Abstract
Objectives:
Pediatric severe sepsis is a major cause of morbidity and mortality worldwide, and hematopoietic cell transplant patients represent a high-risk population. We assessed the epidemiology of severe sepsis in hematopoietic cell transplant patients, describing patient outcomes compared with children with no history of hematopoietic cell transplant.
Design:
Secondary analysis of the Sepsis PRevalence, OUtcomes, and Therapies point prevalence study, comparing demographics, sepsis etiology, illness severity, organ dysfunction, and sepsis-related treatments in patients with and without hematopoietic cell transplant. The primary outcome was hospital mortality. Multivariable logistic regression models were used to determine adjusted differences in mortality.
Setting:
International; 128 PICUs in 26 countries.
Patients:
Pediatric patients with severe sepsis prospectively identified over a 1-year period.
Interventions:
None.
Measurements and Main Results:
In patients with severe sepsis, 37/567 (6.5%) had a history of hematopoietic cell transplant. Compared with patients without hematopoietic cell transplant, hematopoietic cell transplant patients had significantly higher hospital mortality (68% vs 23%; p < 0.001). Hematopoietic cell transplant patients were more likely to have hospital acquired sepsis and had more preexisting renal and hepatic dysfunction than non–hematopoietic cell transplant patients with severe sepsis. History of hematopoietic cell transplant, renal replacement therapy, admission from inpatient floor, and number of organ dysfunctions at severe sepsis recognition were independently associated with hospital mortality in multivariable analysis; hematopoietic cell transplant conferred the highest odds of mortality (odds ratio, 4.00; 95% CI, 1.78–8.98). In secondary analysis of hematopoietic cell transplant patients compared with other immunocompromised patients with severe sepsis, history of hematopoietic cell transplant remained independently associated with hospital mortality (odds ratio, 3.03; 95% CI, 1.11–8.27).
Conclusions:
In an international study of pediatric severe sepsis, history of hematopoietic cell transplant is associated with a four-fold increased odds of hospital mortality after adjustment for potential measured confounders. Hematopoietic cell transplant patients more often originated from within the hospital compared to children with severe sepsis without hematopoietic cell transplant, possibly providing an earlier opportunity for sepsis recognition and intervention in this high-risk population.
Keywords: epidemiology, hematopoietic cell transplantation, immunocompromised host, multiple organ dysfunction syndrome, severe sepsiss
Pediatric severe sepsis is a common indication for admission to the PICU, and it remains a significant cause of morbidity and mortality in children worldwide (1, 2). Recent data estimate that 8% of children receiving critical care around the world have severe sepsis at any given time (3), and mortality estimates for children with severe sepsis range between 10% and 25% (3, 4). The prevalence of severe sepsis in children is increasing (5), and consensus guidelines emphasizing prompt recognition and treatment of severe sepsis (6) have led to improvements in sepsis-related morbidity and mortality (5, 7, 8).
Hematopoietic cell transplant (HCT) patients represent a particularly high-risk cohort of PICU patients; recipients have poor outcomes regardless of their indication for PICU admission (9–11). Data from retrospective cohorts have demonstrated increased morbidity and mortality in subsets of septic patients with malignancy (12), congenital immunodeficiency (13), and HCT (14). Unfortunately, retrospective identification of patients with severe sepsis is problematic (15), which may limit the reliability of retrospective reports. Several studies have prospectively enrolled children with severe sepsis (16–21), although none have specifically evaluated patients for comorbidities associated with immune dysfunction, including history of HCT, and some specifically exclude those with immune compromise or history of HCT (4). Based on the available literature, it is difficult to draw broad generalizations about the epidemiology of severe sepsis in HCT patients, to determine if the treatments and supportive care used for HCT patients with severe sepsis are different than those used for immunocompetent children, and to assess clinically relevant outcomes in this population.
The Sepsis PRevalence, OUtcomes, and Therapies (SPROUT) point prevalence study was a large, multicenter, prospective study to assess the burden of pediatric severe sepsis worldwide. Results from that study demonstrated 25% hospital mortality among patients with severe sepsis and new moderate-to-severe disability at hospital discharge in 17% of survivors (3). In our analysis, we assessed the prevalence of HCT among children with severe sepsis in the SPROUT study and described outcomes in HCT patients versus patients without HCT, comparing demographics, sepsis etiology, illness severity, organ dysfunction, sepsis-related treatments, disability, and mortality. We hypothesized that prior HCT would be an independent risk factor for mortality and that HCT recipients would develop more end-organ dysfunction, receive more intensive therapies, and experience higher levels of new moderate-to-severe disability than SPROUT subjects without a history of HCT. We also hypothesized that HCT would remain an independent risk factor for mortality in a subanalysis of immunocompromised patients with sepsis.
MATERIALS AND METHODS
Study Design
The SPROUT study was a prospective, cross-sectional point prevalence study with 90-day follow-up for measurement of outcomes performed in 128 PICUs in 26 countries on 5 study days spaced throughout 2013 and 2014 (3). Sites were recruited by open invitation, and participation was voluntary. Ethical approval was obtained at all study sites, and waiver of informed consent was granted at all but three sites, at which written consent was required for data collection. The details of the SPROUT study methodology have been published previously (3).
Data Collection
On each study day, PICU patients less than 18 years old were screened for severe sepsis according to consensus criteria (22) using data from the 24 hours preceding the study day, yielding a study cohort of patients with active severe sepsis. Infants less than 42 weeks corrected gestational age, and patients who received cardiopulmonary bypass in the preceding 5 days were excluded. For patients meeting inclusion criteria, demographics were collected from the time of PICU admission. Vital signs, laboratory results, and therapeutic interventions were captured within a 48-hour period around the study day (from 9:00 am the day prior to 9:00 am the day after). Detailed HCT-related data, including date of transplant, indication for transplant, type of transplant, source of cells, and conditioning regimen, were not collected. The date of severe sepsis recognition was determined by retrospective medical record review at the time of study enrollment. To determine severity of illness, the Pediatric Index of Mortality (PIM)-2 score (23) and PIM-3 score (24) were calculated from PICU admission. The Pediatric Logistic Organ Dysfunction score (25) was calculated on the study day. The presence of multiple organ dysfunction syndrome was assessed using published definitions (26) on the day of severe sepsis recognition, and the development of organ dysfunction was assessed for the next 7 days. The Pediatric Overall Performance Category (POPC) ordinal scale (27) was used to calculate baseline disability and disability at hospital discharge for all hospital survivors. Patients were followed until hospital discharge (censored at 90 d if still hospitalized) to determine clinical outcomes. Details of the Goldstein criteria for severe sepsis and the Proulx criteria for multiple organ dysfunction syndrome are shown in eTable 1 (Supplemental Digital Content 1, http://links.lww.com/PCC/A530) and eTable 2 (Supplemental Digital Content 1, http://links.lww.com/PCC/A530), respectively.
Exposure
Patients with a documented history of HCT were assigned to the HCT cohort. History of HCT was identified through medical record review at the time of study enrollment and included patients who received autologous or allogeneic HCT at any time prior to study enrollment, regardless of indication for transplant and source of cells. All other patients with severe sepsis were assigned to the reference cohort. A secondary analysis was performed in the subset of immunocompromised SPROUT patients, comparing those with a history of HCT to immunocompromised patients with no history of HCT. Immunocompromised diagnoses included malignancies, solid organ transplants, and congenital immunodeficiencies.
