Abstract
Tubeimoside-1 (TBMS1), a traditional Chinese herb extracted from Bolbostemma paniculatum (Maxim.), induces apoptosis in a number of human cancer cell lines. TBMS1 has been reported to induce apoptosis in human glioma cells, however the mechanism remains to be elucidated. The present study explored TBMS1-induced PI3K/Akt-related pathways in human glioma cells. The human glioma U251 and the human astrocyte (HA) cell lines were treated with various concentrations of TBMS1. MTT assays were conducted to analyze cell viability. Cell cycle distribution and the rate of apoptosis were assessed using flow cytometry. BrdU incorporation and Hoechst 33342 staining were performed to analyze the cell cycle and apoptosis, respectively. Western blotting was performed to investigate protein expression levels. The results demonstrated that TBMS1 reduced cell viability in human glioma cells U251 by suppressing Akt phosphorylation. Subsequently, TBMS1 inhibited DNA synthesis and induced G2/M phase arrest by targeting the PI3K/Akt/p21 and the cyclin-dependent kinase 1/cyclin B1 signaling cascades. In addition, TBMS1 triggered apoptosis via the PI3K/Akt-mediated Bcl-2 signaling pathway. These results demonstrated that TBMS1 prevented the progression of gliomas via the PI3K/Akt-dependent pathway, which provided a theoretical basis for in vivo studies to use TBMS1 as potential therapy for the prevention of cancer.
Keywords: apoptosis, cell cycle, glioma, tubeimoside-1, PI3K/Akt
Introduction
Gliomas have been recognized as one of the most common invasive malignancies of the central nervous system, with high recurrence rates, high mortality and low rates of cure (1,2). Although the diagnosis and treatment of glioma has made great progress in recent years, the overall survival of glioma patients remains low (3,4). Currently, there is no effective treatment for gliomas; therefore, novel treatments are required. Elucidating the possible molecular mechanisms of action is also an important aspect for the development of treatments for gliomas.
Tubeimoside-1 (TBMS1) is a triterpenoid saponin the sugar chains of which are connected by 3-hydroxy-3-methylglutaric acid to form a unique macrocyclic structure. TBMS1 is extracted from the tubers of a traditional Chinese medicinal plant, Bolbostemma paniculatum (Maxim.): Franquet (Cucurbitaceae), as recorded in the Supplement to the Compendium of Materia Medica (5). It is conventionally used as a natural medicine to treat a variety of diseases by producing anti-inflammatory and immunosuppressive activities (6,7). In addition, TBMS1 has been revealed to have antitumor effects and is considered a candidate for treating various types of cancer (8). Notably, TBMS1 was revealed to exert a direct cytotoxic effect on glioma cell lines and induce apoptosis (9). However, the exact role TBMS1 plays in glioma cells remains to be elucidated.
Serine/threonine kinase Akt kinase regulates diverse cellular processes, including cell survival, proliferation, angiogenesis and migration (10). Akt is a main downstream effector of PI3K whose dysregulation causes aberrant Akt activity. Therefore, targeting this pathway may have an effect on cancer treatment (11). In addition, dysfunction of the PI3K/Akt pathway has been revealed to cause human glioma cell apoptosis (12). TBMS1 has been also reported to act as a potent apoptosis inducer by modulating PI3K/Akt signaling (13). PI3K/Akt has not been reported to be modified in TBMS1-treated glioma cancer, to the best of the authors' knowledge.
In the present study, the mechanisms of action underlying TBMS1-induced cytotoxicity, apoptosis and cell cycle arrest in human glioma cells were investigated. It was also demonstrated that the PI3K/Akt-mediated signaling pathway was involved in the anti-glioma effects of TBMS1 in human glioma cells.
Materials and methods
TBMS1
TBMS1 with HPLC ≥98% was obtained from the National Institute for the Control of Pharmaceutical and Biological Products. TBMS1 powder was processed into a 1-mg/ml stock solution with DMEM (Gibco; Thermo Fisher Scientific, Inc.) and stored at −20°C.
Cell culture
The human glioma U251 cell line was obtained from the Type Culture Collection of the Chinese Academy of Sciences. Normal human astrocyte HA cell line was obtained from Lonza Group Ltd. All cell lines were incubated in DMEM supplemented with 10% FBS with antibiotics 100 U/ml penicillin and 100 µg/ml streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.) in a humidified incubator containing 5% CO2 at 37°C.
Cell proliferation assay
The cell viability was detected using MTT assays (Sigma-Aldrich; Merck KGaA). U251 and HA cell lines were seeded (5×103 cells/well) for 24 h. The cells were treated with TBMS1 (0–50 µg/ml) at 37°C for 24, 48 or 72 h or TBMS1 (30 µg/ml) in the presence or absence of PI3K/Akt inhibitor LY294002 (20 µM; cat. no. L9908; Sigma-Aldrich; Merck KGaA) for 24 h. Then, 20 µl MTT solution (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each cell sample for 4 h at 37°C. The cells were then dissolved using 100 µl DMSO, shaken for 10 min on a mini shaker and analyzed using a multi-well plate reader at 570 nm on a microplate reader (Thermo Fisher Scientific, Inc.). The proliferation rate (%) was calculated using the following formula: Proliferation rate = ODadministrated/ODcontrol × 100%.
BrdU incorporation assay
DNA synthesis was assessed via the incorporation of BrdU into newly synthesized strands. U251 cells (5×103 cells/well) were seeded in 96-well plates, cultured for 24 h and treated with 0, 20, 30 or 40 µg/ml TBMS1 at 37°C for 24 h. BrdU labelling was commenced through the addition of 10 µl/ml labelling solution, at a final concentration of 10 µM, to the medium. After the cells were incubated at 37°C for 6 h, labelling was stopped and the uptake of BrdU was measured using a Cell Proliferation ELISA kit (Roche Diagnostics) according to the manufacturer's protocol.
