Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Helicoverpa zea (Lepidoptera: Noctuidae) (American cotton bollworm, corn earworm) for the EU. H. zea is a polyphagous species that feeds on over 100 plant species. The crops most frequently recorded as host plants are maize, sorghum, cotton, beans, peas, chickpeas, tomatoes, aubergines, peppers and, to a lesser extent, clover, okra, cabbages, lettuces, strawberries, tobacco, sunflowers, cucurbits and ornamentals. H. zea preferentially feeds on flowers and fruits of the host. Eggs are laid mostly on maize silks. Larvae feed on the silks and kernels. Pupation takes place in the soil. Hibernation and estivation as pupa are reported. Adults are nocturnal. H. zea is a strong flier, able to fly up to 400 km during migration. Commission Implementing Regulation (EU) 2019/2072 (Annex IIA) regulates H. zea. Fruits and plants for planting, with and without soil, provide potential pathways for entry into the EU. Climatic conditions and the availability of host plants provide conditions to support establishment in the EU. The introduction of H. zea could have an economic impact in the EU through qualitative and quantitative effects on agricultural production (e.g. tomatoes, soybean, sweet corn). Phytosanitary measures are available to reduce the likelihood of entry. H. zea satisfies the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. H. zea does not meet the criteria of (a) occurring in the EU, and (b) plants for planting being the principal means of spread for it to satisfy the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union regulated non‐quarantine pest.
Keywords: American cotton bollworm, corn earworm, European Union, migration, pest risk, plant health, plant pest, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community established the previous European Union plant health regime. The Directive laid down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union was prohibited, was detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and applied from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorisations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/ pest categorisation is not available.
1.1.2. Terms of reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/20023, to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pest categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocanthus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| urtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 5) Dacus ciliatus Loew | 15) Rhacochlaena japonica Ito |
| 4) Anastrepha suspensa (Loew) | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Heliothis zea (Boddie) is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest (RNQP) for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States (MS) referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
Following the adoption of Regulation (EU) 2016/20314 on 14 December 2019 and the Commission Implementing Regulation (EU) 2019/2072 for the listing of EU regulated pests, the Plant Health Panel interpreted the original request (ToR in Section 1.1.2) as a request to provide pest categorisations for the pests in the Annexes of Commission Implementing Regulation (EU) 2019/20725.
A taxonomic revision by Hardwick (1965) placed Heliothis zea in the genus Helicoverpa. The current valid senior synonym of Helicoverpa zea (Boddie) is used in this opinion.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on H. zea was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name, synonyms and common names of the pest as search terms. Relevant papers were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, 2019) and relevant publications.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission, and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the MS and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for H. zea following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018) and in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
This work was initiated following an evaluation of the EU plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union RNQP in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required in accordance with the specific terms of reference received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a RNQP. If one of the criteria is not met, the pest will not qualify. A pest that does not qualify as a quarantine pest may still qualify as a RNQP that needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone; thus, the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism | Is the pest present in the EU territory? If not, it cannot be a RNQP. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The protected zone system aligns with the pest free area system under the International Plant Protection Convention (IPPC) The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact as regards the intended use of those plants for planting? |
| Available measures (Section 3.6) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential RNQP were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, whereas addressing social impacts is outside the remit of the Panel.
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? (Yes or No)
Yes. The identity is established and taxonomic keys are available for its identification.
Helicoverpa zea (Boddie) (Lepidoptera; Noctuidae) has many common names, including, but not limited to, American cotton bollworm, corn earworm, cotton bollworm, bollworm, tomato fruitworm, soybean podworm and sorghum headworm (CABI ISC, 2020; Olmstead et al., 2016).
This species was originally described as Heliothis umbrosus Grote. Synonyms include Heliothis zea (Boddie), Bombyx obsoleta Fab., Phalaena zea (Boddie) (Smith et al., 1997; CABI ISC, 2020). Hardwick (1965) reviewed the corn earworm species complex and described the new genus Helicoverpa. Some 80 or more species had been formerly placed in Heliothis sensu lato and Hardwick placed 17 species (including 11 new species) in Helicoverpa. Within this new genus, he identified the zea group containing eight species including H. zea (see Hardwick, 1970).
Because the old name of Heliothis, referred to four major and three minor pest species, is so well established in the literature, and since dissection of genitalia is required for identification, there has been resistance to the name change (e.g. Heath & Emmet, 1983), but Hardwick's work is generally accepted and so the name change must also be accepted (see Matthews, 1991).
The EPPO code6 (Griessinger and Roy, 2015; EPPO, 2019) for this species is HELIZE (EPPO GD, 2020 online).
3.1.2. Biology of the pest
H. zea is a very polyphagous pest (see Section 3.4.1). Female fecundity can be dependent upon the quality and quantity of larval food, and also on the quality of adult nutrition. Up to 3,000 eggs have been laid by a single female in captivity, but 1,000–1,500 per female is more usual in nature. Eggs are laid singly, with a pale green colour shortly after oviposition turning to yellow and then grey before hatching, which occurs after 2–4 days (Hardwick, 1965). The tiny grey larvae first eat the egg shell and after a short rest they wander actively for a while before starting to feed on their host plants. Choice of oviposition site by the female seems to be governed by a combination of physical and chemical cues. For example, in maize, one of its preferred hosts, eggs are laid mostly on the silks of maize female inflorescences in small numbers (one to three). Sweet corn silk is very attractive to female H. zea and silk volatiles stimulate them to produce sex pheromones (Raina et al., 1992). However, oviposition can also occur on the upper leaf surface and on the stalk, particularly if silks are not available (Reay‐Jones, 2019). The larvae usually feed on the silks initially and then on the young tender kernels after entering the tip of the husk. Inside the husk, they feed exclusively on corn kernels and do not exit the husk until they prepare for pupation (Waldbauer et al., 1984; Cohen et al., 1988). In cotton, H. zea moths oviposit more frequently within the terminal foliage, or in the top third of the canopy. In other crops, such as tobacco (Nicotiana tabacum L.), H. zea eggs are deposited away from the terminal (Braswell et al., 2019). H. zea larvae prefer to feed on squares, flowers and bolls within a cotton plant, and may feed on these structures throughout the entire plant canopy, regardless of where oviposition occurs (Braswell et al., 2019). The life cycle of H. zea comprises six larval instars. By the third instar the larvae become cannibalistic and usually only one larva survives per cob. Feeding damage is typically confined to the tip of the cob. Butler (1976) cultured earworm on corn at several temperatures, reporting total larval development times of 31.8, 28.9, 22.4, 15.3, 13.6, and 12.6 days at 20.0, 22.5, 25.0, 30.0, 32.0 and 34.0°C, respectively (CABI, ISC). The lower developmental temperature threshold has been calculated as 12.5°C for H. zea reared on sweet corn (Mangat and Apple, 1966). Maximum developmental threshold temperatures for eggs, larvae, pupae, and adults are 34°C, 36°C, 35°C and 42°C, respectively (Butler, 1976). Mangat and Apple (1966) calculated that 690.2 degree‐days are required from oviposition to 75% adult emergence, using a base 12.5°C model. In the final instar (usually sixth) feeding ceases, and the fully fed caterpillar leaves the cob and descends to the ground. It then burrows into the soil for some 10–12 cm and forms an earthen cell, where it rests in a prepupal state for a day or two, before finally pupating. Two basic types of pupal diapause are recognised, one in relation to cold and the other in response to arid conditions. In the tropics pupation takes 13 (10–14) days; the male takes 1 day longer than the female. Adults are nocturnal in habit and emerge in the evenings. They are attracted to light traps (Hardwick, 1968), especially the ultraviolet (UV) light, in company with many other local Noctuidae. Sex pheromones have been identified and synthesised for most of the Heliothis/Helicoverpa pest species, and pheromone traps can be used for population monitoring. Adult longevity is recorded as being about 17 days in captivity; they drink water and feed on nectar from both floral and extra‐floral nectaries. The moths fly strongly and are regular seasonal migrants, flying hundreds of kilometres from the USA into Canada. They migrate by flying high with prevailing wind currents. Landscape‐level movements occur when moths seek nectar or search for mates and oviposition sites (Latheef et al., 1993; Lingren et al., 1993). An adult H. zea sustains its energy by nectar‐feeding, but must do so by visiting flowering plants other than corn. When females are ready to lay eggs, they are attracted to corn silks (Raina et al., 1992). In a short period, H. zea adults can theoretically migrate from a remote location > 400 km away (Westbrook et al., 1997).
