Abstract
Oxidative stress-induced injury and apoptosis of human lens epithelial cells (HLECs) are early events in the development of age-related cataracts (ARCs). Humanin (HN) is a mitochondrial-related peptide that serves a cytoprotective role in various cell types and animal models. Following HN knockdown or overexpression, the level of reactive oxygen species (ROS), mitochondrial membrane potential and mitochondrial DNA copy number, cell viability, LDH activity and apoptosis of HLECs under oxidative stress were detected, and apoptosis and autophagy were detected via transmission electron microscopy. The results suggested that HN may be involved in the response of HLECs to oxidative stress, and that HN expression was significantly upregulated under oxidative stress conditions. Furthermore, exogenous HN reduced intracellular ROS content and mitochondrial damage, and enhanced mitochondrial biosynthesis; however, this protection was lost in an endogenous HN knockdown cell model. In addition, to the best of our knowledge, the present study was the first to identify that HN increased mitochondrial autophagy, which was involved in reducing ROS production under oxidative stress. The present study indicated a potential mechanism underlying the anti-oxidative damage and apoptotic effects of HN under oxidative stress. In conclusion, HN may be a potential therapeutic target for ARCs as it has a significant cellular protective effect on HLECs under oxidative stress; therefore, further study is required to investigate its role in the occurrence and development of ARCs.
Keywords: ARCs, HN, ROS, oxidative stress, HLECs
Introduction
Age-related cataracts (ARCs) are characterized by an increase in the opacity of the lens with age; however, there are currently no effective drugs that can inhibit or reverse the progression of ARCs (1). The exact mechanism of ARC development is not fully understood, but it has been confirmed that oxidative stress serves a key role in its pathogenesis (2). UV rays, nutrient starvation, H2O2 in aqueous humor, amongst other factors can damage the lens by oxidative stress, particularly via oxidative damage to human lens epithelial cells (HLECs) (3). HLECs are a thin layer of cells in the anterior lens capsule that are responsible for the defense of the lens against oxidative stress and are therefore vulnerable to oxygen free radicals (3). Under oxidative stress, reactive oxygen species (ROS), a byproduct of metabolism, accumulate in HLECs and cause oxidative damage to nucleic acids, lipids and organelles, eventually leading to apoptosis (4–6). Furthermore, apoptosis of HLECs is considered to be an early event in the development of cataracts (4,5,7–9). In response to oxidative stress, particularly the increase in ROS production in HLECs, lens proteins are denatured, modified and aggregated, which eventually leads to cataract formation (10). In addition, oxidative stress has been shown to induce lens opacification in experimental animal models and in cultured rat lenses (11).
Humanin (HN) was the first identified mitochondrial-derived peptide (MDP), which comprises 21–24 amino acids expressed by the open reading frame of mitochondrial 16S rRNA (12). HN is expressed in various tissues, including neuronal cells, skeletal muscle cells (13), retinal pigment epithelial cells (14) and blood-derived cells (15). Previous studies have reported that HN may serve a role in metabolic regulation and antioxidant injury in nerve cells, cardiomyocytes, epidermal stem cells and other cell types, as well as in pathological models, such as Alzheimer's disease, type 2 diabetes mellitus (T2DM) and age-related macular degeneration (AMD). These effects include H2O2-induced cell death (16), β-amyloid toxicity (17), serum starvation-induced neuronal cell death (18) and protective effects of oxidized low-density lipoproteins-induced vascular endothelial cell death (19). Furthermore, knockdown of endogenous HN increased the sensitivity of cells to apoptosis induced by oxidative stress (12). However, to the best of our knowledge, there have been no previous studies examining the expression and function of HN in HLECs, and the role of HN in the prevention or treatment of ARCs.
Considering the important role of oxidative stress and ROS in the pathogenesis of cataracts, the present study investigated the role of HN in HLECs under oxidative stress and its underlying mechanisms. Moreover, the present study examined the relationship between HN and the formation of ARCs, by administering exogenous HN or knocking down endogenous HN to assess whether HN can reduce oxidative damage and apoptosis.
Materials and methods
Cell culture and treatment
HLECs (HLE-B3; American Type Culture Collection) were grown and maintained in 1:1 DMEM and Ham's F-12 medium (DMEM/F-12; cat. no. 10-092-CV; Corning, Inc.), which was supplemented with 10% FBS (Biological Industries) and 100 units penicillin and streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2. HN peptides were chemically synthesized and purified to >95% purity (Nanjing Taiye Chemical Industry Co., Ltd.). The HN group was incubated with DMEM containing 50 µM HN at 37°C for 2 h before receiving type B UV (UVB) radiation. In the small interfering RNA (siRNA) HN group, HN siRNA was transfected into cells using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h, after which, cells received UVB radiation. For the rescue experiment, the siHN group was incubated with DMEM containing 50 µM HN at 37°C for 2 h prior to UVB radiation. For serum starvation, after pretreatment with 50 µM HN for 2 h or transfection with siHN for 48 h, cells were incubated in serum-free medium after being rinsed twice with PBS, for 72 h before experiments. Cells incubated in DMEM containing 10% FBS were considered the nutrients group.
For UVB irradiation, cells were treated as aforementioned, rinsed with PBS and treated with a given dose of UVB (0, 10, 20, 30 and 50 mJ/cm2; 37°C; air). Cells were exposed to UVB light (from the bottom) in PBS (37°C; air) for 2.5 min using a Spectroline ‘medium wave’ UV lamp (Spectroline) at a distance of 0.8 cm. Control cells were treated similarly, but were not exposed to UVB light. UVB levels reaching the cells were determined with a radiometer (UVX Digital; UVP, Inc.) equipped with a UVB sensor at 312 nm (UVX-31). Following UVB exposure, cells were rinsed twice with PBS and cultured for 24 h in DMEM containing 10% FBS. The morphological analysis of HLECs was conducted using a fluorescence microscope (Leica Microsystems GmbH; magnification, ×400).
RNA interference
The siRNAs were designed and synthesized by Guangzhou Ribobio Co., Ltd. The sequence of HN-specific siRNA was: 5′-GGGUUCAGCUGUCUCUUAC-3′. A nonsilencing siRNA (cat. no. SI03650318; Qiagen, Inc.) was used as a negative control. At 50% confluence, cells were transfected with siRNA (5 nmol/l) using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.). A total of 48 h post-transfection, subsequent experiments were performed. Control transfection was also performed using Lipofectamine® 3000 without nucleic acids (Mock). The knockdown of HN was confirmed by reverse transcription-quantitative PCR (RT-qPCR) and western blotting.
RT-qPCR
Total RNA was extracted from cells using TRIzol® reagent (Thermo Fisher Scientific, Inc.). Total RNA concentration was estimated by spectrophotometry. Total RNA was reverse transcribed into cDNA using the PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.). qPCR was performed using Power SYBR Green PCR Master Mix (cat. no. 4367659; Thermo Fisher Scientific, Inc.) and the StepOnePlus RT PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) to investigate the expression levels of apoptosis, autophagy, endoplasmic reticulum (ER) stress and antioxidant-associated genes. Each sample was run in triplicate. The following thermocycling conditions were used for qPCR: Initial denaturation for 1 min at 94°C, followed by 30 cycles of 30 sec at 94°C, 30 sec at 60°C, 2 min at 72°C; and a final extension step at 72°C for 5 min. RT-qPCR data were analyzed using the 2−ΔΔCq method (20). ΔCq was the difference between the quantification cycle (Cq) of the target gene and Cq of the housekeeper gene (reference gene). ΔΔCq was calculated by subtracting ΔCq of each experimental group from ΔCq of the control group. Fold change was calculated using the following formula: Fold-change=2−ΔΔCq.
The primers used for RT-qPCR were as follows: Human NADH dehydrogenase 1 (ND1) forward, 5′-ATACCCATGGCCAACCTCCT-3′ and reverse, 5′-GGGCCTTTGCGTAGTTGTAT-3′; human β-globin forward, 5′-GTGCACCTGACTCCTGAGGAGA-3′ and reverse, 5′-CCTTGATACCAACCTGCCCAG-3′; β-actin, forward, 5′-GAGAGGGAAATCGTGCGTGAC-3′ and reverse, 5′-CTGCTGGAAGGTGGACAGTGAG-3′; HN, forward, 5′-CTCCACGAGGGTTCAGCTGT-3′ and reverse, 5′-TTATGTCCGCCTCTTCACGG-3′; and human GAPDH, forward, 5′-GGTGAAGGTCGGAGTCAACG-3′ and reverse, 5′-CAAAGTTGTCATGGATGHACC-3′.