Cell cycle assay
U251 cells (5×103 cells/well) were seeded in six-well plates and treated with 0, 20, 30 or 40 µg/ml TBMS1 at 37°C for 24 h. Cells were collected, washed twice with cold PBS, fixed with 70% cold alcohol at −20°C overnight and then stained with 10 µl propidium iodide (PI; 1 mg/ml) in the presence of 1% RNase A for 30 min at 37°C, for cell cycle detection. Analysis was performed using Cell Quest and Mod Fit programs (v5.1, BD Biosciences) as described previously (14). The proportions of cells in the G0/G1, S and G2/M phases were presented as DNA histograms.
Hoechst 33342 staining assay
U251 glioma cells (5×103 cells/well) were seeded in 6-well culture plates and then treated with TBMS1 (0, 20, 30 or 40 µg/ml) at 37°C for 24 h. The cells were washed twice with cold PBS and then fixed with cold methanol and acetic acid (3/1, v/v) at 4°C for 30 min. After washing with PBS, a solution of Hoechst 33342 staining dye was added to the cells at 37°C for 30 min in the dark. After a final wash in PBS, a single, randomly selected field of view of the cells was visualized under a fluorescence microscope (magnification, ×400; Nikon Corporation).
Annexin V/PI flow cytometric assay
The effect of TBMS1 on the apoptosis of U251 cells was analyzed using flow cytometry. Cells (5×103 cells/well) were seeded in 6-well plates and treated with 0, 20, 30 or 40 µg/ml TBMS1 at 37°C for 24 h and then washed with cold PBS and resuspended in incubation buffer. The rate of apoptosis was examined by staining the samples with Annexin V-fluorescein 5-isothiocyanate (FITC) and PI for 20 min in the dark at room temperature using an Annexin V-FITC staining kit according to the manufacturer's protocol (BD Biosciences). Subsequently, flow cytometric analyses were performed using a FACS-Canto flow cytometer (Beckman Coulter, Inc.) with Cell Quest software (v5.1, BD Biosciences).
Western blot assay
U251 cells were exposed to TBMS1 (0, 20, 30 or 40 µg/ml) or TBMS1 (30 µg/ml) in the presence or absence of PI3K/Akt inhibitor LY294002 (20 µM) at 37°C for 24 h. The proteins were extracted using a cocktail of protein lysate solution supplemented with protease inhibitors (Beyotime Institute of Biotechnology). The cell lysate was centrifuged at 700 × g for 5 min at 4°C and the supernatant fraction was collected for western blotting. Cell lysates were collected, and the total protein was assessed using the BCA protein assay kit (Tiangen Biotech Co., Ltd.). A total of 20 µg total protein samples were separated on 12% SDS-PAGE, transferred onto PVDF membranes (EMD Millipore) and blocked using Tris-buffered saline containing 5% non-fat milk for 30 min at room temperature. These membranes were incubated with the corresponding primary antibodies overnight at 4°C. After being washed three times, the membranes were incubated with a secondary antibody for 1 h at room temperature. ECL (EMD Millipore) was used to detect protein bands. Band densities were quantified using Image J 1.45s software (National Institutes of Health). The antibodies [Akt (cat. no. sc377457; 1:1,000), phosphorylated (p-)Ser 473-Akt (cat. no. sc52940; 1:1,000), p-Thr 308-Akt (cat. no. sc271966; 1:1,000), p21 (cat. no. sc817; 1:200), cyclin B1 (cat. no. sc245; 1:1,000), Bad (cat. no. sc8044; 1:1,000), Bax (cat. no. sc7480; 1:1,000), Bcl-2 (cat. no. sc509; 1:500), caspase-3 (cat. no. sc7272; 1:1,000), caspase-9 (cat. no. sc56076; 1:1,000) and GAPDH (cat. no. sc365062; 1:1,000), mouse IgG-horseradish peroxidase (HRP; cat. no. sc2748; 1:2,000) and rat IgG-HRP (cat. no. sc2750; 1:2,000)] were purchased from Santa Cruz Biotechnology, Inc. The antibody poly-ADP ribose polymerase (PARP; product no. 9542; 1:1,000) and apoptosis-inducing factor (AIF; cat. no. 4642; 1:1,000) were purchased from Cell Signaling Technology, Inc. The antibody cyclin-dependent kinase 1 (CDK1; cat. no. PA5-14438; 1:1,000) was purchased from Thermo Fisher Scientific, Inc.
