Abstract
Patients with advanced gastric cancer (GC) have a poor prognosis with a median overall survival of 10–12 months. Long non-coding RNA nicotinamide nucleotide transhydrogenase-antisense RNA1 (NNT-AS1) and sex-determining region Y-related high mobility group box 4 (SOX4) have been reported to be associated with the progression of various types of cancer; however, the regulatory mechanism between NNT-AS1 and SOX4 in GC is not completely understood. Reverse transcription-quantitative PCR was used to detect the expression levels of NNT-AS1, microRNA (miR)-142-5p and SOX4. Western blotting was performed to assess the protein expression levels of SOX4, β-catenin, c-Myc, Bcl-2 and E-cadherin. The proliferation, apoptosis, migration and invasion of GC cells were determined using MTT, flow cytometry and Transwell assays. The relationship between miR-142-5p and NNT-AS1 or SOX4 was investigated using a dual-luciferase reporter assay. NNT-AS1 and SOX4 were upregulated, whereas miR-142-5p was downregulated in GC tissues and cells compared with normal tissues and cells. Both NNT-AS1 and SOX4 knockdown inhibited GC cell proliferation, migration and invasion, and enhanced GC cell apoptosis. Moreover, the results indicated that NNT-AS1 modulated SOX4 expression by sponging miR-142-5p. In addition, SOX4 overexpression reversed NNT-AS1 knockdown-mediated effects on GC cell proliferation, apoptosis, migration and invasion. NNT-AS1 knockdown blocked the Wnt/β-catenin signaling pathway via the miR-142-5p/SOX4 axis. Collectively, the present study indicated that NNT-AS1 knockdown decreased GC cell proliferation, migration and invasion, and induced GC cell apoptosis by regulating the miR-142-5p/SOX4/Wnt/β-catenin signaling pathway axis.
Keywords: gastric cancer, long non-coding RNA nicotinamide nucleotide transhydrogenase-antisense RNA1, microRNA-142-5p, sex-determining region Y-related high mobility group box 4
Introduction
Gastric cancer (GC) displays the highest morbidity and mortality among all malignant tumors in the majority of Asian countries, especially in China, where it ranks second among the most common types of cancer (1). With the development of medical technology, surgery is the only radical treatment strategy available for GC. Although the 5-year survival rate of patients with stage I and II GC is >70%, the rate of early diagnosis is low and the majority of patients are diagnosed during the advanced stages of the disease, when surgery is no longer an optimal treatment strategy (2,3). Patients with advanced GC have a poor prognosis, with a median overall survival of 10–12 months (4). At present, there are a number of issues regarding the early detection of GC; therefore, it is important to explore the pathogenesis of GC to provide a theoretical basis for the development of effective diagnostic and therapeutic strategies for GC.
Long non-coding RNAs (lncRNAs) are transcripts that have no protein-coding potential and are >200 nucleotides in length, but display important regulatory roles in tissue physiology and disease processes (5). lncRNAs can be used as potential biomarkers for the diagnosis and prognosis of various types of cancer, but can also enhance the specificity and sensitivity of existing biomarkers (6). The lncRNA nicotinamide nucleotide transhydrogenase-antisense RNA1 (NNT-AS1), located at 5p12 with 3 exons, is a newly identified lncRNA (7). NNT-AS1 has been reported to be involved in the progression of lung, ovarian, breast and gastric cancer, as well as osteosarcoma (8–13). It has also been reported that NNT-AS1 expression was increased in GC tissues and cell lines (14); however, the molecular mechanism underlying NNT-AS1 in GC is not completely understood and requires further investigation.
MicroRNAs (miRNAs) are a class of non-coding RNAs that are ~21 nucleotides in length and are involved in a series of processes associated with human physiology and pathology (15,16). As stable biomarkers in tumors and circulation, miRNAs serve as an attractive option for the development of tumor detection, diagnosis and prognosis assessment strategies (17,18). Moreover, it has been reported that miR-142-5p is associated with the progression of a variety of different types of cancer (19–22). Furthermore, Yan et al (22) reported that miR-142-5p knockdown could promote tumor metastasis in GC; however, the molecular mechanism underlying miR-142-5p in GC has not been previously reported.
Sex-determining region Y-related high mobility group box 4 (SOX4), a member of the sex-determining region Ybox family, is associated with a number of different types of cancer, including GC (23–26). However, the regulatory mechanism between NNT-AS1 and SOX4 in GC has not been previously reported.
The present study demonstrated that NNT AS1 serves a cancerogenic role in GC. NNT AS1 knockdown curbed GC progression via regulating the miR-142-5p/SOX4 axis, providing a potential target for GC treatment.
Materials and methods
Patient samples
A total of 25 paired GC tissues and adjacent non-cancerous tissues were collected from patients with GC who underwent surgery resection at The First People's Hospital of Guangyuan. Participants in the present study had not received chemotherapy, radiation or other anticancer drugs before surgery. The patients included 14 males and 11 females (age, 35–72 years). The histology and pathology of the fresh tissue samples were examined by at least two pathologists. Adjacent non-cancerous tissues were sampled ≥3 cm away from tumor margin. The samples were collected from August 2014 to August 2017. All samples were instantly frozen in liquid nitrogen and stored at −80°C until further analysis. The present study was approved by the Ethics Committee of The First People's Hospital of Guangyuan. Written informed consent was obtained from all participants.
Cell culture and transfection
The human GC AGS and HGC-27 cell lines and the human gastric mucosal epithelial GES-1 cell line were acquired from BeNa Culture Collection (Beijing Beina Chunglian Biotechnology Research Institute). Cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2.