Cell viability assay
Cytotoxicity was assessed using a Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.). Briefly, 24 h before UVB treatment, 1×104 cells were seeded in each well of a 96-well plate. Cells were rinsed twice with PBS and treated with a given dose of UVB (0, 10, 20, 30 and 50 mJ/cm2; 37°C; air). Following incubation for 24 h, the medium was removed, and the cells were washed with PBS. Each well was then refilled with 90 µl DMEM/F12 supplemented with 10% FBS and 10 µl CCK-8 reagents, which was incubated at 37°C for 3 h. Cell viability was evaluated at an optical density of 450 nm using a 96-well microplate reader (Bio-Rad Laboratories, Inc.).
Lactate dehydrogenase (LDH) cytotoxicity assay
The percentage of living cells was determined using a LDH Cytotoxicity assay kit (Beyotime Institute of Biotechnology). Briefly, 1×104 cells were seeded in each well of a 96-well plate and incubated for 24 h. Cells were rinsed twice with PBS and treated with a given dose of UVB (0 or 30 mJ/cm2; 37°C; air). After incubation for 24 h, the culture media was collected and centrifuged for 5 min at 2,442 × g at room temperature. The supernatant was dispensed into a 96-well plate and LDH assay reagent was added to each well. The plate was incubated at room temperature for 30 min. Absorbance values were measured at 450 nm using a microplate reader. The living cells (%) was calculated as follows: Cytotoxicity (%) = (absorbance of test sample-absorbance of low control)/(absorbance of maximum enzyme activity-absorbance of low control) ×100.
Measurement of intracellular ROS
ROS were measured using 2′,7′-dichlorofluorescein diacetate (DCFH-DA, Beyotime Institute of Biotechnology). Following incubation for 24 h after UVB exposure, HLECs were washed three times with PBS. DCFH-DA, diluted to a final concentration of 10 µM, was added to HLECs and incubated for 30 min at 37°C in the dark. After the cells were washed three times with serum-free medium, the fluorescence intensity was detected with a multi-detection microplate reader with excitation at 488 nm and emission at 530 nm within 15 min. Fluorescence signals were captured using a fluorescence microscope (Leica Microsystems GmbH). Intracellular levels of ROS were calculated by the average fluorescence intensity as analyzed by Image-Pro Plus software (version 6.0; National Institutes of Health). The measured fluorescence values were expressed as a percentage of the fluorescence in control cells.
Mitochondrial membrane potential (ΔΨm) measurement
JC-1 was used to measure the ΔΨm of HLECs. Briefly, 5×105 cells were collected into 2 ml tubes and incubated with 10 µg/ml JC-1 for 20 min at 37°C. The fluorescence intensity was detected with a flow cytometer (BD FACSAria I cell sorter; Becton, Dickinson and Company). The wavelengths of excitation and emission were 514 and 529 nm, for detection of the monomeric form of JC-1. In addition, 585 and 590 nm were used to detect aggregation of JC-1. The ratio of aggregated JC-1 and monomeric JC-1 represented the ΔΨm of HLECs. The data were analyzed using Quantity One software (version 4.6.6; National Institutes of Health). Images were captured by fluorescence microscopy (Leica Microsystems GmbH; magnification, ×400).
Transmission electron microscopy (TEM)
Cells were fixed for 2 h at 4°C in glutaraldehyde and paraformaldehyde (3 and 4%, respectively) in 0.1 M cacodylate buffer (pH 7.2). After washing in 0.1 M cacodylate buffer, cells were post-fixed with 1% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.2) for 1 h at room temperature. Cells were dehydrated using a graded acetone series and embedded in EPON-82 resin (Sigma-Aldrich; Merck KGaA) for 72 h at 60°C. Ultra-thin sections (80 nm) were stained with uranyl acetate (5%; 10 min) and lead citrate (2%; 30 min) at room temperature and observed using a JEM-1200EX TEM (magnification, ×25,000; JEOL Ltd.) at 120 V. Data were analyzed using Image-Pro Plus software (version 6.0; National Institutes of Health).
Cell apoptosis assay
Cells of each experimental group (5×106 cells/ml) were cultured in 35 mm2 dishes and incubated for 24 h. Cells were rinsed twice with PBS and treated with a given dose of UVB (0 or 30 mJ/cm2; 37°C; air). After 24 h incubation, cells were collected by trypsinization and centrifugation at 2442 × g for 5 min. The cell pellet was washed twice with cold PBS and resuspended in 1X Annexin-binding buffer (BD Biosciences). Subsequently, the cell suspension (100 µl) was incubated with 5 µl Annexin V and 1 µl 100 µg/ml PI working solution (BD Biosciences) at room temperature for 15 min in the dark. After the incubation, 400 µl 1X Annexin-binding buffer was added. After mixing, the fluorescence intensity was detected with a flow cytometer (CyAn ADP; Beckman Coulter, Inc.) at 530 and 575 nm emission, and 488 nm excitation. BD FACSdiva Software (version 8.0.1; BD Biosciences) was used to analyze the data. The percentage of cells stained by AnnexinV+/PI−, which indicates early apoptosis, was presented as a bar chart.
Determination of mitochondrial DNA (mtDNA) copy number
The mtDNA copy number was defined as the total mtDNA copies divided by the total nuclear DNA (nDNA) copies. qPCR was performed to measure the mtDNA and nDNA copies of each experimental group. Total DNA of the cells in each experimental group was extracted using the DNA/RNA Isolation kit (Qiagen GmbH) according to the manufacturer's instructions. For each qPCR, 1 µl sample DNA (10 ng/µl) was amplified in a 10-µl reaction mixture that contained 0.25 µl each primer (20 µM; human ND1 for mtDNA; human β-globin for nDNA; β-actin reference gene), 5 µl SensiFAST SYBR Hi-ROX premix (Thermo Fisher Scientific, Inc.) and 3.5 µl PCR-grade water. In each experiment, 1 µl HLECs DNA (1 ng/µl) and PCR-grade water were used as the positive and negative controls, respectively. The following thermocycling conditions were used for qPCR: Initial denaturation for 30 sec at 95°C; followed by 45 cycles of denaturation for 15 sec at 95°C, annealing for 20 sec at 60°C, extension for 20 sec at 72°C. After obtaining Cq values, the mtDNA copies and nDNA copies of the sample DNA (10 ng) relative to those of HLECs were determined according to the aforementioned equations.
Western blotting
After treatment, total protein was extracted from HLECs using RIPA lysis buffer (cat. no. r0020; Beijing Solarbio Science & Technology Co, Ltd.) and a bicinchoninic acid protein assay kit (cat. no. B9643-1L; Sigma-Aldrich; Merck KGaA) was used to determine the total protein concentration. Equal concentrations of total protein samples (8 µl/lane) were loaded into the wells of 4–12% Bolt mini gels (Thermo Fisher Scientific, Inc.) followed by SDS-PAGE. The proteins were then transferred onto PVDF membranes. Following transfer, membranes were blocked with 5% fat-free milk in TBS for 30 min at room temperature and then incubated with primary antibodies against HN (cat. no. LS-C109400; 1:2,000; LifeSpan BioSciences), Bcl-2 (cat. no. 4223; 1:2,000; Cell Signaling Technology, Inc), Bax (cat. no. 5023; 1:2,000; Cell Signaling Technology, Inc.), cleaved caspase 3 (cat. no. 9665; 1:2,000; Cell Signaling Technology, Inc.) and β-actin (cat. no. sc-47778; 1:5,000; Santa Cruz Biotechnology, Inc.) overnight at 4°C. Subsequently, goat anti-mouse IgG H&L (cat. no. ab205719; 1:5,000; Abcam) and goat anti-rabbit IgG H&L (cat. no. ab205718; 1:5,000; Abcam) horseradish peroxidase-conjugated secondary antibodies or 2 h at room temperature. Protein bands were visualized using the SuperSignal™ Western Blot Substrate bundle (Pierce; Thermo Fisher Scientific, Inc.). Data were analyzed using Image-Pro Plus 6.0 software (version 6.0; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using one-way ANOVA followed by Tukey post hoc test using GraphPad InStat (version 3.05; GraphPad Software, Inc.). Data are presented as the mean ± SD. P<0.05 was considered to indicate a statistically significant difference. Each experiment was repeated at least three times.