The life cycle can be completed in 28–30 days at 25°C, and in the tropics there may be up to 10–11 generations per year. All stages of the insect are to be found throughout the year if food is available, but development is slowed or stopped by either drought or cold. In the northern USA, there are only two generations per year, in Canada only one generation.
3.1.3. Intraspecific diversity
No intraspecific diversity has been reported. However, possible interspecific crosses between H. armigera and H. zea were highlighted by Lopes et al. (2017). Introgression and recombination phenomena could allow the transfer of insecticide resistance genes from H. armigera to H. zea (Leite et al., 2014). Hybridisation may enable the selection of breeds with enhanced hybrid vigor and the ability to rapidly adapt to current management and suppression methods (Lopes et al., 2017).
3.1.4. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, the identity is established, taxonomic keys are available for its identification, although the species is hard to discriminate using only morphological features. Molecular methods have been developed.
Feeding damage is usually visible and the larvae can be seen on the surface of plants but often they are hidden within plant organs such as flowers and fruits. Bore holes may be visible, but otherwise it is necessary to open the plant organs to detect the pest. Based on morphological features, it is not possible to distinguish the larvae of H. zea from those of H. armigera, which is already present in the EU. Positive identification can be done by rearing the larvae and examining the genitalia of the adult males. Differentiating the two species is difficult; adults can only be distinguished by dissection, and larvae cannot be identified to species using morphology, necessitating the use of geographic origin for identification in most instances (Gilligan et al., 2015).
Molecular techniques (e.g. DNA barcoding; restriction fragment length polymorphism, RFLP) have been used to diagnose H. zea and other congeneric species based on variation in the mitochondrial genome (Mastrangelo et al., 2014; Behere et al., 2008). Real‐time polymerase chain reaction (PCR) assays using the internal transcribed spacer region 2 (ITS2) locus as a diagnostic marker were developed for the diagnosis of H. armigera and H. zea larvae and adults (Gilligan et al., 2015; Perera et al., 2015). Diagnostic assays were also developed using Tpi gene as a genetic marker together with CO1 barcoding for congeneric species identification (Nagoshi et al., 2016).
Adults are 20–25 mm in length with 35‐40 mm wing span and have brown (females) to brown‐green (males) colouration (Smith et al., 1997; CABI ISC, 2020) (Figure 1). Small spots are sometimes visible on the forewings, while dark outer‐marginal bands and brown disc‐shaped spots are found on the dorsal surfaces of the underwings (Hardwick, 1965). Eggs are laid singly, have an approximate dimension of 0.5 mm length by 0.5 mm width, and vary in colour from white after they are laid to yellow near larval hatch (CABI ISC, 2020).
Figure 1.

Adult corn earworm (Helicoverpa zea). Wingspan approximately 38 mm, colour varies from light greyish brown to green and brown. (Robert J. Bauernfeind, Kansas State University, Bugwood.org)
First instars are small grey caterpillars, 1–2 mm in length, with a black head capsule. Third instars undergo a change in colour to either brown or green morphs and develop distinct white or yellow longitudinal lines. Fifth and sixth instars change to pink, orange, brown, or green morphs that average 40 mm in length (CABI ISC, 2020) (Figure 2). Pupae are reddish‐brown and are approximately 20 mm in length.
Figure 2.

Corn earworm larva (Helicoverpa zea) near tip of an ear of field corn. (R.L. Croissant, Bugwood.org)
H. zea sex pheromone has been identified and synthesised and pheromone traps can be used for population monitoring (CABI ISC, 2020, Olmstead et al., 2016)
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
Helicoverpa zea is confined to the American continent. It occurs throughout the Americas from Canada to Argentina (CABI ISC, 2020).
Table 2.
Distribution of Helicoverpa zea (Source: EPPO Global database, 2020)
| Continent | Country | Sub‐national area e.g. State | Status |
|---|---|---|---|
| America | Antigua and Barbuda | Present, no details | |
| Argentina | Present, no details | ||
| Bahamas | Present, no details | ||
| Barbados | Present, no details | ||
| Bermuda | Present, no details | ||
| Bolivia | Present, no details | ||
| Brazil | Present, widespread | ||
| Bahia, Ceara, Distrito Federal, Goias, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Para, Parana, Pernambuco, Rio de Janeiro, Rio Grande do Sul, Roraima, Santa Catarina, São Paulo | Present, no details | ||
| Canada | Present, restricted distribution | ||
| British Columbia, Manitoba, New Brunswick, Nova Scotia, Ontario, Québec, Saskatchewan | Present, no details | ||
| Chile | Present, widespread | ||
| Colombia | Present, no details | ||
| Costa Rica | Present, no details | ||
| Cuba | Present, no details | ||
| Dominica | Present, no details | ||
| Dominican Republic | Present, no details | ||
| Ecuador | Present, widespread | ||
| El Salvador | Present, no details | ||
| Falkland Islands | Present, few occurrences | ||
| French Guiana | Present, no details | ||
| Guadeloupe | Present, no details | ||
| Guatemala | Present, no details | ||
| Guyana | Present, no details | ||
| Haiti | Present, no details | ||
| Honduras | Present, no details | ||
| Jamaica | Present, no details | ||
| Martinique | Present, widespread | ||
| Mexico | Present, widespread | ||
| Montserrat | Present, no details | ||
| Nicaragua | Present, no details | ||
| Panama | Present, no details | ||
| Paraguay | Present, widespread | ||
| Peru | Present, no details | ||
| Puerto Rico | Present, no details | ||
| Saint Lucia | Present, no details | ||
| St Kitts‐Nevis | Present, restricted distribution | ||
| St Vincent and the Grenadines | Present, no details | ||
| Suriname | Present, no details | ||
| Trinidad and Tobago | Present, widespread | ||
| United States of America | Present, widespread | ||
| Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, Wyoming | Present, no details | ||
| Uruguay | Present, widespread | ||
| Venezuela | Present, no details | ||
| Virgin Islands (US) | Present, no details |
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
No, the pest is not known to occur in the EU.
The pest is not known to occur in the EU. The Netherlands NPPO declares that H. zea is absent on the basis of surveys (EPPO, 2020).
3.3. Regulatory status
3.3.1. Commission Implementing Regulation 2019/2072
As noted in the interpretation of TOR, Helicoverpa zea is listed in Annex II of Commission Implementing Regulation (EU) 2019/2072 using the synonym Heliothis zea. Details are presented in Tables 3 and 4.
Table 3.
Helicoverpa zea in Commission Implementing Regulation 2019/2072
| Annex II | List of Union quarantine pests and their respective codes |
|---|---|
| Part A | Pests not known to occur in the Union territory |
| C | Insects and mites |
| 33 | Heliothis zea (Boddie) [HELIZE] |
Table 4.