For transfection, short hairpin (sh)RNAs targeting NNT-AS1 (sh-NNT-AS1: 5′-GCAATGATGGGCCACTAAATTG-3′) or SOX4 (sh-SOX4: 5′-AGCAAACCGCACGCCAAGCTCATCCTGGC-3′), and a shRNA negative control (sh-NC) were synthesized by Shanghai GenePharma Co., Ltd. miR-142-5p mimic (miR-142-5p: 5′-CAUAAAGUAGAAAGGAGCACUACU-3′), miR-142-5p inhibitor (anti-miR-142-5p: 5′-AGTAGTGCTCCTTTCTACTTTATG-3′) and their corresponding negative controls (miR-NC or anti-miR-NC) were purchased from Shanghai GenePharma Co., Ltd. The sequences of NNT-AS1 and SOX4 were cloned into the pcDNA 3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.) to establish NNT-AS1 and SOX4 overexpression vectors, respectively. The pcDNA 3.1 vectors (pcDNA) were used as a negative control for overexpression vectors. AGS and HGC-27 cells were transfected with sh-NC (500 ng/µl), sh-NNT-AS1 (500 ng/µl), sh-SOX4 (500 ng/µl), miR-142-5p (40 nM), anti-miR-142-5p miR-NC (40 nM), anti-miR-NC (40 nM), pcDNA (15 nM), NNT-AS1 (15 nM) or SOX4 (15 nM) using Lipofectamine® 2000 reagent (Thermo Fisher Scientific, Inc.) when cell confluence reached 80%. According to the manufacturer's protocol. The subsequent experiments were performed 48 h post-transfection.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from GC tissues and cells using TRlzol® reagent (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Total RNA was reverse-transcribed into cDNA using the RT Reagent kit (Takara Biotechnology Co., Ltd.) or the miRNA Reverse Transcription kit (Thermo Fisher Scientific, Inc.). Subsequently, qPCR was performed using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.). The following primer pairs were used for qPCR: NNT-AS1 forward, 5′-AGTTCCACCAAGTTTCTTCA-3′ and reverse, 5′-AGGTTTTGCCAGCATAGAC-3′; SOX4 forward, 5′-GTGAGCGAGATCTCGGG-3′ and reverse, 5′-CAGGTTGGAGATGCTGGACTC-3′; GAPDH forward, 5′-CACATCGCTCAGACACCATG-3′ and reverse, 5′-TGACGGTGCCATTGGAATTT-3′; U6: Forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; and miR-142-5p: Forward, 5′-AACTCCAGCTGGTCCTTAG-3′ and reverse, 5′-TCTTGAACCCTCATCCTGT-3′. The amplification parameters were as follows: Denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and extension at 72°C for 1 min. mRNA and miRNA expression levels were quantified using the 2−ΔΔCq method (27) and normalized to the internal reference genes GAPDH and U6, respectively.
Western blotting
Total protein was isolated from GC tissues and cells using RIPA buffer (Beyotime Institute of Biotechnology) with a protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Total protein was quantified via a BCA Protein Aassay kit (Beyotime Institute of Biotechnology). Total protein (30 µg) was separated via 10% SDS-PAGE and transferred onto PVDF membranes. Subsequently the membranes were blocked with 5% non-fat milk in PBS for 2 h at room temperature. The membranes were incubated at 4°C overnight with the following primary antibodies: Anti-SOX4 (1:1,000; cat. no. sc-518016; Santa Cruz Biotechnology, Inc.), anti-E-cadherin (1:500; cat. no. sc-21791 Santa Cruz Biotechnology, Inc.), anti-GAPDH (1:2,000; cat. no. sc-32233; Santa Cruz Biotechnology, Inc.), anti-β-catenin (1:200; cat. no. ab16051; Abcam), anti-c-Myc (1:200; cat. no. ab168727; Abcam) and anti-Bcl-2 (1:100; cat. no. ab185002; Abcam). Following primary incubation, the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse (1:2,000; cat. no. sc-2354; Santa Cruz Biotechnology, Inc.) or anti-rabbit (1:2,000; cat. no. ab97051; Abcam) antibodies for 1 h at room temperature. Protein bands were visualized using Immobilon Western Chemiluminescent HRP Substrate (EMD Millipore). Protein expression levels were semi-quantified using ImageJ software (version 1.50; National Institutes of Health) with GAPDH as the loading control.
MTT assay
Cell proliferation was detected using the MTT assay (Promega Corporation), according to the manufacturer's instructions. AGS and HGC-27 cells were seeded (5×103 cells/well) into 96-well plates and cultured for 24, 48 or 72 h at 37°C. Subsequently, AGS and HGC-27 cells were transfected. Following transfection, 20 µl MTT (5 mg/ml) was added to each well and incubated for 4 h at 37°C. The purple formazan crystals were dissolved using 200 µl DMSO. The optical density of each well was measured at a wavelength of 490 nm using a microplate reader.
Migration and invasion assays
The migration and invasion of AGS and HGC-27 cells were determined using Transwell plates (pore size, 8 mm; BD Biosciences). Transfected AGS and HGC-27 cells were seeded (1×105 cells/well) into the upper chambers of Transwell plates with serum-free RPMI-1640 medium. RPMI-1640 medium containing 10% FBS was plated into the lower chambers of Transwell plates. Following incubation for 48 h in humidified air at 37°C with 5% CO2, cells on the lower surface of the Transwell membrane were stained using 0.1% crystal violet at room temperature for 10 min. Stained cells were observed using an inverted microscope (magnification, ×100). For the invasion assay, the upper chambers of Transwell plates were precoated with Matrigel® (BD Biosciences): Transwell plates were covered with cold Matrigel, and then kept at 37°C for 30 min.
Flow cytometry assay
Following transfection, AGS and HGC-27 cell apoptosis was detected using the FITC Annexin V Apoptosis Detection kit I (BD Biosciences). Briefly, transfected AGS and HGC-27 cells were collected and re-suspended in 500 µl binding buffer. Subsequently, cells were incubated with 5 µl Annexin V-FITC specific antibodies and 5 µl propidium iodide for 15 min in the dark at room temperature. Apoptotic cells were detected by flow cytometry using a FACScan® flow cytometer (BD Biosciences). The data were analyzed with the CellQuest software (version 5.1; BD Biosciences).
Dual-luciferase reporter assay
The binding sites between miR-142-5p and NNT-AS1 or SOX4 were predicted by starBase (version 2.0) (starbase.sysu.edu.cn/starbase2). The wild-type (WT) and mutant (MUT) sequences of NNT-AS1 and the 3′-untranslated region (UTR) of SOX4 were synthesized and inserted downstream of pGL3-control luciferase reporter vectors (Promega Corporation) to construct the following luciferase reporter vectors: WT-NNT-AS1, MUT-NNT-AS1, SOX4 3′UTR-WT and SOX4 3′UTR-MUT. AGS and HGC-27 cells were co-transfected with the luciferase reporter vectors and miR-142-5p or miR-NC using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) when cell growth reached 70% and cultured for 48 h at 37°C. Lipofectamine® 2000 reagent was used according to the manufacturer's protocol. Luciferase activities were detected using the Dual-Luciferase Reporter assay kit (Promega Corporation). The luciferase intensities were determined by normalizing the firefly luminescence to Renilla luminescence.
