Abstract
Pain is one of the most common reasons to seek medical attention and chronic pain is a worldwide epidemic. There are currently no relevant biomarkers for the diagnosis of chronic pain, and new therapeutic strategies for chronic pain treatment are desperately needed. The chronic constriction injury (CCI) of the sciatic nerve is a widely used preclinical model of pathological neuropathic pain. Over the past decade, investigators have come to appreciate the many contributions of noncoding RNA including microRNA (miRNA), and other long and short noncoding (nc) RNAs. The development and/or maintenance of chronic pain could be controlled epigenetically through ncRNAs. Here we seek to characterize CNS tissues in a mouse model of neuropathic pain as this may serve to elucidate potential biomarkers relevant to pathological pain in humans. Male C57BL6/J mice (6 CCI and 6 sham procedure) underwent surgery for sciatic nerve ligation with chromic gut sutures. Following 7 days, mechanical allodynia was quantified using the von Frey assay. Mice were then euthanized for collection of spinal cord and sciatic nerve. cDNA was synthesized to 627 unique mature miRNAs from the total RNA. In the CCI mice that displayed mechanical allodynia, 11 and 125 miRNAs were differentially expressed (i.e., greater than 1.5-fold increase or decrease; P < 0.05) in the spinal cord and sciatic nerve, respectively, as compared to sham controls. Among those differentially expressed miRNAs in the sciatic nerve of CCI mice, the following passed the more stringent Bonfferoni correction: miR-138–3p, miR-138–5p and miR-676–3p, reduced and miR-142–5p, increased. Our data support miRNAs as promising therapeutic targets for the treatment of pathological pain.
Keywords: Noncoding RNA, miRNA, allodynia
Introduction
Pain is one of the most common reasons to seek medical attention and chronic pain is a worldwide epidemic [17]. Under some circumstances, incoming protective nociceptive signaling is prolonged leading to clinical manifestations of pathological neuronal signaling [6, 26]. Examples of such pathological states are hyperalgesia, or “increased pain from a stimulus that normally provokes pain” and allodynia, or “pain due to a stimulus that does not normally provoke pain” as defined by the International Association of the Study of Pain (IASP). The chronic constriction injury (CCI) of the sciatic nerve is a widely used preclinical model of pathological neuropathic pain.
Over the past decade, investigators have come to appreciate the many contributions of noncoding RNA including microRNA (miRNA) and long noncoding RNAs (lncRNAs). While the field is still in its infancy, the development and/or maintenance of chronic pain could be partially controlled epigenetically through ncRNAs. miRNAs such as miR-155 play a role in the regulation of inflammation which is often associated with neuropathic pain [14, 15]. miR-155 directly regulates pain in the mouse CCI model by targeting the suppressor of cytokine signaling 1 (SOCS1) mRNA [22]. miRNAs including miR-1, miR-16 and miR-206 are differentially regulated in several of the important anatomical sites for pain processing, such as the dorsal root ganglion (DRG) and the dorsal horn of the spinal cord under different pain states in mice [12]. Several hundred long noncoding RNAs were differentially expressed in the spinal cord of mice with pain induced by spinal cord ligation [10]. It was recently reported that neuropathic pain is controlled by the miR-183 cluster (miR-182–5p, miR-183–5p and miR-96–5p) [16].
Although numerous publications have examined the role of specific miRNAs due to pathological pain [12, 14, 15], with many reports of changes in the CCI model [8, 21], these reports examine a single miRNA or a small handful of miRNAs with pain. Two reports have used RNA sequencing of miRNAs from RNA isolated from the dorsal horn of the spinal cord in the CCI model [4, 7]. Thus, here we sought to examine the extent to which miRNAs are differentially expressed in the spinal cord and sciatic nerve following CCI by profiling over 600 hundred mature miRNAs.
Materials and Methods
Animals
A total of 12 adult male C57BL/6J mice (18–35 grams, Jackson Laboratory, Bar Harbor, ME) served as subjects in these experiments. Mice were housed four per cage in a temperature (20–22 °C), humidity (55 ± 10 %), and light-controlled (12 hour light/dark; lights on at 0600) AAALAC approved facility, with standard rodent chow and water available ad libitum. All procedures adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain as well as ARRIVE Guidelines, [11] and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Florida.