Patient Outcomes
The primary outcome was all-cause hospital mortality at hospital discharge, censored at 90 days. Secondary outcomes included PICU mortality, PICU length of stay (LOS), hospital LOS, PICU-free days, vasoactive-free and ventilator-free days out of 28 from the day of severe sepsis recognition, and new mild or moderate disability measured by change in POPC.
Statistical Analysis
Data are presented as medians with interquartile ranges (IQRs) for continuous variables and frequencies with proportions for categorical variables. Comparisons between groups were performed using the Wilcoxon rank-sum test for continuous variables and Fisher exact test or chi-square test as appropriate categorical variables. Multivariable logistic regression was performed to test the association of HCT with the binary outcome of hospital mortality. Covariates were identified a priori based on biologic plausibility and observed differences between patients with and without HCT. We defined a confounder as a covariate that changed the odds ratio (OR) of the association between HCT and mortality by greater than or equal to 10%. The following covariates were tested for confounding: age, geographic region, number of organ dysfunctions at the time of severe sepsis recognition, viral sepsis etiology, presence of bacteremia, treatment with renal replacement therapy (RRT), and admission from an inpatient floor. Treatments that were expected to differ between groups on the basis of a history of HCT (e.g., blood product and IV immune globulin [IVIG] administration) were not included in the multivariable model. Although age did not meet our a priori criteria for confounding, it was included in all final models. A second multivariable model was constructed for the subset of immunocompromised patients using the same methodology. Data were analyzed using Stata (v12.1; StataCorp LLC, College Station, TX).
RESULTS
Prevalence of HCT and Associated Conditions
In the SPROUT study, 6,925 patients were screened and 569 were identified who met consensus criteria for active severe sepsis (3). Two patients declined consent for data collection, and detailed data were collected on 567 patients (Table 1). Of these, 37 (6.5%) had a history of HCT. HCT patients were older, were more frequently admitted from the inpatient ward, and were more likely to have renal and hematologic dysfunctions (26) at the onset of severe sepsis (Table 2). HCT patients had significantly higher median PIM-3 score (10.6 vs 3.9; p < 0.001); however, PIM-2 score, which does not adjust for HCT, was not significantly different between groups. Although there was no significant difference in the primary site of infection between cohorts, HCT patients were more likely than non-HCT patients to have documented bacteremia (41% vs 23%; p = 0.018) and viral infections (41% vs 20%, p = 0.003), as shown in Table 3.
TABLE 1.
Characteristics of Patients With Hematopoietic Cell Transplant Compared to Patients Without Hematopoietic Cell Transplant
| Characteristics | HCT (n = 37) | No HCT (n = 530) | P |
|---|---|---|---|
| Age (yr), median (IQR) | 9 (3–11) | 3 (0.7–10) | 0.001 |
| Weight (kg), median (IQR)a | 26 (14–37) | 14 (7–31) | 0.001 |
| Male gender, n (%) | 22 (59) | 280 (53) | 0.435 |
| Race, n (%) | 0.193 | ||
| White | 23 (62) | 222 (42) | |
| Black | 2 (5) | 77 (15) | |
| Asian | 3 (8) | 72 (14) | |
| Other | 8 (22) | 128 (24) | |
| Unknown | 1 (3) | 31 (6) | |
| Hispanic ethnicity, n (%) | 5 (14) | 97 (18) | 0.657 |
| Geographic region, n (%) | 0.591 | ||
| North America | 24 (65) | 318 (60) | |
| South America | 1 (3) | 43 (8) | |
| Europe | 8 (22) | 78 (15) | |
| Asia | 2 (5) | 53 (10) | |
| Africa and Middle East | 0 (0) | 15 (3) | |
| Australia and New Zealand | 2 (5) | 23 (4) | |
| Admission source, n (%) | < 0.001 | ||
| Emergency department | 4 (11) | 163 (31) | |
| Inpatient ward | 27 (73) | 131 (25) | |
| Operating room | 1 (3) | 49 (9) | |
| Another hospital | 2 (5) | 164 (31) | |
| Other | 3 (8) | 23 (4) | |
| Admission type, n (%) | 0.289 | ||
| Medical | 34 (92) | 426 (80) | |
| Surgical | 3 (8) | 84 (16) | |
| Trauma | 0 (0) | 10 (4) | |
| Comorbid conditions, n (%) | |||
| None | 0 (0) | 117 (22) | < 0.001 |
| Respiratory | 11 (30) | 161 (30) | 0.934 |
| Cardiovascular | 5 (14) | 131 (25) | 0.162 |
| Gastrointestinal | 9 (24) | 109 (21) | 0.537 |
| Congenital/genetic | 8 (22) | 107 (20) | 0.833 |
| Hematologic/immunologic | 32 (86) | 82 (15) | < 0.001 |
| Neuromuscular | 4 (11) | 93 (18) | 0.371 |
| Prematurity | 0 (0) | 76 (14) | 0.006 |
| Malignancy | 24 (65) | 56 (11) | < 0.001 |
| Solid organ transplant | 0 (0) | 17 (3) | 0.618 |
| Dialysis dependent | 2 (5) | 10 (2) | 0.181 |
| PIM-2 score, median (IQR) | 4.5 (1.3–6.6) | 4.5 (1.5–12.0) | 0.445 |
| PIM-3 score, median (IQR) | 10.6 (7.4–19.3) | 3.9 (1.7–7.4) | < 0.001 |
| Pediatric Logistic Organ Dysfunction score, median (IQR) | 12 (3–21) | 11 (2–12) | 0.015 |
| WBC count < 0.5, n (%) | 7 (19) | 16 (3) | < 0.001 |
| Baseline Pediatric Overall Performance Category, n (%) | 0.075 | ||
| Good | 16 (43) | 274 (52) | |
| Mild disability | 10 (27) | 75 (14) | |
| Moderate disability | 6 (16) | 84 (16) | |
| Severe disability | 4 (11) | 95 (18) | |
| Coma or vegetative state | 1 (3) | 2 (< 1) |
HCT = hematopoietic cell transplant, IQR = interquartile range, PIM = Pediatric Index of Mortality.
Weight data were available in 563 patients.
Boldface values indicate values where p < 0.05.
TABLE 2.