Statistical analysis
The experiments were repeated at least three times. Statistical analyses were performed using GraphPad Prism (version 6.00; GraphPad Software, Inc.) and SPSS (version 13.0; SPSS, Inc.) software. Spearman's correlation analysis was used to analyze the correlation between NNT-AS1 and SOX4 or miR-142-5p in GC tissues. Differences between two groups were analyzed using unpaired Student's t-test. The differences between GC tissues and adjacent non-cancerous tissues were analyzed using paired Student's t-test. Differences among multiple groups were analyzed using one-way ANOVA followed by Sidak's post hoc test. Data are presented as the mean ± SD. P<0.05 was considered to indicate a statistically significant difference.
Results
NNT-AS1 and SOX4 are upregulated in GC tissues
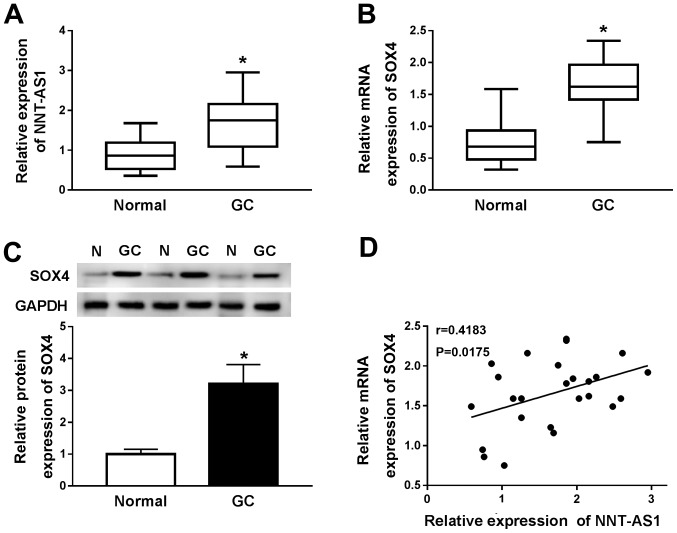
RT-qPCR was performed to measure the expression levels of NNT-AS1 and SOX4 in 25 paired GC and normal adjacent tissues to investigate the role of NNT-AS1 and SOX4 in GC. NNT-AS1 and SOX4 expression levels were significantly increased in GC tissues compared with adjacent normal tissues (Fig. 1A and B). Subsequently, the protein expression levels of SOX4 in GC and adjacent normal tissues were determined by western blotting. Compared with adjacent normal tissues, SOX4 protein expression levels were significantly increased in GC tissues (Fig. 1C). The relationship between NNT-AS1 and SOX4 was further investigated using Spearman's rank test. The results indicated that the expression levels of NNT-AS1 and SOX4 in GC tissues were positively correlated (Fig. 1D). Collectively, the results indicated that NNT-AS1 and SOX4 may serve an oncogenic role in GC.
Figure 1.
Expression levels of NNT-AS1 and SOX4 in GC tissues. mRNA expression levels of (A) NNT-AS1 and (B) SOX4 in 25 paired GC and adjacent normal tissues. (C) Protein expression levels of SOX4 in GC and adjacent normal tissues. (D) Spearman's correlation analysis was performed to assess the relationship between NNT-AS1 and SOX4 expression in GC tissues. *P<0.05 vs. normal. NNT-AS1, nicotinamide nucleotide transhydrogenase-antisense RNA1; SOX4, sex-determining region Y-related high mobility group box 4; GC, gastric cancer; N, normal.
NNT-AS1 knockdown inhibits GC cell proliferation, migration and invasion, and promotes GC cell apoptosis
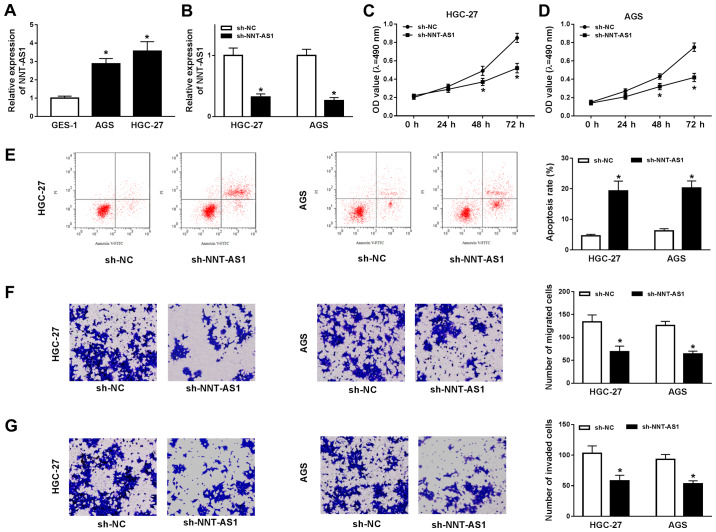
To investigate the role of NNT-AS1 during GC progression, the expression levels of NNT-AS1 in AGS and HGC-27 cells were assessed. NNT-AS1 expression was significantly increased in AGS and HGC-27 cells compared with GES-1 cells (Fig. 2A). Based on the aforementioned finding, the possible role of NNT-AS1 in GC cells was further investigated using loss-of-function experiments. NNT-AS1 expression was significantly reduced in the sh-NNT-AS1 group compared with the sh-NC group in AGS and HGC-27 cells (Fig. 2B). Subsequently, the MTT assay indicated that NNT-AS1 knockdown significantly reduced the proliferation of AGS and HGC-27 cells compared with the sh-NC group (Fig. 2C and D). The flow cytometry results indicated that NNT-AS1 knockdown significantly increased AGS and HGC-27 cell apoptosis compared with the sh-NC group (Fig. 2E). The migration and invasion of AGS and HGC-27 cells was then determined using Transwell assays. The results indicated that the migration and invasion of AGS and HGC-27 cells were significantly decreased in the sh-NNT-AS1 group compared with the sh-NC group (Fig. 2F and G). Overall, the results suggested that NNT-AS1 knockdown inhibited GC cell proliferation, migration and invasion, and induced GC cell apoptosis.
Figure 2.
NNT-AS1 knockdown alters GC cell proliferation, migration, invasion and apoptosis. AGS and HGC-27 cells were transfected with sh-NNT-AS1 or sh-NC. (A) Expression of NNT-AS1 in GES-1, AGS and HGC-27 cells. (B) Transfection efficiency of NNT-AS1 in AGS and HGC-27 cells. The MTT assay was performed to determine (C) HGC-27 and (D) AGS cell proliferation. (E) Flow cytometry was performed to detect AGS and HGC-27 cell apoptosis. Transwell assays were performed to assess AGS and HGC-27 cell (F) migration and (G) invasion. The cells were observed using an inverted microscope (magnification, ×100). *P<0.05 vs. sh-NC. NNT-AS1, nicotinamide nucleotide transhydrogenase-antisense RNA1; GC, gastric cancer; sh, short hairpin RNA; NC, negative control; PI, propidium iodide; OD, optical density.