Regulation of maize (preferred host of Helicoverpa zea) regulated in Annex XI of Commission Implementing Regulation (EU) 2019/2074
| Annex XI | List of plants, plant products and other objects subject to phytosanitary certificates and those for which such certificates are not required for their introduction into the Union territory | |
|---|---|---|
| Part A | List of plants, plant products and other objects, as well as the respective third countries of origin or dispatch, for which, pursuant to Article 72(1) of Regulation (EU) 2016/2031 phytosanitary certificates are required for their introduction into the Union territory | |
| Plants, plant products and other objects | CN code and its respective description under Council Regulation (EEC) No 2658/87 | Country of origin or dispatch |
| 3. Parts of plants, other than fruits and seeds, of: | ||
| Zea mays L. |
Other vegetables, fresh or chilled: Sweetcorn: ex 0709 99 60 Maize (corn), other: 1005 90 00 Vegetable products of maize (Zea mays), not elsewhere specified or included, fresh: ex 1404 90 00 |
Third countries other than Switzerland |
3.3.2. Legislation addressing the hosts of Helicoverpa zea
H. zea is a polyphagous pest listed in Annex II A. Therefore, it is banned from introduction into the EU irrespective of the plant where it may be found on. It is not specifically named in relation to specific measures on hosts listed in pertinent Annexes of Commission Implementing Regulation (EU) 2019/2072.
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
H. zea is regarded as being polyphagous. Most hosts are recorded from the family Poaceae, Malvaceae, Fabaceae and Solanaceae; in total more than 100 plant species are recorded as hosts. The crops most frequently recorded as host plants are maize, sorghum, cotton, beans, peas, chickpeas, tomatoes, aubergines, peppers, and, to a lesser extent, clover, okra, cabbages, lettuces, strawberries, tobacco, sunflowers, cucurbits and many of the other legumes. Damage to fruits and to trees has also been recorded. Buds and flowers of a wide range of ornamentals are attacked. Feeding preference is shown for flowers and fruits of the host plant. Most infestations are of field and garden crops, but invasion of greenhouses is recorded and protected crops are clearly at risk (Smith et al., 1997).
The expression of host preference depends upon a complex of factors including spatial and temporal availability of the hosts at the preferred stage of development. Maize and grain sorghum are commonly attacked in most locations, although legumes are widely infested (Kennedy and Storer, 2000; Hardwick, 1965; Johnson et al., 1975). Corn kernels have multiple nutritional components, and H. zea larvae take advantage of these different parts to satisfy their developmental requirements (Waldbauer et al., 1984). For this reason, H. zea will preferentially choose corn over other plant hosts (Johnson et al., 1975) because there is a higher likelihood of completing development (Olmstead et al., 2016). Cotton is a crop particularly susceptible to damage by H. zea but is clearly not a preferred host, because in many places it is only heavily attacked after alternative hosts have senesced or been harvested. Trifolium and other legumes are often important host plants in the spring before the annual crops are established. Crop harvesting may often result in larvae abandoning the field, and then many less desirable hosts may be attacked (trees) (Smith et al., 1997). A variety of uncultivated, weedy plants can also serve as hosts to H. zea (Neunzig, 1963; Hardwick, 1965; Sudbrink and Grant, 1995; Kennedy and Storer, 2000).
Many host crops for H. zea are available in the EU, and especially in the southern part. Many wild host species could act as sources of infestation of commercial crops. Further north in the EU region, greenhouse crops could be attacked.
3.4.2. Entry
Is the pest able to enter into the EU territory? (Yes or No) If yes, identify and list the pathways.
Yes, the pest can enter into the EU territory via cut flowers, fruits, plants for planting with and without soil attached and soil/growing media (closed pathway)
The Europhyt database lists 30 records of interception of H. zea since 1995 (accessed on 7/4/2020). For three interceptions the host plant was not reported in the interception file. Further details on the host plants on which the pest was intercepted, on the country of origin of the consignment and the interception date are provided in Figures 4, 5 and in Appendix B.
Figure 4.
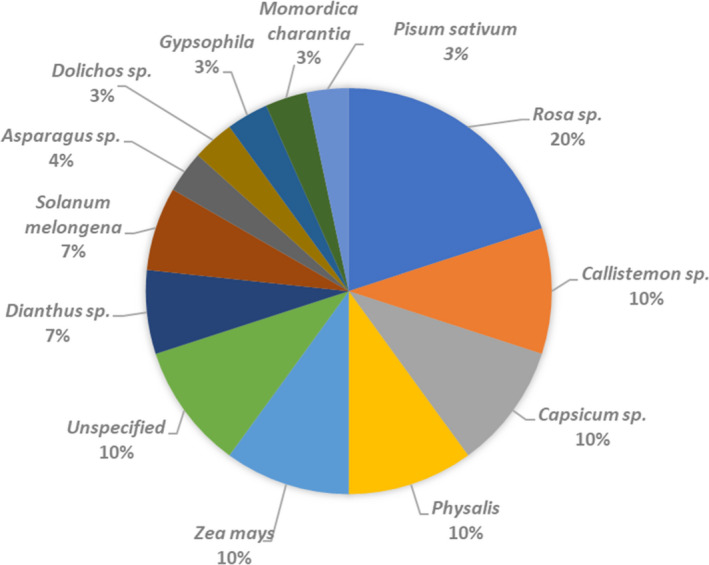
Host plants on which Helicoverpa zea was intercepted (for details, see Appendix B)
Figure 5.
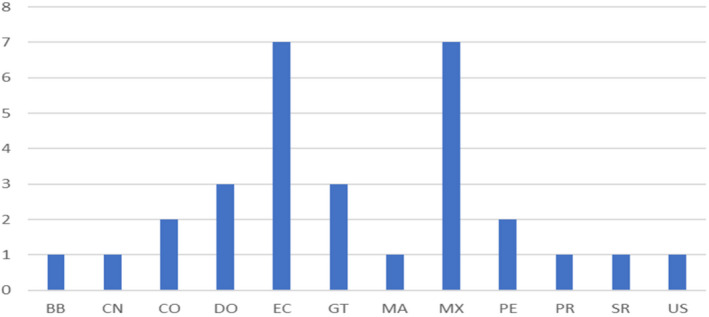
Number of interceptions per country of origin. (BB = Barbados, CN = China, CO = Colombia, DO = Dominican Republic, EC = Ecuador, GT = Guatemala, MA = Morocco, MX = Mexico, PE = Peru, PR = Puerto Rico, SR = Suriname, US = United States of America). The interception from Morocco does not match its distribution
Table 5.
Potential pathways for Helicoverpa zea and existing mitigations (if any)
| Pathways | Life stage | Relevant mitigations [prohibitions (Annex VI) or special requirements (Annex VII)] from third countries |
|---|---|---|
| Plants for planting (excluding seeds) | Adults*, eggs, larvae | |
| Cut flowers and branches with foliage | Adults*, eggs, larvae | |
| Fruits and vegetables | Larvae | |
| Plants for planting already planted (i.e., with soil attached) | Adults*, eggs, larvae and pupae | Annex VII of Regulation 2016/2031 regulates the introduction of soil and growing medium when attached to plants for planting into the Union from third countries other than Switzerland |
| Soil/growing medium | Pupae | Annex VI of Regulation 2016/2031 prohibits the introduction of soil and growing medium as such into the Union from third countries other than Switzerland |
Adults could be hitchhikers or developing during transport.