Organ Dysfunction Among Patients With Severe Sepsis, by Time Point, Criteria, and History of Hematopoietic Cell Transplant
| Goldstein Criteria (22), Assessed at Study Screening | |||
|---|---|---|---|
| HCT (n = 37) | No HCT (n = 530) | P | |
| No. of organ dysfunctions, median (IQR) | 3 (2–4) | 2 (2–3) | 0.018 |
| Respiratory dysfunction, n (%) | 29 (78) | 440 (83) | 0.499 |
| Cardiovascular dysfunction, n (%) | 21 (57) | 377 (71) | 0.065 |
| Kidney dysfunction, n (%) | 14 (38) | 79 (15) | < 0.001 |
| Liver dysfunction, n (%) | 14 (38) | 129 (24) | 0.068 |
| Hematologic dysfunction, n (%) | 26 (70) | 149 (28) | < 0.001 |
| Neurologic dysfunction, n (%) | 5 (14) | 114 (22) | 0.301 |
| Proulx Criteria (26), Assessed on Day of Severe Sepsis Recognition | |||
| HCT (n = 37) | No HCT (n = 530) | P | |
| No. of organ dysfunctions, median (IQR) | 3 (2–3) | 2 (1–3) | 0.001 |
| Cardiovascular dysfunction, n (%) | 26 (70) | 339 (64) | 0.439 |
| Respiratory dysfunction, n (%) | 31 (84) | 435 (82) | 1.000 |
| Neurologic dysfunction, n (%) | 6 (16) | 81 (15) | 0.816 |
| Gastrointestinal dysfunction, n (%) | 5 (14) | 43 (8) | 0.228 |
| Hematologic dysfunction, n (%) | 31 (84) | 182 (34) | < 0.001 |
| “Preexisting” hematologic dysfunction, n (%) | 29 (78) | 62 (12) | < 0.001 |
| “New” hematologic dysfunction, n (%) | 2 (5) | 121 (23) | 0.012 |
| Hepatic dysfunction, n (%) | 14 (38) | 82 (15) | < 0.001 |
| “Preexisting” hepatic dysfunction, n (%) | 8 (22) | 36 (7) | 0.005 |
| “New” hepatic dysfunction, n (%) | 6 (16) | 46 (9) | 0.137 |
| Renal dysfunction, n (%) | 20 (54) | 97 (18) | < 0.001 |
| “Preexisting” renal dysfunction, n (%) | 9 (24) | 38 (7) | 0.002 |
| “New” renal dysfunction, n (%) | 11 (30) | 59 (11) | 0.001 |
HCT = hematopoietic cell transplant, IQR = interquartile range.
TABLE 3.
Comparison of Site of Infection and Distribution of Pathogens in Hematopoietic Cell Transplant Patients Versus Patients Without Hematopoietic Cell Transplant
| Characteristics of Infection | HCT (n = 37) | No HCT (n = 530) | P |
|---|---|---|---|
| Primary site of infection, n (%) | 0.071 | ||
| Respiratory | 13 (35) | 215 (41) | |
| Bloodstream | 15 (41) | 93 (18) | |
| Abdominal | 2 (5) | 45 (8) | |
| CNS | 0 (0) | 25 (5) | |
| Skin | 0 (0) | 20 (4) | |
| Urinary tract | 1 (3) | 20 (4) | |
| Other | 0 (0) | 29 (5) | |
| Unknown | 6 (16) | 83 (16) | |
| Pathogen identified, n (%) | 26 (70) | 345 (65) | 0.522 |
| Bacteremia present, n (%) | 15 (41) | 123 (23) | 0.018 |
| Gram-positive infection, n (%) | 9 (24) | 141 (27) | 0.849 |
| Gram-negative infection, n (%) | 9 (24) | 149 (28) | 0.707 |
| Fungal infection, n (%) | 6 (16) | 70 (13) | 0.616 |
| Viral infection, n (%) | 15 (41) | 104 (20) | 0.003 |
| Parasitic infection, n (%) | 0 (0) | 3 (< 1) | 1.000 |
HCT = hematopoietic cell transplant.
Boldface values indicate values where p < 0.05.
HCT Was Associated With Hospital Mortality and Prolonged LOS
The primary outcome, hospital mortality, occurred significantly more frequently in HCT patients in our cohort (68% vs 23%; p < 0.001) (Table 4). PICU and hospital mortality were high among HCT patients despite similar PICU LOS and similar ventilator-free days and vasoactive infusion-free days in the 28 days following severe sepsis recognition. Among non-survivors, median hospital LOS was significantly higher in the HCT group, though PICU LOS was similar between groups. Stratification by mortality revealed a bimodal distribution of PICU LOS in HCT patients (eTable 3, Supplemental Digital Content 1, http://links.lww.com/PCC/A530), with a much higher median LOS in children who died in the PICU (23 d [IQR, 12–56]) compared with those who were discharged alive (6.5 d [IQR, 3.5–23]). Median PICU-free days for HCT patients were significantly fewer than the reference cohort, and Figure 1 shows the cumulative incidence of being discharged from the PICU alive among HCT patients versus patients without HCT (Fig. 1A) and among HCT patients versus immunocompromised patients without HCT versus immunocompetent patients (Fig. 1B). No difference in moderate-to-severe disability among survivors was noted between the cohorts (Table 4). Multivariable logistic regression identified history of HCT, number of organ dysfunctions at recognition of severe sepsis, RRT, and admission from an inpatient floor as independent variables associated with hospital mortality in patients with severe sepsis (Table 5). After adjustment for confounders, the odds of hospital mortality were four-fold higher in HCT patients compared to patients without HCT (adjusted OR, 4.00; 95% CI, 1.78–8.98; p = 0.001).
TABLE 4.
Comparison of Outcomes of Patients With Hematopoietic Cell Transplant and Patients Without Hematopoietic Cell Transplant
| Outcomes | HCT (n = 37) | No HCT (n = 530) | P |
|---|---|---|---|
| PICU mortality, n (%) | 24 (65) | 115 (22) | < 0.001 |
| Hospital mortality, n (%) | 25 (68) | 120 (23) | < 0.001 |
| PICU LOS (d), median (IQR) | 19 (10–36) | 15 (7–35) | 0.222 |
| PICU-free daysa out of 28, median (IQR) | 0 (0–5) | 7 (0–19) | 0.009 |
| PICU-free daysa out of 60, median (IQR) | 0 (0–37) | 39 (0–51) | 0.001 |
| Hospital LOS (d), median (IQR) | 42 (19–74) | 26 (13–53) | 0.034 |
| Ventilator-free daysb, median (IQR) | 15 (0–25) | 19 (1–25) | 0.255 |
| Vasoactive-free daysb, median (IQR) | 23 (17–27) | 25 (18–28) | 0.212 |
| New mild disability in survivorsc, n (%) | 1 (8) | 115 (28) | 0.193 |
| New moderate disability in survivorsc, n (%) | 1 (8) | 72 (18) | 0.700 |
HCT = hematopoietic cell transplant, IQR = interquartile range, LOS = length of stay.
Excludes six patients who survived the PICU but died in the hospital.
Out of 28 d.
Out of 422 hospital survivors.
Boldface values indicate values where p < 0.05.
Figure 1.