SOX4 knockdown decreases GC cell proliferation, migration and invasion, and induces GC cell apoptosis
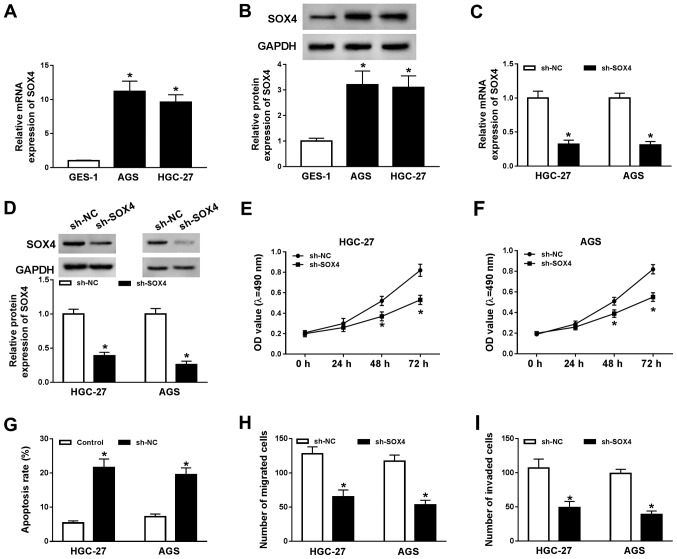
To explore the function of SOX4 during GC progression, the mRNA and protein expression levels of SOX4 in GES-1, AGS and HGC-27 cells were determined by RT-qPCR and western blotting, respectively. The mRNA and protein expression levels of SOX4 were significantly increased in AGS and HGC-27 cells compared with GES-1 cells (Fig. 3A and B). Subsequently, AGS and HGC-227 cells were transfected with sh-SOX4 or sh-NC to knockdown SOX4 expression. Compared with the sh-NC group, the mRNA and protein expression levels of SOX4 were significantly decreased in the sh-SOX4 group (Fig. 3C and D). Subsequently, the effects of SOX4 knockdown on AGS and HGC-27 cell proliferation, migration, invasion and apoptosis were investigated. The MTT assay indicated that SOX4 knockdown significantly decreased AGS and HGC-27 cell proliferation compared with the sh-NC group (Fig. 3E and F). The flow cytometry results suggested that SOX4 knockdown significantly increased AGS and HGC-27 cell apoptosis compared with the sh-NC group (Fig. 3G). Moreover, the results of the Transwell assays indicated that SOX4 knockdown significantly reduced AGS and HGC-27 cell migration and invasion (Fig. 3H and I). In summary, the results indicated that SOX4 knockdown inhibited GC cell proliferation, migration and invasion, and induced GC cell apoptosis.
Figure 3.
Effects of SOX4 knockdown on GC cell proliferation, migration, invasion and apoptosis. AGS and HGC-27 cells were transfected with sh-SOX4 or sh-NC. (A) mRNA and (B) protein expression levels of SOX4 in GES-1, AGS and HGC-27 cells. The transfection efficiency of sh-SOX4 was determined by (C) reverse transcription-quantitative PCR and (D) western blotting in AGS and HGC-27 cells. Effect of SOX4 knockdown on GC cell proliferation in (E) HGC-27 and (F) AGS cells. Effect of SOX4 knockdown on GC cell (G) apoptosis, (H) migration and (I) invasion. *P<0.05 vs. sh-NC. SOX4, sex-determining region Y-related high mobility group box 4; GC, gastric cancer; sh, short hairpin RNA; NC, negative control; OD, optical density.
SOX4 overexpression reverses NNT-AS1 knockdown- mediated effects on GC cell proliferation, apoptosis, migration and invasion
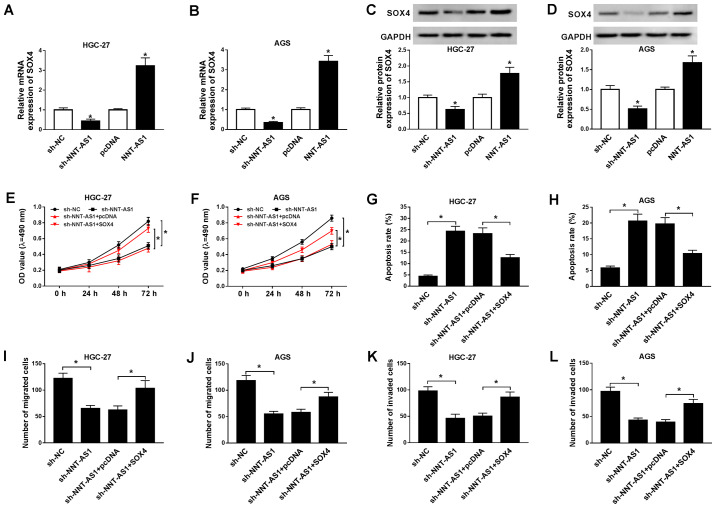
To further investigate the relationship between NNT-AS1 and SOX4 during GC progression, the expression levels of NNT-AS1 in AGS and HGC-27 cells transfected with pcDNA-NNT-AS1 or pcDNA were determined. NNT-AS1 expression was significantly upregulated in AGS and HGC-27 cells transfected with pcDNA-NNT-AS1 compared with the pcDNA group (Fig. S1). The mRNA and protein expression levels of SOX4 in AGS and HGC-27 cells transfected with sh-NNT-AS1, sh-NC, pcDNA or NNT-AS1 were determined by RT-qPCR and western blotting, respectively. NNT-AS1 knockdown significantly decreased the mRNA and protein expression levels of SOX4 in AGS and HGC-27 cells, whereas NNT-AS1 overexpression significantly increased the mRNA and protein expression levels of SOX4 in AGS and HGC-27 cells, compared with the corresponding control groups (Fig. 4A-D). Moreover, SOX4 mRNA and protein expression levels were upregulated in AGS and HGC-27 cells transfected with SOX4 compared with the pcDNA group (Fig. S2). Furthermore, the effects of SOX4 overexpression on AGS and HGC-27 cell proliferation, migration, invasion and apoptosis following NNT-AS1 knockdown were investigated. NNT-AS1 knockdown-mediated inhibition of AGS and HGC-27 cell proliferation was reversed by SOX4 overexpression (Fig. 4E and F). Furthermore, SOX4 overexpression reversed NNT-AS1 knockdown-mediated AGS and HGC-27 cell apoptosis induction (Fig. 4G and H). The results of the Transwell assays also indicated that SOX4 overexpression reversed NNT-AS1 knockdown-mediated inhibition of AGS and HGC-27 cell migration and invasion (Fig. 4I-L). Collectively, the results suggested that SOX4 overexpression reversed NNT-AS1 knockdown-mediated effects on GC cell proliferation, apoptosis, migration and invasion.