Chronic constriction injury (CCI) surgery
Following baseline behavioral assessment, the surgical procedure for chronic constriction of the sciatic nerve was completed as previously described [9]. In brief, the mice were anesthetized with isoflurane (induction 5% vol. followed by 2.0% in oxygen), and the mid to lower back and the dorsal left thigh were shaved and cleaned with 75% ethanol. Using aseptic procedures, the sciatic nerve was carefully isolated, and loosely ligated with three segments of 5–0 chromic gut sutures (Ethicon, Somerville, NJ). Sham surgery was identical to CCI surgery, but without the loose nerve ligation. The overlying muscle was closed with (1) 4–0 sterile silk suture (Ethicon, Somerville, NJ), and animals recovered from anesthesia within approximately 5 min. Use of chromic gut sutures in this model has been well characterized to produce bilateral allodynia, as well as robust upregulation of bilateral markers of inflammation in the dorsal horn of the spinal cord and the corresponding dorsal root ganglia [23, 24].
Assessment of allodynia
The mice were placed inside ventilated polycarbonate chambers on an elevated aluminum mesh table and allowed to acclimate to the apparatus for 60 min before testing. Pre-surgical baseline measurements and mechanical allodynia was assessed with von Frey filaments (North Coast Medical, Morgan Hill, CA), using the “up-down” method [5] and again 7 days after CCI or sham surgery. The plantar surface of each hind paw was stimulated five times with each filament (0.16 – 4.0 g), starting with the 0.6 g filament and increasing until the mouse responded by licking and/or lifting the paw off the surface of the test apparatus. Three or more responses out of five stimulations were coded as a positive response. Once a positive response was detected, sequentially lower weight filaments were used to assess the sensory threshold for each paw. As this CCI model is well-characterized to produce bilateral allodynia, both left and right hindpaws were tested post-surgery, and were analyzed together.
Tissue collection, cDNA generation, and qPCR analysis
After behavioral testing on day 7 post surgery, mice were then euthanized for collection of the region of the lumbar (L) spinal cord that the sciatic nerve innervates, L4-L6, ipsilateral sciatic nerve from the site of surgery. Trizol reagent was used to isolate between 10 and 20 μg of total RNA from the spinal cord and sciatic nerve. cDNA was synthesized to 627 unique mature miRNAs from the total RNA using miRNA specific stem loop primers [20]. qPCR (TaqMan™ MicroRNA Assays Thermo) was performed in 384 well plates [19] to quantify the miRNAs on total RNA isolated from the spinal cord or sciatic nerve of six sham and six CCI mice. One set of 384 wells plates were used per mouse per tissue sample (i.e. single technical replicates for screening). For the miRNA expression profiling experiments, normalization was performed against the mean expression value of all expressed miRNAs (CT ≤ 36) [3]. For the qPCR validation, the cDNA from the profiling experiment was amplified using triplicate qPCRs per mouse tissue, data was normalized to miR-29a-3p and data are presented as the fold change using the formula 2-ΔΔCT. Where ΔΔCT = (CTmiRNA-CmiR-29a)CCI - (CTmiRNA-CmiR-29a)Sham [13].
Data Analysis
Behavioral data were analyzed using an unpaired T test for pre-surgical analysis, and two-way ANOVA for pre- and post-surgical analysis. Bonferroni’s test was used for post hoc analysis following a significant two-way ANOVA. A P-value of < 0.05 was considered statistically significant. The computer program GraphPad Prism version 6.0 (GraphPad Software Inc., San Diego, CA, USA) was used in the statistical analyses of behavior. Principal component analysis (PCA) was generated using the prcomp command in the R (version 3.6) package [21] using the statistics from qPCR relative expression values. Difference in the clustering of the two group’s samples based on their miRNA expression was tested using an unpaired t-test in R [18, 25]. Briefly, we compared the distances between the two groups using either PCA axis 1 or axis 2 (i.e. we only tested the first two components because they explain most of the variance between those samples). Data are expressed as mean ± SD as mentioned in the figure legend.
