (28.3%) | 16 (26.7%) | ||
| 2 | 15 (25.0%) | 8 (13.3%) | ||
| ≥ 3 | 3 (5.0%) | 0 | ||
| Oral ibuprofen, times | 0 | 0 | 60 (100%) | < 0.001* |
| 1 | 44 (73.3%) | 0 | ||
| 2 | 16 (26.7%) | 0 | ||
| ≥ 3 | 0 | 0 | ||
| Additional analgesic | 15 (25.0%) | 0 | < 0.001* | |
PACU postanesthesia care unit; FLACC Face, Leg, Activity, Cry, and Consolability; FPS-R Faces Pain Scale-Revised; NRS numeric rate scale
Data shown as mean ± SD or n (%)
* < 0.05
As for the pain response after corrective osteotomy for cubitus varus deformity (Table 5), there were 23 patients (12/11, male/female) in the GA group and 22 patients (15/7, male/female) in the GA + BPB group. There existed no significant difference between the two groups concerning age, sex, fracture side, duration of surgery, and application of a tourniquet. The FLACC pain scale was significantly higher for those in the GA group (6.7 ± 0.7) when compared to the GA + BPB group (2.1 ± 0.7) (P < 0.001), and all patients in GA group required additional analgesics, whereas 54.5% (12/22) in the GA + BPB group required analgesics in PACU. The pain response from the patient, caregiver, and HCW was significantly better in the GA + BPB group. The frequency of waking up from the sleep, calling nurse/doctor, and utilization of oral ibuprofen was significantly higher in the GA group than the GA + BPB group. Seventy-four percent (17/23) of patients in the GA group required additional analgesics during the night shift, whereas only 27.3% (6/22) in the GA + BPB group required additional analgesics.
Table 5.
Pain response in children with cubitus varus
| Parameters | GA (n = 23) | GA + BPB (n = 22) | P value | |
|---|---|---|---|---|
| Age, years | 8.3 ± 1.4 | 6.8 ± 1.8 | 0.004* | |
| Sex, male/female | 12/11 | 15/7 | 0.128 | |
| Fracture side, L/R | 16/7 | 16/6 | 0.843 | |
| Duration of surgery, min | 58.6 ± 6.1 | 61.8 ± 6.3 | 0.097 | |
| Tourniquet | 23 (100%) | 22 (100%) | > 0.99 | |
| FLACC in PACU | 6.7 ± 0.7 | 2.1 ± 0.7 | < 0.001* | |
| Analgesic in PACU | 23 (100%) | 12 (54.5%) | < 0.001* | |
| FPS-R, patient | 5.5 ± 0.8 | 3.9 ± 1.1 | < 0.001* | |
| NRS, caregiver | 4.8 ± 0.5 | 2.7 ± 0.5 | < 0.001* | |
| FLACC in ward | 3.9 ± 0.7 | 2.1 ± 0.7 | < 0.001* | |
| Wake from sleeping, times | 0 | 0 | 3 (13.6%) | < 0.001* |
| 1 | 0 | 7 (31.8%) | ||
| 2 | 4 (17.4%) | 12 (54.5%) | ||
| ≥ 3 | 19 (82.6%) | 0 | ||
| Call nurse/doctor, times | 0 | 2 (8.7%) | 10 (45.5%) | 0.002* |
| 1 | 7 (30.4%) | 10 (45.5%) | ||
| 2 | 8 (34.8%) | 2 (9.1%) | ||
| ≥ 3 | 6 (26.1%) | 0 | ||
| Oral ibuprofen, times | 0 | 0 | 4 (18.2%) | < 0.001* |
| 1 | 0 | 9 (40.9%) | ||
| 2 | 6 (26.1%) | 9 (40.9%) | ||
| ≥ 3 | 17 (73.9%) | 0 | ||
| Additional analgesic | 17 (73.9%) | 6 (27.3%) | < 0.001* | |
PACU postanesthesia care unit; FLACC Face, Leg, Activity, Cry, and Consolability; FPS-R Faces Pain Scale-Revised; NRS numeric rate scale
Data shown as mean ± SD or n (%)
* < 0.05
None of the patients reported BPB-related complications during the postoperative follow-up visit.