The soil/growing medium pathway can be considered as closed, as soil from third countries other than Switzerland is banned from entering into the EU (Annex VI), and regulated when attached to plants for planting or machinery (Annex VII). The plants for planting (excluding seeds), cut flowers and branches with foliage pathways are not specifically regulated for this pest.
3.4.3. Establishment
Is the pest able to become established in the EU territory? (Yes or No)
Yes, H. zea could establish in the EU; hosts are widely available and there are areas where environmental conditions are suitable for reproduction and population development
3.4.3.1. EU distribution of main host plants
H zea is a polyphagous plant pest that can feed on over 100 plant species in 4 families. Many potential hosts occur widely over the EU. Cultivated hosts such as maize, cotton and sorghum are grown as field crops with maize occurring widely. Some hosts such as tomatoes and peppers are grown both in the field and in greenhouses as well as in home‐gardens (de Rougemont, 1989). Tables 6 and 7 show the area of key hosts cultivated in the EU in recent years.
Table 6.
EU 28 crop production (2015–2019) of maize (grain maize and corn‐cob‐mix and green maize), sorghum, tomatoes, eggplants, peppers and fresh beans (in 1,000 ha). Source: Eurostat, data extracted on 7/4/2020
| Crop/year | Eurostat code | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| Grain maize and corn‐cob mix | 9,255.56 | 8,563.21 | 8,271.64 | 8,282.57 | 8,904.03 | |
| Green maize | 6,267.95 | 6,256.88 | 6,183.30 | 6,355.91 | : | |
| Sorghum | 139.15 | 123.18 | 135.66 | 150.85 | 197.07 | |
| Tomatoes | V3100 | 254.43 | 247.00 | 241.07 | 239.71 | : |
| Eggplants | V3410 | 22.27 | 21.58 | 20.73 | 21.44 | : |
| Peppers (Capsicum) | V3600 | 58.61 | 57.69 | 57.57 | 56.36 | : |
| Fresh beans* | V5200 | 93.41 | 99.17 | 102.66 | 98.04 | : |
‘:’ Data not available.
Fresh beans include Phaseolus vulgaris which is a major host plant.
Table 7.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways on which interceptions have occurred. Control measures are measures that have a direct effect on pest abundance
| Information sheet title (with hyperlink to information sheet if available) | Control measure summary | Risk component (entry/establishment/spread/impact) |
|---|---|---|
| Growing plants in isolation | To prevent introduction of the pest to the production place, plants could be grown in a dedicated greenhouse | Entry |
| Chemical treatments on consignments or during processing |
Use of chemical compounds that may be applied to plants or to plant products after harvest, during process or packaging operations and storage The treatments addressed in this information sheet are: a) fumigation; b) spraying/dipping pesticides |
Entry |
| Soil treatment | The control of pupae in the soil may be possible with a chemical or physical treatment of the soil | Entry, Impact |
| Controlled atmosphere | Treatment of plants and plant products by storage in a modified atmosphere (including modified humidity, O2, CO2, temperature, pressure) | Entry |
| Crop rotation, associations and density, weed/volunteer control |
Various cultural practices can be used to kill the different instars, including deep ploughing, discing and other methods of mechanical destruction (Smith et al., 1997). Trap crops or push–pull strategies were tested (Olmstead et al., 2016) Corn has been used as the trap crop for H. zea in other crops, including soybean and cotton (Javaid et al., 2005; Lincoln and Isely, 1947) |
Establishment, Impact |
| Timing of planting and harvesting | Differences in sweet corn attraction could be created by manipulating the planting date or using early maturing cultivars. For example, smaller plantings of noncash‐crop sweet corn could be planted in proximity to the large plantings of cash‐crop sweet corn. Earlier plantings of the non‐cash crop corn would produce silks earlier and be more attractive than those in the later plantings of cash‐crop sweet corn, thereby luring ovipositing H. zea away from the cash crop (Olmstead et al., 2016) | Establishment, Impact |
| Heat and cold treatments | Consignments treated by refrigeration for 2‐4 days at 1.7°C followed by chemical fumigation is considered effective against the congeneric H. armigera (Smith et al., 1997) | Entry |
| Use of resistant and tolerant plant species/varieties |
Tolerance, non‐preference, and antibiosis have been identified as mechanisms of resistance to H. zea in corn (Wiseman and Davis, 1990) Insect‐resistant genetically engineered sweet corn and cotton was proved effective alone or in combination with foliar applications of insecticides (Flood et al., 2005; Allen et al., 2019) |
Establishment, spread and impact |
| Biological control and behavioural manipulation |
Other pest control techniques not covered by 1.03 and 1.13
Many insects have been identified in the literature as control agents of H. zea in sweet corn fields. Species of Coccinellidae, Diptera, Hemiptera, and Hymenoptera either parasitise or are predators of eggs and larval stages (Olmstead et al., 2016). The parasitoid Archytas marmoratas (Diptera: Tachinidae) reached a parasitism rate of 58% when adult females were released in fields with low‐density H. zea larval populations (Gross, 1990). Inundative release of T. pretiosum is a potentially more viable management tool for H. zea control in corn (Manandhar and Wright, 2015) A reduction in pest pressure might be possible at the field level where H. zea is a resident, using mating disruption in a coordinated area‐wide effort across the agricultural landscape would have the highest likelihood of area‐wide pest suppression (Cardé and Minks, 1995) |
Establishment and impact |
3.4.3.2. Climatic conditions affecting establishment
Morey et al. (2012) cites Hardwick (1965) when suggesting that pupae of H. zea overwinter south of the 40th parallel in the USA. This may indicate the range of cold tolerance and the area where climate favours permanent establishment. Köppen‐Geiger climate zones (Kottek et al., 2006) that occur south of the 40th parallel in North America and which also occur in the EU, and elsewhere around the world, are shown in Figure 3.
Figure 3.
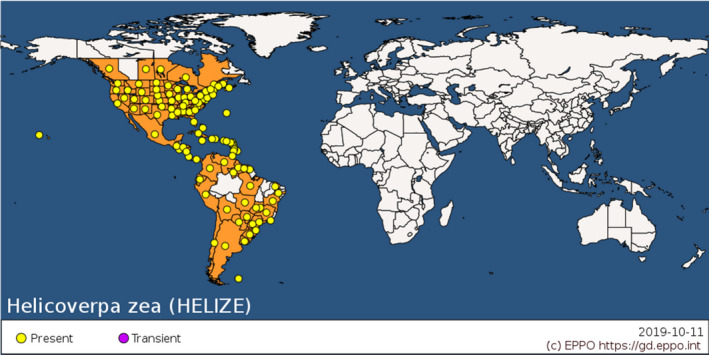
Global distribution of Helicoverpa zea (extracted from the EPPO Global Database accessed on 16/3/2020)
H zea occurs in a number of zones south of the 40th parallel in North America, such as Cfa, Cfb and Cfc. These climate zones also occur in the EU where many hosts are grown. We assume that climatic conditions will not limit the ability of H. zea to establish in the EU.
Figure 6.