Statistical analysis
All data represent at least 3 independent experiments and are expressed as the mean ± standard deviation. Statistical analysis with multiple comparisons were performed using one-way ANOVAs followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
TBMS1 suppresses the growth of glioma cells through the PI3K/Akt signaling pathway
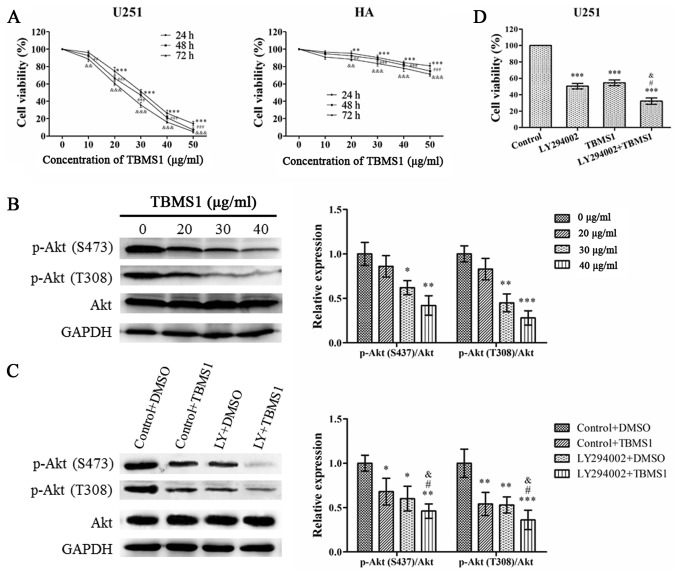
To investigate the effect of TBMS1 on glioma cells, MTT was used to analyze changes in cell viability. As revealed in Fig. 1A, the viability of U251 cells decreased in a time- and concentration-dependent manner. However, the effect of TBMS1 on HA cells was less pronounced than in U251 cells. The IC50 concentrations of TBMS1-treated U251 cells at 24, 48 and 72 h were 31.55±1.60, 28.38±1.21 and 25.30±1.26, respectively. When U251 cells were treated with TBMS1 for 24 h, the average viability of the cells at concentrations of 20, 30 and 40 µg/ml was reduced to 74.62%, 51.40% and 26.32%, respectively, compared with the control group (0 µg/ml). Therefore, concentrations of 20, 30 and 40 µg/m at 24 h were selected for further experiments to research the mechanism of action of TBMS1 in glioma cells.
Figure 1.
TBMS1 suppresses the growth of glioma cells by inhibiting the PI3K/Akt signaling pathway. U251 and HA cells were incubated with 0–50 µg/ml of TBMS1 for 24, 48 or 72 h. (A) Cell viability was determined by MTT. **P<0.01 and ***P<0.001 vs. TBMS1 (at 24h, 0 µg/ml); ##P<0.01 and ###P<0.001 vs. TBMS1 (at 48 h, 0 µg/ml); &&P<0.01 and &&&P<0.001 vs. TBMS1 (at 72h, 0 µg/ml). (B) The expression levels and quantitative values of p-Akt (S473), p-Akt (T308) and total Akt in U251 cells were monitored by western blot analysis. U251 cells were treated with TBMS1 (30 µg/ml) in the presence or absence of PI3K/Akt inhibitor LY294002 for 24 h. *P<0.05, **P<0.01 and ***P<0.001 vs. TBMS1 (0 µg/ml). (C) The expression levels and quantitative values of p-Akt (S473), p-Akt (T308) and total Akt in U251 cells were monitored by western blot analysis. *P<0.05, **P<0.01 and ***P<0.001 vs. Control+DMSO; #P<0.05 vs. LY294002+DMSO; &P<0.05 vs. Control+TBMS1. (D) Cell viability was determined by MTT. ***P<0.001 vs. Control; #P<0.05 vs. LY294002; &P<0.05 vs. TBMS1. TBMS1, tubeimoside-1; p-, phosphorylated.
In order to evaluate the effect of TBMS1 on the PI3K/Akt pathway in U251 cells, Akt phosphorylation and total Akt expression in U251 cells were also examined. As revealed in Fig. 1B, TBMS1 inhibited the phosphorylation of Akt in a concentration-dependent manner, while expression of total Akt was unchanged. To confirm the results, LY294002 was used to treat U251 cells alone or in combination with TBMS1 (30 µg/ml). As revealed in Fig. 1C, the phosphorylation of Akt at Thr308 and Ser473 significantly decreased in cells treated with TBMS1 alone, LY294002 alone and TBMS1 combined with LY294002 compared with the control group, which was treated only with DMSO. As revealed in Fig. 1D, compared with the control group (0 µg/ml), cell viability was significantly decreased in cells exposed to TBMS1 alone, LY294002 alone or the combination treatment. These results indicated that TBMS1 had the potential to inhibit human glioma cell growth through the PI3K/Akt signaling pathway.
TBMS1 arrests the G2/M phase of glioma cells by regulating the p21/CDK1/cyclin B1 signaling pathway
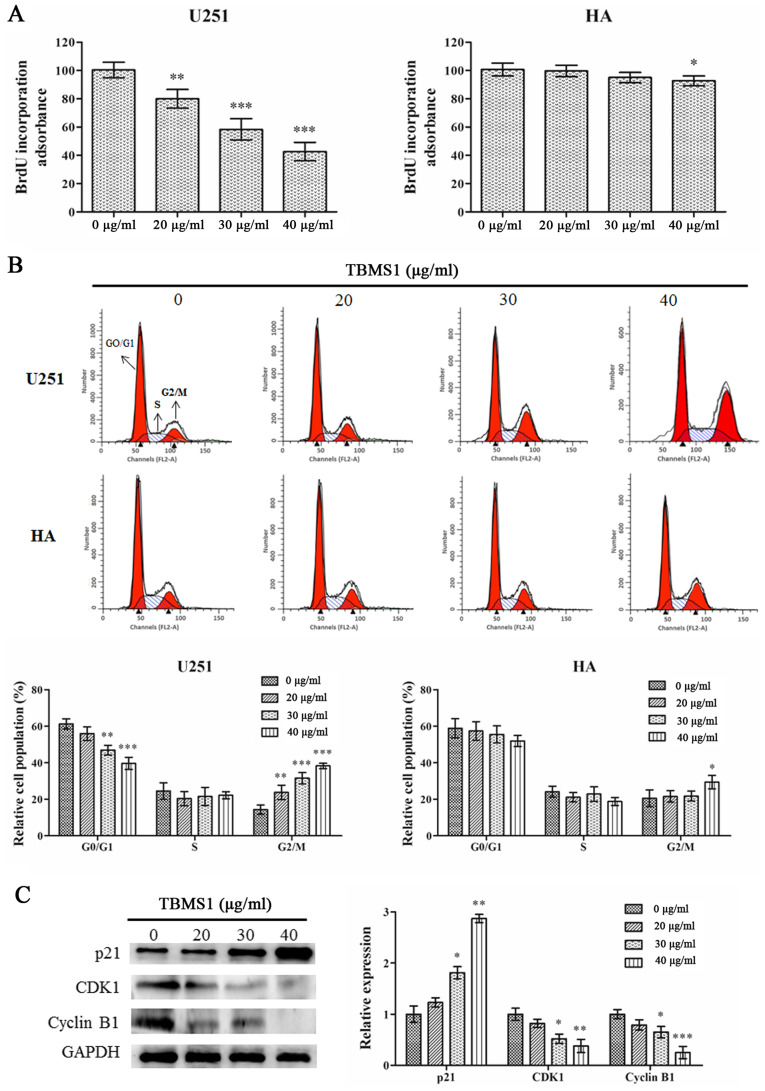
To explore the mechanisms of action underlying the inhibition of cell viability in the TBMS1 treatment group, cell cycle changes in U251 cells and HA cells were investigated. BrdU incorporation was used to examine the effect of TBMS1 on DNA synthesis. As revealed in Fig. 2A, TBMS1 suppressed DNA synthesis in U251 cells in a dose-dependent manner (P<0.01) while having a reduced effect in HA cells, with a significant response only observed at the highest dose (40 µg/ml; P<0.05).