Discussion
Additional BPB for elbow surgeries resulted in better postoperative pain response without significant BPB-related complications.
Elbow surgeries for fractures and cubitus varus deformity correction are common in the pediatric population [16, 17]. Although clinical outcomes of surgeries following fractures around the elbow are usually satisfactory, however, postoperative pain management remains challenging [2, 5, 6]. Pain is a significant parameter influencing recovery, early mobilization, and hospital stay [18]. BPB is an effective choice in the management of shoulder or humeral surgery in children [19–22]. Guidance with ultrasonography (US) improves the accuracy of needle advancement and anatomic identification of neural structures [23]. Although US-guided BPB is gaining popularity in the pediatric population [24, 25], the application of BPB in younger kids is still limited [26]. In our hospital, GA remains the preferred choice in pediatric surgeries. However, the results of this study indicated that the GA + BPB was more effective in reducing the pain following surgeries around the elbow.
In displaced LCFH and MCFH, open reduction and internal fixation (ORIF) is our preferred choice and usually yields satisfactory outcomes [27, 28]. Whereas, closed reduction and percutaneous pinning (CRPP) remains the primary choice for displaced SCFH [29]. Regardless of the fracture type and operative choice, the pain response was significantly lower in the GA + BPB group in our study. However, some patients receiving GA + BPB for corrective osteotomy and fixation for cubitus varus deformity complained of significant pain, possibly due to the application of a tourniquet.
Additionally, the cost for BPB is about 120 US dollars at our institute, and it is affordable for most patients and their families. There have been reports regarding the complications, including pneumothorax and neuropathy, related to the BPB [30, 31]; however, no BPB-related complications were apparent on the second postoperative day in our study.
An additional BPB is recommended for patients undergoing ORIF for elbow fractures; however, it might not be necessary for patients requiring CRPP only. More attention should be paid for postoperative pain management for patients undergoing corrective osteotomy and fixation with the application of a tourniquet for cubitus varus deformity. It is because the corrective osteotomy and fixation usually takes longer surgical time and prolonged use of the tourniquet that might result in tourniquet-related pain [32, 33].
There were certain limitations in our study. Firstly, it is a retrospective study with modest sample size; secondly, the cost of BPB is different in different countries, and cost-effective analysis remains to be investigated.
Conclusion
An additional shot of BPB for patients undergoing surgeries for elbow surgeries resulted in better postoperative pain response in younger children without significant BPB-related complications. Those treated with GA + BPB had significantly less pain regardless of the fracture type. Patients treated with GA + BPB also woke up from sleep much less and utilized oral ibuprofen significantly less than those treated with GA alone regardless of fracture type.
Acknowledgements
Not applicable.
Abbreviations
- HCW
Healthcare worker
- GA
General anesthesia
- PACU
Postanesthesia care unit
- BPB
Brachial plexus block
- FLACC
Face, Leg, Activity, Cry, and Consolability pain scale
- FPS-R
Faces Pain Scale-Revised
- NRS
Numeric rate scale
- ORIF
Open reduction and internal fixation
- CRPP
Closed reduction and percutaneous pinning
Authors’ contributions
PH* is in charge of the main idea and is the guarantor of integrity of the entire clinical study; JL and PH are in charge of the study concepts, design, manuscript preparation, and editing; PH and SR are in charge of the language polishing and the grammar revision; RKL and RJX are in charge of the collection of the study data. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets supporting the conclusion of this article are included within the article. Upon request, raw data can be provided by the corresponding author.
Ethics approval and consent to participate
Not applicable. Since this study was a retrospective investigation, the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology ruled that no formal ethics approval was required. Written consents to participate in this study were obtained from the legal guardians of every patient.