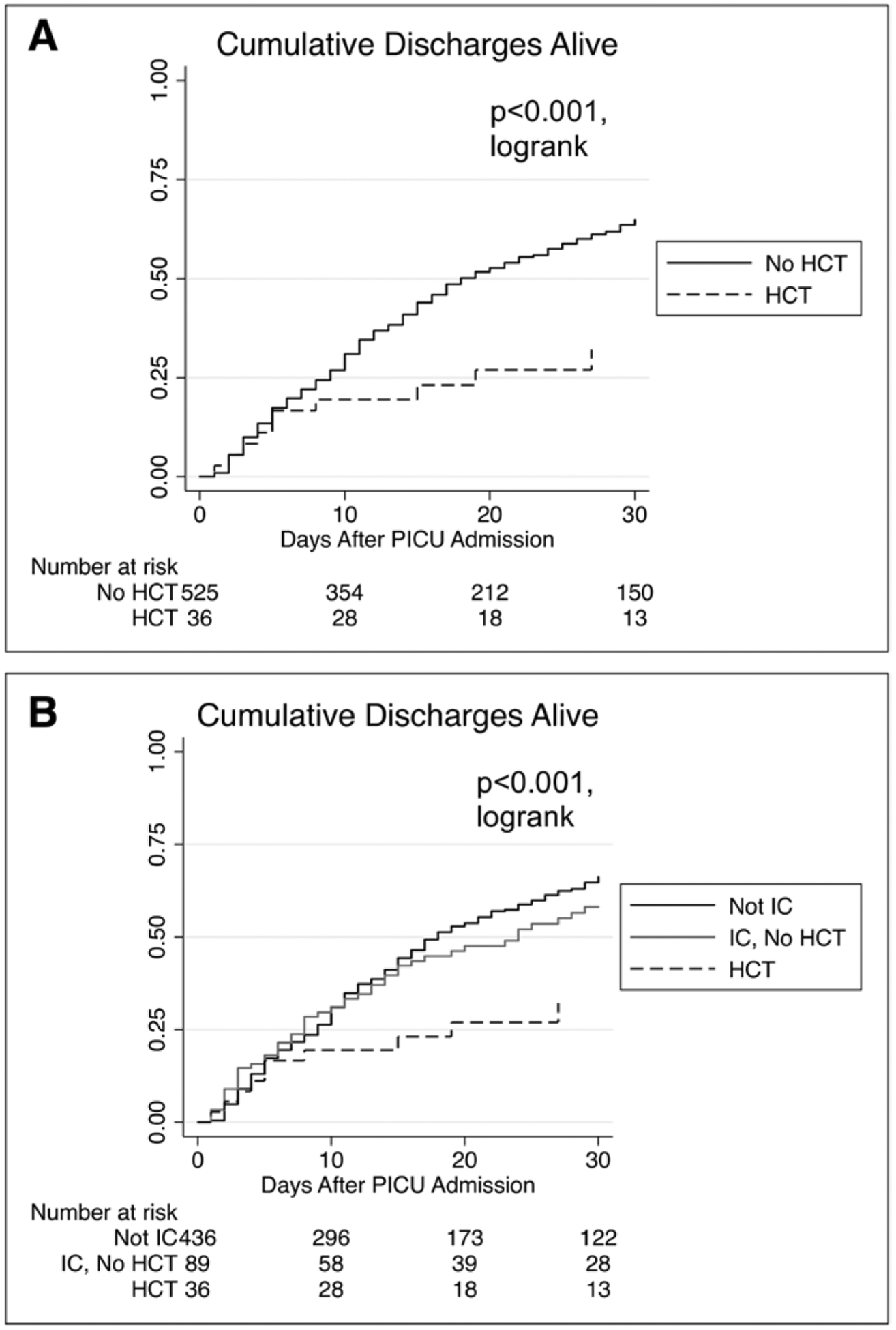
Cumulative discharges alive by cohort, from PICU admission to discharge. Lines represent the cumulative rate of survival to discharge. The x-axis shows PICU length of stay. A, Compares hematopoietic cell transplant (HCT) patients to patients without HCT. B, Divides the cohort into HCT patients, immunocompromised patients without HCT, and nonimmunocompromised patients. Six patients who survived PICU admission but later died in the hospital could not be assessed based on the method of data collection and are excluded from this figure. IC = immunocompromised.
TABLE 5.
Multivariable Logistic Regression Analysis for Risk of Hospital Mortality, Complete Cohort
| Exposure | OR for Hospital Mortality | 95% CI | P |
|---|---|---|---|
| Hematopoietic cell transplant | 4.00 | 1.78–8.98 | 0.001 |
| Age (yr) | 1.00 | 0.96–1.04 | 0.933 |
| Region | |||
| North America | Reference | Reference | Reference |
| South America | 0.50 | 0.19–1.33 | 0.166 |
| Europe | 1.00 | 0.54–1.83 | 0.988 |
| Asia | 2.31 | 1.17–4.58 | 0.016 |
| Africa | 2.82 | 0.89–8.97 | 0.079 |
| Australia/New Zealand | 1.20 | 0.44–3.27 | 0.727 |
| No. of organ dysfunctions at severe sepsis recognition (Proulx criteria) | 1.59 | 1.31–1.93 | < 0.001 |
| Renal replacement therapy | 2.77 | 1.56–4.94 | 0.001 |
| Admission from inpatient floor | 1.98 | 1.24–3.16 | 0.004 |
OR = odds ratio.
Boldface values indicate values where p < 0.05.
HCT Patients Developed Sepsis While Hospitalized
In our cohort, HCT patients were more than twice as likely to be admitted from an inpatient ward compared to patients without HCT (73% vs 25%) (Table 1). Among HCT patients with severe sepsis, median PIM-3 score was higher in those patients admitted from an inpatient ward than those admitted from another source (4.6 [IQR, 2.1–10.6] vs 3.8 [IQR, 1.5–7.7]; p = 0.001). In the multivariable analysis, admission from an inpatient ward was independently associated with mortality among patients with severe sepsis (Table 5).
New Organ Dysfunction Is More Common in HCT Patients
Rates of organ dysfunction were assessed at multiple times and by multiple definitions, as shown in Table 2. When compared with the reference cohort, there was more preexisting renal dysfunction and hepatic dysfunction defined by Proulx criteria (26) in HCT patients, while rates of cardiovascular and respiratory dysfunctions were similar across groups. The total number of organ dysfunctions defined by Proulx criteria at the time of severe sepsis recognition was higher among HCT patients, accounted for by preexisting hematologic, hepatic, or renal dysfunction. In all categories of organ dysfunction, there was no difference in the timing of new organ dysfunction onset relative to severe sepsis recognition in those who had organ dysfunction (not shown). HCT patients had significantly more renal dysfunction present at the time of severe sepsis recognition than the reference cohort (54% vs 18%; p < 0.001), both new renal dysfunction (30% vs 11%; p = 0.001) and preexisting renal dysfunction (24% vs 7%; p = 0.002).
HCT Patients Were Exposed to High-Risk Therapies During Treatment for Sepsis
Despite similar PICU LOS, ventilator-free days, and vasoactive infusion-free days in the 28 days following severe sepsis recognition, HCT patients were more frequently exposed to RRT, corticosteroids, insulin, blood products, colony-stimulating factors, and IVIG. As noted above, renal dysfunction was more common among HCT patients, and 38% of HCT patients were treated with RRT. HCT patients also received less enteral nutrition (EN) (38% vs 60%; p = 0.009) and more parenteral nutrition (PN) (78% vs 36%; p < 0.001) than non-HCT patients. There were not differences, however, between the use of mechanical ventilation, vasoactive infusion, or central, arterial, and bladder catheters between patients with and without HCT (eTable 4, Supplemental Digital Content 1, http://links.lww.com/PCC/A530).
Subanalysis of Immunocompromised Patients With Severe Sepsis
Secondary analysis restricted to the subpopulation of immunocompromised SPROUT patients shows similar results to the overall cohort. When compared with immunocompromised patients without HCT (n = 91), HCT patients were more likely to be admitted from an inpatient ward and had more organ dysfunction at the time of severe sepsis recognition but did not have higher PIM-2 scores (eTable 5, Supplemental Digital Content 1, http://links.lww.com/PCC/A530). HCT patients did not have bacteremia more frequently but had a higher proportion of viral infections when compared with other immunocompromised patients (eTable 6, Supplemental Digital Content 1, http://links.lww.com/PCC/A530). Hospital mortality among HCT patients was much higher than among other immunocompromised patients (68% vs 31%; p < 0.001) despite similar ventilator-free days and vasoactive infusion-free days (eTable 7, Supplemental Digital Content 1, http://links.lww.com/PCC/A530).