Figure 2.
TBMS1 induces the G2/M phase arrest of glioma cells by regulating p21/CDK1/cyclin B1 signaling pathway. U251 and HA cells were incubated with 20, 30 or 40 µg/ml of TBMS1 for 24 h. (A) The DNA synthesis ability was detected via BrdU incorporation. (B) Representative profiles and percentage of cell cycle distribution was determined via flow cytometry. (C) The expression levels and quantitative values of p21, CDK1 and cyclin B1 were monitored by western blot analysis. *P<0.05, **P<0.01 and ***P<0.001 vs. TBMS1 (0 µg/ml). TBMS1, tubeimoside-1; CDK1, cyclin-dependent kinase 1.
Cell cycle progression was assessed using flow cytometry. Flow cytometric analysis demonstrated that with TBMS1 treatment at 20, 30 or 40 µg/ml, the percentage of U251 cells in the G2/M phase increased from 14.30% to 23.76%, 31.56% and 38.24%, respectively (Fig. 2B). The percentage of HA cells slightly increased only at the highest dose of TBMS1 (40 µg/ml), indicating that TBMS1 induced cell cycle arrest in the G2/M phase in glioma U251 cells.
To investigate the molecular basis of the effect of TBMS1 on cell cycle arrest, western blotting was used to study the expression levels of regulatory proteins. As a checkpoint of G2/M phase, the expression levels of CDK1 and cyclin B1 were studied. Fig. 2C demonstrated that the protein expression levels of CDK1 and cyclin B1 were significantly decreased in the TBMS1-treated group, in a dose-dependent manner. As a major regulator of the CDK1/cyclin B1 signaling pathway, p21 protein expression was significantly increased. These data further demonstrated that TBMS1 could induce G2/M arrest in glioma cells by regulating the p21/CDK1/cyclin B1 signaling pathway.
TBMS1 induces apoptosis of glioma cells by blocking the Bcl-2 signaling pathway
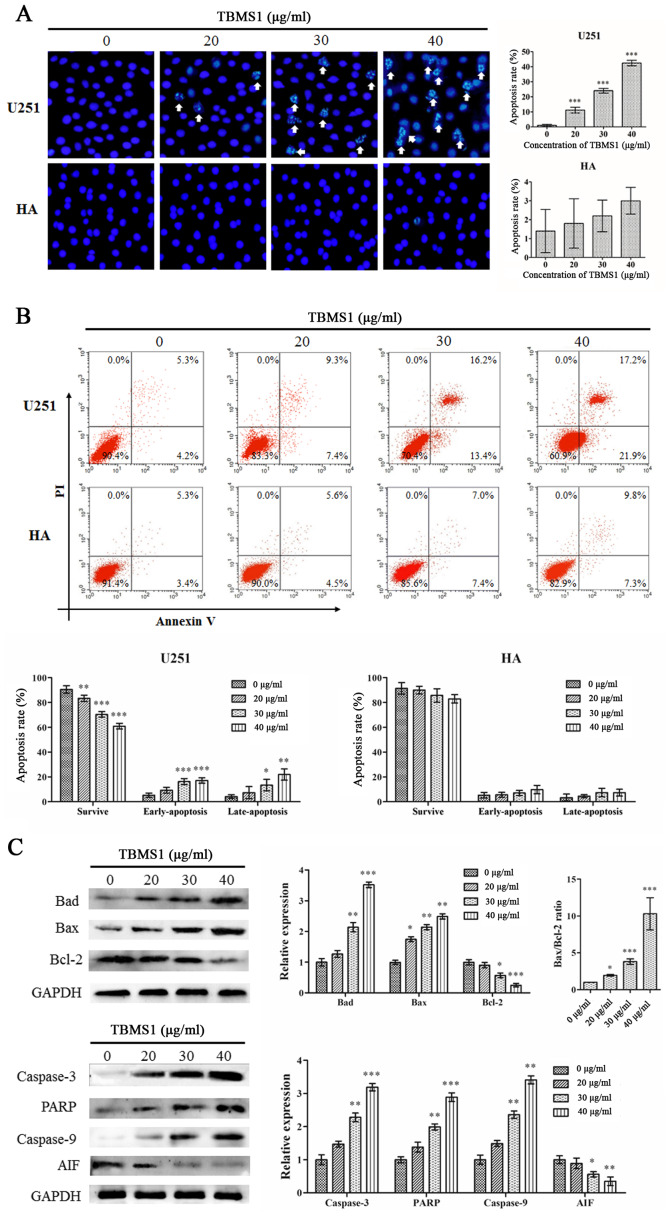
The effects on apoptosis of treatment with TBMS1 were investigated using Hoechst 33342 staining and Annexin V-FITC/PI staining. As revealed in Fig. 3A, the Hoechst 33342 staining assay demonstrated that following treatment with TBMS1 for 24 h, chromatin was agglutinated, nuclear fragmentation was observed and apoptotic bodies were formed in U251 cells, but there was no significant change in HA cells. Flow cytometric results demonstrated that in the treated U251 cells, the cells experienced typical morphological changes associated with apoptosis. The results of flow cytometric analysis demonstrated that rates of apoptosis were 16.67, 29.65 and 39.07% in the cells treated with 20, 30 or 40 µg/ml of TBMS1 respectively for 24 h as compared to 9.51% in the control cells (Fig. 3B). No effect was observed in the HA cells. These results indicated that TBMS1 could induce apoptosis in glioma cells.