Results
Figure 1 shows that CCI induces allodynia and alters spinal as well as sciatic nerve miRNA. Prior to surgery, both groups of mice had similar mechanical stimulus threshold values (P = 0.72). CCI produced robust allodynia 7 days after surgery, (main effect of surgery, F(1,22) = 20.46, P < 0.001; main effect of time, F(1,22) = 40.10, P < 0.0001; main effect of interaction, F(1,22) 14.08, P < 0.05, Figure 1A). Immediately after day 7 behavioral testing mice were sacrificed, spinal cord and sciatic nerves were collected and analyzed. We found that CCI alters sciatic nerve miRNA expression, with PCA analysis of the ipsilateral sciatic nerve from the profiling of 627 different miRNAs revealing P = 1.856e-05 (Figure 1B). CCI alters L4-L6 of spinal cord miRNA expression as well however the P value, though statistically significant, was higher than in the sciatic nerve data P = 0.024 (Figure 1C).
Figure 1.
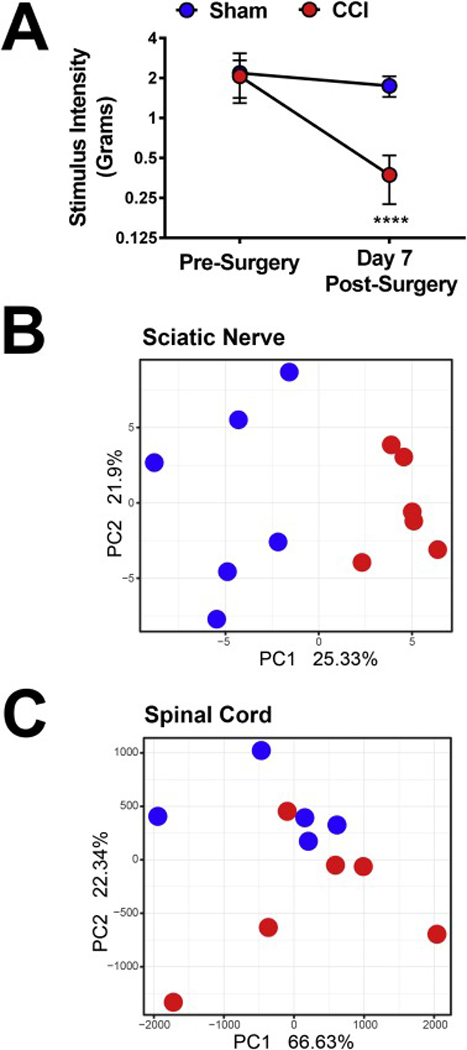
CCI induces allodynia and alters spinal as well as sciatic nerve miRNA. A.) Mechanical stimulus intensity thresholds were determined immediately before and 7 days after CCI surgery. Compared to pre-surgical baselines, CCI induces mechanical allodynia. B.) PCA analysis reveals that CCI alters spinal cord (p-value = 0.02349) and C.) Sciatic nerve (p-value = 1.86×10−5) miRNA 7 days after surgery. **** P < 0.0001 vs. sham. Data reflect mean ± SD, n = 6 mice per group.
Figures 2 and 3 show the volcano plots of the up- and down-regulated miRNAs in the L4-L6 spinal cord and sciatic nerve, respectively, due to CCI surgery. In behaviorally verified CCI mice that displayed mechanical allodynia, 11 and 125 miRNAs were differentially expressed (i.e., greater or less than 1.5-fold change; P < 0.05) in the spinal cord and sciatic nerve, respectively, as compared to sham controls. Of these differentially expressed miRNAs in spinal cord and sciatic nerve, only 4 passed the more stringent Bonferroni correction. These include miR-138–5p, miR-138–3p, miR-142–5p and miR-676–3p in sciatic nerve (Figure 3). Independent qPCR validation of these 4 miRNAs support the profiling data (Figure 4). We were interested in the miR-183 gene cluster, as recent studies show that this gene cluster controls more than 80% of neuropathic pain-regulated genes [16]. The miR-183 gene cluster (miR-183–5p miR-182–5p and miR-96–5p) are down-regulated in CCI-injured sciatic nerve 7 days following surgery (Figures 3–4). miRNAs from the miR-183 cluster were not differentially expressed with CCI in the spinal cord (Figure 2).