Results
HN expression in HLECs is associated with oxidative stress
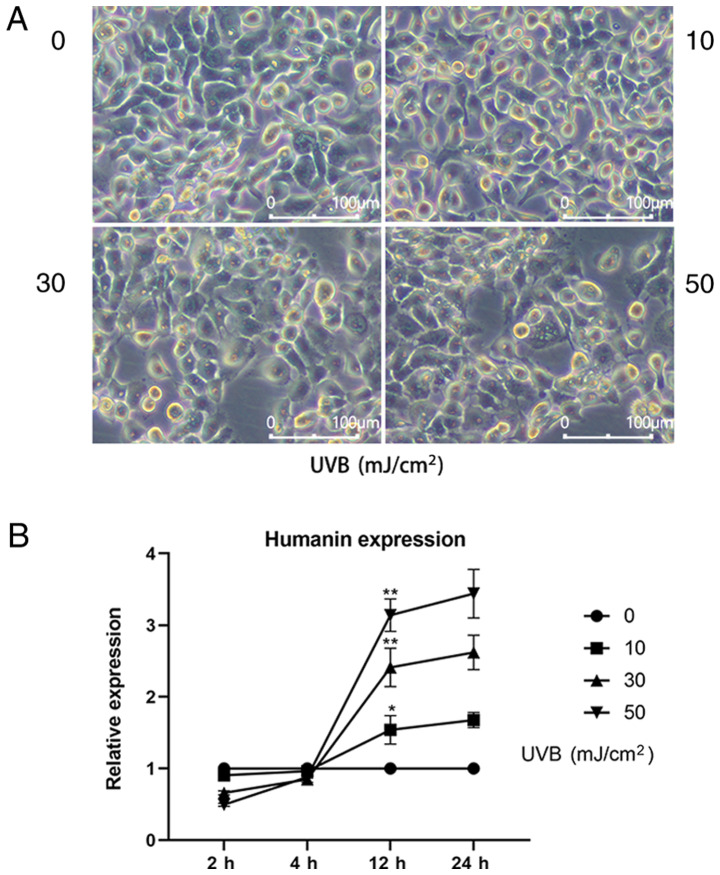
It was revealed that 24 h after UVB treatment, the morphology of HLECs changed (Fig. 1A); UVB induced cell loss, disordered intracellular structure and enhanced the appearance of apoptotic bodies. Moreover, the higher the UVB dose used to treat HLECs, the more significant the change in cell morphology, indicating that the cells were under high oxidative stress. To further investigate the relationship between endogenous HN expression and oxidative stress, RT-qPCR was used to detect the mRNA expression levels of HN in HLECs treated with various doses of UVB (0, 10, 30 and 50 mJ/cm2; Fig. 1B). It was demonstrated that the expression levels of HN were increased 12 h after UVB irradiation and HN expression was positively associated with UVB exposure. Moreover, the mRNA expression levels of HN were upregulated by 62% (P<0.05) at the dose of 10 mJ/cm2, 138% (P<0.01) at 30 mJ/cm2 and 219% (P<0.01) at 50 mJ/cm2 compared with the control group. Therefore, the present results suggested that UVB irradiation may induce upregulation of HN in HLECs, and that HN may be involved in the response of cells to oxidative stress.
Figure 1.
Expression of HN in HLECs at different levels of oxidative stress. (A) Images of HLECs 24 h after exposure to a 0, 10, 30 or 50 mJ/cm2 dose of UVB. (B) HLECs were subjected to different doses of UVB (0, 10, 30 or 50 mJ/cm2), and the mRNA expression levels of HN were detected by reverse transcription-quantitative PCR at 2, 4, 12 and 24 h. Data are presented as the mean ± SD, n=3. *P<0.05, **P<0.01 vs. 12 h 0 mJ/cm2 UVB. HN, humanin; HLECs, human lens epithelial cells; UVB, type B UV.
HN protects HLECs from oxidative damage
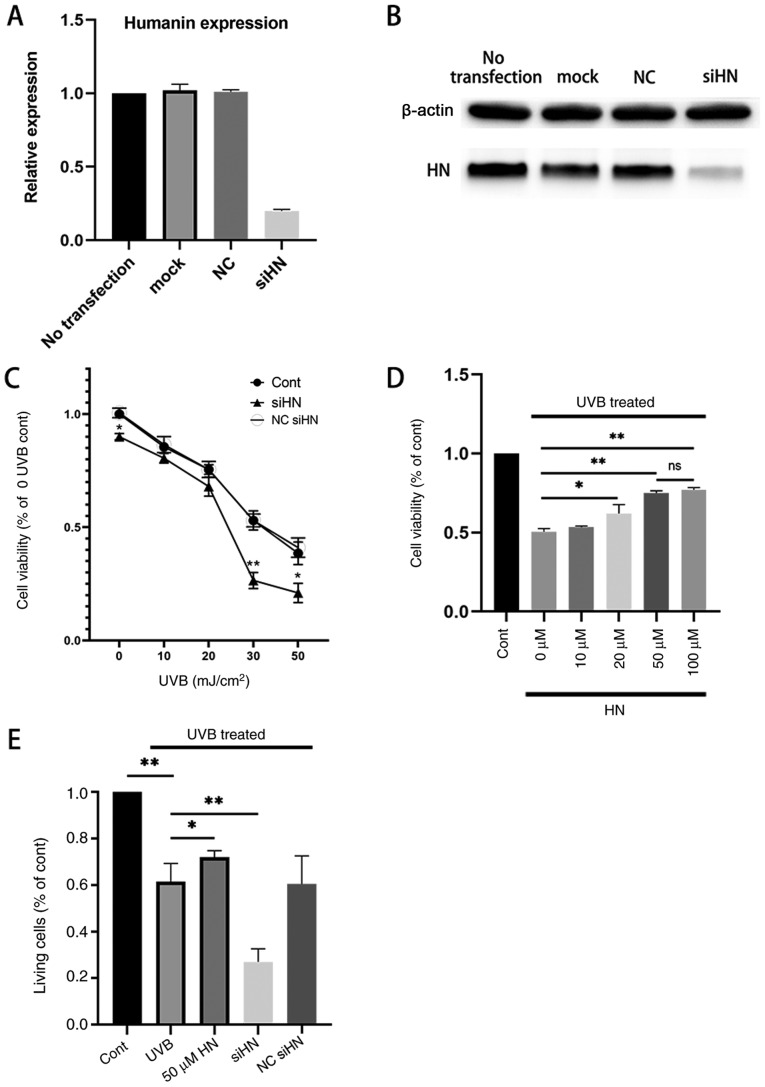
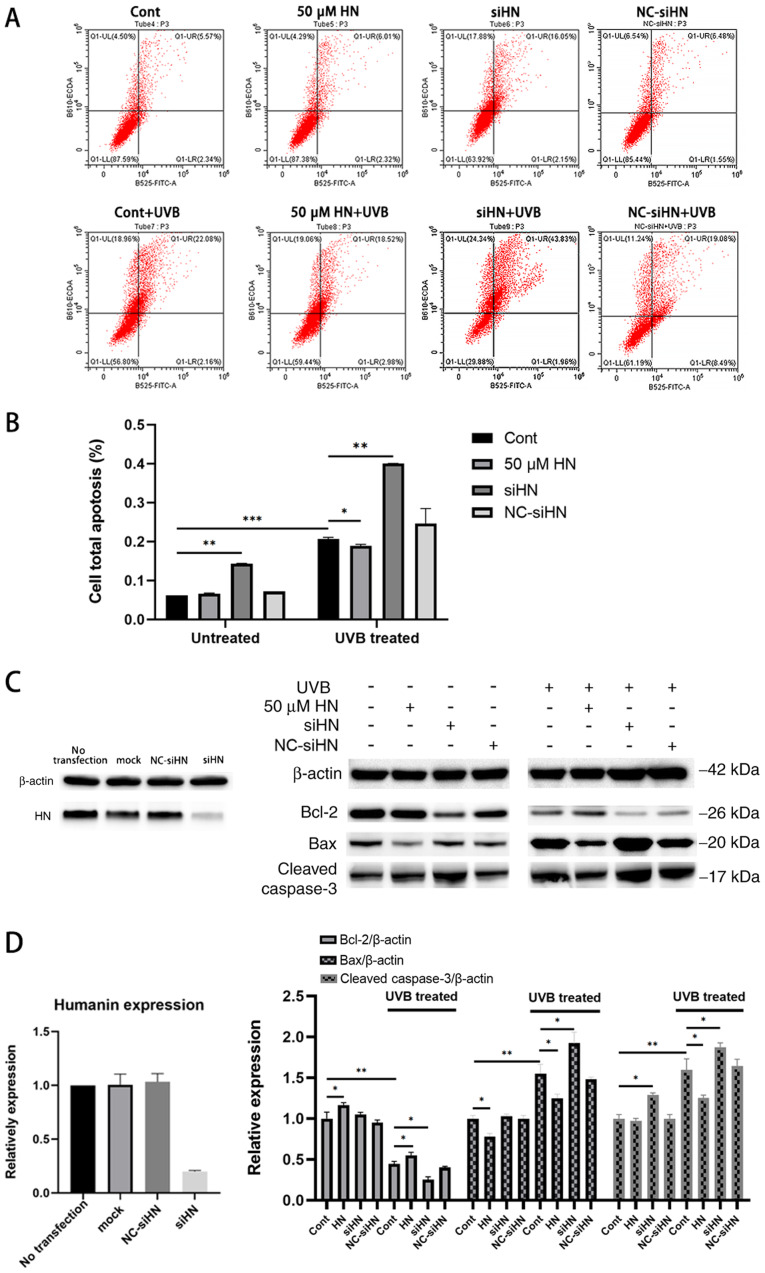
To investigate whether HN has a protective effect on HLECs under oxidative stress, a HN knockdown cell line was established by gene silencing. qPCR and western blot analysis of HN were performed and it was confirmed by qPCR analysis that the expression of HN was downregulated by 83% compared with the notransfection group (Fig. 2A and B). In addition, a CCK-8 assay was used to detect the viability of HLECs (Fig. 2C). The results revealed that knockdown of HN significantly decreased the viability of HLECs (P<0.05). In addition, the present study treated the HN knockdown group with different levels of UVB; the results demonstrated that cell viability was reduced by ~50% in the control group treated with UVB irradiation (30 mJ/cm2) and by 77% (P<0.01) in the HN knockdown group compared with the control group (0 UVB; Fig. 2C). Therefore, 30 mJ/cm2 was used as the UVB irradiation dose in follow-up experiments. The viability of HLECs pretreated with various doses of exogenous HN 24 h after UVB irradiation was then measured (Fig. 2D). It was demonstrated that the cellular protective effect of HN was dose-dependent, but there was no significant increase in response to 100 µM HN compared with 50 µM HN; therefore, the present study used 50 µM HN (P<0.01) as the concentration of exogenous HN in the follow-up experiments. Furthermore, 30 mJ/cm2 UVB exerted significant cytotoxicity on HLECs (P<0.01). However, pretreatment with HN reduced UVB cytotoxicity (P<0.05) and reduced LDH activity by 20% (Fig. 2E), whereas knockdown of HN significantly increased the sensitivity of HLECs to UVB cytotoxicity (P<0.01). Collectively, the present results indicated that HN may effectively protect HLECs from oxidative damage induced by UVB.