Figure 3.
TBMS1 induces the apoptosis of glioma cells by blocking Bcl-2. U251 and HA cells were incubated with 20, 30 or 40 µg/ml of TBMS1 for 24 h. The cells were stained using (A) Hoechst 33342 staining and (B) Annexin V-FITC/PI double staining. A statistical analysis was performed for apoptosis. (C) The expression levels and quantitative values of Bad, Bax, PARP, caspase-3, caspase-9, AIF and Bcl-2 were monitored via western blot analysis. *P<0.05, **P<0.01 and ***P<0.001 vs. TBMS1 (0 µg/ml). TBMS1, tubeimoside-1; FITC, fluorescein 5-isothiocyanate; PI, propidium iodide; PARP, poly-ADP ribose polymerase; AIF, apoptosis-inducing factor.
To confirm the results, expression changes of the apoptosis regulators Bad, Bax, caspase-3, PARP, caspase-9, AIF and Bcl-2 were analyzed by immunoblotting. As revealed in Fig. 3C, with increasing doses of TBMS1, the protein expression levels of Bad, Bax, caspase-3, PARP and caspase-9 were increased while the expression levels of AIF and Bcl-2 proteins were decreased. In addition, TBMS1 increased the Bax/Bcl-2 ratio. These results demonstrated that TBMS1 increased cell death by suppressing the Bcl-2 signaling pathway.
TBMS1 attenuates the progression of glioma cells through the PI3K/Akt-mediated signaling pathways
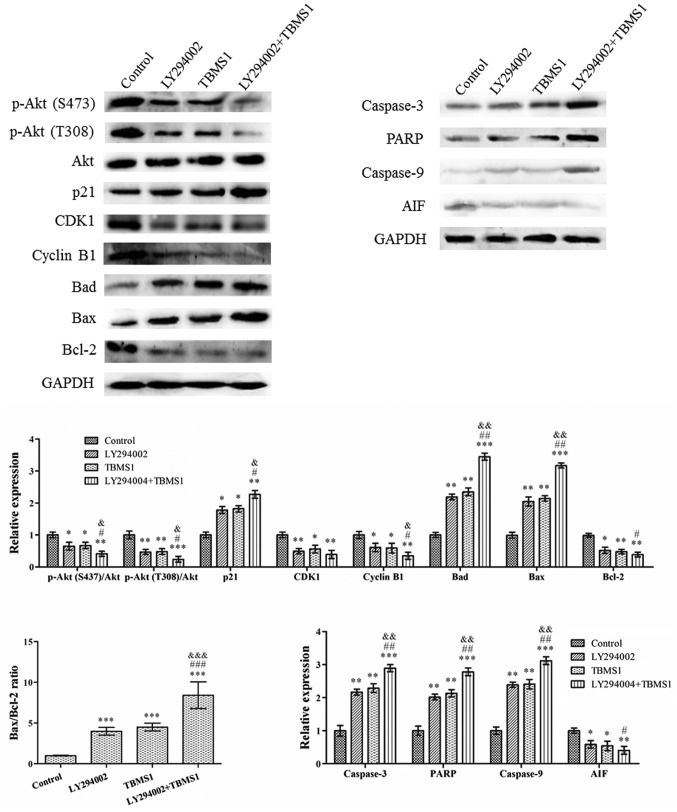
The PI3K/Akt signaling pathway is a potential survival signaling pathway in a number of systems and its inactivation can inhibit growth and induce apoptosis. In order to detect whether gliomas are inhibited by TBMS1 inducing the inactivation of the PI3K/Akt signaling pathway, U251 cells were treated with TBMS1 (30 µg/ml) in the presence or absence of 20 µM LY294002. Western blotting was then performed to explore alterations in the protein expression levels. The results indicated that the use of TBMS1 alone, LY294002 alone or a combination treatment for 24 h, amplified the effect of Akt dephosphorylation in U251 cells (Fig. 4), while total Akt protein levels remained constant in all of the treatments. In addition, the protein expression levels of p-Akt was significantly reduced in cells treated with TBMS1 and LY294002 together, compared with that of TBMS1 or LY294002 alone. Compared with TBMS1 (30 µg/ml) treatment, the CDK-interacting protein, p21, expression levels demonstrated the greatest increase whereas CDK1 and cyclin B1 levels were reduced the most in cells that were treated with TBMS1 and LY294002 combined. Following combined treatment, the Bad, Bax, caspase-3, PARP and caspase-9 levels increased, while the AIF and Bcl-2 levels decreased. In addition, the Bax/Bcl-2 ratio also demonstrated the largest increase following combination therapy, compared with single therapies alone.
Figure 4.
