Abstract
Myocardial ischemia-reperfusion (MI/R) injury is a complex pathological process that occurs when tissues are reperfused following a prolonged period of ischemia. Troxerutin has been reported to have cardioprotective functions. However, the underlying mechanism by which troxerutin protects against MI/R injury has not been fully elucidated. The aim of the present study was to explore whether troxerutin-mediated protection against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced H9C2 cell injury was associated with the inhibition of oxidative stress and the inflammatory response by regulating the PI3K/AKT/hypoxia-inducible factor-1α (HIF-1α) signaling pathway. The results of the present study suggested that troxerutin pretreatment prevented the OGD/R-induced reduction in cell viability, and the increase in lactate dehydrogenase activity and apoptosis. Troxerutin reversed OGD/R-induced the inhibition of the PI3K/AKT/HIF-1α signaling pathway as demonstrated by the increased expression of PI3K and HIF-1α, and the increased ratio of phosphorylated AKT/AKT. LY294002, a selective PI3K inhibitor, inhibited the PI3K/AKT/HIF-1α signaling pathway and further attenuated the protective effect of troxerutin against OGD/R-induced H9C2 cell damage. Furthermore, small interfering (si)RNA-mediated knockdown of HIF-1α reduced troxerutin-induced protection against OGD/R injury. Troxerutin pretreatment alleviated OGD/R-induced oxidative stress, as demonstrated by the reduced generation of reactive oxygen species and malonaldehyde content, and the increased activities of superoxide dismutase and glutathione peroxidase, which were reduced by HIF-1α-siRNA. Troxerutin-induced decreases in the levels of interleukin (IL)-1β, IL-6 and tumor necrosis factor-α in OGD/R conditions were also reduced by HIF-1α-siRNA. The results from the present study indicated that troxerutin aggravated OGD/R-induced H9C2 cell injury by inhibiting oxidative stress and the inflammatory response. The primary underlying protective mechanism of troxerutin was mediated by the activation of the PI3K/AKT/HIF-1α signaling pathway.
Keywords: troxerutin, myocardial ischemia-reperfusion injury, oxidative stress, inflammation, PI3K/AKT/hypoxia-inducible factor-1α signaling pathway
Introduction
Myocardial ischemia/reperfusion (MI/R) injury is an acute adverse cardiac event, resulting from the deterioration or loss of cardiac function, thereby reducing the efficacy of reperfusion therapy (1). Despite recent improvements in medical knowledge and management, MI/R and related heart failure are still significant health problems worldwide (2). The mechanisms of MI/R pathogenesis are complex and multifactorial, including endothelial cell injury, apoptosis, marked oxidative/nitrosative stress, inflammation, mitochondrial dysfunction, dysregulated vascular relaxation and intracellular calcium overload (3). Due to the lack of a comprehensive understanding of the pathological processes occurring during I/R injury, there are no definitive interventions to eliminate MI/R injury (4,5). Therefore, it is important to explore novel therapeutic strategies to alleviate MI/R injury and elucidate the underlying mechanisms.
Troxerutin is a trihydroxyethylated derivative of the natural bioflavonoid rutin and is found in tea, corn buds, coffee, fruit and green vegetables (6,7). Troxerutin has been found to have multiple biological properties, including having anti-oxidative, anti-inflammatory, anti-fibrinolytic, anti-neoplastic and neuroprotective activities (7–9). Recently, emerging evidence has revealed the cardioprotective potential of troxerutin (10,11). Mokhtari et al (12) reported that troxerutin attenuates myocardial reperfusion injury in diabetic rats, which is dependent on its anti-apoptotic function. Another previous study demonstrated that troxerutin treatment, as well as ischemic postconditioning, inhibited the activation of leukocyte-endothelial cell interactions and prevented inflammatory-pathological changes under I/R insults in myocardial cells (13). Although there are studies that have reported the protective effect of troxerutin preconditioning following MI/R injury, the specific effects of troxerutin on MI/R injury, and the underlying mechanisms, have not been fully elucidated under healthy and disease conditions.
Cardiomyocyte oxidative stress and inflammatory responses have been recognized as hallmarks of MI/R injury (14,15). Previous studies reported that oxidative stress is increased or accelerated during I/R, and partially contributes to the overall level of apoptosis and cardiomyocyte death (16,17). Similarly, inflammation plays an important role in the pathophysiology of MI/R injury (18). Reducing oxidative stress and the inflammatory response minimizes cardiac injury induced by I/R (19). In addition, the PI3K/AKT signaling pathway has been identified as a potential therapeutic target in the treatment of MI/R injury (20,21). Hypoxia-inducible factor-1α (HIF-1α), a transcription factor that is a central component of the oxygen sensing mechanism in mammalian cells, has been shown to be an important regulator in the response to hypoxia and ischemia (22,23). Previous studies have revealed that enhancing the expression of HIF-1α reduces infarct size, promotes angiogenesis and improves cardiac function (24,25). PI3K/AKT and HIF-1α also play a substantial role in mediating the inflammatory response and oxidative stress (26,27). A previous study showed that HIF-1α is a downstream target of the PI3K/AKT pathway and is involved in lung I/R injury (28), cerebral infarction (29) and oral squamous cell carcinoma (30). Nevertheless, the role of the PI3K/AKT/HIF-1α signaling pathway in the pathogenesis of MI/R injury and the protective effect of troxerutin on MI/R injury remains unclear.
The aim of the present study was to investigate the effects of troxerutin on oxidative stress and inflammation following MI/R injury in H9C2 cells, with an emphasis on the role of the PI3K/AKT/HIF-1α signaling pathway.
Materials and methods
H9C2 cardiomyocyte culture
The H9C2 cardiomyocyte line, a subclone of the original clonal cell line derived from embryonic rat heart tissue, was obtained from the American Type Cell Culture Collection. The cells were cultured in DMEM (Sigma-Aldrich; Merck KGaA) supplemented with (v/v) 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 U/ml streptomycin in a humidified incubator at 37°C with an atmosphere of 5% CO2 and 95% air.
Establishment of the Oxygen-Glucose Deprivation and Reoxygenation (OGD/R) injury model
To create an in vitro model of MI/R injury, H9C2 cells were maintained in glucose-free Hanks' Balanced Salt Solution (Invitrogen; Thermo Fisher Scientific, Inc.) within an anaerobic chamber containing 95% N2 and 5% CO2 at 37°C for 6 h, which induced OGD. Subsequently, H9C2 cells from the OGD-treated groups were removed from the anoxic incubator and cultured under normal condition as aforementioned for a further 18 h to allow reoxygenation to occur. This process was called MI/R injury.