Consent for publication
Written consents were obtained from the legal guardians of every patient for publication of this paper.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104(3):509–520. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- 2.Rømsing J, Møller-Sonnergaard J, Hertel S, Rasmussen M. Postoperative pain in children: comparison between ratings of children and nurses. J Pain Symptom Manage. 1996;11(1):42–46. doi: 10.1016/0885-3924(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 3.Hamers JP, Abu-Saad HH, van den Hout MA, Halfens RJ. Are children given insufficient pain-relieving medication postoperatively? J Adv Nurs. 1998;27(1):37–44. doi: 10.1046/j.1365-2648.1998.00493.x. [DOI] [PubMed] [Google Scholar]
- 4.Moyer SM, Howe CJ. Pediatric pain intervention in the PACU. Crit Care Nurs Clin North Am. 1991;3(1):49–57. doi: 10.1016/S0899-5885(18)30756-1. [DOI] [PubMed] [Google Scholar]
- 5.Rheiner JG, Megel ME, Hiatt M, Halbach R, Cyronek DA, Quinn J. Nurses’ assessments and management of pain in children having orthopedic surgery. Issues Compr Pediatr Nurs. 1998;21(1):1–18. doi: 10.1080/01460869808951124. [DOI] [PubMed] [Google Scholar]
- 6.Chieng YJ, Chan WC, Klainin-Yobas P, He HG. Perioperative anxiety and postoperative pain in children and adolescents undergoing elective surgical procedures: a quantitative systematic review. J Adv Nurs. 2014;70(2):243–255. doi: 10.1111/jan.12205. [DOI] [PubMed] [Google Scholar]
- 7.Joestlein L. Pain, pain, go away! Evidence-based review of developmentally appropriate pain assessment for children in a postoperative setting. Orthop Nurs. 2015;34(5):252–261. doi: 10.1097/NOR.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 8.Mesko PJ, Eliades AB. Using pictures to assess pain location in children. J Perianesth Nurs. 2018;33(3):319–324. doi: 10.1016/j.jopan.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Makhlouf MM, Garibay ER, Jenkins BN, Kain ZN, Fortier MA. Postoperative pain: factors and tools to improve pain management in children. Pain Manag. 2019;9(4):389–397. doi: 10.2217/pmt-2018-0079. [DOI] [PubMed] [Google Scholar]
- 10.Olsen SW, Rosenkilde C, Lauridsen J, Hasfeldt D. Effects of nonpharmacologic distraction methods on children’s postoperative pain-a nonmatched case-control study. J Perianesth Nurs. 2020;35(2):147–154. doi: 10.1016/j.jopan.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Kavak Akelma F, Altınsoy S, Arslan MT, Ergil J. Effect of favorite music on postoperative anxiety and pain. Wirkung von Lieblingsmusik auf postoperative Angst und Schmerz. Anaesthesist. 2020;69(3):198–204. doi: 10.1007/s00101-020-00731-8. [DOI] [PubMed] [Google Scholar]
- 12.Lin CL, Hwang SL, Jiang P, Hsiung NH. Effect of music therapy on pain after orthopedic surgery-a systematic review and meta-analysis. Pain Pract. 2020;20(4):422–436. doi: 10.1111/papr.12864. [DOI] [PubMed] [Google Scholar]
- 13.Chambers CT, Giesbrecht K, Craig KD, Bennett SM, Huntsman E. A comparison of faces scales for the measurement of pediatric pain: children’s and parents’ ratings. Pain. 1999;83:25. doi: 10.1016/S0304-3959(99)00086-X. [DOI] [PubMed] [Google Scholar]
- 14.Hicks CL, von Baeyer C, Spafford PA, van Korlaar I, Goodenough B. The faces pain scale-revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 15.Voepel-Lewis T, Malviya S, Tait AR, et al. A comparison of the clinical utility of pain assessment tools for children with cognitive impairment. Anesth Analg. 2008;106:72–78. doi: 10.1213/01.ane.0000287680.21212.d0. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Rai S, Tang X, Ze R, Liu R, Hong P. Surgical management of delayed Gartland type III supracondylar humeral fractures in children: a retrospective comparison of radial external fixator and crossed pinning. Medicine (Baltimore). 2020;99(10):e19449. doi: 10.1097/MD.0000000000019449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang X, Wang J, Slongo T, et al. Comparison of internal fixation vs. external fixation after corrective osteotomy in children with cubitus varus. J Shoulder Elbow Surg. 2020;29(4):845–852. doi: 10.1016/j.jse.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Touray ST, de Leeuw MA, Zuurmond WW, Perez RS. Psoas compartment block for lower extremity surgery: a meta-analysis. Br J Anaesth. 2008;101(6):750–760. doi: 10.1093/bja/aen298. [DOI] [PubMed] [Google Scholar]
- 19.Gerrard C, Roberts S. Ultrasound-guided regional anaesthesia in the paediatric population. ISRN Anesthesiol. 2012:1–7.