A second multivariable logistic regression model evaluated the independent association of HCT with hospital mortality within the subpopulation of SPROUT patients with immunocompromising conditions. In this analysis, history of HCT remained a significant independent risk factor for hospital mortality (OR, 3.03; 95% CI, 1.11–8.27), along with RRT, insulin therapy, and admission from an inpatient floor (Table 6). Differences were also seen in the cumulative incidence of being discharged alive between HCT patients and other immunocompromised patients without HCT (eFig. 1, Supplemental Digital Content 2, http://links.lww.com/PCC/A531; legend, Supplemental Digital Content 1, http://links.lww.com/PCC/A530).
TABLE 6.
Multivariable Logistic Regression Analysis for Risk of Hospital Mortality, Limited to Immunocompromised Patients
| Exposure | OR for Hospital Mortality | 95% CI | P |
|---|---|---|---|
| Hematopoietic cell transplant | 3.03 | 1.11–8.27 | 0.031 |
| Age (yr) | 1.03 | 0.95–1.12 | 0.481 |
| Region | |||
| North America | Reference | Reference | Reference |
| South America | 0.97 | 0.07–12.68 | 0.980 |
| Europe | 1.40 | 0.38–5.22 | 0.613 |
| Asia | 1.72 | 0.40–7.44 | 0.467 |
| Africa | 10.33 | 0.73–146.26 | 0.084 |
| Australia/New Zealand | 0.84 | 0.07–10.00 | 0.891 |
| No. of organ dysfunctions at severe sepsis recognition (Proulx criteria) | 1.39 | 0.95–2.03 | 0.089 |
| Insulin infusion | 4.08 | 1.15–14.53 | 0.030 |
| Renal replacement therapy | 5.02 | 1.68–14.98 | 0.004 |
| Admission from inpatient floor | 3.08 | 1.16–8.19 | 0.024 |
OR = odds ratio.
Boldface values indicate values where p < 0.05.
DISCUSSION
A history of HCT was present in approximately 7% of patients with severe sepsis and was independently associated with a four-fold increased odds of hospital mortality in this cohort. This association remained significant in secondary analysis restricted to only immunocompromised patients. Notably, in this study of severe sepsis, the reference cohort is a medically complex patient population, with 22% being previously healthy patients. Thus, the elevated mortality risk associated with a history of HCT is significant in comparison to other patients with complex chronic conditions frequently cared for in PICUs around the world. Our secondary multivariable logistic regression analysis suggests that the mortality risk associated with HCT is not merely explained by immune dysfunction, as there is a significantly increased risk of mortality associated with HCT even compared with other immunocompromised patients with severe sepsis.
Poor outcomes among HCT patients with sepsis are consistent with previously published literature (14). Although this finding has been consistently reported, its precise cause remains poorly understood. HCT patients are exposed to multiple antibiotics placing them at risk for multidrug resistant organisms (28). Our study did not collect antibiotic sensitivity data and thus cannot assess questions of antibiotic resistance, but it is plausible that the bacterial organisms causing sepsis in HCT patients were resistant to the initial parenteral antibiotic. However, many of the patients in this study had sepsis due to viral infections, and their clinical outcomes were not different than patients with bacterial infections.
Although differences in type and site of infection were not independently associated with mortality in this study, the higher rates of viral infection and bacteremia in the HCT cohort likely reflect differences in risk factors for infection specific to patients with prior HCT. T-cell depletion following HCT is well documented (29) and could explain the higher prevalence of viral illnesses in HCT patients in this study. Of note, HCT patients had higher rates of leukopenia than non-HCT patients in our cohort. The higher rates of bacteremia observed in our HCT patients may be related to the higher rates of indwelling central venous catheters, which have also been described as clear risk factors for bacteremia among immunocompromised patients (30).
Another possible factor leading to inferior outcomes among HCT patients with severe sepsis is that sepsis can serve as a trigger for inflammation-associated complications of HCT, including graft versus host disease (GVHD) and thrombotic microangiopathy (TMA). GVHD is a common complication of HCT, with up to 50% of allogeneic HCT patients developing grade II–IV GVHD. Steroid-refractory GVHD has a very poor survival rate of less than 30% (31), and one of the described triggers for GVHD is infection. The intestinal microbiome seems to also play an important role in GVHD (32), and HCT patients with sepsis may have alterations in the microbiome secondary to antibiotics and lack of enteral feeds, among other factors. Broad-spectrum antibiotics have been shown to increase the mortality in patients with GVHD (31). Due to emerging antibiotic resistance, the increased risk of mortality associated with exposure to broad-spectrum antibiotics, and the high rates of mortality associated with sepsis in HCT patients, proper antimicrobial stewardship is both difficult and imperative for HCT patients.
TMA is an increasingly recognized complication of HCT. TMA is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and thrombi in the microcirculation. Severe TMA carries a mortality rate up to 80%, may cause multiple organ failure, and is associated with complement activation (33). The etiology is thought to be due to endothelial damage caused during transplant conditioning, immunosuppressive agents, GVHD, and/or infection (34). It is possible that the increased mortality seen in HCT patients with sepsis could in part be due to the infection triggering TMA or GVHD, both significant complications with very high mortality rates.
This study also identified admission from an inpatient ward as an independent risk factor for mortality in patients with severe sepsis. Patients with HCT were more likely to be admitted from inpatient wards than patients without HCT, and HCT patients admitted from inpatient wards had higher PIM-3 scores than HCT patients admitted from other locations. In multivariable analysis, source of admission was independently associated with mortality in patients with severe sepsis. These findings support results from a recent prospective study from South America which demonstrated an association between admission from an inpatient ward and hospital mortality in pediatric severe sepsis (18). These observations suggest that future research priorities should focus on the impact of earlier recognition and intervention of severe sepsis in the inpatient setting. Earlier transfer to the PICU for hemodynamic monitoring and comprehensive sepsis care by a team of pediatric intensivists and transplant physicians may be beneficial in these high-risk patients.
The diagnosis of sepsis may also be more challenging in HCT patients than in other populations, and delayed recognition and initiation of sepsis therapies may also contribute to the high mortality rates seen in this population. In our cohort, HCT patients had more preexisting organ dysfunction, likely due to their primary disease, prior chemotherapy, transplant conditioning, and transplant-related complications. In these high-risk, medically complex children, delayed diagnosis due to multiple other active medical problems must be considered in addition to their comorbid immune dysfunction when considering potential explanations for sepsis-related mortality among HCT patients.
Data from this study also demonstrate that children with sepsis and HCT experience a long LOS in PICU, particularly among nonsurvivors. This pattern is consistent with a recently described epidemiologic trend within the PICU population; although crude mortality in PICUs is falling, the PICU LOS of nonsurvivors is steadily increasing (35). The combination of high PICU mortality rate (> 60%) and long PICU LOS in patients with HCT raises questions about the experience of patients and family. Priorities for further research should focus on identifying interventions to reduce mortality and identifying cases in which treatment may be futile. Traditional PICU admission illness severity scores, designed for population not individual mortality risk assessment, are likely not useful in this regard. Although PIM-3 score was more accurate than PIM-2 score in predicting the increased mortality risk associated with HCT patients with severe sepsis, both scores significantly underestimated the risk of hospital mortality in patients with severe sepsis, particularly in those with prior HCT.
Baseline organ dysfunction was more frequent in HCT patients, and new renal dysfunction was also more common in this patient population. Renal dysfunction has been previously described as a risk factor for death in patients with severe sepsis (36). In our cohort, nearly all HCT patients with renal dysfunction received RRT. Based on these findings, septic patients with prior HCT and new renal dysfunction are very likely to require RRT. Further investigation into early initiation of RRT may be warranted in these patients.