Cell transfection
Small interfering (si)RNAs against HIF-1α (HIF-1α-siRNA) and non-specific control siRNA (NS-siRNA) were synthesized by Shanghai GenePharma Co., Ltd. The sense strand siRNA sequences are as follows: HIF-1α-siRNA, 5′-GCCGCUCAAUUUAUGAAUATT-3′ and NS-siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′. H9C2 cells were transfected with HIF-1α-siRNA (50 nM) or NS-siRNA (50 nM) using Lipofectamine® RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. Briefly, the cells were seeded in 6-well plates at a density of 5×105 cells/per well. HIF-1α-siRNA, NS-siRNA or Lipofectamine® RNAiMAX transfection reagent was diluted in Opti-MEM medium (Gibco; Thermo Fisher Scientific, Inc.). The diluted siRNA was added to the diluted Lipofectamine® RNAiMAX reagent in a 1:1 ratio. Following incubation at room temperature for 5 min, the siRNA-lipid complexes were added to the cells and incubated for a further 6 h, after which the media was changed to complete media. The cells were harvested 48 h post-transfection and the transfection efficiency was determined using reverse transcription-quantitative (RT-q)PCR.
Experimental protocols
A previous study and preliminary experimental results indicated that troxerutin (10 µM) addition to cultured myocardial cells for 1 h before OGD exerts cardioprotective effects (31). LY294002 (20 µM; Merck KGaA), a specific inhibitor of the PI3K/AKT signaling pathway, was used to pretreat H9C2 cardiomyocytes for 2 h before troxerutin treatment (32).
In the present study, cells were divided into the following 6 groups: i) Control group, H9C2 cells were cultured under normal conditions in high-glucose DMEM; ii) OGD/R group, H9C2 cells were subjected to OGD for 6 h and reoxygenation for 18 h; iii) troxerutin + OGD/R group, H9C2 cells were incubated with troxerutin (10 µM) for 1 h followed by co-treatment with OGD/R; iv) LY294002 + troxerutin + OGD/R group, cells were pre-conditioned with LY2940021 (20 µM) for 2 h, troxerutin (10 µM) for 1 h and then subjected to OGD/R; v) siRNA+ troxerutin + OGD/R group, H9C2 cells were transfected with HIF-1α-siRNA (50 nM) or NS-siRNA (50 nM) for 24 h and then treated with troxerutin (10 µM) for 1 h followed by OGD/R; and vi) LY294002 + siRNA, H9C2 cells were incubated with LY2940021 (20 µM) for 24 h and treated with HIF-1α-siRNA (50 nM) or NS-siRNA (50 nM) for 24 h.
Cell viability assay
The cell viability of H9C2 cells treated as aforementioned was analyzed using an MTT assay. Briefly, the cells were seeded in 96-well plates at a density of 1×104 cells/well. Following the aforementioned treatments, MTT solution (0.5 mg/ml) was added to the each well and incubated with the cells at 37°C for 4 h. Following this, the supernatants were removed and 150 µl DMSO was added to each well to dissolve the formazan crystals. The absorbance at 570 nm was measured using a microplate reader (Bio-Rad Laboratories, Inc.).
Cell cytotoxicity measurement
H9C2 cells were cultured in 96-well plates at a density of 1×104 cells/well and subjected to the aforementioned treatments. Extracellular lactate dehydrogenase (LDH) activity was determined using a commercial LDH assay kit (Nanjing KeyGen Biotech Co., Ltd.), according to the manufacturer's instructions. The absorbance at 490 nm was measured using a microplate reader.
Cell apoptosis assay
H9c2 cells were treated as aforementioned and the level of apoptosis was determined using an Annexin V-FITC/PI kit (Becton, Dickinson and Company), according to the manufacturer's instructions. After treatment, the cells were harvested, washed twice with PBS and stained with annexin V-FITC (5 µl) and propidium iodide (10 µl) solution for 15 min at 37°C in the dark. The level of apoptosis was determined using a flow cytometer (FACScan; BD Biosciences) and quantified using CELL Quest 3.0 software (BD Biosciences).
RT-qPCR
Total RNA was isolated from H9C2 cells using TRIzol Reagent (Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. First-strand complementary DNA was synthesized using 1 µg RNA using the PrimeScript RT Reagent kit with gDNA Eraser (Takara Bio, Inc.). The temperature protocol used for the RT reaction consisted of 37°C for 15 min and at 90°C for 5 sec. The mRNA levels of HIF-1α, interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α were analyzed using the SYBR Premix Ex Taq kit (Takara Bio, Inc.) and an ABI PRISM 7500 PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR thermocycling conditions were: 95°C for 90 sec for an initial denaturation, 45 denaturation cycles at 94°C for 6 sec, followed by 60°C for 30 sec for annealing and elongation, and final 72°C for 8 min for extension. β-actin was used as an internal control and the relative level of mRNA was analyzed using the 2−ΔΔCq method (33). The primer sequences used are as follows: HIF-1α forward, 5′-GATGACGGCGACATGGTTTAC-3′ and reverse, 5′-CTCACTGGGCCATTTCTGTGT-3′; IL-1β forward, 5′-GCCTCGTGCTGTCGGACCCATAT-3′ and reverse, 5′-TCCTTTGAGGCCCAAGGCCACA-3′; IL-6 forward, 5′-ACAACCACGGCCTTCCCTACTT-3′ and reverse, 5′-CACGATTTCCCAGAGAACATGTG-3′; TNF-α forward, 5′-CCCTCCTGGCCAACGGCATG-3′ and reverse, 5′-TCGGGGCAGCCTTGTCCCTT-3′); and β-actin forward, 5′-CGTGCGTGACATCAAAGAGAAG-3′ and reverse, 5′-CCAAGAAGGAAGGCTGGAAAA-3′.
Intracellular reactive oxygen species (ROS) generation
H9C2 cells were seeded at a density of 1×105 cells/ml in 6-well plates and the production of intracellular ROS was determined using an ROS assay kit (Beyotime Institute of Biotechnology). Briefly, following the aforementioned treatments, H9C2 cells were washed twice with PBS and incubated with 2′,7′-dichlorodihydrofluorescein diacetate (10 µM) at 37°C for 20 min. The fluorescence intensity was observed under a fluorescence microscope (Olympus Corporation, magnification, ×200). For the quantitative assay, the fluorescence intensity was measured using a flow cytometer at an excitation wavelength of 485 nm and an emission wavelength of 525 nm.
Measurement of oxidative stress biomarkers
The H9C2 cells from each group were homogenized with Scientz-IID Ultrasound Cell lysis Instrument (Changchun, Hangzhou, 15 sec/3 times pyrolysis and 15 sec for interval) on ice to determine the malondialdehyde (MDA) content, and the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px). The MDA content was determined using the thiobarbituric acid method, the activity of was measured using the xanthine oxidase method and the activity of GSH-Px was determined using the dithio-dinitrotoluidine method (all Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's instructions.