Figure 4.
SOX4 overexpression reverses NNT-AS1 knockdown-mediated effects on GC cell proliferation, apoptosis, migration and invasion. (A-D) AGS and HGC-27 cells were transfected with sh-NNT-AS1, sh-NC, pcDNA, or NNT-AS1. The mRNA expression levels of SOX4 in (A) HGC-27 and (B) AGS cells. Protein expression levels of SOX4 in (C) HGC-27 and (D) AGS cells. *P<0.05 vs. sh-NC or pcDNA. (E-L) AGS and HGC-27 cells were transfected with sh-NNT-AS1, sh-NC, sh-NNT-AS1 + pcDNA or sh-NNT-AS1 + SOX4. Effect of NNT-AS1 knockdown and SOX4 overexpression on (E) HGC-27 and (F) AGS cell proliferation. Effect of NNT-AS1 knockdown and SOX4 overexpression on (G) HGC-27 and (H) AGS cell apoptosis. Effect of NNT-AS1 knockdown and SOX4 overexpression on (I) HGC-27 and (J) AGS cell migration. Effect of NNT-AS1 knockdown and SOX4 overexpression on (K) HGC-27 and (L) AGS cell invasion. *P<0.05 vs. sh-NC or sh-NNT-AS1 + SOX4. SOX4, sex-determining region Y-related high mobility group box 4; NNT-AS1, nicotinamide nucleotide transhydrogenase-antisense RNA1; GC, gastric cancer; sh, short hairpin RNA; NC, negative control; OD, optical density.
NNT-AS1 regulates SOX4 expression by sponging miR-142-5p in GC cells
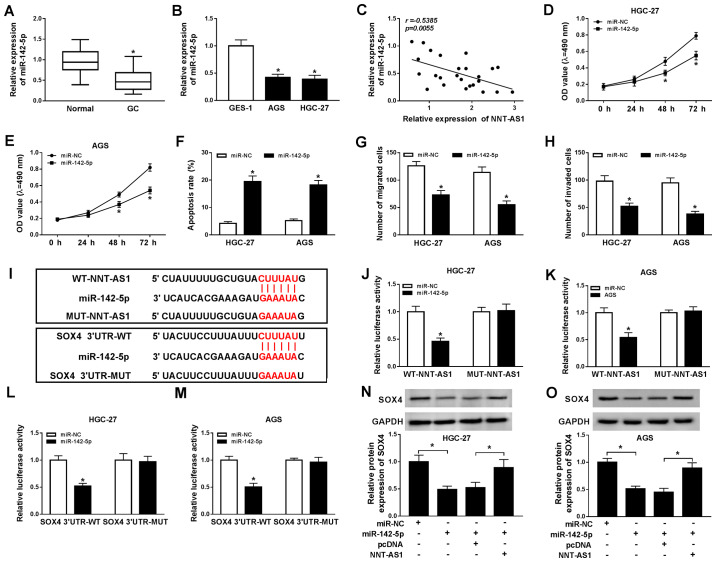
It has been reported that miR-142-5p is associated with the recurrence and poor prognosis of GC (22). The expression of miR-142-5p in GC tissues and cells was determined using RT-qPCR. Compared with the adjacent normal tissues, miR-142-5p expression was significantly reduced in GC tissues (Fig. 5A). Similarly, miR-142-5p expression was significantly decreased in GC cells compared with GES-1 cells (Fig. 5B). Moreover, a negative correlation between NNT-AS1 and miR-142-5p expression was identified in GC tissues (Fig. 5C). Additionally, miR-142-5p expression was significantly increased in AGS and HGC-27 cells transfected with miR-142-5p compared with the control group, and significantly decreased in AGS and HGC-27 cells transfected with anti-miR-142-5p, compared with the corresponding control cells (Fig. S3). Subsequently, AGS and HGC-27 cells were transfected with miR-142-5p or miR-NC to explore the effects of miR-142-5p overexpression on GC cell proliferation, migration, invasion and apoptosis. The MTT assay indicated that miR-142-5p overexpression significantly reduced AGS and HGC-27 cell proliferation compared with the miR-NC group (Fig. 5D and E). Moreover, AGS and HGC-27 cell apoptosis was significantly increased by miR-142-5p overexpression compared with the miR-NC group (Fig. 5F). The Transwell assays indicated that miR-142-5p overexpression significantly inhibited AGS and HGC-27 cell migration and invasion compared with the miR-NC group (Fig. 5G and H). It has been reported that lncRNAs can act as sponges for miRNAs to modulate miRNA expression (28); therefore, the binding sites between miR-142-5p and NNT-AS1 or SOX4 were predicted using starBase. miR-142-5p displayed binding sites for NNT-AS1 and SOX4 (Fig. 5I). Furthermore, the dual-luciferase reporter assay indicated that the luciferase activities of the WT-NNT-AS1 and SOX4 3′UTR-WT reporter vectors were significantly reduced in the miR-142-5p overexpression group compared with the miR-NC group in AGS and HGC-27 cells. By contrast, the luciferase activities of the MUT-NNT-AS1 and SOX4 3′UTR-MUT reporter vectors were not significantly altered by miR-142-5p overexpression in AGS and HGC-27 cells (Fig. 5J-M). In addition, the protein expression levels of SOX4 in the miR-142-5p-overexpression group were significantly decreased compared with the miR-NC group in AGS and HGC-27 cells. MiR-142-5p overexpression-induced effects on SOX4 expression were reversed by NNT-AS1 overexpression (Fig. 5N and O). Collectively, the results indicated that NNT-AS1 regulated SOX4 expression via miR-142-5p in GC cells.
Figure 5.