Figure 2.
Effects of HN on the viability and LDH cytotoxicity of HLECs subjected to UVB. (A and B) Cells were transfected with siHN for 48 h. NC siHN group cells were transfected with NC siRNA for 48 h. Mock transfection was performed using Lipofectamine® 3000 without nucleic acids. (A) mRNA expression levels of HN were detected by reverse transcription-quantitative PCR. (B) Protein expression levels of HN were examined using western blotting. (C and D) Cell Counting Kit-8 assay. (C) After transfection with siHN and NC siRNA for 48 h, the cells were washed twice with PBS, and HLECs were subjected to different doses (0, 10, 20, 30 and 50 mJ/cm2) of UVB. (D) After incubation with a given concentration (0, 10, 20, 50 or 100 µM) of HN for 2 h, the cells were washed twice with PBS, and HLECs were treated with UVB at a dose of 30 mJ/cm2. (E) After pretreatment with 50 µM HN for 2 h or transfection with siHN or NC siRNA for 48 h, the cells were washed twice with PBS, and then HLECs were subjected to UVB radiation at a dose of 30 mJ/cm2. After 24 h, LDH release was determined using a commercial kit. Data are presented as the mean ± SD, n=3. *P<0.05 and **P<0.01 vs. Cont. HN, humanin; LDH, lactate dehydrogenase; HLECs, human lens epithelial cells; UVB, type B UV; siHN, HN siRNA; siRNA, small interfering RNA; NC, negative control; Cont, control.
HN inhibits ROS production in HLECs
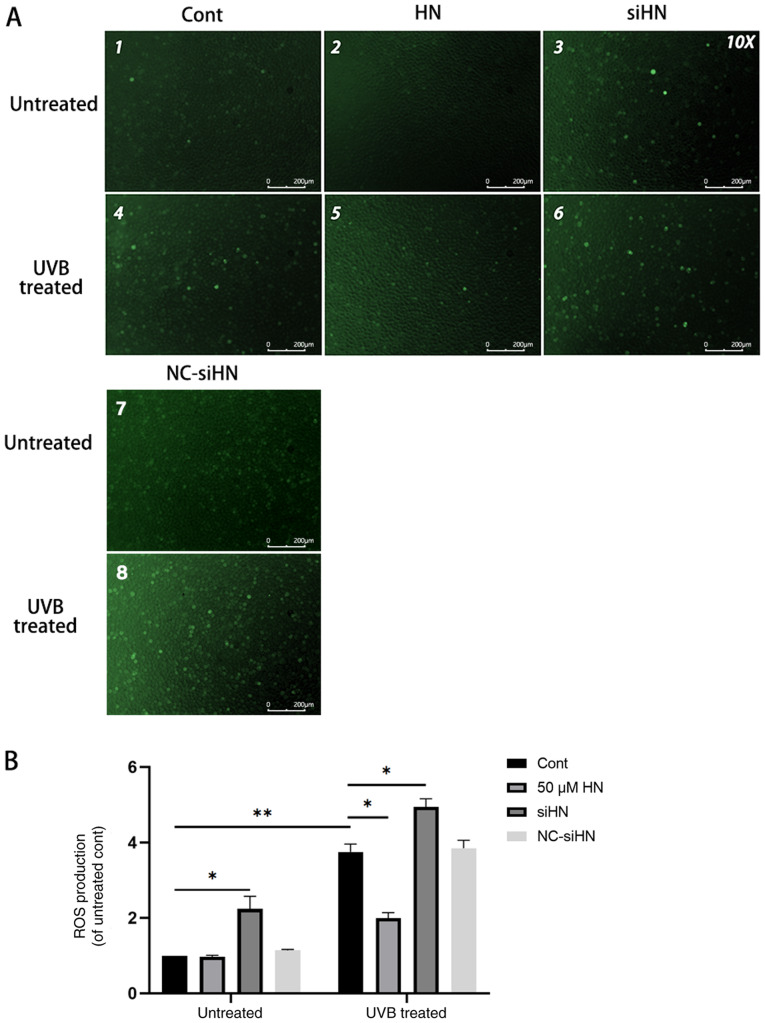
Under oxidative stress, the level of ROS in cells increases significantly (4). ROS derived from damaged mitochondria are important physiological signaling molecules that regulate gene expression, apoptosis and cell proliferation (5,6). Excessive ROS can further impair mitochondrial function and affect cell viability (21,22). Therefore, the present study hypothesized that HN may reduce the production of ROS in HLECs and act as an antioxidant. The present study detected intracellular ROS using the fluorescent probe DCFH-DA, and it was found that the level of ROS in HLECs increased under oxidative stress (Fig. 3). In addition, the level of ROS in the HN siRNA group was significantly higher compared with the control group, regardless of whether there was UVB treatment. However, HLECs pretreated with 50 µM HN under oxidative stress showed lower ROS levels compared with the UVB-treated control group (P<0.05). Thus, the present results suggested that HN can significantly reduce ROS production in HLECs under oxidative stress.
Figure 3.
Endogenous HN inhibits UVB-induced ROS production in HLECs. After pretreatment with 50 µM HN for 2 h or transfection with siHN or NC siRNA for 48 h, HLECs were subjected to 30 mJ/cm2 UVB radiation. After 24 h, intracellular ROS levels were determined by 2′,7′-dichlorofluorescein diacetate. (A) Observation under a fluorescence microscope and (B) detection by a fluorescence microplate reader. Data are presented as the mean ± SD, n=3. *P<0.05, **P<0.01. HN, humanin; HLECs, human lens epithelial cells; UVB, type B UV; ROS, reactive oxygen species; siHN, HN siRNA; siRNA, small interfering RNA; NC, negative control; Cont, control.