Western blot analysis
Following the aforementioned treatments, cells were lysed in ice-cold RIPA Buffer (Beyotime Institute of Biotechnology) containing 1% (v/v) Combined protease and phosphatase inhibitors (Thermo Fisher Scientific, Inc.). The cell lysates were centrifuged at 13,000 × g at 4°C for 10 min. The protein concentration in each sample was determined using the bicinchoninic acid method. Equal amounts of protein (30 µg) were separated on 10% SDS-PAGE and subsequently transferred to PVDF membranes. After blocking with 5% (w/v) non-fat dry milk in PBS with Tween (PBST) at room temperature for 2 h. The following primary antibodies were diluted 1:2,000 and incubated overnight at 4°C with the membranes (all Cell Signaling Technology, Inc.): PI3K (cat. no. 4249), phosphorylated AKT (p-AKT, cat. no. 9271), AKT (cat. no. 4691), HIF-1α (cat. no. 36169) and GAPDH (cat. no. 5174). After washing with TBST three times, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Inc., cat. no. 7074) at room temperature for 2 h. The membranes were washed and the protein bands were visualized using enhanced chemiluminescence (ECL; Pierce; Thermo Fisher Scientific, Inc.). Optical density values for each group were normalized to GAPDH and quantified using Quantity One 4.6.2 software (Bio-Rad, Laboratories, Inc.).
ELISA
Following the aforementioned treatments, the levels of TNF-α (Thermo Fisher Scientific, Inc., cat. no. BMS622), IL-6 (Thermo Fisher Scientific, Inc., cat. no. BMS603-2) and IL-1β (E-EL-R0012c, Elabscience) in the supernatant were evaluated using ELISA, according to the manufacturer's instructions. The results of the ELISAs were measured using a plate reader at 450 nm.
Statistical analysis
Statistical analysis was performed using a one-way ANOVA followed by the least significant difference test using SPSS software, version 17.0 (SPSS, Inc.). All data are presented as the mean ± SD from three experiments. P<0.05 was considered to indicate a statistically significant difference.
Results
Troxerutin alleviates OGD/R-induced H9C2 cell injury
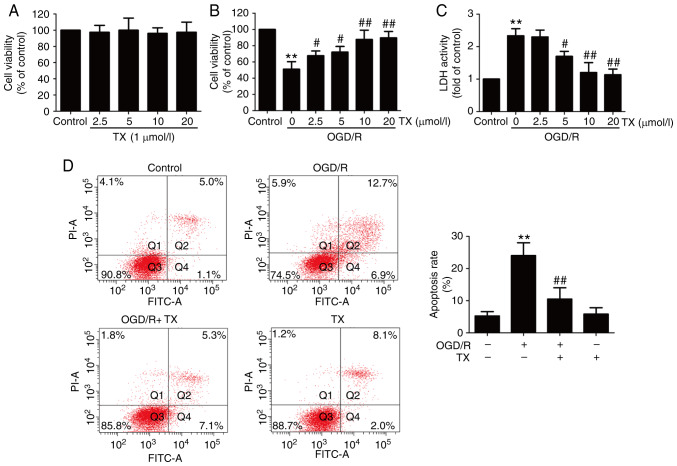
To examine the protective effect of troxerutin on OGD/R injury, cell viability was assessed. The results revealed that the different concentrations of troxerutin treatment had no effect on cell viability (Fig. 1A), and Results from the MTT assay demonstrated that OGD/R treatment resulted in reduced cell viability, which was reversed by troxerutin (Fig. 1B). In addition, ODG/R-induced an increase in LDH activity that was also blocked by troxerutin (Fig. 1C). Subsequently, the effect of troxerutin on apoptosis in OGD/R-treated H9C2 cells was also measured. The results indicated that OGD/R treatment induced apoptosis (Fig. 1D) in H9C2 cells. However, these effects were attenuated by troxerutin treatment. These data demonstrated that troxerutin exerted cardioprotective effects against OGD/R injury in H9C2 cells.
Figure 1.
Effects of ΤX on cytotoxicity and apoptosis in OGD/R-treated H9C2 cells. H9C2 cells were pretreated with ΤX (10 µM), and then co-treated with or without OGD (6 h)/R (18 h). (A) The cell viability in each group was determined using the MTT assay following treatment with different doses of ΤX. (B) LDH activity was measured using a commercial LDH assay kit. (C) Cell apoptosis percentage was detected by Annexin V-FITC/PI staining kit. (D) Flow cytometry analysis of H9C2 cell apoptosis. Data are presented as the mean ± SD (n=3). **P<0.01 vs. control; #P<0.05, ##P<0.01 vs. OGD/R 0 µmol/l troxerutin group. OGD/R, oxygen-glucose deprivation and reoxygenation; LDH, lactate dehydrogenase; TX, troxerutin.
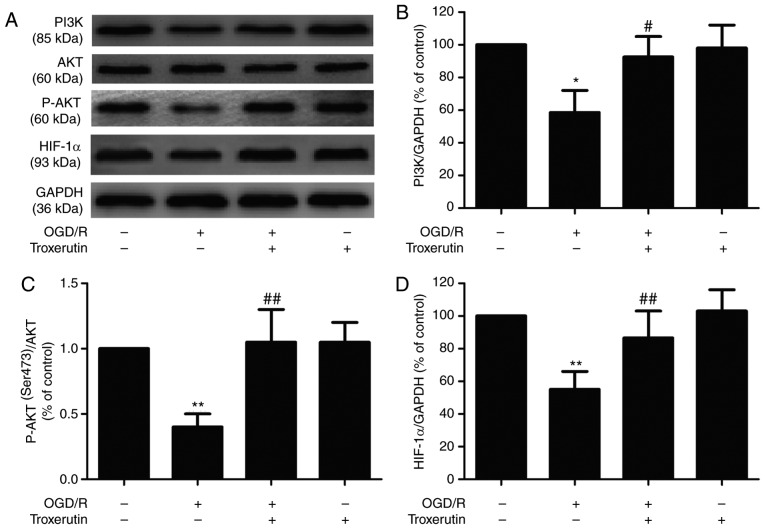
Troxerutin activates the PI3K/AKT/HIF-1α signaling pathway in OGD/R-treated H9C2 cells
To elucidate the potential underlying mechanism of troxerutin, the impact of troxerutin on the PI3K/AKT/HIF-1α signaling pathway was evaluated in OGD/R-injured H9C2 cells. OGD/R treatment significantly reduced the expressions of PI3K (Fig. 2A and B) and p-AKT (Fig. 2A and C), indicating that OGD/R inhibited the PI3K/AKT pathway. However, the expression of PI3K and p-AKT was reversed by troxerutin. In addition, troxerutin also significantly reversed the OGD/R-induced decrease in the expression of HIF-1α (Fig. 2A and D). Taken together, these data suggested that the PI3K/AKT/HIF-1α signaling pathway may play an important role in the cardioprotective effect of troxerutin against myocardial OGD/R damage.
Figure 2.
Effects of troxerutin on PI3K/AKT/HIF-1α signaling pathway under OGD/R condition in H9C2 cells. H9C2 cells were pretreated with troxerutin (10 µM) and then co-treated with or without OGD (6 h)/R (18 h). (A) The expression levels of PI3K, p-AKT, AKT and HIF-1α were determined using western blot analysis. Quantitative analysis of (B) PI3K, (C) p-AKT/AKT ratio and (D) HIF-1α expression in H9C2 cells. Data are presented as the mean ± SD (n=3). *P<0.05, **P<0.01 vs. -OGD/R-troxerutin; #P<0.05, ##P<0.01 vs. +OGD/R-troxerutin. OGD/R, oxygen-glucose deprivation and reoxygenation; HIF-1α, hypoxia-inducible factor-1α; p-, phosphorylated.