In this study, HCT patients received significantly more PN and less EN than the comparison group. Prior studies have demonstrated a beneficial association with early EN and survival in critically ill children (37) and potential harm with early PN (38). There are likely a multitude of reasons that the HCT patients received less EN than the comparison group, though this study did not capture those reasons. The HCT population may not have received EN due to GVHD of the gastrointestinal tract, a common post-HCT complication; frequently, children post HCT have poor oral intake and are placed on PN to supplement their caloric intake; and enteral tract dysfunction requiring PN is common post HCT. Although there were similar amounts of gastrointestinal dysfunction by Proulx criteria between groups, enteral feeding intolerance may not meet the strict gastrointestinal dysfunction criteria used in this study. As a majority of HCT patients originated from another hospital ward, they may have presented in severe sepsis while already receiving PN. This study is limited in that is does not track the timing of EN versus PN, and some patients likely received both simultaneously. The safety of enteral feeding in HCT patients has been established (39), though enteral feeding practices vary by center. Future studies of nutrition in children with severe sepsis could assess the safety of targeted EN and PN support in HCT patients.
Although the SPROUT study has several methodologic strengths, including international scope, prospective clinical diagnosis of severe sepsis based on consensus criteria, and a 90-day follow-up period allowing for analysis of meaningful, patient-centered outcomes, there are some important limitations of this study. The SPROUT study did not collect information regarding indication for HCT, type of transplant, source of cells, conditioning regimen, and transplant complications. Inclusion of these variables could allow for further risk-stratification of HCT patients, as recent reports have highlighted the heterogeneity of risk for transplant-related mortality based on patient comorbidities and HCT characteristics (40). Because of the international scope of our study, around half of the participating sites do not provide care for HCT patients, and thus HCT patients represent a relatively small proportion of patients with severe sepsis in our cohort. In our subanalysis of immunocompromised patients, immunocompromising diagnoses were identified by chart review and did not include robust measures of immune dysfunction. Finally, limited sample size did not allow for analysis of variations in supportive care among geographic regions, which could impact outcomes for children with severe sepsis after HCT.
CONCLUSIONS
In this international study of pediatric severe sepsis, a history of HCT, after adjustment for potentially measured cofounders, is independently associated with a four-fold increased odds of hospital mortality. HCT patients also had more organ dysfunction and were treated with more RRT. Due to the small number of survivors in the HCT group, comparison of new disability in severe sepsis survivors was limited. Notably, HCT recipients with severe sepsis were more likely to be hospitalized and have preexisting organ dysfunction at recognition of sepsis, which may provide a unique opportunity for earlier monitoring, recognition, and intervention. Future investigation of severe sepsis in HCT should include HCT-specific monitoring approaches and interventions. Physicians caring for HCT patients must continue to engage in collaborative investigations and therapeutic strategies to improve recognition and earlier intervention for this high-risk population.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all local investigators at participating Sepsis PRevalence, OUtcomes, and Therapies (SPROUT) study sites for their contributions to this study. SPROUT Study Investigators: North America: Canada: P. Fontela (Montreal Children’s Hospital-McGill); M. Tucci and M. Dumitrascu (Sainte Justine Hospital); and P. Skippen and G. Krahn (BC Children’s Hospital). United States: M. Bigham, T. Polanski, S. Latifi, D. Giebner, and H. Anthony (Akron Children’s Hospital); J. Hume, A. Galster, and L. Linnerud (Amplatz Children’s Hospital); R. Sanders, G. Hefley (Arkansas Children’s Hospital); K. Madden (Boston Children’s Hospital); A. Thompson and S. Shein (Children’s Hospital of Pittsburgh); S. Gertz (Children’s Hospital-Hackensack); Y. Han, T. Williams, and A. Hughes-Schalk (Children’s Mercy Hospital); H. Chandler (Children’s Health-care of Atlanta); A. Orioles, E. Zielinski, and A. Doucette (Children’s Hospital in Minnesota); A. Orioles, E. Zielinski, and A. Doucette (Children’s Hospital St. Paul); C. Zebuhr and T. Wilson (Children’s Hospital Colorado); C. Dimitriades, J. Ascani, S. Layburn, and S. Valley (Children’s Hospital New Orleans); B. Markowitz, J. Terry, and R. Morzov (Children’s Hospital of Los Angeles); A. McInnes (Children’s Hospital of Monmouth); J. McArthur, K. Woods, and K. Murkowski (Children’s Hospital of Wisconsin); M. Spaeder and M. Sharron (Children’s National Medical Center); D. Wheeler, E. Beckman, E. Frank, and K. Howard (Cincinnati Children’s Medical Center); C. Carroll (Connecticut Children’s Medical Center); S. Nett and D. Jarvis (Dartmouth Hitchcock Medical Center); V. Patel (Dayton Children’s Hospital); R. Higgerson and L. Christie (Dell Children’s Medical Center); K. Typpo and J. Deschenes (Diamond Children’s Hospital); A. Kirby (Doernbecher Children’s Hospital); T. Uhl, K. Rehder, I. Cheifetz, and S. Wrenn (Duke Children’s Hospital); K. Kypuros (El Paso Children’s Hospital); K. Ackerman (Golisano Children’s Hospital); E. Bezares (Hospital Cardiovascular de Puerto Rico y el Caribe); F. Maffei and G. Bloomquist (Janet Weis Children’s Hospital/Geisinger); N. Rizkalla (Johns Hopkins Hospital); D. Kimura, S. Shah, and C. Tigges (Le Bonheur Children’s Hospital); F. Su and C. Barlow (Lucile Packard Children’s Hospital); K. Michelson, K. Wolfe, D. Goodman, L. Campbell, and L. Sorce (Lurie Children’s Hospital of Chicago); K. Bysani and T. Monjure (Medical City Children’s-Dallas); M. Evans (Medical University of South Carolina); B. Totapally, M. Chegondi, and