HN protects mitochondria from oxidative stress in HLECs
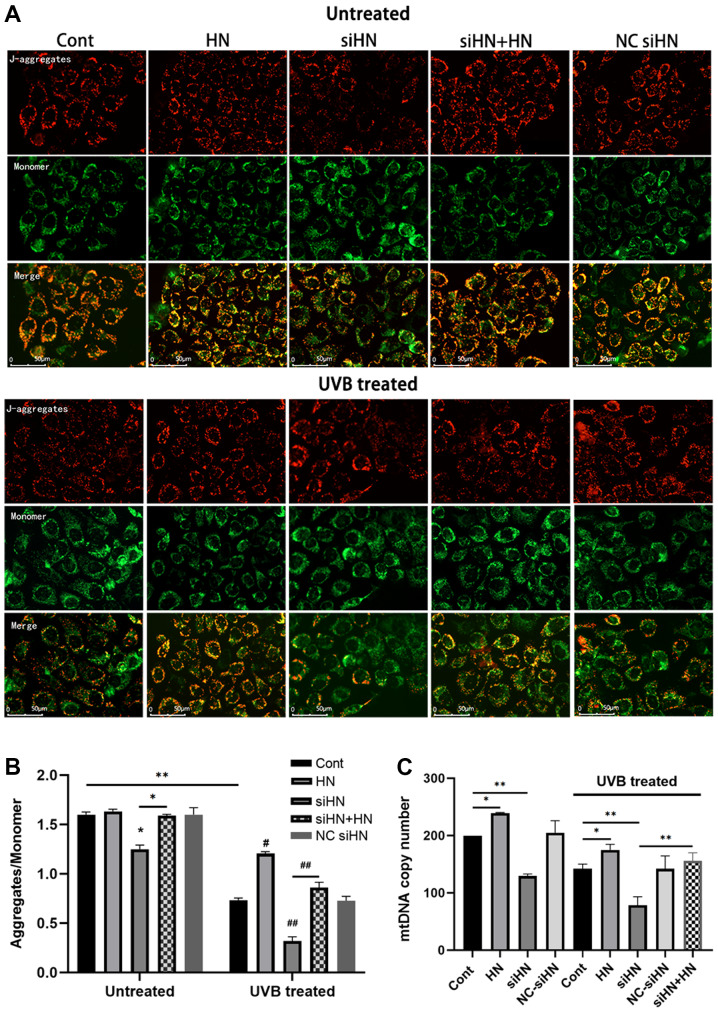
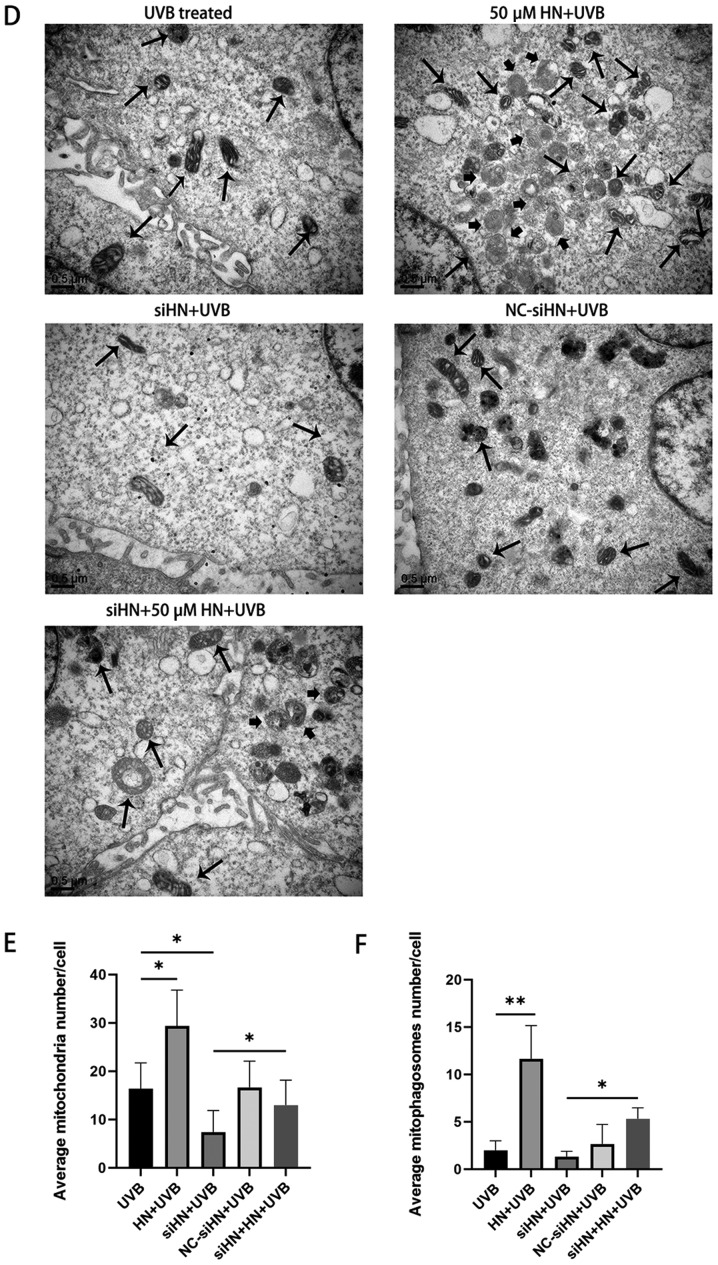
The present study identified a significant increase in ROS levels in UVB-treated HLECs, which aggravated mitochondrial damage under oxidative stress. Due to the poor repair ability of mtDNA, damage to mtDNA can cause energy metabolism disorders, apoptosis and cell necrosis (23). To further investigate whether HN is involved in mitochondrial protection, the present study detected the Δψm using JC-1 staining. JC-1 aggregates in normal mitochondria and has red fluorescence. It was found that UVB treatment increased green fluorescence in HLECs, indicating a decrease in Δψm and an increase in depolarizing mitochondria (Fig. 4A). A decrease in Δψm is also considered an important event during early apoptosis (23). Moreover, the HN-administered group exhibited a higher red-green fluorescence ratio after UVB treatment compared with the control group (P<0.01), whereas the HN siRNA group had a significantly lower red-green fluorescence ratio compared with the control group and the HN knockdown group supplemented with exogenous HN (P<0.01; Fig. 4B). The present study also examined the mtDNA copy number to assess the degree of mtDNA damage and biosynthesis (Fig. 4C). It was demonstrated that HN knockdown resulted in a decrease in mitochondrial copy number under oxidative stress (P<0.01). However, exogenous HN significantly reversed the damage to mitochondria in the siHN group (P<0.01). Moreover, the number of mitochondria per cell was counted after 24 h of UVB irradiation by TEM (Fig. 4D). Consistent with the results of mtDNA copy number, exogenous HN could increase mitochondrial number in HLECs induced by oxidative stress (Fig. 4E; P<0.05). Furthermore, a significant increase in mitochondrial autophagosomes was identified (Fig. 4D) in HLECs administered with exogenous HN compared with the UVB control cells, thus HN may enhance mitophagy (Fig. 4F). This allows HLECs to remove damaged mitochondria in time to prevent ROS accumulation within cells (24). In summary, the present results indicated that HN has a beneficial effect on mitochondrial damage and biosynthesis in HLECs under oxidative stress.
Figure 4.