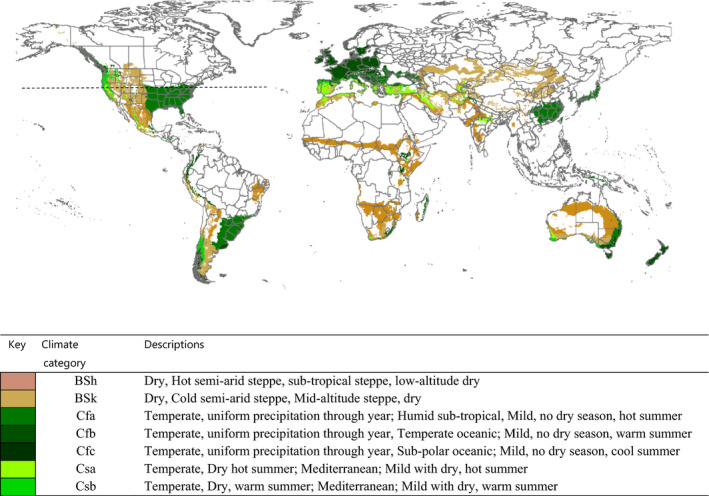
Köppen–Geiger climate types occurring south of the 40th parallel in USA and elsewhere around the world. (40th parallel indicated by broken line) (Map based on MacLeod and Korycinska, 2019)
3.4.4. Spread
Is the pest able to spread within the EU territory following establishment?
Yes, adults are strong migrant fliers
RNQPs: Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects?
No, the spread is mainly via migratory flight of adults.
H. zea can fly long distances. Adults migrate in response to poor local conditions for reproduction when weather conditions are suitable. Migratory flights occur at higher altitudes and may last for several hours. Migratory flights of hundreds of kilometres are common (Smith et al.,1997, CABI ISC, 2020, Westbrook et al., 1998, Sandstrom et al., 2007, Westbrook and Lopez, 2010). Eggs and larvae can be attached to plants or plant parts such as fruits (larvae feed inside fruits and vegetables) and pupae can be found in soil. Therefore, human‐assisted movement of infested host plant material and soil can be an additional means of long‐range dispersal.
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, the introduction of H. zea could have an economic impact in the EU through qualitative and quantitative effects on agricultural production (e.g. tomatoes, soybean, sweet corn)
RNQPs: Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting?
Yes, should H. zea be present in plants for planting, an economic impact on their intended use would be expected
Larvae prefer to feed on reproductive plant structures and growing points. It often attacks valuable crops (Fitt, 1989). Larvae are often found on plant structures such as blossoms, buds and fruits (Hardwick, 1965; Capinera, 2017). Many of the crops attacked are of high value such as cotton, maize and tomatoes. In cotton for example, the pest leads to delayed maturity and reduction of yields in cotton (Gore and Adamczyk, 2004). In maize plants, the pest feeds on ears. As the damage is limited to the tip of the ears, this does not lead to major yield losses in maize when corn is planted within the recommended planting windows in the USA (Reay‐Jones and Reisig, 2014). However, for sweet corn, H. zea is considered a major economic pest species of increasing importance (Olmstead et al., 2016). In addition to impacts on yield, corn earworm injury to ears may provide a pathway for fungal colonisation, leading to greater levels of the two major types of mycotoxins, fumonisins and aflatoxins, which are a major concern for human and animal health (Widstrom, 1996; Munkvold, 2003). However, association between ear injury and concentrations of fumonisins and aflatoxins is generally weak (Bibb et al., 2018). It is also an important insect pest for sorghum in the USA (Knutson and Cronholm, 2007). H. zea was identified as the most important insect pest in fresh tomatoes in North Carolina causing losses in profits of $ 3,385 and $ 941 per ha in 1988 and 1989 (Walgenbach and Estes, 1992). It is also an important pest species in soybeans (Swenson et al., 2013) and was observed as the second most important soybean insect pest in Mississippi, Tennessee and Arkansas (Musser et al., 2011).
3.6. Availability and limits of mitigation measures
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes, the existing measures (see Sections 3.3 and 3.4.2) can mitigate the risks of entry, establishment, and spread within the EU. As a pest listed in Annex IIA, its introduction and spread in the EU is banned irrespective of what it may be found on.
RNQPs: Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated?
Yes, sourcing plants and plant parts from PFA would mitigate the risk.
3.6.1. Identification of additional measures
3.6.1.1. Additional control measures
Potential additional control measures are listed in Table 7.
3.6.1.2. Additional supporting measures
Potential additional supporting measures are listed in Table 8.
Table 8.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance
| Information sheet title (with hyperlink to information sheet if available) | Supporting measure summary | Risk component (entry/establishment/spread/impact) |
|---|---|---|
| Inspection and trapping |
Feeding damage is usually visible and the larvae can be seen on the surface of plants but often they are hidden within plant organs (flowers, fruits, etc.). Bore holes may be visible, but otherwise it is necessary to cut open the plant organs to detect the pest. Because of morphological similarity, it is impossible to identify the larvae. Positive identification can also be done by rearing the larvae and examining the genitalia of the adult Pheromone traps are available |
Entry |
| Laboratory testing | Molecular techniques (e.g. DNA barcoding; restriction fragment length polymorphism, RFLP) are available for H. zea diagnosis | Entry |
| Surveillance | Surveillance to guarantee that plants and produce originate from a Pest Free Area could be an option | Entry |
3.6.1.3. Biological or technical factors limiting the effectiveness of measures to prevent the entry, establishment and spread of the pest
Mobility of adults with migratory flights of several hundred kilometres.
Pupal stage in the soil.
Larvae can be hidden inside fruits.
Control with insecticides is usually complicated by the insect's biology.
3.6.1.4. Biological or technical factors limiting the ability to prevent the presence of the pest on plants for planting
Polyphagous nature of the organism.
High dispersal ability of adults.
High fecundity.
3.7. Uncertainty
Larvae of H zea cannot be distinguished morphologically from larvae of H armigera and adults can be distinguished only by dissection of reproductive organs. This might delay the detection of the species if it arrived into Europe. However, this does not affect the overall conclusion. There are no other uncertainties affecting the conclusions of this pest categorisation.
4. Conclusions
H. zea satisfies the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. H. zea does not meet the criteria of (a) occurring in the EU and (b) plants for planting being the principal means of spread for it to satisfy the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union RNQP.
Table 9.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pests (Section 3.1) |
The identity of Helicoverpa zea is well established and there are taxonomic keys available for its identification to species level In the current EU legislation Helicoverpa zea is referred to with its synonym Heliothis zea |
The identity of Helicoverpa zea is well established and there are taxonomic keys available for its identification to species level | |
| Absence/presence of the pest in the EU territory (Section 3.2) | H. zea is not known to be present in the EU | H. zea is not known to be present in the EU | |
| Regulatory status (Section 3.3) | The pest is listed in Commission Implementing Regulation (EU) 2019/2072, Annex II, Part A, list of Union quarantine pests and their respective codes of Pests not known to occur in the Union territory | There are no grounds to consider its status as a quarantine pest to be revoked | |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | H. zea could enter into, become established in, and spread within, the EU territory. The main entry pathways are: via cut flowers, fruits, plants for planting with and without soil attached and soil/growing media | Adults are strong flyers and plants for planting would not be the main means of spread in the EU | |
| Potential for consequences in the EU territory (Section 3.5) | The pests’ introduction would most probably have an economic impact in the EU | Should the pest be present on plants for planting, an economic impact on its intended use would be expected | |
| Available measures (Section 3.6) | Measures exist which can mitigate the risks of entry, establishment, and spread within the EU. As a pest listed in Annex IIA, its introduction and spread in the EU is banned irrespective of what it may be found on | Because of the polyphagous nature of the organism on hosts which are widely available outdoors and its high dispersal ability it would be difficult for measures to be effective | |
| Conclusion on pest categorisation (Section 4) | All criteria assessed by EFSA above for consideration as a potential quarantine pest are met with no uncertainties | The criteria of the pest being present in the EU territory and plants for planting being the main means of spread, which are the pre‐requisites for consideration as a potential RNQP, are not met | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | |||
Abbreviations
- DG SANTÉ
Directorate General for Health and Food Safety
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- ITS2
internal transcribed spacer region 2
- MS
Member State
- PCR
polymerase chain reaction
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- RFLP
restriction fragment length polymorphism
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
- UV
ultraviolet
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 1995, 2017)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2017)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017)
- Greenhouse
The term ‘greenhouse’ is used in the current opinion as defined by EPPO (https://gd.eppo.int/taxon/3GREEL) as a walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment. A similar definition is also given in EFSA Guidance Document on protected crops (2014) https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2014.3615 .