C. Rodriguez (Miami Children’s Hospital); J. Frazier and L. Steele (Nationwide Children’s Hospital); S. Viteri and A. Costarino (Nemours/Alfred I. duPont Children’s Hospital); N. Thomas and D. Spear (Penn State Hershey Medical Center); E. Hirshberg and J. Lilley (Primary Children’s Medical Center); C. Rowan and C. Rider (Riley Hospital for Children); J. Kane (Rush Children’s Hospital); G. Puig and A. Puig-Ramos (San Jorge Children’s Hospital); J. Zimmerman and C. Greeley (Seattle Children’s Hospital); J. Lin and R. Jacobs (St. Louis Children’s Hospital); M. Parker and K. Culver (Stony Brook University); L. Loftis, N. Jaimon, and M. Goldsworthy (Texas Children’s Hospital); J. Fitzgerald, S. Weiss, V. Nadkarni, J. Bush, and M. Diliberto (The Children’s Hospital of Philadelphia); C. Alen and M. Gessouroun (Oklahoma University Medical Center); A. Sapru, T. Lang, and M. Alkhouli (University of California San Francisco); S. Kamath, D. Friel, and J. Daufeldt (University of Iowa); R. Garcia and M. Villar (University Pediatric Hospital); D. Hsing, C. Carlo, and S. Pon (Weill Cornell Medical Center); J. Scimeme and A. Shaheen (Wolfson Children’s Hospital); A. Hassinger and H. Qiao (Women and Children’s Hospital of Buffalo); and J. Giuliano and J. Tala (Yale Children’s Hospital). South America: Argentina: D. Vinciguerra and A. Fernandez (Hospital Durand). Colombia: R. Carrero (Clínica Infantil Colsubsidio); P. Hoyos (Hospital de San Jose); J. Jaramillo and A. Posada (Hospital General de Medellín); L. Izquiierdo (Hospital Military Central); and B. E. Piñeres Olave and J. Donado (Pablo Tobón Uribe). Chile: R. Dalmazzo and S. Rendich (Clínica Las Condes); L. Palma and M. Lapadula (Clínica Santa María); C. Acuña (Hospital Luis Calvo Mackenna); and P. Cruces (Hospital Padre Hurtado). Europe: Belgium: S. Clément De Cléty, M. Dujardin, C. Berghe, and S. Renard (St. Luc University Hospital). Czech Republic: J. Zurek (Masaryk University). Germany: H. Steinherr (Klinikum Augsburg). Greece: K. Mougkou (Aghia Sophia Children’s Hospital); and E. Critselis and K. Mougkou (P. & A. Kyriakou Children’s Hospital). Italy: M. Di Nardo, S. Picardo, and F. Tortora (Bambino Gesu Area Rossa); E. Rossetti (Bambino Gesu Children’s Hospital); T. Fragasso, P. Cogo, and R. Netto (Bambino Gesu Pediatrico). Lithuania: A. Dagys, V. Gurskis, and R. Kevalas (Lithuanian University of Health Sciences). Netherlands: C. Neeleman, J. Lemson, and C. Luijten (Radboud University Medical Centre). Poland: K. Wojciech and I. Pagowska-Klimek (Polish Mother Memorial Hospital); and M. Szczepanska and J. Karpe (Szyszko Ślaskiego University). Portugal: P. Nunes and H. Almeida (Hospital Professor Dr. Fernando Fonseca); and J. Rios and M. Vieira (Centrol Hospitalar Lisboa Norte). Spain: J.P. Garcia Iniguez and P. Revilla (Children’s Hospital Miguel Servet); J. Urbano, J. Lopez-Herce, and A. Bustinza (Hospital General Universitario Gregorio Marañón); A. Palacios and S. Hofheinz (Hospital 12 de Octubre); A. Rodriguez-Nunez (Hospital Clínico Universitario); S. Sana-gustin and E. Gonzalez (Hospital de la Sant Creu Sant Pau); M. Riaza and R. Piaya (Hospital Universitario Madrid); P. Soler (Hospital Carlos Haya Materno Infantil); E. Esteban (Hospital Sant Joan de Déu); J. Laraudogoitia and C. Monge (Hospital Universitario Donostia); V. Herrera and J. Granados (Hospital Universitario Salamanca); and C. Gonzalez (Hospital Virgen de la Arrixaca). Turkey: T. Koroglu and E. Ozcelik (Dokuz Eylul University). United Kingdom: P. Baines (Alder Hey Children’s Hospital); and A. Plunkett (Birmingham Children’s Hospital); P. Davis and S. George (Bristol Royal Hospital for Children); S. Tibby and J. Harris (Evelina Children’s Hospital); R. Agbeko and R. Lampitt (Great North Children’s Hospital-Newcastle); J. Brierly, M. Peters, A. Jones, T. Dominguez, and T. Thiruchelvam (Great Ormond Street Hospital); A. Deep, L. Ridley, and W. Bowen (King’s College Hospital); R. Levin and I. Macleod (Royal Hospital for Sick Children); M. Gray and N. Hemat (St George’s Hospital); J. Alexander and S. Ali (University Hospital of North Staffordshire NHS Trust); J. Pappachan and J. McCorkell (University Hospital Southampton NHS Foundation Trust); and P.Fortune, M. MacDonald, and P. Hudnott (Royal Manchester Children’s Hospital). Asia: China: Q. Suyun (Beijing Children’s Hospital). India: S. Singhi and K. Nallasamy (Advanced Pediatrics); and R. Lodha (All India Institute of Medical Sciences). Japan: N. Shime and Y. Tabata (Kyoto Prefectural University of Medicine); O. Saito and T. Ikeyama (Tokyo Metropolitan Children’s Hospital); and T. Kawasaki (Shizuoka Children’s Hospital). Malaysia: L. Lum, A. Abidin, and S. Kee (University Malaya Medical Center); S. Tang and R. Jalil (Kebangsaan Malaysia Medical Center). Singapore: Y. Guan and L. Yao (KK Women’s and Children’s Hospital); and K. Lin and J. Ong (National University Hospital). Africa: South Africa: A. Salloo, L. Doedens, and L. Mathivha (Chris Hani Baragwanath Hospital); G. Reubenson and S. Moaisi (Rahima Moosa Mother and Child Hospital); and A. Pentz and R. Green (Steve Biko Academic Hospital). Australia: A. Schibler and A. Fernandez (Mater Children’s Hospital); S. Erickson (Princess Margaret Hospital); J. McEniery, D. Long, T. Dorofaeff, and M. Coulthard (Royal Children’s Hospital Brisbane); J. Millar and C. Delzoppo (Royal Children’s Hospital Melbourne); G. Williams and M. Morritt (Sydney Children’s Hospital); and N. Watts and M. Morritt (Children’s Hospital Westmead). New Zealand: J. Beca, C. Sherring, and T. Bushell (Starship Children’s Hospital).
The Sepsis PRevalence, OUtcomes, and Therapies study was supported by the Endowed Chair, Department of Anesthesiology and Critical Care Medicine, University of Pennsylvania Perelman School of Medicine, and the Center for Pediatric Clinical Effectiveness at The Children’s Hospital of Philadelphia. Financial support for data collection in all U.K. centers was provided by the National Institute for Health Research (NIHR) Clinical Research Network and in Southampton by the Southampton NIHR Wellcome Trust Clinical Research Facility. None of the funders participated in the design and conduct of study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the article.