TBMS1 attenuates the progression of glioma cells by the PI3K/Akt-mediated signaling pathways. U251 cells were incubated with TBMS1 (30 µg/ml) in the presence or absence of PI3K/Akt inhibitor LY294002 (20 µM) for 24 h. The expression levels and quantitative values of proteins were monitored by western blotting. *P<0.05, **P<0.01 and ***P<0.001 vs. the control; #P<0.05, ##P<0.01 and ###P<0.001 vs. LY294002; &P<0.05, &&P<0.01 and &&&P<0.001 vs. TBMS1. TBMS1, tubeimoside-1; PARP, poly-ADP ribose polymerase; AIF, apoptosis-inducing factor; CDK1, cyclin-dependent kinase 1.
Discussion
As a common intracranial tumor, gliomas account for ~40% of intracranial tumors (15). Despite recent advances in the surgical and medical treatment of glioma, the prognosis of patients with malignant glioma is still poor (16). Previous research has revealed that a traditional Chinese herb, TBMS1, inhibits proliferation and promotes apoptosis in U251 glioma cancer cells (9). This apoptotic response is associated with the regulation of the expression of the Bcl-2 gene family and the mitochondrial pathway of apoptosis by inducing intracellular reactive oxygen species (ROS) (9). Based on previous research, the present study further explored changes in the PI3K/Akt pathway proteins that regulate apoptosis and changes in related pathways (9). The development and progression of glioma is usually associated with molecular changes in the PI3K/Akt signaling pathway (17), which is responsible for a variety of biological processes, including cell metabolism, survival, cell cycle progression, regulation of apoptosis, protein synthesis and genomic instability (18). It is an important intracellular pathway which is frequently aberrantly activated in various types of human cancer (19). Therefore, targeting the PI3K/Akt pathway can have implications for the treatment of numerous cancer types (20). Akt is a downstream protein of PI3K, a lipid kinase consisting of a catalytic subunit (21). The Akt protein contains two phosphorylation sites, Thr308 and Ser473, whose activation requires phosphorylation by the phosphoinositide-dependent kinase 1 and rapamycin-insensitive complex (21). Thr308, located in the core activation region of the catalytic protein kinase, and Ser473, located in the C-terminal hydrophobic motif, can fully activate Akt (22). Akt phosphorylation is widely considered as a marker for the activation of the PI3K/Akt signaling pathway (23). Aberrant activation of the PI3K/Akt signaling pathway has been reported in gliomas (23). The present study demonstrated that TBMS1 inhibited the viability of U251 cells and the phosphorylation of Akt. LY294002 demonstrated the same effect. In addition, the combination of TBMS1 and LY294002 enhanced these inhibitions. These results demonstrated that TBMS1 can inhibit human glioma cell activity through Akt phosphorylation.
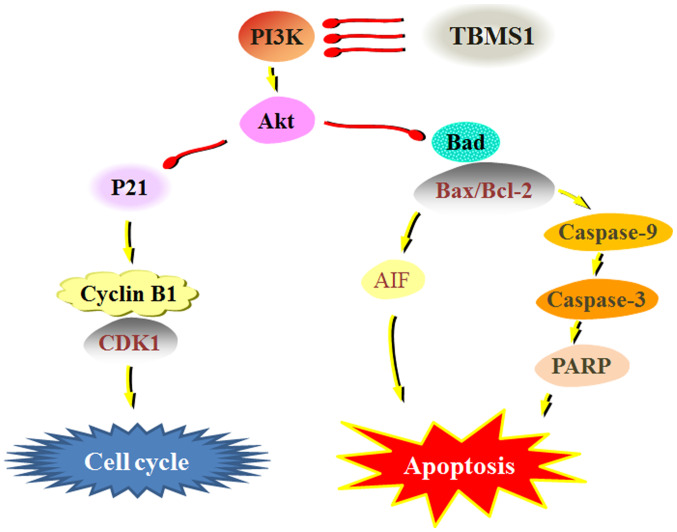
Progression through the cell cycle in normal cells is regulated through various checkpoints during cell division and impaired regulation of checkpoints may lead to uncontrolled cell proliferation (24). Cell cycle dysregulation is the basis for characterizing abnormal cell proliferation in cancer (25). It has been reported that maintaining proper cell cycle progression is an effective therapeutic strategy to prevent tumor growth (25). In the present study, BrdU incorporation and flow cytometry demonstrated that TBMS1 could inhibit DNA synthesis and lead to the accumulation of U251 cells in the G2/M phase in a concentration-dependent manner. The cell cycle control system is based on two major protein families, the cyclins and CDKs (26). Typically, the expression levels of cyclins are dynamic, while CDKs act as catalytic subunits and stabilize the expression levels of the cyclins (26). During the transition from G2 to M phase, cyclin B1 activates CDK1, resulting in an increase in activated CDK1 (26). In glioma cells, the constitutive activation of the PI3K/Akt signaling cascade and a decrease in p21 protein expression levels are common, leading to flawed cell cycle progression (27). The present study indicated that TBMS1 decreased cyclin B1, CDK1 and Akt phosphorylation levels, and increased the levels of the CKD1 inhibitor p21. Therefore, the PI3K/Akt/p21/CDK1/cyclinB1 signaling pathways were involved in TBMS1-induced glioma cell cycle G2/M arrest (Fig. 5).
Figure 5.
Mechanisms of TBMS1 allow it to suppress glioma cells via the PI3K/Akt signaling pathways. TBMS1, tubeimoside-1; PARP, poly-ADP ribose polymerase; AIF, apoptosis inducing factor; CDK1, cyclin-dependent kinase 1.