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017)
- Measures
Control (of a pest) is defined in ISPM 5 (FAO 2017) as “Suppression, containment or eradication of a pest population” (FAO, 1995). Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate Risk Reduction Options that do not directly affect pest abundance
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017)
- Protected zones (PZ)
A Protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union.
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017)
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017)
Appendix A – Host plants for Helicoverpa zea
1.
| Host category | Host | Common name | Family | Reference |
|---|---|---|---|---|
| Major/Main | Capsicum annuum | Bell pepper | Solanaceae | EPPO (2020)/CABI ISC (2020) |
| Major/Main | Gossypium hirsutum | Cotton | Malvaceae | EPPO (2020)/CABI ISC (2020) |
| Major/Main | Phaseolus vulgaris | Common bean | Fabaceae | EPPO (2020)/CABI ISC (2020) |
| Major/Main | Solanum lycopersicum | Tomato | Solanaceae | EPPO (2020)/CABI ISC (2020) |
| Major/Main | Solanum melongena | Aubergine | Solanaceae | EPPO (2020)/CABI ISC (2020) |
| Major/Main | Sorghum bicolor | Sorghum | Poaceae | EPPO (2020)/CABI ISC (2020) |
| Major/Main | Zea mays | Maize | Poaceae | EPPO (2020)/CABI ISC (2020) |
| Main/Minor | Abelmoschus esculentus | Okra | Malvacease | EPPO (2020)/CABI ISC (2020) |
| Main/Minor | Cajanus cajan | Pigeon pea | Fabaceae | EPPO (2020)/CABI ISC (2020) |
| Main | Glyzine max | Soybean | Fabaceae | CABI ISC (2020) |
| Main/Minor | Phaseolus | Beans | Fabaceae | EPPO (2020)/CABI ISC (2020) |
| Main | Zea mays subsp. mays | Sweet corn | Poaceae | CABI ISC (2020) |
| Minor | Brassica | EPPO (2020) | ||
| Minor/Other | Cicer arietinum | EPPO (2020)/CABI ISC (2020) | ||
| Minor | Cucurbitaceae | EPPO (2020) | ||
| Minor/Other | Fragaria x ananassa | Rosaceae | EPPO (2020)/CABI ISC (2020) | |
| Minor | Helianthus annuus | Asteraceae | EPPO (2020) | |
| Minor/Other | Lactuca sativa | Lettuce | Asteraceae | EPPO (2020)/CABI ISC (2020) |
| Minor | Malvaceae | EPPO (2020) | ||
| Minor/Other | Nicotiana tabacum | Tobacco | Solanaceae | EPPO (2020)/CABI ISC (2020) |
| Minor | Pisum sativum | Peas | Fabaceae | EPPO (2020) |
| Minor | Poaceae | EPPO (2020) | ||
| Minor | Solanaceae | EPPO (2020) | ||
| Minor/Other | Trifolium | Clover | Fabaceae | EPPO (2020)/CABI ISC (2020) |
| Minor | Vegetable plants | EPPO (2020) | ||
| Minor | Vicia faba | Faba bean | Fabaceae | EPPO (2020) |
| Other | Abutilon theophrasti | Velvet leaf | Malvaceae | CABI ISC (2020) |
| Other | Amaranthus | Amaranth | Amaranthaceae | CABI ISC (2020) |
| Other | Arachis hypogaea | Groundnut | Fabaceae | CABI ISC (2020) |
| Other | Brassica oleracea | Cabbages, cauliflowers | Brassicaceae | CABI ISC (2020) |
| Other | Capsicum | Peppers | Solanaceae | CABI ISC (2020) |
| Other | Chenopodium quinoa | Quinoa | Chenopodiaceae | CABI ISC (2020) |
| Other | Citrus | Rutaceae | CABI ISC (2020) | |
| Other | Cucumis melo | Melon | Cucurbitaceae | CABI ISC (2020) |
| Other | Cucumis sativus | Cucumber | Cucurbitaceae | CABI ISC (2020) |
| Other | Fragaria | Strawberry | Rosaceae | CABI ISC (2020) |
| Other | Geranium carolinianum | Carolina geranium | Geraniaceae | CABI ISC (2020) |
| Other | Gerbera | Barbeton daisy | Asteraceae | CABI ISC (2020) |
| Other | Ipomoea purpurea | Tall morning glory | Convolvulaceae | CABI ISC (2020) |
| Other | Lamium amplexicaule | Henbit deadnettle | Lamiaceae | CABI ISC (2020) |
| Other | Lespedeza juncea var. sericea | Sericea lespedeza | Fabaceae | CABI ISC (2020) |
| Other | Medicago lupulina | Black medick | Fabaceae | CABI ISC (2020) |
| Other | Medicago sativa | Lucerne | Fabaceae | CABI ISC (2020) |
| Other | Panicum miliaceum | Millet | Poaceae | CABI ISC (2020) |
| Other | Salix | Willows | Salicaceae | CABI ISC (2020) |
| Other | Securigera varia | Crown vetch | Fabaceae | CABI ISC (2020) |
| Other | Spinacia oleracea | Spinach | Chenopodiaceae | CABI ISC (2020) |
| Other | Trifolium incarnatum | Crimson clover | Fabaceae | CABI ISC (2020) |
| Other | Vicia sativa | Common vetch | Fabaceae | CABI ISC (2020) |
| Other | Vigna unguiculata | Cowpea | Fabaceae | CABI ISC (2020) |
| Wild host | Lonicera japonica | Japanese honeysuckle | Fabaceae | CABI ISC (2020) |
| Wild host | Vicia villosa | Hairy vetch | Fabaceae | CABI ISC (2020) |
Appendix B – Interceptions of Helicoverpa zea in the EU since 1995 according to Europhyt database (accessed on 7/4/2020)
1.