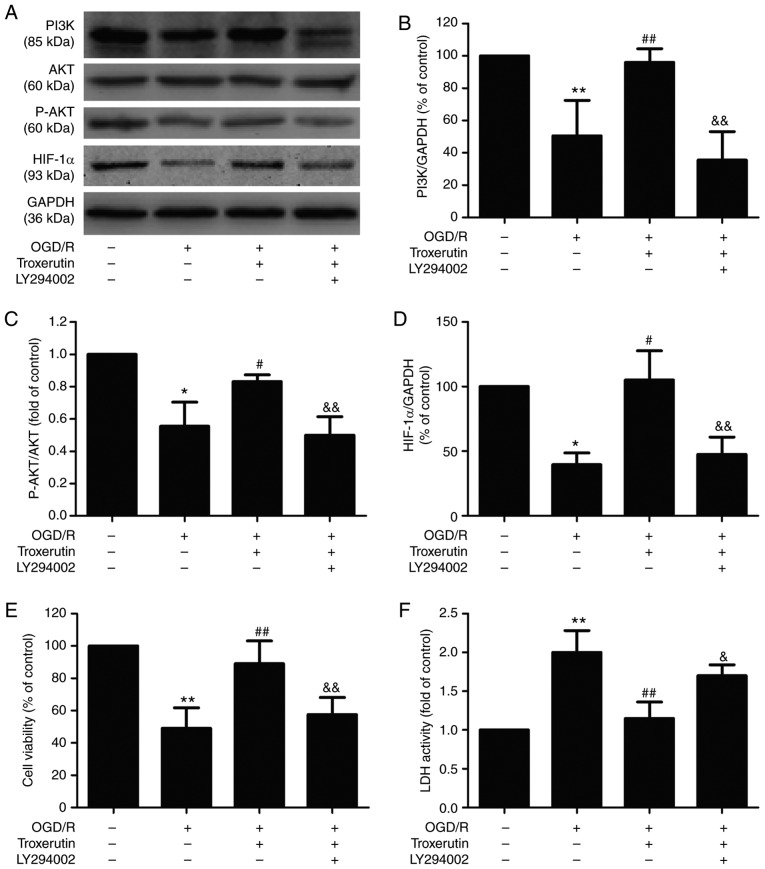
PI3K/AKT/HIF-1α signaling pathway mediates the troxerutin-induced suppression of H9C2 cell damage under OGD/R conditions
Based on the aforementioned results, the effect of the PI3K/AKT pathway inhibitor LY294002 on the cardioprotective effects of troxerutin was investigated. LY294002 pretreatment significantly mitigated the troxerutin-induced upregulation of PI3K (Fig. 3A and B), p-AKT (Fig. 3A and C) and HIF-1α (Fig. 3A and D) in OGD/R-treated H9C2 cells, indicating that LY294002 results in the inhibition of the PI3K/AKT/HIF-1α signaling pathway. Meanwhile, compared with troxerutin + OGD/R group, pretreatment with LY294002 reversed troxerutin-induced increased cell viability (Fig. 3E) and decreased LDH activity (Fig. 3F) in OGD/R-treated H9C2 cells. These results suggested that troxerutin exerted its cardioprotective effects against OGD/R injury by enhancing PI3K/AKT-mediated HIF-1α signaling pathway.
Figure 3.
Effects of LY294002 on the PI3K/AKT/HIF-1α signaling pathway, cell viability and LDH activity in the presence or absence of troxerutin in OGD/R-treated H9C2 cells. H9C2 cells were pretreated with PI3K pathway inhibitor LY294002 (20 µM) for 2 h and then treated with troxerutin (10 µM) followed by OGD (6 h)/R (18 h) treatment. (A) The expression levels of PI3K, AKT, p-AKT and HIF-1α were determined using western blot analysis. Quantitative analysis of (B) PI3K, (C) p-AKT/AKT and (D) HIF-1α expression in H9C2 cells. (E) Cell viability in each group was determined using the MTT assay. (F) LDH activity was determined using a commercial LDH assay kit. Data are presented as the mean ± SD (n=3). *P<0.05, **P<0.01 vs. -OGD/R-troxerutin-LY294002 group; #P<0.05, ##P<0.01 vs. +OGD/R-troxerutin-LY294002 group; &P<0.05, &&P<0.01 vs. +OGD/R +troxerutin-LY294002 group. OGD/R, oxygen-glucose deprivation and reoxygenation; LDH, lactate dehydrogenase; HIF-1α, hypoxia-inducible factor-1α.
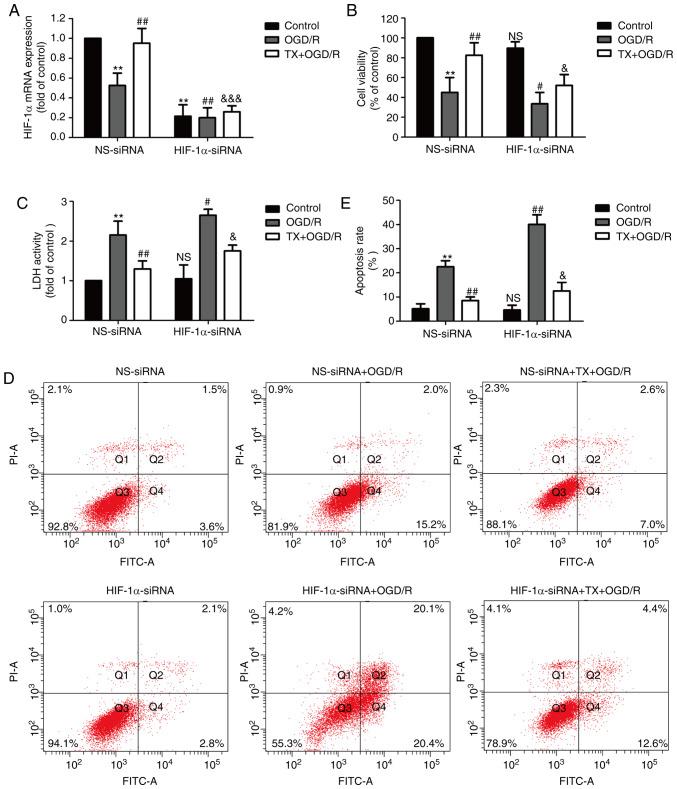
HIF-1α knockdown blocks the cardioprotective effects of troxerutin against OGD/R injury in H9C2 cells
Next, an siRNA against HIF-1α was used to further investigate the underlying mechanism of troxerutin. H9C2 cells were transfected with HIF-1α-siRNA or NS-siRNA and the transfection efficiency was determined using RT-qPCR. The results showed that compared with NS-siRNA transfection group, HIF-1α-siRNA transfection significantly decreased the level of HIF-1α mRNA in the control, OGD/R group and troxerutin + OGD/R groups (Fig. 4A). HIF-1α-siRNA further reduced cell viability and reduced the troxerutin-induced increase in cell viability in OGD/R-treated H9C2 cells (Fig. 4B). HIF-1α-siRNA also exacerbated the OGD/R-mediated increase in LDH activity, and, notably, HIF-1α-siRNA did not eliminate the inhibition of troxerutin on LDH activity in troxerutin + OGD/R-treated H9C2 cells (Fig. 4C). Additionally, HIF-1α-siRNA aggravated apoptosis in the OGD/R-treated group (compared with the NS-siRNA + OGD/R group) and reduced the troxerutin-induced decrease in apoptosis rate in the troxerutin + OGD/R-treated group (compared with the troxerutin + OGD/R group) (Fig. 4D and E). Notably, the effects of troxerutin on apoptosis were not abolished by HIF-1α-siRNA, meaning other pathways may contribute to the cardioprotection of troxerutin against apoptosis. These results indicated that troxerutin protected against OGD/R-induced cytotoxicity partly depending on the PI3K/AKT/HIF-1α signaling pathway, while the effects of troxerutin attenuated apoptosis independent of the PI3K/AKT/HIF-1α signaling pathway.