Apoptosis defects play an important role in the pathogenesis of tumors: The cytotoxic effects of a number of antitumor drugs are usually accompanied by the induction of apoptosis (28). The present study demonstrated that TBMS1 induced apoptosis, as revealed by the typical morphologic features such as clear chromatin condensation, nuclear fragmentation and apoptotic bodies in the treated U251 cells. Cell apoptosis signaling pathways are classified into the death receptor (extrinsic) pathway and the mitochondrial (intrinsic) pathway (29). The Bcl-2 family, including anti-apoptotic components such as Bcl-2 and Bcl-xL, and pro-apoptotic components such as Bax, Bak and Bad, are involved in the regulation of apoptosis through the mitochondrial pathway (30). The promotion of Bad expression and the increase in the Bax/Bcl-2 ratio are associated with apoptosis in a variety of human cancer types (31). The ratio of Bax/Bcl-2 is involved in the release of AIF and endonuclease G from mitochondria, also referred to as the caspase-independent pathway (32). Mitochondria-dependent signaling occurs through the cleavage of caspase-9, which subsequently activates downstream caspase-3, leading to the cleavage of various key cellular substrates (including PARP), thereby inducing apoptosis (33). Previous studies have demonstrated that TBMS1 exerts anti-growth and induces apoptotic activity against cancer cells by activating caspases and Bcl-2 family proteins (34,35). The present study demonstrated that after treatment with TBMS1, p-Akt, AIF, Bad, caspase-3, caspase-8 and caspase-9 expression levels were significantly decreased and that the Bax/Bcl-2 ratio was increased, indicating that TBMS1 promoted cell death. Inhibition of Akt phosphorylation results in downregulation of Akt-regulated anti-apoptotic proteins, thereby promoting the death of apoptotic cells (36). Inhibition of interference with Akt phosphorylation has been revealed to be sufficient to promote mitochondrial membrane depolarization and apoptosis in numerous types of cancer cells (17). To further explore the relationship between apoptotic regulator proteins and Akt phosphorylation, glioma cell lines were treated with TBMS1, LY294002 or a combination treatment of the two drugs. The results demonstrated that the PI3K/Akt inhibitor, LY294002, reduced Akt phosphorylation and AIF expression, and also increased Bad, caspase-3, caspase-8 and caspase-9 expression levels as well as the Bax/Bcl-2 ratio. TBMS1 and LY294002 enhanced these responses. These data revealed that the mechanism of action by which TBMS1 triggered mitochondria-mediated apoptosis may be through the PI3K/Akt signaling pathway.
Collectively, TBMS1 exhibited an inhibitory effect on cellular growth, promoted apoptosis and induced cell cycle arrest by suppressing the PI3K/Akt-mediated signaling pathways in glioma cells. This provides the rationale for in vivo studies on the utilization of TBMS1 as a potential cancer therapeutic compound.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
LJC and ZS contributed to the conception and design of the study. HTX and ZXC performed experimental procedures. YM and MMW conducted data analysis. LJC and ZS were involved in revising the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Diamandis P, Aldape K. World Health Organization 2016 Classification of Central Nervous System Tumors. Neurol Clin. 2018;36:439–447. doi: 10.1016/j.ncl.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Shi J, Dong B, Cao J, Mao Y, Guan W, Peng Y, Wang S. Long non-coding RNA in glioma: Signaling pathways. Oncotarget. 2017;8:27582–27592. doi: 10.18632/oncotarget.15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: From molecular pathology to targeted treatment. Annu Rev Pathol. 2014;9:1–25. doi: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- 4.Olar A, Aldape KD. Using the molecular classification of glioblastoma to inform personalized treatment. J Pathol. 2014;232:165–177. doi: 10.1002/path.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu L, Ma R, Wang Y, Nishino H. Potent anti-tumor activity and low toxicity of tubeimoside 1 isolated from Bolbostemma paniculatum. Planta Med. 1994;60:204–208. doi: 10.1055/s-2006-959459. [DOI] [PubMed] [Google Scholar]
- 6.Zhang XH, Sun NX, Guo RX, Xing JL, Liu XN. Efficacy research of tubeimoside against the experimental herpes simplex keratitis. Rec Adv Ophthalmol. 2002;22:373–376. [Google Scholar]
- 7.He D, Huang B, Fu S, Li Y, Ran X, Liu Y, Chen G, Liu J, Liu D, Tubeimoside I. Tubeimoside I protects dopaminergic neurons against inflammation-mediated damage in lipopolysaccharide (LPS)-evoked model of Parkinson's disease in rats. Int J Mol Sci. 2018;19:E2242. doi: 10.3390/ijms19082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam MS, Wang C, Zheng J, Paudyal N, Zhu Y, Sun H. The potential role of tubeimosides in cancer prevention and treatment. Eur J Med Chem. 2019;162:109–121. doi: 10.1016/j.ejmech.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Jia G, Wang Q, Wang R, Deng D, Xue L, Shao N, Zhang Y, Xia X, Zhi F, Yang Y. Tubeimoside-1 induces glioma apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome C/Caspase-3 pathway. Onco Targets Ther. 2015;8:303–311. doi: 10.2147/OTT.S76063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu T, Cui H, Xu Y, Du Q, Zhao E, Cao J, Nie L, Fu G, Ren A. The effect of tubeimoside-1 on the proliferation, metastasis and apoptosis of oral squamous cell carcinoma in vitro. OncoTargets Ther. 2018;11:3989–4000. doi: 10.2147/OTT.S164503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang SL, Guan YD, Chen XS, Ge P, Wang XL, Lao YZ, Xiao SS, Zhang Y, Yang JM, Xu XJ, et al. Tubeimoside-1, a triterpenoid saponin, induces cytoprotective autophagy in human breast cancer cells in vitro via Akt-mediated pathway. Acta Pharmacol Sin. 2019;40:919–928. doi: 10.1038/s41401-018-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baryawno N, Sveinbjörnsson B, Eksborg S, Chen CS, Kogner P, Johnsen JI. Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma growth. Cancer Res. 2010;70:266–276. doi: 10.1158/0008-5472.CAN-09-0578. [DOI] [PubMed] [Google Scholar]