HN protects mitochondria from oxidative stress in HLECs. After pretreatment with 50 µM HN for 2 h or transfection with siRNA for 48 h, HLECs were subjected to 30 mJ/cm2 UVB radiation. (A) Red fluorescence represents the mitochondrial aggregate form of JC-1, indicating an intact ΔΨm. Green fluorescence represents the monomeric form of JC-1, indicating dissipation of ΔΨm. (B) Detection by a fluorescence microplate reader. The ratio of red to green fluorescence indicates the ratio of JC-1 aggregates/monomer. Data are presented as the mean ± SD, n = 6. (C) Determination of mtDNA copy number. After 24 h of cultivation, ND1 and β-actin expression levels were detected by reverse transcription-quantitative PCR. HN protects mitochondria from oxidative stress in HLECs. After pretreatment with 50 µM HN for 2 h or transfection with siRNA for 48 h, HLECs were subjected to 30 mJ/cm2 UVB radiation. ((D) Mitochondria and mitophagosomes were detected by TEM (magnification, ×25,000X). The thin arrow indicates mitochondria and the thick arrow indicates mitochondrial autophagosomes. (E) Average number of mitochondria per cell. Record the number of mitochondria in 15 cells and get the average value. (F) Average number of mitophagosomes per cell. Record the number of mitochondria in 15 cells and get the average value. Data are presented as the mean ± SD, n=3. *P<0.05, **P<0.01. ΔΨm, mitochondrial membrane potential; mtDNA, mitochondrial DNA; HN, humanin; HLECs, human lens epithelial cells; UVB, type B UV; siHN, HN siRNA; siRNA, small interfering RNA; NC, negative control; Cont, control.
HN protects HLECs from oxidative stress-induced apoptosis
Under oxidative stress, elevated levels of intracellular ROS and damaged mitochondria can lead to apoptosis, which is also considered to be an early event in the development of cataracts (2). To investigate the effect of HN on oxidative stress-induced apoptosis of HLECs, cells were stained with Annexin V-FITC/PI to analyze the apoptotic rate of HLECs induced by UVB (Fig. 5A) or serum starvation (Fig. S1) using flow cytometry. HLECs exhibited significant apoptosis after UVB irradiation or serum starvation (P<0.001), and apoptosis in the HN siRNA group was more significant compared with the control group with or without oxidative stress (Fig. 5A and B; P<0.01). Under UVB irradiation, HN-pretreated cell apoptosis was decreased by 11% compared with the control group (P<0.05), while under serum starvation, HN-pretreated cell apoptosis was decreased by 42% compared with the control group (P<0.01). Thus, exogenous HN may serve a moderate protective role in UVB-induced HLEC apoptosis. Compared with the control group, oxidative stress significantly increased the protein expression levels of Bax and cleaved caspase-3, and decreased the expression levels of Bcl-2 (P<0.01; Fig. 5C). Furthermore, under UVB treatment, the HN-administered group displayed higher Bcl-2 expression levels compared with the control group (37.5%; P<0.05; Fig. 5D), but 17 (P<0.05) and 18% (P<0.05) lower Bax and cleaved caspase3 expression levels compared with the control group. Collectively, the present results suggested that HN may inhibit apoptosis by regulating the expression levels of Bcl-2 and Bax, and the activation of caspase-3.
Figure 5.
Apoptosis assay of HLECs under oxidative stress induced by UVB. After pretreatment with 50 µM HN for 2 h or transfection with siRNA for 48 h, HLECs were subjected to 30 mJ/cm2 UVB radiation. (A) Annexin V/PI staining detected by flow cytometry. (B) Quantification of flow cytometry results. (C) Western blotting and (D) semi-quantification of the expression levels of the apoptosis-related proteins Bcl-2, Bax and cleaved caspase-3. Data are presented as the mean ± SD, n=3. *P<0.05, **P<0.01, ***P<0.001. HN, humanin; HLECs, human lens epithelial cells; UVB, type B UV; siHN, HN siRNA; siRNA, small interfering RNA; NC, negative control; Cont, control.
Discussion
Epidemiology has linked oxidative stress to the development of cataracts, and oxidative stress-induced apoptosis of HLECs has been reported as a key early event of ARC development (22,25,26). The in vitro experiments suggested that HN may act as an antioxidant MDP in HLECs. To the best of our knowledge, the present study was the first to identify that exogenous HN enhanced the resistance of HLECs to oxidative stress and reduced apoptosis by inhibiting the production of ROS, preventing the decrease of Δψm, increasing mitochondrial membrane biosynthesis and enhancing the autophagy of mitochondria. Furthermore, it was revealed that HN knockdown increased the sensitivity of HLECs to oxidative damage and led to increased apoptosis.
The present study revealed that HN was expressed in HLECs and responded to oxidative stress. The level of ROS in HLECs has previously been reported to be positively associated with the dose of UVB and induced different degrees of oxidative stress in cells (27). The results indicated that 4–12 h after UVB irradiation, HN expression increased, which was associated with UVB irradiation. Thus, HN, as a highly conserved cytoprotective peptide, may have an important role in the survival mechanism of HLECs under oxidative stress. HN expression in various tissues and cells, as well as age-related pathological models, has been reported to be associated with oxidative stress resistance (13,19,28). It was previously shown that HN expression was elevated in polarized retinal pigment epithelium monolayers, which increased their resistance to oxidative stress (14). Moreover, HN levels were downregulated in patients with T2DM, which reduced their antioxidant capacity (29).
Under oxidative pressure (29,30), characteristic changes in cells include a significant increase in intracellular ROS and mitochondrial depolarization (31). High concentrations of ROS in cells may cause oxidative injury to lipids, proteins and nucleic acids, and could damage the integrity of various biomolecules (23). In addition, excessive ROS may have a central role in the pathogenesis of various human diseases, such as cataracts, cardiovascular disease, AMD, diabetes and aging (32,33). The oxidative phosphorylation process of depolarized mitochondria is a major source of intracellular ROS (34,35). Furthermore, mitochondria are sensitive to oxidative stress, and the repair of mtDNA is slower compared with that of nDNA (23). Decreased resistance to oxidative damage or lack of rapid removal of damaged mitochondria may result in a significant accumulation of ROS within the cell, which may cause more severe oxidative damage to mitochondria and various biomolecules (36), and eventually lead to apoptotic cell death (31,34). Therefore, reducing ROS production and protecting mitochondrial function are critical for HLEC resistance to oxidative stress. The present study revealed that knockdown of HN resulted in a significant increase in the levels of ROS in HLECs, particularly under oxidative stress, whereas exogenous HN significantly reduced the levels of ROS in HLECs under oxidative stress. The present study hypothesized that the primary reason for this was that the expression of endogenous HN may be important for cells to maintain a low level of ROS, and under oxidative stress HN reduces the production of endogenous ROS via its protective effect against mitochondrial damage. Moreover, it was found that HN effectively enhanced mitochondrial resistance to oxidative stress, and that exogenous HN pretreatment significantly increased Δψm in UVB-treated HLECs. In addition, it was demonstrated that HN co-treatment rescued the increased sensitivity of mitochondria to oxidative stress. Furthermore, the present results suggested that the changes in HN altered mitochondrial biosynthesis and mitochondrial numbers. In HLECs with HN knockdown, the mtDNA copy number was significantly reduced compared with the control group, whereas exogenous HN could rescue this reduction in mtDNA copy number, as verified by the TEM results. Sreekumar et al (15) reported that HN upregulated the expression of mitochondrial transcription factor A, a key biogenesis regulator protein, to regulate mitochondrial transcription initiation (37,38). Similarly, the present results indicated that HN increased the copy number of mtDNA with or without oxidative stress. Therefore, HN may maintain mitochondrial germination and normal function by promoting the initiation of mtDNA transcription, thereby increasing the resistance of HLECs to oxidative stress and preventing oxidation of lipids and proteins, which is important for maintaining the transparency of the lens (39).
HN may promote the removal of damaged depolarized mitochondria from cells (15). Mitochondrial damage caused by oxidative stress induces mitophagy to restore and maintain cellular energy metabolism, reduces mitochondria-mediated cell and tissue damage, and induces apoptosis (40). The present TEM results identified that exogenous HN pretreatment significantly enhanced mitophagy in HLECs under oxidative stress. However, as shown by results from previous studies, the relationship between HN and autophagy is controversial. Gong et al (41) reported that HN induced chaperone-mediated autophagy as opposed to macroautophagy. However, Han et al (42) found that HN induced macroautophagy in the nervous system. Cataracts are associated with lens epithelial cells and lens fiber cells. Moreover, lens fibroblasts are differentiated from lens epithelial cells. The programmed removal of organelles from differentiating lens fiber cells contributes towards lens transparency via the formation of an organelle-free zone (OFZ). Disruptions in OFZ formation are accompanied by the persistence of organelles in lens fiber cells and can contribute towards cataracts (43). Furthermore, mitochondrial autophagy can eliminate damaged or unnecessary mitochondria in HLECs, which ensures the continuous differentiation of lens epithelial cells into lens fibroblasts, and plays an important role in lens development and the maintenance of lens transparency (44). Costello et al (45) reported that the loss of mitophagy may result in retention of mitochondria in the OFZ, leading to light scattering and cataract formation. Mutations in the autophagy gene FYVE and coiled coil domain containing 1 have been confirmed to cause congenital cataracts (46). In addition, in lens fiber cells, crystallin is denatured, modified and aggregated, which can eventually lead to cataracts (10). Therefore, further research on lens fiber cells is necessary. To the best of our knowledge, the present study is the first to use TEM to demonstrate that HN significantly promoted mitochondrial autophagy in HLECs under oxidative stress. However, further investigation is required into the relationship between HN and mitochondrial autophagy, and its role in cataract formation. Future studies will detect autophagy-related proteins, such as microtubule-associated protein 1A/1B-light chain 3, and study the specific molecular pathways of HN to promote mitochondrial autophagy via the use of various autophagy regulators. Overall, the present results identified the mechanisms of HN in reducing intracellular ROS and clearing damaged mitochondria, which is of great significance for understanding the repair of oxidative damage and maintaining cell homeostasis.