NNT-AS1 regulates SOX4 expression via miR-142-5p. miR-142-5p expression levels in GC (A) tissues and (B) cells. (C) The relationship between NNT-AS1 and miR-142-5p expression in GC tissues. Effect of miR-143-5p overexpression on (D) HGC-27 and (E) AGS cell proliferation. Effect of miR-143-5p overexpression on HGC-27 and AGS cell (F) apoptosis, (G) migration and (H) invasion. (I) The binding sites between miR-142-5p and NNT-AS1 or SOX4 were predicted using starBase. The luciferase activities of WT-NNT-AS1, MUT-NNT-AS1 in (J) HGC-27 and (K) AGS cells. The luciferase activities of SOX4 3′UTR-WT and SOX4 3′UTR-MUT in (L) HGC-27 and (M) AGS cells. Protein expression levels of SOX4 in (N) HGC-27 and (O) AGS cells. *P<0.05 vs. corresponding control. NNT-AS1, nicotinamide nucleotide transhydrogenase-antisense RNA1; SOX4, sex-determining region Y-related high mobility group box 4; miR, microRNA; GC, gastric cancer; WT, wild-type; MUT, mutant; 3′UTR, 3′-untranslated region; NC, negative control; OD, optical density.
NNT-AS1 knockdown blocks the Wnt/β-catenin signaling pathway via the miR-142-5p/SOX4 axis
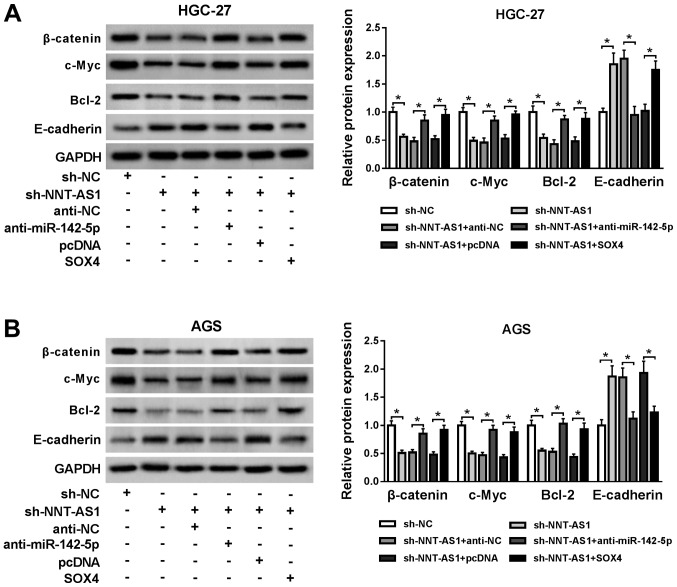
To investigate whether the Wnt/β-catenin signaling was associated with the regulatory mechanism underlying NNT-AS1 during GC, the expression levels of the Wnt/β-catenin signaling pathway-associated proteins, β-catenin, c-Myc, Bcl-2 and E-cadherin, were assessed by western blotting. NNT-AS1 knockdown reduced the protein expression levels of β-catenin, c-Myc and Bcl-2, but enhanced the protein expression level of E-cadherin compared with the sh-NC group in AGS and HGC-27 cells (Fig. 6A and B). However, miR-142-5p knockdown and SOX4 overexpression reversed NNT-AS1 knockdown-mediated effects on the protein expression levels of β-catenin, c-Myc, Bcl-2 and E-cadherin in AGS and HGC-27 cells (Fig. 6A and B). The results suggested NNT-AS1 knockdown blocked the Wnt/β-catenin signaling pathway via the miR-142-5p/SOX4 axis.
Figure 6.
NNT-AS1 knockdown blocks the Wnt/β-catenin signaling pathway via the miR-142-5p/SOX4 axis. Protein expression levels of β-catenin, c-Myc, Bcl-2 and E-cadherin in (A) HGC-27 and (B) AGS cells transfected with sh-NC, sh-NNT-AS1, sh-NNT-AS1 + anti-NC, sh-NNT-AS1 + anti-miR-142-5p, sh-NNT-AS1 + pcDNA or sh-NNT-AS1 + SOX4. *P<0.05, as indicated. NNT-AS1, nicotinamide nucleotide transhydrogenase-antisense RNA1; miR, microRNA; SOX4, sex-determining region Y-related high mobility group box 4; sh, short hairpin RNA; NC, negative control.
Discussion
GC is a malignant tumor with high morbidity and mortality, which seriously threatens human health and life worldwide (29). Recent studies have indicated that lncRNA dysregulation participates in a number of different types of human cancers, including GC (14,30,31); therefore, identifying the regulatory mechanisms underlying lncRNAs is important for the diagnosis and treatment of GC.
It has been reported that NNT-AS1 dysregulation displays vital roles during the progression of various types of cancer. For example, Hua et al (32) reported that NNT-AS1 overexpression promoted cervical cancer cell proliferation and invasion. Another study reported that NNT-AS1 overexpression was related to osteosarcoma progression and poor prognosis (12). Chen et al (14) also revealed that NNT-AS1 expression was notably elevated in GC tissues and cells. In the present study, NNT-AS1 expression levels were upregulated in GC tissues and cells compared with normal tissues and cells. Moreover, NNT-AS1 knockdown inhibited GC cell proliferation, migration and invasion, and promoted GC cell apoptosis. The study of Chen et al (14) also demonstrated that NNT-AS1 knockdown decreased GC cell proliferation and invasion, and induced cell-cycle progression arrest at G0/G1 phase in GC cells. The results of the present study were therefore consistent with the aforementioned studies, which indicated that NNT-AS1 may serve a carcinogenic role during GC.
lncRNAs bind to miRNAs as a competitive endogenous RNA to regulate the expression of miRNA target genes (28). Li et al (9) reported that NNT-AS1 accelerated breast cancer cell proliferation and metastasis by upregulating zinc finger E-box binding homeobox 1 (ZEB1) expression via sponging miR-142-3p (9). Increasing evidence has suggested that abnormal miRNA expression displays essential roles in tumorigenesis (33,34). In hepatocellular cancer cells, miR-142-5p enhances cell apoptosis and suppresses cell proliferation (35). Moreover, enhanced miR-142-5p expression induced cell apoptosis and inhibited cell proliferation in osteosarcoma (36). In the present study, the results indicated that miR-142-5p acted as a target for NNT-AS1. Moreover, miR-142-5p expression was decreased in GC tissues and cells compared with normal tissues and cells, and the expression of miR-142-5p in GC tissues was negatively correlated with NNT-AS1. In addition, miR-142-5p overexpression induced cell apoptosis and decreased cell proliferation, migration and invasion. A previous study demonstrated that miR-142-5p inhibition enhanced GC cell migration (22); therefore, it was hypothesized that miR-142-5p may display an anticancer role during GC. By contrast, miR-142-5p promotes tumor growth in renal cell and colorectal cancer, which might be related to tissue specificity (20,37). Subsequently, the results of the present study indicated that miR-142-5p directly targeted SOX4 in GC cells. Moreover, SOX4 expression was upregulated in GC tissues and cells compared with normal tissues and cells, and SOX4 knockdown promoted GC cell apoptosis and decreased GC cell proliferation, migration, and invasion. A previous study reported that SOX4 overexpression reversed miR-138-mediated inhibition of GC cell proliferation in vitro and in vivo (38). Zhang et al (39) demonstrated that SOX4 knockdown decreased GC cell migration and invasion, indicating that SOX4 served as a carcinogen during GC. In addition, the results of the present study suggested that NNT-AS1 regulated SOX4 expression via miR-142-5p in GC cells. Furthermore, SOX4 overexpression reversed NNT-AS1 knockdown-mediated effect on GC cell proliferation, apoptosis, migration and invasion. Therefore, the present study indicated that NNT-AS1 exerted its effects via the miR-142-5p/SOX4 axis in GC.