Figure 4.
Effects of HIF-1α-siRNA or NS-siRNA on TX-induced inhibition of cytotoxicity and apoptosis in OGD/R-treatedH9C2 cells. H9C2 cells were transfected with HIF-1α-siRNA or NS-siRNA for 6 h and then treated with TX (10 µM) followed by OGD (6 h)/R (18 h) treatment. (A) The relative level of HIF-1α mRNA was determined using reverse transcription-quantitative PCR and the relative level of mRNA was analyzed using the 2−ΔΔCq method. (B) Cell viability was determined using the MTT assay. (C) LDH activity was determined using a commercial LDH assay kit. (D) The percentage of apoptosis was determined using an Annexin V-FITC/propidium iodide staining kit. (E) Quantification of flow cytometry analysis of H9C2 cell apoptosis. Data are presented as the mean ± SD (n=3). **P<0.01 vs. NS-siRNA + control group; #P<0.05, ##P<0.01 vs. NS-siRNA + OGD/R group; &P<0.05, &&&P<0.001 vs. NS-siRNA +TX + OGD/R group. OGD/R, oxygen-glucose deprivation and reoxygenation; siRNA, small interfering RNA; HIF-1α, hypoxia-inducible factor-1α; NS, non-specific; TX, troxerutin; LDH, lactate dehydrogenase.
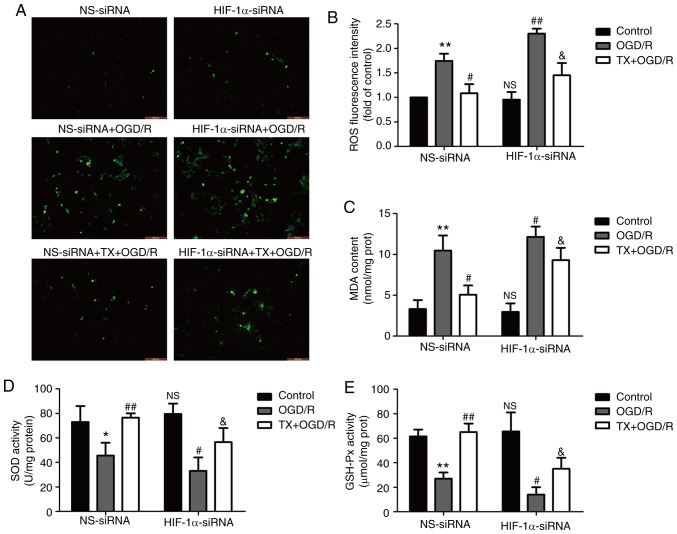
HIF-1α knockdown mitigates troxerutin-induced inhibition of oxidative stress in OGD/R-treated H9C2 cells
To further explore the effect of troxerutin on oxidative stress and the role of the PI3K/AKT/HIF-1α signaling pathway in this process, oxidative stress markers were assessed in H9C2 cells. Compared with the control group, OGD/R treatment increased ROS generation (Fig. 5A and B) and the MDA content (Fig. 5C) in H9C2 cells, while compared with OGD/R group, troxerutin pretreatment decreased ROS production (Fig. 5A and B) and the MDA content (Fig. 5C) in OGD/R-treated H9C2 cells, highlighting the anti-oxidative properties of troxerutin under OGD/R condition in H9C2 cells. However, compared with NS-siRNA group, the inhibition of troxerutin on oxidative stress was partly reversed by HIF-1α-siRNA in TX + OGD/R group. In addition, troxerutin alleviated the OGD/R-induced decreases in antioxidant stress indicators, including SOD (Fig. 5D) and GSH-Px activities (Fig. 5E), while these effects were reduced by HIF-1α-siRNA. Compared with the NS-siRNA transfection group, HIF-1α-siRNA induced oxidative stress in the OGD/R treatment group as shown by the increased generation of ROS and MDA content, and the reduced activities of SOD and GSH-Px. Notably, the effect of troxerutin on oxidative stress was not totally abolished by the knockdown of HIF-1α, the effect was only reduced, meaning that other molecule or pathways may be involved in the protection of troxerutin against oxidative stress. These results suggested that troxerutin attenuated OGD/R-induced oxidative stress, partly by enhancing PI3K/AKT/HIF-1α signaling pathway.
Figure 5.
Effects of HIF-1α-siRNA on oxidative and antioxidative products in the presence or absence of TX in OGD/R-treated H9C2 cells. H9C2 cells were transfected with HIF-1α-siRNA or NS-siRNA for 6 h, and then treated with TX (10 µM) followed by OGD (6 h)/R (18 h) treatment. (A) Intracellular ROS generation was measured using a DCFH-DA assay and the fluorescence intensity was observed under a fluorescent microscope. (B) The DCFH-DA assay was quantitatively analyzed using a flow cytometer. The (C) MDA content, (D) SOD activity and (E) GSH-Px activity were measured using commercial kits. Data are presented as the mean ± SD (n=3). *P<0.05, **P<0.01 vs. NS-siRNA + control; #P<0.05, ##P<0.01 vs. NS-siRNA + OGD/R; &P<0.05 vs. NS-siRNA + TX + OGD/R group. OGD/R, oxygen-glucose deprivation and reoxygenation; siRNA, small interfering RNA; HIF-1α, hypoxia-inducible factor-1α; NS, non-specific; TX, troxerutin; ROS, reactive oxygen species; MDA, malonaldehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; DCFH-DA, 2′,7′-dichlorodihydrofluorescein diacetate.