Figure 2.
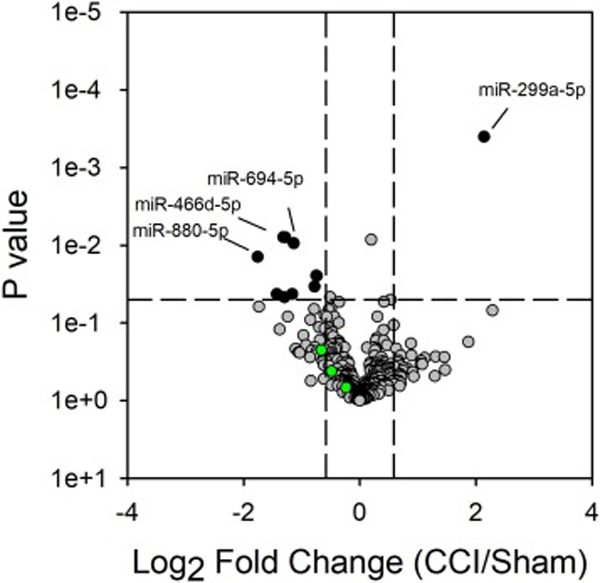
CCI produces alterations in spinal cord miRNA expression. Volcano plot of gene expression from profiling mature miRNAs by qPCR. The Log2 of the fold change in expression between the CCI and sham treated mice are shown. Grey data points represent those miRNAs with fold change less than or greater than 1.5 and P < 0.05. Green data points represent the three miRNAs of the miR-183 cluster (miR-182–5p, miR-183–5p and miR-96–5p).
Figure 3.
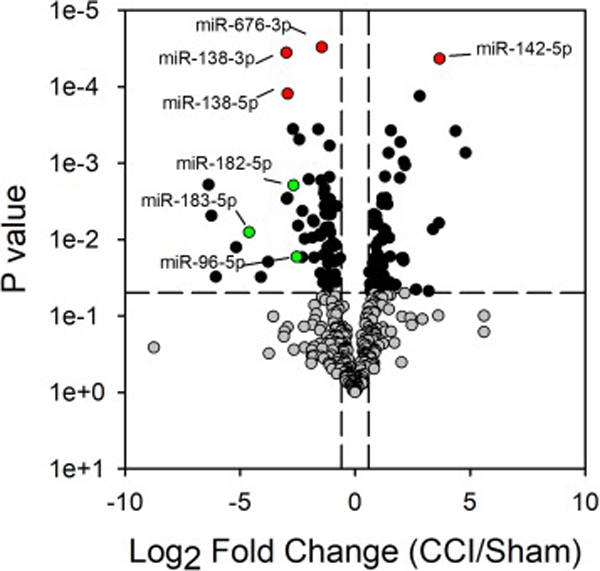
CCI produces alterations in sciatic nerve miRNA expression. Volcano plot of gene expression from profiling mature miRNAs by qPCR. The Log2 of the fold change in expression between the CCI and sham treated mice are shown. Grey, green and red data points represent those miRNAs with fold change less than or greater than 1.5 and P < 0.05. Red data points are those miRNAs that passed the Bonferoni correction. Green data points represent the three miRNAs of the miR-183 cluster.
Figure 4.