- 13.Shi H, Bi H, Sun X, Dong H, Jiang Y, Mu H, Li W, Liu G, Gao R, Su J. Tubeimoside-1 inhibits the proliferation and metastasis by promoting miR-126-5p expression in non-small cell lung cancer cells. Oncol Lett. 2018;16:3126–3134. doi: 10.3892/ol.2018.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastav AK, Dubey D, Chopra D, Singh J, Negi S, Mujtaba SF, Dwivedi A, Ray RS. Oxidative stress-mediated photoactivation of carbazole inhibits human skin cell physiology. J Cell Biochem. 2020;121:1273–1282. doi: 10.1002/jcb.29360. [DOI] [PubMed] [Google Scholar]
- 15.Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist. 2006;11:681–693. doi: 10.1634/theoncologist.11-6-681. [DOI] [PubMed] [Google Scholar]
- 16.Bush NA, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 2017;40:1–14. doi: 10.1007/s10143-016-0709-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Gao W, Zhang L, Zhang D, Zhao Z, Bao Y. Deoxypodophyllotoxin inhibits cell viability and invasion by blocking the PI3K/Akt signaling pathway in human glioblastoma cells. Oncol Rep. 2019;41:2453–2463. doi: 10.3892/or.2019.7016. [DOI] [PubMed] [Google Scholar]
- 18.Ebrahimi S, Hosseini M, Shahidsales S, Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM, Avan A. Targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer. Curr Med Chem. 2017;24:1321–1331. doi: 10.2174/0929867324666170206142658. [DOI] [PubMed] [Google Scholar]
- 19.Yang Q, Jiang W, Hou P. Emerging role of PI3K/AKT in tumor-related epigenetic regulation. Semin Cancer Biol pii. 2019 doi: 10.1016/j.semcancer.2019.04.001. S1044-579X(18)30136-6. [DOI] [PubMed] [Google Scholar]
- 20.Fleischer A, Ghadiri A, Dessauge F, Duhamel M, Rebollo MP, Alvarez-Franco F, Rebollo A. Modulating apoptosis as a target for effective therapy. Mol Immunol. 2006;43:1065–1079. doi: 10.1016/j.molimm.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Wu C, Chen N, Gu H, Yen A, Cao L, Wang E, Wang L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7:33440–33450. doi: 10.18632/oncotarget.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manning BD, Toker A. AKT/PKB signaling: Navigating the network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiman KG, Zhivotovsky B. Understanding cell cycle and cell death regulation provides novel weapons against human diseases. J Intern Med. 2017;281:483–495. doi: 10.1111/joim.12609. [DOI] [PubMed] [Google Scholar]
- 25.Williams GH, Stoeber K. The cell cycle and cancer. J Pathol. 2012;226:352–364. doi: 10.1002/path.3022. [DOI] [PubMed] [Google Scholar]
- 26.Arellano M, Moreno S. Regulation of CDK/cyclin complexes during the cell cycle. Int J Biochem Cell Biol. 1997;29:559–573. doi: 10.1016/S1357-2725(96)00178-1. [DOI] [PubMed] [Google Scholar]
- 27.Yang R, Yi L, Dong Z, Ouyang Q, Zhou J, Pang Y, Wu Y, Xu L, Cui H. Tigecycline inhibits glioma growth by regulating miRNA-199b-5p-HES1-AKT pathway. Mol Cancer Ther. 2016;15:421–429. doi: 10.1158/1535-7163.MCT-15-0709. [DOI] [PubMed] [Google Scholar]
- 28.Robertson JD, Orrenius S. Molecular mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev Toxicol. 2000;30:609–627. doi: 10.1080/10408440008951122. [DOI] [PubMed] [Google Scholar]
- 29.Creagh EM. Caspase crosstalk: Integration of apoptotic and innate immune signalling pathways. Trends Immunol. 2014;35:631–640. doi: 10.1016/j.it.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Kvansakul M, Hinds MG. Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis. 2013;4:e909. doi: 10.1038/cddis.2013.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soriano ME, Scorrano L. The interplay between BCL-2 family proteins and mitochondrial morphology in the regulation of apoptosis. Adv Exp Med Biol. 2010;687:97–114. doi: 10.1007/978-1-4419-6706-0_6. [DOI] [PubMed] [Google Scholar]
- 32.Forbes-Hernández TY, Giampieri F, Gasparrini M, Mazzoni L, Quiles JL, Alvarez-Suarez JM, Battino M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem Toxicol. 2014;68:154–182. doi: 10.1016/j.fct.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: Intricate mechanisms and dynamic machinery. Cell Signal. 2011;23:1534–1545. doi: 10.1016/j.cellsig.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Xu X, He P. Tubeimoside-1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549 cells. Mol Med Rep. 2011;4:25–29. doi: 10.3892/mmr.2010.379. [DOI] [PubMed] [Google Scholar]
- 35.Chen WJ, Yu C, Yang Z, He JL, Yin J, Liu HZ, Liu HT, Wang YX. Tubeimoside-1 induces G2/M phase arrest and apoptosis in SKOV-3 cells through increase of intracellular Ca2+ and caspase-dependent signaling pathways. Int J Oncol. 2012;40:535–543. doi: 10.3892/ijo.2011.1218. [DOI] [PubMed] [Google Scholar]
- 36.Chao Y, Wang Y, Liu X, Ma P, Shi Y, Gao J, Shi Q, Hu J, Yu R, Zhou X. Mst1 regulates glioma cell proliferation via the AKT/mTOR signaling pathway. J Neurooncol. 2015;121:279–288. doi: 10.1007/s11060-014-1654-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.