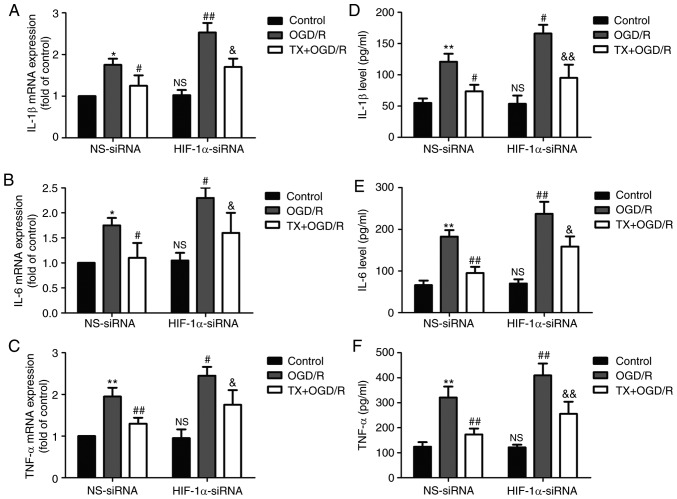
HIF-1α knockdown eliminates the protective effect of troxerutin on the inflammatory response in OGD/R-treated H9C2 cells
The effect of troxerutin on inflammation in OGD/R-treated H9C2 cells was investigated. Troxerutin treatment mitigated OGD/R-induced increases in inflammatory markers including IL-1β (Fig. 6A), IL-6 (Fig. 6B) and TNF-α (Fig. 6C) mRNA expression in H9C2 cells, while these effects were reduced by HIF-1α-siRNA. In addition, OGD/R-induced an increase in the levels of IL-1β (Fig. 6D), IL-6 (Fig. 6E), and TNF-α (Fig. 6F) in the culture supernatant, which were also reversed by troxerutin treatment. However, these effects of troxerutin on the inflammatory markers reduced by HIF-1α-siRNA. These results indicated that troxerutin attenuated OGD/R-induced inflammation by promoting the PI3K/AKT/HIF-1α signaling pathway.
Figure 6.
Effects of HIF-1α-siRNA on inflammatory cytokines in the presence or absence of TX in OGD/R-treated H9C2 cells. H9C2 cells were transfected with HIF-1α-siRNA or NS-siRNA for 6 h and then treated with TX (10 µM) followed by OGD (6 h)/R (18 h) treatment. The expression levels of (A) IL-1β, (B) IL-6 and (C) TNF-α were determined using reverse transcription-quantitative PCR. The levels of (D) IL-1β, (E) IL-6 and (F) TNF-α in the culture supernatant were analyzed using ELISA kits. Data are presented as the mean ± SD (n=3). *P<0.05, **P<0.01 vs. NS-siRNA + control; #P<0.05, ##P<0.01 vs. NS-siRNA + OGD/R; &P<0.05, &&P<0.01 vs. NS-siRNA + TX + OGD/R group. OGD/R, oxygen-glucose deprivation and reoxygenation; siRNA, small interfering RNA; HIF-1α, hypoxia-inducible factor-1α; NS, non-specific; TX, troxerutin; IL, interleukin; TNF-α, tumor necrosis factor-α.
Discussion
Mortality from MI/R remains high and there is an unmet need for exploring innovative and effective therapeutic strategies for MI/R injury (1,2). Chinese herbal medicines have long been used as alternative therapies against MI/R injury because of their anti-apoptotic, anti-oxidative stress, and anti-inflammatory activities (33). Troxerutin, a trihydroxyethylated derivative of the natural bioflavonoid rutin, elicits a therapeutic action in cardiovascular disease (6,7). However, the impacts of troxerutin on MI/R injury remain unclear. The present study, for the first time to the best of our knowledge, demonstrated that troxerutin attenuated MI/R injury by suppressing oxidative stress and inflammation associated with enhancing the activation of the PI3K/AKT/HIF-1α signaling pathway.
In recent years, emerging evidence has identified the cardioprotective properties of troxerutin in MI/R (10,31). Badalzadeh et al (13) reported that troxerutin attenuates MI/R injury through pharmacological preconditioning. In addition, preconditioning with troxerutin significantly reduced myocardial infarct size, the levels of inflammatory cytokines and inhibited cardiomyocyte apoptosis, ameliorating MI/R injury (34). Consistent with these previous studies, the present study revealed that troxerutin increased H9C2 cell viability, reduced LDH release and apoptosis under OGD/R condition, attenuating OGD/R-induced H9C2 cell injury.
HIF-1α is an important transcription factor that modulates the cellular response to hypoxia; an increase in the expression of HIF-1α is one of the first adaptations of the myocardium to ischemic injury (35,36). Previous studies confirm that the PI3K/AKT pathway can activate HIF-1α, thus playing an important role in cell growth and cell survival (22,30). Research has shown that the activation of the PI3K/AKT/HIF-1α pathway mediates protection against lung I/R injury (28), and the upregulation and activation of the PI3K/AKT/HIF-1α pathway is involved in the protective effects of microRNAs on cardiomyocytes (37). Troxerutin preconditioning has been shown to protect against MI/R injury by inhibiting apoptosis and activating the PI3K/AKT pathway (34). In present study, It was found that OGD/R treatment significantly reduced the expression of PI3K, p-AKT and HIF-1α, indicating that OGD/R led to the inhibition of the PI3K/AKT/HIF-1α pathway. However, troxerutin pretreatment reversed these effects. In addition, LY294002, an inhibitor of the PI3K pathway, attenuated troxerutin-induced upregulation of the PI3K/AKT/HIF-1α pathway and the protection against OGD/R-induced H9C2 cell injury. HIF-1α-siRNA transfections partially reversed troxerutin-induced protection against OGD/R injury. These results indicated that the PI3K/AKT/HIF-1α signaling pathway mediated the cardioprotective effect of troxerutin against OGD/R injury.
Oxidative damage plays a causal role in the pathogenesis of MI/R (17), oxidative stress induces injury through the accumulation of ROS in I/R-injured hearts and plays important roles in cardiac apoptosis (38). Although previous studies have demonstrated that troxerutin has many different biological activities, including antioxidant and anti-inflammatory activities, in diabetic male rats (7), cerebral ischemia (39) and cardiac disease in metabolic syndrome patients (40), the effects of troxerutin on oxidative stress in MI/R injury remain unclear. A number of enzymes, such as SOD and GSH, provide cellular protection against damage from oxygen-derived free radicals (41). MDA is the end-product of the oxygen-derived free radicals and lipid oxidation, which reflects the damage caused by ROS (42). In present study, the results indicated that troxerutin pretreatment eliminated OGD/R-induced oxidative stress as illustrated by the enhancement of antioxidant systems and the inhibition of oxidative stress. In addition, HIF-1α-siRNA partially reversed the inhibition of troxerutin on oxidative stress under OGD/R condition. To the best of our knowledge, the present study is the first to report that troxerutin attenuates OGD/R-induced oxidative stress by promoting the PI3K/AKT/HIF-1α signaling pathway.
Inflammation is an important contributor to MI/R injury, which is triggered and aggravated during the process of I/R, resulting in cardiomyocyte death (43). Cardiac inflammation is characterized by the upregulation of pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α, which are major contributors to MI/R injury (43). Previous studies revealed that troxerutin can regulate the inflammatory response in a variety of diseases, such as cerebral I/R injuries (44), kidney damage (45) and MI/R injury (13). Although, the PI3K/AKT signaling pathway has been reported to be implicated in human malignancies, it can suppress inflammation, however, the effects of HIF-1α signaling on inflammation remain to be explored. The present study demonstrated that troxerutin pretreatment mitigated OGD/R-induced inflammatory response as shown by the decreases in the levels of IL-1β, IL-6 and TNF-α. Furthermore, the present study was the first, to the best of our knowledge, to find that HIF-1α knockdown partially reversed the inhibition of troxerutin on inflammation in OGD/R-treated H9C2 cells. Taken together, these results indicated that the PI3K/AKT/HIF-1α signaling pathway contributes to the protection of troxerutin against ODG/R injury by inhibiting the inflammatory response.