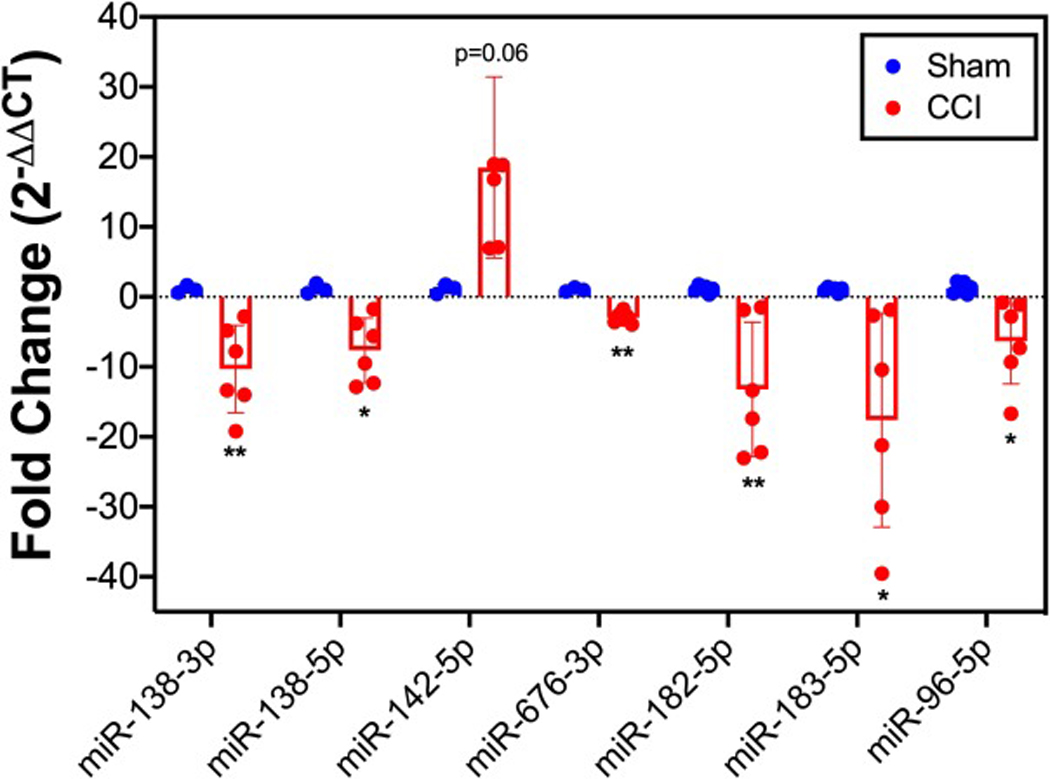
Differentially expressed miRNAs in sciatic nerve of CCI mice. qPCR validation of the expression of miR-138–5p, miR-138–3p, miR-142–5p, miR-676–3p, miR-182–5p, miR-183–5p and miR-96–5p are reported for the sciatic nerve of both sham and CCI mice. Data are normalized to miR-29a-3p and are presented as the fold change relative to sham treated mice. Data reflect mean ± SD. ** P < 0.01, * P < 0.05.
Discussion
Here we show that miRNA in the sciatic nerve is more dynamically regulated than miRNA in the spinal cord after CCI surgery. This is not surprising, as the sciatic nerve is the site of injury, meanwhile the spinal cord is distal to the site of injury. One important caveat of this study is that here we analyzed both dorsal and ventral horn together. Although it is common after CCI for alterations to be seen in both dorsal and ventral spinal cord, these alterations might not be observed at the same magnitude. Thus, it is a possibility that these data mask more discrete changes at the level of the dorsal horn of the spinal cord and explain why we do not observe as many differentially expressed miRNAs in the spinal cord (Figure 2). Additionally, here we only examined male mice after CCI surgery, and thus these results may not be generalizable to results obtained in a similar study of female mice.
We found that CCI alters sciatic nerve miRNA expression, with PCA analysis of the ipsilateral sciatic nerve from the profiling of 627 different miRNAs revealing P (PC1) = 1.856e-05 (Figure 1C). CCI alters L4-L6 of spinal cord miRNA expression as well however the P value, though statistically significant, was higher than in the sciatic nerve data P (PC2) = 0.024 (Figure 1B). Several of the differentially expressed miRNAs in our study have been previously reported with pain. miR-138 was reduced in sciatic nerve injury-induced neuropathic pain and upregulation of the miRNA alleviated the pain by inhibiting proinflammatory cytokine production [27]. The miR-183 cluster (miR-182–5p, miR-183–5p and miR-96–5p) were reduced in our study (Figure 4) and while our results are confirmatory of the literature [16], to our knowledge, this is the first description of reduced miR-183 family member expression in the sciatic nerve of the CCI model. In addition, a non-biased deep sequencing study that examined rat spinal cord tissue found time-dependent decreases in several miRNAs which were expressed 4 hours after CCI surgery, with only mir-720 RNA remaining significantly decreased 24 hours after CCI surgery [2]. Other non-biased deep sequencing studies of rat dorsal root ganglia tissue found that 3 days after spinal nerve ligation significant alterations in the miRNAs miR-30d-5p, miR-125b-5p and miR-379–5p [1]. These findings in combination with our own suggest it is likely that there is a temporal and spatial impact of miRNA expression after nerve injury which can be leveraged in clinically relevant manners.