| Date of interception | Country of origin | Plant species |
|---|---|---|
| 25‐JAN‐96 | Barbados | Callistemon sp. |
| 07‐NOV‐96 | China | Unspecified |
| 27‐APR‐95 | Colombia | Dianthus sp. |
| 03‐NOV‐97 | Dianthus sp. | |
| 04‐APR‐00 | Dominican Republic | Solanum melongena |
| 11‐JUN‐15 | Capsicum sp. | |
| 13‐MAY‐16 | Capsicum chinense | |
| 16‐OCT‐14 | Ecuador | Rosa sp. |
| 11‐AUG‐17 | Rosa Tea hybrids | |
| 28‐MAY‐18 | Rosa | |
| 15‐AUG‐18 | Gypsophila | |
| 22‐OCT‐18 | Rosa | |
| 30‐SEP‐19 | Rosa | |
| 08‐DEC‐19 | Rosa | |
| 23‐MAY‐02 | Guatemala | Unspecified |
| 24‐MAR‐03 | Unspecified | |
| 06‐JAN‐16 | Pisum sativum | |
| 12‐AUG‐98 | Marocco | Zea mays |
| 30‐OCT‐95 | Mexico | Callistemon sp. |
| 06‐MAR‐15 | Physalis | |
| 22‐JUN‐16 | Physalis | |
| 03‐NOV‐16 | Physalis | |
| 03‐FEB‐17 | Solanum melongena | |
| 04‐DEC‐17 | Momordica charantia | |
| 24‐MAY‐19 | Capsicum chinense | |
| 16‐MAR‐09 | Peru | Asparagus sp. |
| 18‐DEC‐18 | Zea mays subsp. saccharata | |
| 31‐MAR‐99 | Puerto Rico | Zea mays |
| 23‐AUG‐16 | Suriname | Dolichos sp. |
| 01‐NOV‐95 | United States of America | Callistemon sp. |
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Yuen J, Zappalà L, Czwienczek E, Streissl F and MacLeod A, 2020. Scientific Opinion on the pest categorisation of Helicoverpa zea . EFSA Journal 2020;18(7):6177, 31 pp. 10.2903/j.efsa.2020.6177
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00123
Panel members: Claude Bragard, Katharina Dehnen‐Schmutz, Francesco Di Serio, Paolo Gonthier, Marie‐Agnès Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent, Jonathan Yuen and Lucia Zappalà.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figures 1, 2: © Bugwood.org, Figure 3: © EPPO
Adopted: 10 June 2020
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) No 228/2013, (EU) No 652/2014 and (EU) No 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC.
Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019.
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed, the EPPO code remains the same. This provides a harmonized system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (Griessinger and Roy, 2015; EPPO, 2019).
References
- Allen KC, Luttrell RG, Little NS, Parys KA and Perera OP, 2019. Response of Bt and non‐Bt cottons to high infestations of Bollworm (Helicoverpa zea Boddie) and Tobacco Budworm (Heliothis virescens (F.)) under sprayed and unsprayed conditions. Agronomy, 9, 759 10.3390/agronomy9110759 [DOI] [Google Scholar]
- Behere GT, Tay WT, Russell DA and Batterham P, 2008. Molecular markers to discriminate among four pest species of Helicoverpa (Lepidoptera: Noctuidae). Bulletin of Entomological Research, 98, 599–603. 10.1017/s0007485308005956 [DOI] [PubMed] [Google Scholar]
- Bibb JL, Cook D, Catchot A, Musser F, Stewart SD, Leonard BR, Buntin GD, Kerns D, Allen TW and Gore J, 2018. Impact of corn earworm (Lepidoptera: Noctuidae) on field corn (Poales: Poaceae) yield and grain quality. Journal of Economic Entomology, 111, 1249–1255. [DOI] [PubMed] [Google Scholar]
- Butler GD, 1976. Bollworm: development in relation to temperature and larval food. Environmental Entomology, 5, 520–522. [Google Scholar]
- CABI ISC (Invasive Species Compendium), 2020. online. Helicoverpa zea datasheet. Available online: https://www.cabi.org/isc/datasheet/26776 [Accessed: 7 April 2020].
- Capinera JL, 2017. Featured Creatures. Corn earworm–Helicoverpa zea. Available online http://entnemdept.ufl.edu/creatures/veg/corn_earworm.htm [Accessed: 20 April 2020].
- Cardé RT and Minks AK, 1995. Control of moth pests by mating disruption: successes and constraints. Annual Review of Entomology, 40, 559–585. [Google Scholar]
- Cohen RW, Waldbauer GP and Friedman S, 1988. Natural diets and self‐selection: Heliothis zea larvae and maize. Entomologia Experimentalis et Applicata, 46, 161–171. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertesz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. Available online: 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO , 2019. EPPO codes. Available online: https://www.eppo.int/RESOURCES/eppo_databases/eppo_codes
- EPPO , 2020. (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed 7 April 2020].
- FAO (Food and Agriculture Organization of the United Nations), 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2017. ISPM (International standards for phytosanitary measures) No 5. Glossary of phytosanitary terms. Available online: https://www.ippc.int/en/publications/622/
- Fitt GP, 1989. The ecology of heliothis species in relation to agroecosystems. Annual Review of Entomology, 34, 17–52. 10.1146/annurev.en.34.010189.000313 [DOI] [Google Scholar]
- Flood BR, Foster R, Hutchison WD and Pataky S, 2005. Sweet corn In: Foster R. and Flood BR. (eds). Vegetable insect management. Meister Media Worldwide, Willoughby, OH: pp. 39–61. [Google Scholar]
- Gilligan TM, Tembrock LR, Farris RE, Barr NB, van der Straten MJ, van de Vossenberg BTLH and Metz‐Verschure E, 2015. A multiplex real‐time PCR assay to diagnose and separate Helicoverpa armigera and H. zea (Lepidoptera: Noctuidae) in the New World. PLoS ONE, 10, e0142912 10.1371/journal.pone.0142912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore J and Adamczyk JJJ, 2004. Impact of bollworms [Helicoverpa zea (Boddie)] on maturity and yield of bollgard cotton. Journal of Cotton Science, v. 8:pp. 617‐610‐2004 v.2008 no.2004 [Google Scholar]
- Griessinger D and Roy A‐S, 2015. EPPO codes: a brief description. Available online: https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Gross HR, 1990. Field release and evaluation of Archytas marmoratus (Diptera: Tachinidae) against larvae of Heliothis zea (Lepidoptera: Noctuidae) in whorl stage corn. Environmental Entomology, 19, 1122–1129. [Google Scholar]
- Hardwick DF, 1965. The corn earworm complex. Memoirs of the Entomological Society of Canada, 97, 5–247. 10.4039/entm9740fv [DOI] [Google Scholar]
- Hardwick DF, 1968. A brief review of the principles of light trap design with a description of an efficient trap for collecting noctuid moths. Journal of the Lepidopterists' Society, 22, 65–75. [Google Scholar]
- Hardwick DF, 1970. A generic revision of the North American Heliothidinae (Lepidoptera: Noctuidae). Memoirs of the Entomological Society of Canada, 73, 1–59. [Google Scholar]
- Heath J and Emmet AM, 1983. The moths and butterflies of Great Britain and Ireland. Vol 10 Harley Books, Colchester, UK, Noctuidae and Agaristidae: pp. 296–301. [Google Scholar]
- Javaid I, Joshi J, Dadson RB, Hashem FM and Allen AL, 2005. The potential of Bt corn as a trap crop for the control of corn earworm, Helicoverpa zea Boddie, in soybean. Journal of Sustainable Agriculture, 26, 115–121. [Google Scholar]
- Johnson MW, Stinner RE and Rabb RL, 1975. Ovipositional response of Heliothis zea (Boddie) to its major hosts in North Carolina. Environmental Entomology, 4, 291–297. [Google Scholar]
- Kennedy GG and Storer NP, 2000. Life systems of polyphagous arthropod pests in temporally unstable cropping systems. Annual Review of Entomology, 45, 467–493. [DOI] [PubMed] [Google Scholar]