The results of the present study suggested that HIF-1a knockdown further increased apoptosis, oxidative stress and inflammation compared with NS-siRNA, and in these cases, although troxerutin still had a partial protective effect against MI/R-induced apoptosis, oxidative stress and inflammation in H9C2 cell, the beneficial effects were reduced compared with the NS-siRNA group. A previous study reported that troxerutin reduced 2,2,4,4-tetrabromodiphenyl ether-induced oxidative stress and inflammatory injury, which was dependent on increasing nuclear factor erythroid 2-reated factor 2 (Nrf2) activity and inhibiting NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome signaling in kidney damage (46). ROS- NLRP3 inflammasome activation and the Nrf2-antioxidant response element both play an important role in MI/R injury (47,48). In addition, microRNAs was also reported to be involved in the cardioprotective effects of troxerutin during I/R injury (31). Hence, these pathways may also contribute to the antioxidant, anti-inflammatory and cardioprotective properties of troxerutin in MI/R injury, and deserves further investigation in future experiments.
In conclusion, troxerutin exerted protective effects against MI/R injury by inhibiting oxidative stress and inflammation. The underlying mechanisms for these phenomena involved the activation of the PI3K/AKT/HIF-1α signaling pathway. The findings of the present study facilitate finding new molecular therapeutic targets for MI/R injury and provide a novel insight into therapeutic development as an adjuvant therapy for MI/R injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZPY participated in designing the research and writing of the paper. HQY and JL contributed equally to data analysis. CL, XH and XSS were involved in designing the study, performing the experiments and revising the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
References
- 1.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang CF. Clinical manifestations and basic mechanisms of myocardial ischemia/reperfusion injury. Ci Ji Yi Xue Za Zhi. 2018;30:209–215. doi: 10.4103/tcmj.tcmj_33_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donato M, Evelson P, Gelpi RJ. Protecting the heart from ischemia/reperfusion injury: An update on remote ischemic preconditioning and postconditioning. Curr Opin Cardiol. 2017;32:784–790. doi: 10.1097/HCO.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 5.Hentia C, Rizzato A, Camporesi E, Yang Z, Muntean DM, Săndesc D, Bosco G. An overview of protective strategies against ischemia/reperfusion injury: The role of hyperbaric oxygen preconditioning. Brain Behav. 2018;8:e00959. doi: 10.1002/brb3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Z, Zhang L, Wang Y, Gao H, Li X, Huang X, Huang T. Effects of rutin and its derivatives on citrinin production by Monascus aurantiacus Li AS3.4384 in liquid fermentation using different types of media. Food Chem. 2019;284:205–212. doi: 10.1016/j.foodchem.2019.01.109. [DOI] [PubMed] [Google Scholar]
- 7.Zavvari Oskuye Z, Mirzaei Bavil F, Hamidian GR, Mehri K, Qadiri A, Ahmadi M, Oghbaei H, Vatankhah AM, Keyhanmanesh R. Troxerutin affects the male fertility in prepubertal type 1 diabetic male rats. Iran J Basic Med Sci. 2019;22:197–205. doi: 10.22038/ijbms.2018.32678.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raja B, Saranya D, Prabhu R. Role of flavonoid troxerutin on blood pressure, oxidative stress and regulation of lipid metabolism. Front Biosci (Elite Ed) 2019;11:121–129. doi: 10.2741/e851. [DOI] [PubMed] [Google Scholar]
- 9.Xin X, Zhang M, Li X, Lai F, Zhao G. Biocatalytic synthesis of acylated derivatives of troxerutin: Their bioavailability and antioxidant properties in vitro. Microb Cell Fact. 2018;17:130. doi: 10.1186/s12934-018-0976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najafi M, Noroozi E, Javadi A, Badalzadeh R. Anti-arrhythmogenic and anti-inflammatory effects of troxerutin in ischemia/reperfusion injury of diabetic myocardium. Biomed Pharmacother. 2018;102:385–391. doi: 10.1016/j.biopha.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 11.Yu Y, Zheng G. Troxerutin protects against diabetic cardiomyopathy through NFkappaB/AKT/IRS1 in a rat model of type 2 diabetes. Mol Med Rep. 2017;15:3473–3478. doi: 10.3892/mmr.2017.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mokhtari B, Badalzadeh R, Alihemmati A, Mohammadi M. Phosphorylation of GSK-3β and reduction of apoptosis as targets of troxerutin effect on reperfusion injury of diabetic myocardium. Eur J Pharmacol. 2015;765:316–321. doi: 10.1016/j.ejphar.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 13.Badalzadeh R, Baradaran B, Alihemmati A, Yousefi B, Abbaszadeh A. Troxerutin preconditioning and ischemic postconditioning modulate inflammatory response after myocardial ischemia/reperfusion injury in rat model. Inflammation. 2017;40:136–143. doi: 10.1007/s10753-016-0462-8. [DOI] [PubMed] [Google Scholar]
- 14.Gielis JF, Beckers PAJ, Briede JJ, Cos P, Van Schil PE. Oxidative and nitrosative stress during pulmonary ischemia-reperfusion injury: From the lab to the OR. Ann Transl Med. 2017;5:131. doi: 10.21037/atm.2017.03.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Resendiz S, Chinda K, Ong SB, Cabrera-Fuentes H, Zazueta C, Hausenloy DJ. The role of redox dysregulation in the inflammatory response to Acute myocardial ischaemia-reperfusion injury-adding fuel to the fire. Curr Med Chem. 2018;25:1275–1293. doi: 10.2174/0929867324666170329100619. [DOI] [PubMed] [Google Scholar]
- 16.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Montero J, Brito R, Gajardo AI, Rodrigo R. Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J Cardiol. 2018;10:74–86. doi: 10.4330/wjc.v10.i9.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsuki S, Matoba T, Koga JI, Nakano K, Egashira K. Anti-inflammatory nanomedicine for cardiovascular disease. Front Cardiovasc Med. 2017;4:87. doi: 10.3389/fcvm.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinning C, Westermann D, Clemmensen P. Oxidative stress in ischemia and reperfusion: Current concepts, novel ideas and future perspectives. Biomark Med. 2017;11:11031–11040. doi: 10.2217/bmm-2017-0110. [DOI] [PubMed] [Google Scholar]
- 20.Sun XH, Wang X, Zhang Y, Hui J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 2019;177:23–32. doi: 10.1016/j.thromres.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Yao H, Han X, Han X. The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. Am J Cardiovasc Drugs. 2014;14:433–442. doi: 10.1007/s40256-014-0089-9. [DOI] [PubMed] [Google Scholar]