It has been reported that aberrant activation of the Wnt/β-catenin signaling pathway is associated with the occurrence and development of various types of cancer, such as colorectal and prostate cancer (30,31,40). Β-catenin is a component of the Wnt signaling cascade that accumulates in the cytoplasm and transfers to the nucleus to activate downstream target genes, including c-Myc (41). E-cadherin is a calcium-dependent cell-cell adhesion molecule that displays vital roles in epithelial cell behavior, tissue formation and cancer inhibition (42). Bcl-2 is an apoptosis suppressor gene that reduces cadherin-mediated expression of cell adhesion, leading to unregulated cell proliferation and tumorigenesis (43). Previous studies have demonstrated that activation of the Wnt/β-catenin signaling pathway promoted GC progression (44,45). In the present study, NNT-AS1 knockdown blocked the Wnt/β-catenin signaling pathway via the miR-142-5p/SOX4 axis in GC cells. The results indicated that the Wnt/β-catenin signaling pathway was involved in the development of GC, which was consistent with the results of previous studies.
In summary, NNT-AS1 and SOX4 expression levels were upregulated in GC tissues and cells compared with normal tissues and cells. NNT-AS1 knockdown and SOX4 knockdown inhibited GC cell proliferation, migration and invasion, and promoted GC cell apoptosis. NNT-AS1 regulated SOX4 expression via sponging miR-142-5p. SOX4 overexpression reversed NNT-AS1 knockdown-mediated effects on GC cell proliferation, migration, invasion and apoptosis. Moreover, NNT-AS1 knockdown inhibited the Wnt/β-catenin signaling pathway via the miR-142-5p/SOX4 axis. Collectively, the results indicated that NNT-AS1 knockdown inhibited GC cell proliferation, migration and invasion, and promoted GC cell apoptosis by modulating the miR-142-5p/SOX4/Wnt/β-catenin signaling pathway. Therefore, NNT-AS1 may serve as a potential biomarker and target for the diagnosis, prognosis assessment and treatment of GC.
Supplementary Material
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JZ and KZ conceived and designed the study. YH performed the experiments. KZ and YH analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y, Kaminishi M. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–316. doi: 10.1007/s10120-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: Prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842–13862. doi: 10.3748/wjg.v20.i38.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22:2403–2414. doi: 10.3748/wjg.v22.i8.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra Gupta S, Nandan Tripathi Y. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int J Cancer. 2017;140:1955–1967. doi: 10.1002/ijc.30546. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Yang L, Hu X, Jiang Y, Hu Y, Liu Z, Liu J, Wen T, Ma Y, An G, Feng G. Upregulated NNT-AS1, a long noncoding RNA, contributes to proliferation and migration of colorectal cancer cells in vitro and in vivo. Oncotarget. 2017;8:3441–3453. doi: 10.18632/oncotarget.13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Zhang S, Qiu T, Wang Y, Ricketts DM, Qi C. Upregulation of long non-coding RNA NNT-AS1 promotes osteosarcoma progression by inhibiting the tumor suppressive miR-320a. Cancer Biol Ther. 2019;20:413–422. doi: 10.1080/15384047.2018.1538612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Lv M, Song Z, Lou Z, Wang R, Zhuang M. Long non-coding RNA NNT-AS1 affects progression of breast cancer through miR-142-3p/ZEB1 axis. Biomed Pharmacother. 2018;103:939–946. doi: 10.1016/j.biopha.2018.04.087. [DOI] [PubMed] [Google Scholar]
- 10.Shen Q, Jiang Y. LncRNA NNT-AS1 promotes the proliferation, and invasion of lung cancer cells via regulating miR-129-5p expression. Biomed Pharmacother. 2018;105:176–181. doi: 10.1016/j.biopha.2018.05.123. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Ren M, Li Y, Hu J, Lu G, Ma W, Guo D, Lu X, He S. Long noncoding RNA NNT-AS1 promotes gastric cancer proliferation and invasion by regulating microRNA-363 expression. J Cell Biochem. 2019;120:5704–5712. doi: 10.1002/jcb.27855. [DOI] [PubMed] [Google Scholar]
- 12.Ye H, Lin J, Yao X, Li Y, Lin X, Lu H. Overexpression of long non-coding RNA NNT-AS1 correlates with tumor progression and poor prognosis in osteosarcoma. Cell Physiol Biochem. 2018;45:1904–1914. doi: 10.1159/000487966. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Shi J, Xu Y. Long non-coding RNA NNT-AS1 contributes to cell proliferation, metastasis and apoptosis in human ovarian cancer. Oncol Lett. 2018;15:9264–9270. doi: 10.3892/ol.2018.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen B, Zhao Q, Guan L, Lv H, Bie L, Huang J, Chen XB. Long non-coding RNA NNT-AS1 sponges miR-424/E2F1 to promote the tumorigenesis and cell cycle progression of gastric cancer. J Cell Mol Med. 2018;22:4751–4759. doi: 10.1111/jcmm.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhaskaran M, Mohan M. MicroRNAs: History, biogenesis, and their evolving role in animal development and disease. Vet Pathol. 2014;51:759–774. doi: 10.1177/0300985813502820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Liu A, Hou C, Chen H, Zong X, Zong P. Genetics and epigenetics of glioblastoma: Applications and overall incidence of IDH1 mutation. Front Oncol. 2016;6:16. doi: 10.3389/fonc.2016.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Chen W, Jin Y, Xue R, Su J, Mu Z, Li J, Jiang S. miR-142-5p enhances cisplatin-induced apoptosis in ovarian cancer cells by targeting multiple anti-apoptotic genes. Biochem Pharmacol. 2019;161:98–112. doi: 10.1016/j.bcp.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Xiao Z, Ai F, Liu F, Chen X, Cao K, Ren W, Zhang X, Shu P, Zhang D. miR-142-5p promotes development of colorectal cancer through targeting SDHB and facilitating generation of aerobic glycolysis. Biomed Pharmacother. 2017;92:1119–1127. doi: 10.1016/j.biopha.2017.05.134. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Liu Z, Fang X, Yang H. miR-142-5p suppresses tumorigenesis by targeting PIK3CA in non-small cell lung cancer. Cell Physiol Biochem. 2017;43:2505–2515. doi: 10.1159/000484459. [DOI] [PubMed] [Google Scholar]