- 20.Delvi MB. Ultrasound-guided peripheral and truncal blocks in pediatric patients. Saudi J Anaesth. 2011;5(2):208–216. doi: 10.4103/1658-354X.82805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J-H, Kim Y-R, Yu H-K, Cho S-H, Kim S-H, Chae WS. Ultrasound-guided interscalene brachial plexus block in a pediatric patient with acute hepatitis - a case report. Korean J Anesthesiol. 2012;62(6):568–570. doi: 10.4097/kjae.2012.62.6.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ecoffey C. Safety in pediatric regional anesthesia. PediatrAnesth. 2012;22(1):25–30. doi: 10.1111/j.1460-9592.2011.03705.x. [DOI] [PubMed] [Google Scholar]
- 23.Ganesh A, Gurnaney HG. Ultrasound guidance for pediatric peripheral nerve blockade. Anesthesiol Clin. 2009;27(2):197–212. doi: 10.1016/j.anclin.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Yayik AM, Cesur S, Öztürk F, Celik EC, Ahiskalioglu A. Ultrasound guided costoclavicular approach to brachial plexus: first pediatric report. J Clin Anesth. 2019;55:136–137. doi: 10.1016/j.jclinane.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Xu C, Wang B, Yang A, et al. The efficacy of pediatric ultrasound guided brachial plexus block anesthesia and determination of optimal anesthetic drug dosage [published online ahead of print, 2016 Nov 9]. Minerva Pediatr. 2016.. [DOI] [PubMed]
- 26.Yanal H, Gürkan Y, Kuş A, Balaban O, Solak M, Toker K. Awake hand surgery under ultrasound-guided infraclavicular block is possible for cooperative children. Agri. 2016;28(4):190–193. doi: 10.5505/agri.2015.09327. [DOI] [PubMed] [Google Scholar]
- 27.Tomori Y, Nanno M, Takai S. Posteromedial elbow dislocation with lateral humeral condylar fracture in children: three case reports and a literature review. Medicine (Baltimore). 2018;97(36):e12182. doi: 10.1097/MD.0000000000012182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Y, Nan G. Treatment of medial humeral epicondyle fractures in children using absorbable self-reinforced polylactide pins. Medicine (Baltimore). 2020;99(17):e19861. doi: 10.1097/MD.0000000000019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okubo H, Nakasone M, Kinjo M, Onaka K, Futenma C, Kanaya F. Epidemiology of paediatric elbow fractures: a retrospective multi-centre study of 488 fractures. J Child Orthop. 2019;13(5):516–521. doi: 10.1302/1863-2548.13.190043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell HG, Tsao JW. Phantom sensations following brachial plexus nerve block: a case report. Front Neurol. 2018;9:436. doi: 10.3389/fneur.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta A, Talwar V, Kamal G, Gupta N. Delayed onset and prolonged Horner syndrome in two children after single-shot ultrasound guided infraclavicular and subclavian perivascular brachial plexus blocks for upper extremity surgery: case reports. AANA J. 2019;87(4):313–316. [PubMed] [Google Scholar]
- 32.Inal S, Er M, Ozsoy M, Cavusoglu A, Dincel V, Sakaogullari A. Comparison of two different anesthesia techniques for tourniquet pain with the use of forearm tourniquet. Iowa Orthop J. 2009;29:55–59. [PMC free article] [PubMed] [Google Scholar]
- 33.Crews JC, Cahall MA. An investigation of the neurophysiologic mechanisms of tourniquet-related pain: changes in spontaneous activity and receptive field size in spinal dorsal horn neurons. Reg Anesth Pain Med. 1999;24(2):102–109. doi: 10.1016/s1098-7339(99)90069-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusion of this article are included within the article. Upon request, raw data can be provided by the corresponding author.