Apoptotic cell death of HLECs under oxidative stress may involve complex mechanisms, including the mitochondrial pathway, Bcl-2 protein family and caspase-3 activation. In damaged mitochondria, the decrease in Δψm, massive production of ROS and the release of nucleotide-modified mitochondrial proteins from mitochondria into the cytosol or nucleus play a key role in apoptosis (47). The present study demonstrated that under oxidative stress, HN may have an important role in maintaining HLEC viability, reducing cytotoxic sensitivity and preventing apoptosis. Moreover, it was found that exogenous HN pretreatment alleviated apoptosis of HLECs induced by UVB or serum starvation, while knockdown of HN increased apoptosis in HLECs. In addition, Gross et al (48) found a common checkpoint in the mammalian cell death pathway, which is at the level of the pro-apoptotic Bax and anti-apoptotic Bcl-2. The present study found that exogenous HN treatment upregulated Bcl-2 protein levels and reduced the expression of Bax in HLECs treated with UVB. Furthermore, in untreated HLECs, HN pretreatment did not further reduce the apoptotic rate, but the levels of Bcl-2 and Bax proteins were altered. Thus, it is hypothesized that exogenous HN may inhibit apoptosis at the protein level in untreated conditions, but it is difficult to further reduce apoptosis due to its low basal rate. Gottardo et al (49) revealed that in untreated GH3 cells, HN was able to upregulate Bcl-2 expression, reduce Bax expression and significantly increase the ratio of Bcl-2 to Bax, which is a good index of antiapoptotic cell behavior. Therefore, HN may be involved in the regulation of Bcl-2 family proteins and have a complex relationship with apoptosis-related proteins involved in the mechanism of preventing apoptosis; the binding between HN and Bax has been previously reported (12). Furthermore, caspase-3 plays a key role in the execution of apoptosis and the apoptotic pathway in the development of cataracts (50–52), and caspase activities may be activated by the release of cytochrome c from mitochondria (48). Moreover, the present results suggested that HN reduced the levels of cleaved caspase-3 in UVB-treated HLECs.
In conclusion, the present results indicated that HN may reduce the damage and apoptosis of HLECs under oxidative stress by reducing the production of intracellular ROS and protecting the function of mitochondria. To the best of our knowledge, the present study was the first to demonstrate that exogenous HN may enhance the occurrence of intracellular mitochondrial autophagy to remove dysfunctional mitochondria, and remove harmful byproducts and oxidants to help maintain intracellular environmental balance and cell survival. Moreover, the expression levels of HN have been reported to decrease with age, thus a deficiency in endogenous HN may lead to insufficient oxidative resistance and may be involved in the pathogenesis of age-related diseases (53). The present study investigated the protective effect of HN on HLECs under in vitro oxidative stress. The lack of in vivo studies or clinical data is the main limitation of the present study. To examine the role of HN in the process of cataracts, further in vivo study of HN is required. Given the multiple protective effects of HN in HLECs under oxidative stress, HN may be a valuable potential protective molecule in the prevention and treatment of ARCs.
Supplementary Material
Acknowledgements
The authors would like to thank the Dr Chenqi Luo of the Eye Center of the Second Affiliated Hospital for excellent technical assistance.
Glossary
Abbreviations
- HLECs
human lens epithelial cells
- ARCs
age-related cataracts
- HN
humanin
- MDP
mitochondrial-derived peptide
- ROS
reactive oxygen species
- T2DM
type 2 diabetes mellitus
- AMD
age-related macular degeneration
- CCK-8
Cell Counting Kit-8
- mtDNA
mitochondrial DNA
- Δψm
mitochondrial membrane potential
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81670834), the National Natural Science Foundation of China (grant no. 81970781), the Natural Science Foundation of Zhejiang Province (gran no. LY17H090004), the National Natural Science Foundation of China (grant no. 81800807) and the National Natural Science Foundation of China (grant no. 81800869).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HY wrote the manuscript. HY and YC performed the experiments. HY and QY analyzed the data. YT performed the flow cytometry analysis. JZ, XT, XY and XS designed the study and interpreted the data. XS modified the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Skinner C, Miraldi Utz V. Pharmacological approaches to restoring lens transparency: Real world applications. Ophthalmic Genet. 2017;38:201–205. doi: 10.1080/13816810.2016.1214971. [DOI] [PubMed] [Google Scholar]
- 2.Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010;44:155–165. doi: 10.1159/000316481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu D, Zhao J, Wu D, Zhang J. Ultraviolet A exposure induces reversible disruption of gap junction intercellular communication in lens epithelial cells. Int J Mol Med. 2011;28:239–245. doi: 10.3892/ijmm.2011.665. [DOI] [PubMed] [Google Scholar]
- 4.Li WC, Spector A. Lens epithelial cell apoptosis is an early event in the development of UVB-induced cataract. Free Radic Biol Med. 1996;20:301–311. doi: 10.1016/0891-5849(96)02050-3. [DOI] [PubMed] [Google Scholar]
- 5.Löfgren S. Solar ultraviolet radiation cataract. Exp Eye Res. 2017;156:112–116. doi: 10.1016/j.exer.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Wolfle U, Esser PR, Simon-Haarhaus B, Martin SF, Lademann J, Schempp CM. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic Biol Med. 2011;50:1081–1093. doi: 10.1016/j.freeradbiomed.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Hightower KR. A review of the evidence that ultraviolet irradiation is a risk factor in cataractogenesis. Doc Ophthalmol. 1994;88:205–220. doi: 10.1007/BF01203675. [DOI] [PubMed] [Google Scholar]
- 8.Dillon J. Sunlight exposure and cataract. JAMA. 1999;28:229. doi: 10.1001/jama.281.3.229. [DOI] [PubMed] [Google Scholar]
- 9.Ji Y, Cai L, Zheng T, Ye H, Rong X, Rao J, Lu Y. The mechanism of UVB irradiation induced-apoptosis in cataract. Mol Cell Biochem. 2015;401:87–95. doi: 10.1007/s11010-014-2294-x. [DOI] [PubMed] [Google Scholar]
- 10.Berthoud VM, Beyer EC. Oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal. 2009;11:339–353. doi: 10.1089/ars.2008.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta SK, Trivedi D, Srivastava S, Joshi S, Halder N, Verma SD. Lycopene attenuates oxidative stress induced experimental cataract development: An in vitro and in vivo study. Nutrition. 2003;19:794–799. doi: 10.1016/S0899-9007(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 12.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Human in peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 13.Kin T, Sugie K, Hirano M, Goto YI, Nishino I, Ueno S. Humanin expression in skeletal muscles of patients with chronic progressive external ophthalmoplegia. J Hum Genet. 2006;51:555–558. doi: 10.1007/s10038-006-0397-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Li H, Yuan H, Zheng M, Bai C, Chen L, Pei X. Human in delays apoptosis in K562 cells by downregulation of P38 MAP kinase. Apoptosis. 2005;10:963–971. doi: 10.1007/s10495-005-1191-x. [DOI] [PubMed] [Google Scholar]