- Knutson AE and Cronholm G, 2007. Economic injury levels for sorghum midge, Stenodiplosis sorghicola and corn earworm, Helicoverpa zea, feeding on panicles of sorghum, Sorghum bicolor. Southwestern Entomologist, 32, 75–85. 10.3958/0147-1724-32.2.75 [DOI] [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of Köppen‐ Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- Latheef MA, Lopez JD and Witz JA, 1993. Capture of corn earworm (Lepidoptera: Noctuidae) in pheromone traps and hand nets: relationship to egg and adult densities in field corn, Texas Brazos River Valley. Journal of Economic Entomology, 86, 407–415. [Google Scholar]
- Leite NA, Alves‐Pereira A, Corrêa AS, Zucchi MI and Omoto C, 2014. Demographics and genetic variability of the new world bollworm (Helicoverpa zea) and the old world bollworm (Helicoverpa armigera) in Brazil. PLoS ONE, 9, e113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C and Isely D, 1947. Corn as a trap crop for the cotton bollworm. Journal of Economic Entomology, 40, 437. [DOI] [PubMed] [Google Scholar]
- Lingren PD, Bryant VM, Raulston JR, Pendleton M, Westbrook J and Jones GD, 1993. Adult feeding host range and migratory activities of corn earworm, cabbage looper, and celery looper (Lepidoptera: Noctuidae) moths as evidenced by attached pollen. Journal of Economic Entomology, 86, 1429–1439. [Google Scholar]
- Lopes HM, Bastos CS, Boiteux LS, Foresti J and Suinaga FA, 2017. A RAPD‐PCR‐based genetic diversity analysis of Helicoverpa armigera and H. zea populations in Brazil. Genetics and Molecular Research, 16, gmr16038757. [DOI] [PubMed]
- MacLeod A and Korycinska A, 2019. Detailing Koppen‐Geiger climate zones at a country and regional level: a resource for pest risk analysis. EPPO Bulletin, 49, 73–82. [Google Scholar]
- Manandhar R and Wright MG, 2015. Enhancing biological control of corn earworm, Helicoverpa zea and thrips through habitat management and inundative release of Trichogramma pretiosum in corn cropping systems. Biological Control, 89, 84–90. [Google Scholar]
- Mangat BS and Apple JW, 1966. Corn earworm development in relation to temperature. Journal of Economic Entomology, 59, 1005–1006. [Google Scholar]
- Mastrangelo T, Paulo DF, Bergamo LW, Morais EGF, Silva M, Bezerra‐Silva G and Azeredo‐Espin G, 2014. Detection and genetic diversity of a heliothine invader (Lepidoptera: Noctuidae) from North and Northeast of Brazil. Journal of Economic Entomology, 107, 970–980 PMID: 25026655. [DOI] [PubMed] [Google Scholar]
- Matthews M, 1991. Classification of the Heliothinae. Bulletin of the Natural Resources Institute No. 44. Natural Resources Institute, Chatham, UK. [Google Scholar]
- Morey A, Hutchison W, Venette R and Burkness E, 2012. Cold hardiness of Helicoverpa zea (Lepidoptera: Noctuidae) pupae. Environmental Entomology, 41, 172–179. [DOI] [PubMed] [Google Scholar]
- Munkvold GP, 2003. Epidemiology of Fusarium diseases and their mycotoxinsin maize ears. European Journal of Plant Pathology, 109, 705–713. [Google Scholar]
- Musser FR, Lorenz GM, Stewart SD and Catchot AL Jr, 2011. 2010 soybean insect losses for Mississippi, Tennessee and Arkansas. Midsouth Entomologist, 4, 22–28. [Google Scholar]
- Nagoshi RN, Gilligan TM and Brambila J, 2016. Combining Tpi and CO1 Genetic Markers to Discriminate Invasive Helicoverpa armigera From Local Helicoverpa zea (Lepidoptera: Noctuidae) Populations in the Southeastern United States. Journal of Economic Entomology, 109, 2115–2124. 10.1093/jee/tow177 [DOI] [PubMed] [Google Scholar]
- Neunzig HH, 1963. Wild host plants of the corn earworm and the tobacco budworm in eastern North Carolina. Journal of Economic Entomology, 56, 135–139. [Google Scholar]
- Olmstead DL, Nault BA and Shelton AM, 2016. Biology, ecology, and evolving management of Helicoverpa zea (Lepidoptera: Noctuidae) in Sweet Corn in the United States. Journal of Economic Entomology, 109, 1667–1676. 10.1093/jee/tow125 [DOI] [PubMed] [Google Scholar]
- Perera OP, Allen KC, Jain D, Purcell M, Little NS and Luttrell RG, 2015. Rapid Identification of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) Using Ribosomal RNA Internal Transcribed Spacer 1. Journal of Infectious Science, 15, 155 10.1093/jisesa/iev137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina AK, Kingan TG and Mattoo AK, 1992. Chemical signals from host plant and sexual behavior in a moth. Sciences, New Ser., 255, 592–594. [DOI] [PubMed] [Google Scholar]
- Reay‐Jones FPF, 2019. Pest status and management of corn earworm (Lepidoptera: Noctuidae) in field corn in the United States. Journal of Integrated Pest Management, 10, 1–9. 10.1093/jipm/pmz017 [DOI] [Google Scholar]
- Reay‐Jones FPF and Reisig DD, 2014. Impact of corn earworm injury on yield of transgenic corn producing BT toxins in the Carolinas. Journal of Economic Entomology, 107, 1101–1109. 10.1603/ec13516 [DOI] [PubMed] [Google Scholar]
- de Rougemont GM, 1989. A Field Guide to the Crops of Britain and Europe. Collins Sons and Co. Ltd., London. [Google Scholar]
- Sandstrom MA, Changnon D and Flood BR, 2007. Improving our understanding of Helicoverpa zea migration in the Midwest: assessment of source populations. Plant Health Progress, 8, 63 10.1094/php-2007-0719-08-rv [DOI] [Google Scholar]
- Smith IM, McNamara DG, Scott PR and Holderness M (eds), 1997. Helicoverpa zea In: Quarantine Pests for Europe, 2nd Edition, CABI/EPPO, Wallingford, 1425 pp. [Google Scholar]
- Sudbrink DL and Grant JF, 1995. Wild host plants of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) in eastern Tennessee. Environmental Entomology, 24, 1080–1085. [Google Scholar]
- Swenson SJ, Prishmann‐Voldseth DA and Musser FR, 2013. Corn Earworms (Lepidoptera: Noctuidae) as Pests of Soybean. Journal of integrated Pest Management, 4. [Google Scholar]
- Waldbauer GP, Cohen RW and Friedman S, 1984. Self‐selection of an optimal nutrient mix from defined diets by larvae of the corn earworm, Heliothis zea (Boddie). Physiological Zoology, 57, 590–597. [Google Scholar]
- Walgenbach JF and Estes EA, 1992. Economics of insecticide use on staked tomatoes in Western North Carolina. Journal of Economic Entomology, 85, 888–894. 10.1093/jee/85.3.888 [DOI] [Google Scholar]
- Westbrook JK and Lopez JD, 2010. Long‐distance migration in Helicoverpa zea what we know and need to know. Southwestern Entomologist, 35, 355–360. 10.3958/059.035.0315 [DOI] [Google Scholar]
- Westbrook JK, Wolf WW, Lingren PD, Raulston JR, Lopez JD, Matis JH, Eyster RS, Esquivel JF and Schleider PG, 1997. Early‐season migratory flights of corn earworm (Lepidoptera: Noctuidae). Environmental Entomology, 26, 12–20. [Google Scholar]
- Westbrook JK, Esquivel JF, Lopez JD, Jones GD, Wolf WW and Raulston JR, 1998. Validation of bollworm (Lepidoptera : Noctuidae) migration across south‐central Texas in 1994‐1996. Southwestern Entomologist, 23, 209–219. [Google Scholar]
- Widstrom NW, 1996. The alfatoxin problem with corn grain. Advances in Agronomy, 56, 219–280. [Google Scholar]
- Wiseman BR and Davis FM, 1990. Plant resistance to insects attacking corn and grain sorghum. The Florida Entomologist, 73, 446–458. [Google Scholar]