This report expands upon the existing literature in favor of miRNA changes as an underlying mechanism in the development and maintenance of pathological pain. Further, here we sought to characterize the full scope of changes that occur in the mouse CCI model of neuropathic pain, both at the site of injury (i.e., the sciatic nerve) and the spinal cord, a key area known to mediate pathological pain processing, in behaviorally verified animals expressing mechanical allodynia. If similar miRNA changes also occur in patient populations within easy-to-obtain patient samples, miRNAs may be used as biomarkers to identify subpopulations of patients with neuropathic pain. Further, targeting miRNAs may hold promise for the treatment of pathological pain.
Highlights.
In the mouse CCI model, 125 miRNAs are differentially expressed in the sciatic nerve
The differentially expressed miRNAs in sciatic nerve include miR-138 (decreased), miR-142–5p (increased) and the three members of the miR-183 cluster (decreased)
Eleven miRNAs are differentially expressed in the spinal cord of mice with CCI
Acknowledgements
Funding: This work was supported by the National Institutes of Health grant number DA25267. RZG was supported by UF Health Cancer Center funds. JKB was supported by NCI fellowship F31CA220937.
Abbreviations
- (CNS)
central nervous system
- CCI
Chronic constriction injury
- (DRG)
dorsal root ganglion
- L
lumbar
- lncRNA
long non-coding RNA
- ncRNA
non-coding RNA
- miRNA
microRNA
- PCA
principal component analysis
- (SOCS1)
suppressor of cytokine signaling 1
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bali KK, Hackenberg M, Lubin A, Kuner R, Devor M, Sources of individual variability: miRNAs that predispose to neuropathic pain identified using genome-wide sequencing, Molecular pain 10 (2014) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brandenburger T, Castoldi M, Brendel M, Grievink H, Schlosser L, Werdehausen R, Bauer I, Hermanns H, Expression of spinal cord microRNAs in a rat model of chronic neuropathic pain, Neurosci Lett 506 (2012) 281–286. [DOI] [PubMed] [Google Scholar]
- [3].Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT, The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments, Clin Chem 55 (2009) 611–622. [DOI] [PubMed] [Google Scholar]
- [4].Cao S, Yuan J, Zhang D, Wen S, Wang J, Li Y, Deng W, Transcriptome Changes In Dorsal Spinal Cord Of Rats With Neuropathic Pain, J Pain Res 12 (2019) 3013–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL, Quantitative assessment of tactile allodynia in the rat paw, Journal of neuroscience methods 53 (1994) 55–63. [DOI] [PubMed] [Google Scholar]
- [6].Costigan M, Scholz J, Woolf CJ, Neuropathic pain: a maladaptive response of the nervous system to damage, Annu Rev Neurosci 32 (2009) 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Genda Y, Arai M, Ishikawa M, Tanaka S, Okabe T, Sakamoto A, microRNA changes in the dorsal horn of the spinal cord of rats with chronic constriction injury: A TaqMan(R) Low Density Array study, Int J Mol Med 31 (2013) 129–137. [DOI] [PubMed] [Google Scholar]
- [8].Grace PM, Strand KA, Galer EL, Maier SF, Watkins LR, MicroRNA-124 and microRNA-146a both attenuate persistent neuropathic pain induced by morphine in male rats, Brain Res 1692 (2018) 9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ignatowska-Jankowska B, Wilkerson JL, Mustafa M, Abdullah R, Niphakis M, Wiley JL, Cravatt BF, Lichtman AH, Selective monoacylglycerol lipase inhibitors: antinociceptive versus cannabimimetic effects in mice, The Journal of pharmacology and experimental therapeutics 353 (2015) 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jiang BC, Sun WX, He LN, Cao DL, Zhang ZJ, Gao YJ, Identification of lncRNA expression profile in the spinal cord of mice following spinal nerve ligation-induced neuropathic pain, Molecular pain 11 (2015) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research, Journal of pharmacology & pharmacotherapeutics 1 (2010) 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kusuda R, Cadetti F, Ravanelli MI, Sousa TA, Zanon S, De Lucca FL, Lucas G, Differential expression of microRNAs in mouse pain models, Molecular pain 7 (2011) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods 25 (2001) 402–408. [DOI] [PubMed] [Google Scholar]