- 22.Yan J, Yang B, Lin S, Xing R, Lu Y. Downregulation of miR-142-5p promotes tumor metastasis through directly regulating CYR61 expression in gastric cancer. Gastric Cancer. 2019;22:302–313. doi: 10.1007/s10120-018-0872-4. [DOI] [PubMed] [Google Scholar]
- 23.Hu B, Zhang H, Wang Z, Zhang F, Wei H, Li L. lncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance in non-small-cell lung cancer cell line by targeting SOX4. Cancer Biol Ther. 2017;18:974–983. doi: 10.1080/15384047.2017.1385679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Goodarzi H, Tavazoie SF, Alarcón CR. TMEM2 is a SOX4-regulated gene that mediates metastatic migration and invasion in breast cancer. Cancer Res. 2016;76:4994–5005. doi: 10.1158/0008-5472.CAN-15-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandbothe M, Buurman R, Reich N, Greiwe L, Vajen B, Gürlevik E, Schäffer V, Eilers M, Kühnel F, Vaquero A, et al. The microRNA-449 family inhibits GF-β-mediated liver cancer cell migration by targeting SOX4. J Hepatol. 2017;66:1012–1021. doi: 10.1016/j.jhep.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Sun R, Jiang B, Qi H, Zhang X, Yang J, Duan J, Li Y, Li G. SOX4 contributes to the progression of cervical cancer and the resistance to the chemotherapeutic drug through ABCG2. Cell Death Dis. 2015;6:e1990. doi: 10.1038/cddis.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Wang Y, Wang H. Use of immunotherapy in the treatment of gastric cancer. Oncol Lett. 2019;18:5681–5690. doi: 10.3892/ol.2019.10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu X, Zhu X, Qin F, Zhang Y, Lin J, Ding Y, Yang Z, Shang Y, Wang L, Zhang Q, Gao Q. Linc00210 drives Wnt/β-catenin signaling activation and liver tumor progression through CTNNBIP1-dependent manner. Mol Cancer. 2018;17:73. doi: 10.1186/s12943-018-0783-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Guo C, Wang X, Chen LP, Li M, Li M, Hu YH, Ding WH, Wang X. Long non-coding RNA MALAT1 regulates ovarian cancer cell proliferation, migration and apoptosis through Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:3703–3712. doi: 10.26355/eurrev_201806_15249. [DOI] [PubMed] [Google Scholar]
- 32.Hua F, Liu S, Zhu L, Ma N, Jiang S, Yang J. Highly expressed long non-coding RNA NNT-AS1 promotes cell proliferation and invasion through Wnt/β-catenin signaling pathway in cervical cancer. Biomed Pharmacother. 2017;92:1128–1134. doi: 10.1016/j.biopha.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 33.Rupaimoole R, Slack FJ. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 34.Deris Zayeri Z, Tahmasebi Birgani M, Mohammadi Asl J, Kashipazha D, Hajjari M. A novel infram deletion in MSH6 gene in glioma: Conversation on MSH6 mutations in brain tumors. J Cell Physiol. 2019;234:11092–11102. doi: 10.1002/jcp.27759. [DOI] [PubMed] [Google Scholar]
- 35.Lou K, Chen N, Li Z, Zhang B, Wang X, Chen Y, Xu H, Wang D, Wang H. MicroRNA-142-5p overexpression inhibits cell growth and induces apoptosis by regulating FOXO in hepatocellular carcinoma cells. Oncol Res. 2017;25:65–73. doi: 10.3727/096504016X14719078133366. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Cheng D, Li J, Zhang L, Hu L. miR-142-5p suppresses proliferation and promotes apoptosis of human osteosarcoma cell line, HOS, by targeting PLA2G16 through the ERK1/2 signaling pathway. Oncol Lett. 2019;17:1363–1371. doi: 10.3892/ol.2018.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Liu S, Duan Q, Chen L, Wu T, Qian H, Yang S, Xin D, He Z, Guo Y. MicroRNA-142-5p promotes cell growth and migration in renal cell carcinoma by targeting BTG3. Am J Transl Res. 2017;9:2394–2402. [PMC free article] [PubMed] [Google Scholar]
- 38.Pang L, Li B, Zheng B, Niu L, Ge L. miR-138 inhibits gastric cancer growth by suppressing SOX4. Oncol Rep. 2017;38:1295–1302. doi: 10.3892/or.2017.5745. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Huang S, Long D. miR-381 inhibits migration and invasion in human gastric carcinoma through downregulatedting SOX4. Oncol Lett. 2017;14:3760–3766. doi: 10.3892/ol.2017.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Shiina H, Igawa M, Shigeno K, Terashima M, Deguchi M, Yamanaka M, Ribeiro-Filho L, Kane CJ, Dahiya R. Beta-catenin mutations correlate with over expression of C-myc and cyclin D1 Genes in bladder cancer. J Urol. 2002;168:2220–2226. doi: 10.1016/S0022-5347(05)64359-5. [DOI] [PubMed] [Google Scholar]
- 42.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Backer J, Wong AS, Schwanke EL, Stewart BG, Pasdar M. Bcl-2 expression decreases cadherin-mediated cell-cell adhesion. J Cell Sci. 2003;116:3687–3700. doi: 10.1242/jcs.00644. [DOI] [PubMed] [Google Scholar]
- 44.Wu F, Li J, Guo N, Wang XH, Liao YQ. miRNA-27a promotes the proliferation and invasion of human gastric cancer MGC803 cells by targeting SFRP1 via Wnt/β-catenin signaling pathway. Am J Cancer Res. 2017;7:405–416. [PMC free article] [PubMed] [Google Scholar]
- 45.Shan Y, Ying R, Jia Z, Kong W, Wu Y, Zheng S, Jin H. LINC00052 promotes gastric cancer cell proliferation and metastasis via activating the Wnt/β-catenin signaling pathway. Oncol Res. 2017;25:1589–1599. doi: 10.3727/096504017X14897896412027. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.