- 15.Sreekumar PG, Ishikawa K, Spee C, Mehta HH, Wan J, Yen K, Cohen P, Kannan R, Hinton DR. The mitochondrial-derived peptide human in protects RPE cells from oxidative stress, senescence, and mitochondrial dysfunction. Invest Ophthalmol Vis Sci. 2016;57:1238–1253. doi: 10.1167/iovs.15-17053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein LE, Cui L, Gong Z, Su K, Muzumdar R. A humanin analog decreases oxidative stress and preserves mitochondrial integrity in cardiac myoblasts. Biochem Biophys Res Commun. 2013;440:197–203. doi: 10.1016/j.bbrc.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and A beta. Proc Natl Acad Sci USA. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kariya S, Takahashi N, Ooba N, Kawahara M, Nakayama H, Ueno S. Humanin inhibits cell death of serum-deprived PC12h cells. Neuroreport. 2002;13:903–907. doi: 10.1097/00001756-200205070-00034. [DOI] [PubMed] [Google Scholar]
- 19.Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88:360–366. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Jiang H, Li H, Song W, Xia X. Alpha-A-crystallin protects lens epithelial cell-derived iPSC-like cells against apoptosis induced by oxidative stress. Cell Reprogram. 2016;18:327–332. doi: 10.1089/cell.2016.0017. [DOI] [PubMed] [Google Scholar]
- 22.Rwei P, Alex Gong CS, Luo LJ, Lin MB, Lai JY, Liu HL. In vitro investigation of ultrasound-induced oxidative stress on human lens epithelial cells. Biochem Biophys Res Commun. 2017;482:954–960. doi: 10.1016/j.bbrc.2016.11.139. [DOI] [PubMed] [Google Scholar]
- 23.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen K, Lee C, Mehta H, Cohen P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. 2013;50:R11–R19. doi: 10.1530/JME-12-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West SK, Longstreth JD, Munoz BE, Pitcher HM, Duncan DD. Model of risk of cortical cataract in the US population with exposure to increased ultraviolet radiation due to stratospheric ozone depletion. Am J Epidemiol. 2005;162:1080–1088. doi: 10.1093/aje/kwi329. [DOI] [PubMed] [Google Scholar]
- 26.Yam JC, Kwok AK. Ultraviolet light and ocular diseases. Int Ophthalmol. 2014;34:383–400. doi: 10.1007/s10792-013-9791-x. [DOI] [PubMed] [Google Scholar]
- 27.Hua H, Yang T, Huang L, Chen R, Li M, Zou Z, Wang N, Yang D, Liu Y. Protective effects of lanosterol synthase up-regulation in UV-B-induced oxidative Stress. Front Pharmacol. 2019;10:947. doi: 10.3389/fphar.2019.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodzioch M, Lapicka-Bodzioch K, Zapala B, Kamysz W, Kiec-Wilk B, Dembinska-Kiec A. Evidence for potential functionality of Nuclearly-encoded humanin isoforms. Genomics. 2009;94:247–256. doi: 10.1016/j.ygeno.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Ramanjaneya M, Bettahi I, Jerobin J, Chandra P, Abi Khalil C, Skarulis M, Atkin SL, Abou-Samra AB. Mitochondrial-derived peptides are down regulated in diabetes subjects. Front Endocrinol (Lausanne) 2019;10:331. doi: 10.3389/fendo.2019.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Yu R, Shi Y, Dai Y, Zeng Z, Guo X, Ji Q, Wang G, Zhong J. Transduced protein transduction domain linked HSP27 protected LECs against UVB radiation-induced damage. Exp Eye Res. 2014;120:36–42. doi: 10.1016/j.exer.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Marchetti MA, Lee W, Cowell TL, Wells TM, Weissbach H, Kantorow M. Silencing of the methionine sulfoxide reductase A gene results in loss of mitochondrial membrane potential and increased ROS production in human lens cells. Exp Eye Res. 2006;83:1281–1286. doi: 10.1016/j.exer.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ylä-Herttuala S. Oxidized LDL and atherogenesis. Ann N Y Acad Sci. 1999;874:134–137. doi: 10.1111/j.1749-6632.1999.tb09231.x. [DOI] [PubMed] [Google Scholar]
- 33.Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, Lin L, Giblin F, Ho YS, Lou MF. Glutaredoxin 2 knockout increases sensitivity to oxidative stress in mouse lense pithelial cells. Free Radic Biol Med. 2011;51:2108–2117. doi: 10.1016/j.freeradbiomed.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooks MM, Neelam S, Fudala R, Gryczynski I, Cammarata PR. Lenticular mitoprotection. Part A: Monitoring mitochondrial depolarization with JC-1 and artifactual fluorescence by the glycogen synthase kinase-3β inhibitor, SB216763. Mol Vis. 2013;19:1406–1412. [PMC free article] [PubMed] [Google Scholar]
- 36.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–8660. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcriptioni nitiation, DNA packaging, and genome copy number. Biochim Biophys Acta. 2012;1819:921–929. doi: 10.1016/j.bbagrm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Lee C, Wu SB, Hong CH, Liao WT, Wu CY, Chen GS, Wei YH, Yu HS. Aberrant cell proliferation by enhanced mitochondrial biogenesis via mtTFA in arsenical skin cancers. Am J Pathol. 2011;178:2066–2076. doi: 10.1016/j.ajpath.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Q, Guo D, Bi H, Wang D, Du Y. UVB irradiation-induced dysregulation of plasma membrane calcium ATPase1 and intracellular calcium homeostasis in human lens epithelial cells. Mol Cell Biochem. 2013;382:263–272. doi: 10.1007/s11010-013-1743-2. [DOI] [PubMed] [Google Scholar]
- 40.Shefa U, Jeong NY, Song IO, Chung HJ, Kim D, Jung J, Huh Y. Mitophagy links oxidative stress conditions and neurodegenerative diseases. Neural Regen Res. 2019;14:749–756. doi: 10.4103/1673-5374.249218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong Z, Tasset I, Diaz A, Anguiano J, Tas E, Cui L, Kuliawat R, Liu H, Kühn B, Cuervo AM, Muzumdar R. Humanin is an endogenous activator of chaperone-mediated autophagy. J Cell Biol. 2018;217:635–647. doi: 10.1083/jcb.201606095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han K, Jia N, Zhong Y, Shang X. S14G-humanin alleviates insulin resistance and increases autophagy in neurons of APP/PS1 transgenic mouse. J Cell Biochem. 2018;119:3111–3117. doi: 10.1002/jcb.26452. [DOI] [PubMed] [Google Scholar]
- 43.Wride MA. Lens fibre cell differentiation and organelle loss: Many paths lead to clarity. Philos Trans R Soc Lond B Biol Sci. 2011;366:1219–1233. doi: 10.1098/rstb.2010.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brennan LA, McGreal-Estrada R, Logan CM, Cvekl A, Menko AS, Kantorow M. BNIP3L/NIX is required for elimination of mitochondria, endoplasmic reticulum and Golgi apparatus during eye lens organelle-free zone formation. Exp Eye Res. 2018;74:173–184. doi: 10.1016/j.exer.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costello MJ, Brennan LA, Basu S, Chauss D, Mohamed A, Gilliland KO, Johnsen S, Menko S, Kantorow M. Autophagy and mitophagy participate in ocular lens organelle degradation. Exp Eye Res. 2013;116:141–150. doi: 10.1016/j.exer.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, Kantorow M, Pras E, Frydman M, Pras E, Riazuddin S, et al. Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am J Hum Genet. 2011;88:827–838. doi: 10.1016/j.ajhg.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 49.Gottardo MF, Ayala MM, Ferraris J, Zárate S, Pisera D, Candolfi M, Jaita G, Seilicovich A. Humanin inhibits apoptosis in pituitary tumor cells through several signaling pathways including NF-κB activation. J Cell Commun Signal. 2017;11:329–340. doi: 10.1007/s12079-017-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy S. Caspases at the heart of the apoptotic cell death pathway. Chemical Res Toxicol. 2000;13:961–962. doi: 10.1021/tx000109k. [DOI] [PubMed] [Google Scholar]
- 51.Andersson M, Honarvar A, Sjöstrand J, Peterson A, Karlsson JO. Decreased caspase-3 activity in human lens epithelium from posterior sub capsular cataracts. Exp Eye Res. 2003;76:175–182. doi: 10.1016/S0014-4835(02)00283-X. [DOI] [PubMed] [Google Scholar]
- 52.Yao H, Tang X, Shao X, Feng L, Wu N, Yao K. Parthenolide protects human lens epithelial cells from oxidative stress-induced apoptosis via inhibition of activation of caspase-3 and caspase-9. Cell Res. 2007;17:565–571. doi: 10.1038/cr.2007.6. [DOI] [PubMed] [Google Scholar]
- 53.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, et al. Humanin: A novel central regulator of peripheral insulin action. PLoS One. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.