- 22.Agani F, Jiang BH. Oxygen-independent regulation of HIF-1: Novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013;13:245–251. doi: 10.2174/1568009611313030003. [DOI] [PubMed] [Google Scholar]
- 23.Brocato J, Chervona Y, Costa M. Molecular responses to hypoxia-inducible factor 1α and beyond. Mol Pharmacol. 2014;85:651–657. doi: 10.1124/mol.113.089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jianqiang P, Ping Z, Xinmin F, Zhenhua Y, Ming Z, Ying G. Expression of hypoxia-inducible factor 1 alpha ameliorate myocardial ischemia in rat. Biochem Biophys Res Commun. 2015;465:691–695. doi: 10.1016/j.bbrc.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Cui Y, He W, Deng X, Wang Y, Cai M, Wang Y, Pei J, Mei X, Wu P. Effects of triple-mutated hypoxia-inducible factor-1α on angiogenesis and cardiac function improvement in rats with myocardial infarction. Cell Physiol Biochem. 2018;50:2329–2340. doi: 10.1159/000495094. [DOI] [PubMed] [Google Scholar]
- 26.Man S, Chai H, Cui J, Yao J, Ma L, Gao W. Antitumor and anti-metastatic mechanisms of Rhizoma paridis saponins in Lewis mice. Environ Toxicol. 2018;33:149–155. doi: 10.1002/tox.22501. [DOI] [PubMed] [Google Scholar]
- 27.Movafagh S, Crook S, Vo K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: New developments in an old debate. J Cell Biochem. 2015;116:696–703. doi: 10.1002/jcb.25074. [DOI] [PubMed] [Google Scholar]
- 28.Liang S, Wang Y, Liu Y. Dexmedetomidine alleviates lung ischemia-reperfusion injury in rats by activating PI3K/Akt pathway. Eur Rev Med Pharmacol Sci. 2019;23:370–377. doi: 10.26355/eurrev_201901_16785. [DOI] [PubMed] [Google Scholar]
- 29.Liang Z, Chi YJ, Lin GQ, Luo SH, Jiang QY, Chen YK. MiRNA-26a promotes angiogenesis in a rat model of cerebral infarction via PI3K/AKT and MAPK/ERK pathway. Eur Rev Med Pharmacol Sci. 2018;22:3485–3492. doi: 10.26355/eurrev_201806_15175. [DOI] [PubMed] [Google Scholar]
- 30.Wei J, Wu J, Xu W, Nie H, Zhou R, Wang R, Liu Y, Tang G, Wu J. Salvianolic acid B inhibits glycolysis in oral squamous cell carcinoma via targeting PI3K/AKT/HIF-1α signaling pathway. Cell Death Dis. 2018;9:599. doi: 10.1038/s41419-018-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shu L, Zhang W, Huang G, Huang C, Zhu X, Su G, Xu J. Troxerutin attenuates myocardial cell apoptosis following myocardial ischemia-reperfusion injury through inhibition of miR-146a-5p expression. J Cell Physiol. 2019;234:9274–9282. doi: 10.1002/jcp.27607. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Sui H, Zhao J, Wang Y. Osmotin protects H9c2 cells from simulated ischemia-reperfusion injury through AdipoR1/PI3K/AKT signaling pathway. Front Physiol. 2017;8:611. doi: 10.3389/fphys.2017.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Li J, Wang J, Li J, Janicki JS, Fan D. Effects and mechanisms of chinese herbal medicine in ameliorating myocardial ischemia-reperfusion injury. Evid Based Complement Alternat Med. 2013;2013:925625. doi: 10.1155/2013/925625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shu L, Zhang W, Huang C, Huang G, Su G. Troxerutin protects against myocardial ischemia/reperfusion injury Via Pi3k/Akt pathway in rats. Cell Physiol Biochem. 2017;44:1939–1948. doi: 10.1159/000485884. [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 37.Semenza GL. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 38.Sun N, Meng F, Xue N, Pang G, Wang Q, Ma H. Inducible miR-145 expression by HIF-1a protects cardiomyocytes against apoptosis via regulating SGK1 in simulated myocardial infarction hypoxic microenvironment. Cardiol J. 2018;25:268–278. doi: 10.5603/CJ.a2017.0105. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, Zhang W, Huang C, Liang Q, Bao H, Gong Z, Xu M, Wang Z, Wen M, Cheng X. FoxO4 promotes myocardial ischemia-reperfusion injury: The role of oxidative stress-induced apoptosis. Am J Transl Res. 2018;10:2890–2900. [PMC free article] [PubMed] [Google Scholar]
- 40.Ma W, Wang S, Liu X, Tang F, Zhao P, Cheng K, Zheng Q, Zhuo Y, Zhao X, Li X, Feng W. Protective effect of troxerutin and cerebroprotein hydrolysate injection on cerebral ischemia through inhibition of oxidative stress and promotion of angiogenesis in rats. Mol Med Rep. 2019;19:3148–3158. doi: 10.3892/mmr.2019.9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geetha R, Sathiya Priya C, Anuradha CV. Troxerutin abrogates mitochondrial oxidative stress and myocardial apoptosis in mice fed calorie-rich diet. Chem Biol Interact. 2017;278:74–83. doi: 10.1016/j.cbi.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czerska M, Mikołajewska K, Zieliński M, Gromadzińska J, Wąsowicz W. Today's oxidative stress markers. Med Pr. 2015;66:393–405. doi: 10.13075/mp.5893.00137. [DOI] [PubMed] [Google Scholar]
- 44.Ong SB, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek XY, Cabrera-Fuentes HA, Hausenloy DJ. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87. doi: 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhào H, Liu Y, Zeng J, Li D, Zhang W, Huang Y. Troxerutin and cerebroprotein hydrolysate injection protects neurovascular units from oxygen-glucose deprivation and reoxygenation-induced injury in vitro. Evid Based Complement Alternat Med. 2018;2018:9859672. doi: 10.1155/2018/9859672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shan Q, Zheng GH, Han XR, Wen X, Wang S, Li MQ, Zhuang J, Zhang ZF, Hu B, Zhang Y, Zheng YL. Troxerutin protects kidney tissue against BDE-47-induced inflammatory damage through CXCR4-TXNIP/NLRP3 signaling. Oxid Med Cell Longev. 2018;2018:9865495. doi: 10.1155/2018/9865495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shan Q, Zhuang J, Zheng G, Zhang Z, Zhang Y, Lu J, Zheng Y. Troxerutin reduces kidney damage against BDE-47-induced apoptosis via inhibiting NOX2 activity and increasing Nrf2 activity. Oxid Med Cell Longev. 2017 doi: 10.1155/2017/6034692. 6034692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu Z, Lei S, Zhao B, Wu Y, Su W, Liu M, Meng Q, Zhou B, Leng Y, Xia ZY. NLRP3 inflammasome activation-mediated pyroptosis aggravates myocardial ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev. 2017;2017:9743280. doi: 10.1155/2017/9743280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen Y, Liu X, Shi J, Wu X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol Macromol. 2019;125:496–502. doi: 10.1016/j.ijbiomac.2018.11.190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.