- [14].O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D, MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development, Immunity 33 (2010) 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D, MicroRNA-155 is induced during the macrophage inflammatory response, Proc Natl Acad Sci U S A 104 (2007) 1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Peng C, Li L, Zhang MD, Bengtsson Gonzales C, Parisien M, Belfer I, Usoskin D, Abdo H, Furlan A, Haring M, Lallemend F, Harkany T, Diatchenko L, Hokfelt T, Hjerling-Leffler J, Ernfors P, miR-183 cluster scales mechanical pain sensitivity by regulating basal and neuropathic pain genes, Science 356 (2017) 1168–1171. [DOI] [PubMed] [Google Scholar]
- [17].C.f.D.C.a. Prevention, Health, United States, 2006, With Special Feature on Pain, Centers for Disease Control and Prevention’s (CDC) National Center for Health Statistics; (2006) 559. [Google Scholar]
- [18].Sanapareddy N, Legge RM, Jovov B, McCoy A, Burcal L, Araujo-Perez F, Randall TA, Galanko J, Benson A, Sandler RS, Rawls JF, Abdo Z, Fodor AA, Keku TO, Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans, The ISME journal 6 (2012) 1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schmittgen TD, Lee EJ, Jiang J, High-throughput real-time PCR, Methods in molecular biology 429 (2008) 89–98. [DOI] [PubMed] [Google Scholar]
- [20].Schmittgen TD, Lee EJ, Jiang J, Sarkar A, Yang L, Elton TS, Chen C, Real-time PCR quantification of precursor and mature microRNA, Methods 44 (2008) 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sun W, Zhang L, Li R, Overexpression of miR-206 ameliorates chronic constriction injury-induced neuropathic pain in rats via the MEK/ERK pathway by targeting brain-derived neurotrophic factor, Neurosci Lett 646 (2017) 68–74. [DOI] [PubMed] [Google Scholar]
- [22].Tan Y, Yang J, Xiang K, Tan Q, Guo Q, Suppression of microRNA-155 attenuates neuropathic pain by regulating SOCS1 signalling pathway, Neurochemical research 40 (2015) 550–560. [DOI] [PubMed] [Google Scholar]
- [23].Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Armijo LM, Kuhn MN, Thakur GA, Makriyannis A, Milligan ED, Intrathecal cannabilactone CB(2)R agonist, AM1710, controls pathological pain and restores basal cytokine levels, Pain 153 (2012) 1091–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Kuhn MN, Zvonok AM, Thakur GA, Makriyannis A, Milligan ED, Immunofluorescent spectral analysis reveals the intrathecal cannabinoid agonist, AM1241, produces spinal anti-inflammatory cytokine responses in neuropathic rats exhibiting relief from allodynia, Brain and behavior 2 (2012) 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, Fleck JS, Voigt AY, Palleja A, Ponnudurai R, Sunagawa S, Coelho LP, Schrotz-King P, Vogtmann E, Habermann N, Nimeus E, Thomas AM, Manghi P, Gandini S, Serrano D, Mizutani S, Shiroma H, Shiba S, Shibata T, Yachida S, Yamada T, Waldron L, Naccarati A, Segata N, Sinha R, Ulrich CM, Brenner H, Arumugam M, Bork P, Zeller G, Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer, Nat Med 25 (2019) 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Woolf CJ, Salter MW, Neuronal plasticity: increasing the gain in pain, Science 288 (2000) 1765–1769. [DOI] [PubMed] [Google Scholar]
- [27].Zhu B, Gao J, Ouyang Y, Hu Z, Chen X, Overexpression Of miR138 Ameliorates Spared Sciatic Nerve Injury-Induced Neuropathic Pain Through The Anti-Inflammatory Response In Mice, J Pain Res 12 (2019) 3135–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]