Dr. Weiss’ institution received funding from National Institute of General Medical Sciences K23GM110496, and he received funding from Bristol-Meyers Squibb Company (Advisory Panel) and ThermoFisher Scientific (honorarium for lecture). Dr. Thomas’ institution received funding from Gene Fluidics, and he received funding from CareFusion and Therabron. Dr. Fitzgerald’s institution received funding from Center for Pediatric Clinical Effectiveness at the Children’s Hospital of Philadelphia. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
REFERENCES
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, et al. : Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013; 14:686–693 [DOI] [PubMed] [Google Scholar]
- 2.Watson RS, Carcillo JA: Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med 2005; 6:S3–S5 [DOI] [PubMed] [Google Scholar]
- 3.Weiss SL, Fitzgerald JC, Pappachan J, et al. ; Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadel S, Goldstein B, Williams MD, et al. ; REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective (RESOLVE) study group: Drotrecogin alfa (activated) in children with severe sepsis: A multicentre phase III randomised controlled trial. Lancet 2007; 369:836–843 [DOI] [PubMed] [Google Scholar]
- 5.Balamuth F, Weiss SL, Neuman MI, et al. : Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med 2014; 15:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brierley J, Carcillo JA, Choong K, et al. : Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 2009; 37:666–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul R, Melendez E, Stack A, et al. : Improving adherence to PALS septic shock guidelines. Pediatrics 2014; 133:e1358–e1366 [DOI] [PubMed] [Google Scholar]
- 8.Workman JK, Ames SG, Reeder RW, et al. : Treatment of pediatric septic shock with the Surviving Sepsis Campaign Guidelines and PICU patient outcomes. Pediatr Crit Care Med 2016; 17:e451–e458 [DOI] [PubMed] [Google Scholar]
- 9.Duncan CN, Lehmann LE, Cheifetz IM, et al. ; Pediatric Acute Lung Injury and Sepsis (PALISI) Network: Clinical outcomes of children receiving intensive cardiopulmonary support during hematopoietic stem cell transplant. Pediatr Crit Care Med 2013; 14:261–267 [DOI] [PubMed] [Google Scholar]
- 10.Chima RS, Daniels RC, Kim MO, et al. : Improved outcomes for stem cell transplant recipients requiring pediatric intensive care. Pediatr Crit Care Med 2012; 13:e336–e342 [DOI] [PubMed] [Google Scholar]
- 11.Rowan CM, Gertz SJ, McArthur J, et al. ; Investigators of the Pediatric Acute Lung Injury and Sepsis Network: Invasive mechanical ventilation and mortality in pediatric hematopoietic stem cell transplantation: A multicenter study. Pediatr Crit Care Med 2016; 17:294–302 [DOI] [PubMed] [Google Scholar]
- 12.Zinter MS, DuBois SG, Spicer A, et al. : Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med 2014; 40:1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faraci M, Bagnasco F, Giardino S, et al. : Intensive care unit admission in children with malignant or nonmalignant disease: Incidence, outcome, and prognostic factors: A single-center experience. J Pediatr Hematol Oncol 2014; 36:e403–e409 [DOI] [PubMed] [Google Scholar]
- 14.Zinter MS, Dvorak CC, Spicer A, et al. : New insights into multicenter picu mortality among pediatric hematopoietic stem cell transplant patients. Crit Care Med 2015; 43:1986–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss SL, Parker B, Bullock ME, et al. : Defining pediatric sepsis by different criteria: Discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med 2012; 13:e219–e226 [DOI] [PubMed] [Google Scholar]
- 16.Jaramillo-Bustamante JC, Marín-Agudelo A, Fernández-Laverde M, et al. : Epidemiology of sepsis in pediatric intensive care units: First Colombian multicenter study. Pediatr Crit Care Med 2012; 13:501–508 [DOI] [PubMed] [Google Scholar]
- 17.Wolfler A, Silvani P, Musicco M, et al. ; Italian Pediatric Sepsis Study (SISPe) group: Incidence of and mortality due to sepsis, severe sepsis and septic shock in Italian Pediatric Intensive Care Units: A prospective national survey. Intensive Care Med 2008; 34:1690–1697 [DOI] [PubMed] [Google Scholar]
- 18.de Souza DC, Shieh HH, Barreira ER, et al. ; LAPSES Group: Epidemiology of sepsis in children admitted to PICUs in South America. Pediatr Crit Care Med 2016; 17:727–734 [DOI] [PubMed] [Google Scholar]
- 19.Shime N, Kawasaki T, Saito O, et al. : Incidence and risk factors for mortality in paediatric severe sepsis: Results from the national paediatric intensive care registry in Japan. Intensive Care Med 2012; 38:1191–1197 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Sun B, Yue H, et al. : An epidemiologic survey of pediatric sepsis in regional hospitals in China. Pediatr Crit Care Med 2014; 15:814–820 [DOI] [PubMed] [Google Scholar]
- 21.van Paridon BM, Sheppard C, G GG, et al. ; Alberta Sepsis Network: Timing of antibiotics, volume, and vasoactive infusions in children with sepsis admitted to intensive care. Crit Care 2015; 19:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis: International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 23.Slater A, Shann F, Pearson G; Paediatric Index of Mortality (PIM) Study Group: PIM2: A revised version of the Paediatric Index of Mortality. Intensive Care Med 2003; 29:278–285 [DOI] [PubMed] [Google Scholar]
- 24.Straney L, Clements A, Parslow RC, et al. ; ANZICS Paediatric Study Group and the Paediatric Intensive Care Audit Network: Paediatric index of mortality 3: An updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Med 2013; 14:673–681 [DOI] [PubMed] [Google Scholar]
- 25.Leteurtre S, Martinot A, Duhamel A, et al. : Validation of the paediatric logistic organ dysfunction (PELOD) score: Prospective, observational, multicentre study. Lancet 2003; 362:192–197 [DOI] [PubMed] [Google Scholar]
- 26.Proulx F, Fayon M, Farrell CA, et al. : Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest 1996; 109:1033–1037 [DOI] [PubMed] [Google Scholar]
- 27.Fiser DH: Assessing the outcome of pediatric intensive care. J Pediatr 1992; 121:68–74 [DOI] [PubMed] [Google Scholar]
- 28.Macesic N, Morrissey CO, Cheng AC, et al. : Changing microbial epidemiology in hematopoietic stem cell transplant recipients: Increasing resistance over a 9-year period. Transpl Infect Dis 2014; 16:887–896 [DOI] [PubMed] [Google Scholar]
- 29.de Koning C, Plantinga M, Besseling P, et al. : Immune reconstitution after allogeneic hematopoietic cell transplantation in children. Biol Blood Marrow Transplant 2016; 22:195–206 [DOI] [PubMed] [Google Scholar]
- 30.Doganis D, Asmar B, Yankelevich M, et al. : Predictive factors for blood stream infections in children with cancer. Pediatr Hematol Oncol 2013; 30:403–415 [DOI] [PubMed] [Google Scholar]
- 31.Zeiser R, Socié G, Blazar BR: Pathogenesis of acute graft-versus-host disease: From intestinal microbiota alterations to donor T cell activation. Br J Haematol 2016; 175:191–207 [DOI] [PubMed] [Google Scholar]
- 32.Holler E, Butzhammer P, Schmid K, et al. : Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: Loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant 2014; 20:640–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jodele S, Dandoy CE, Myers KC, et al. : New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apher Sci 2016; 54:181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shayani S, Palmer J, Stiller T, et al. : Thrombotic microangiopathy associated with sirolimus level after allogeneic hematopoietic cell transplantation with tacrolimus/sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant 2013; 19:298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plunkett A, Parslow RC: Is it taking longer to die in paediatric intensive care in England and Wales? Arch Dis Child 2016; 101:798–802 [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald JC, Basu RK, Akcan-Arikan A, et al. ; Sepsis PRevalence, OUtcomes, and Therapies Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators Network: Acute kidney injury in pediatric severe sepsis: An independent risk factor for death and new disability. Crit Care Med 2016; 44:2241–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta NM, Bechard LJ, Cahill N, et al. : Nutritional practices and their relationship to clinical outcomes in critically ill children-an international multicenter cohort study. Crit Care Med 2012; 40:2204–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fivez T, Kerklaan D, Mesotten D, et al. : Early versus late parenteral nutrition in critically ill children. N Engl J Med 2016; 374:1111–1122 [DOI] [PubMed] [Google Scholar]
- 39.Bicakli DH, Yilmaz MC, Aksoylar S, et al. : Enteral nutrition is feasible in pediatric stem cell transplantation patients. Pediatr Blood Cancer 2012; 59:1327–1329 [DOI] [PubMed] [Google Scholar]
- 40.Sorror ML, Logan BR, Zhu X, et al. : Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: A center for international blood and marrow transplant research study. Biol Blood Marrow Transplant 2015; 21:1479–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


