Abstract
To provide insight into molecular diagnosis and individualized treatment of ischemic stroke (IS), several available datasets in IS were analyzed to identify the differentially expressed genes and microRNAs (miRNAs). Series matrix files from GSE22255 and GSE16561 (mRNA profiles), a well as GSE110993 (miRNA profile) were downloaded from the Gene Expression Omnibus database. System-level clustering was performed with GeneCluster 3.0 software, and gene annotation and pathway enrichment were performed with gene ontology analysis and Database for Annotation, Visualization and Integrated Discovery software. For a protein-protein interaction (PPI) network, Biological General Repository for Interaction Datasets and IntAct interaction information were integrated to determine the interaction of differentially expressed genes. The selected miRNA candidates were imported into the TargetScan, miRDB and miRecords databases for the prediction of target genes. The present study identified 128 upregulated and 231 downregulated genes in female stroke patients, and 604 upregulated and 337 downregulated genes in male stroke patients compared with sex- and age-matched controls. The construction of a PPI network demonstrated that male stroke patients exhibited YWHAE, CUL3 and JUN as network center nodes, and in female patients CYLD, FOS and PIK3R1 interactions were the strongest. Notably, these interactions are mainly involved in immune inflammatory response, apoptosis and other biological pathways, such as blood coagulation. Female and male upregulated genes were cross-validated with another set of Illumina HumanRef-8 v3.0 expression beadchip (GSE16561). Functional item association networks, gene function networks and transcriptional regulatory networks were successfully constructed, and the relationships between miRNAs and target genes were successfully predicted. The present study identified a number of transcription factors, including DEFA1, PDK4, SDPR, TCN1 and MMP9, and miRNAs, including miRNA (miR)-21, miR-143/145, miR-125-5p and miR-122, which may serve important roles in the development of cerebral stroke and may be important molecular indicators for the treatment of IS.
Keywords: bioinformatics, gene, microRNA, signaling pathway, stroke
Introduction
Ischemic stroke (IS), which is a major cause of disability and mortality worldwide, is a cerebrovascular disease caused by cerebral blood supply disorder or hemorrhage and results in the formation of an infarction zone with tissue necrosis and softening (1,2). It can lead to the severe impairment of sensory and motor dysfunction, neurodegenerative diseases and sudden mortality (3). Cerebral infarction not only seriously affects the life quality of patients, but also imposes a huge burden and pressure on public health care and the social economy (4). A previous study reported that the impact caused by IS will become more apparent in the next two decades as the world's population ages and lifestyles change, especially in developing countries (5). An epidemiological study has showed that >70% of patients with IS currently have mental and physical functional disorders (6). Tissue plasminogen activator is the only thrombolytic drug approved by the US Food and Drug Administration for the treatment of acute cerebral infarction, but its clinical application is limited by the timing of stenosis (onset) and side effects such as bleeding (7,8). A number of other previous studies have reported that transient cerebral ischemia/hypoxia can lead to neuronal death, but the mechanism is not fully understood (9,10). Therefore, further understanding of the pathogenesis of cerebral infarction, finding biomarkers that can be used to diagnose and predict prognosis, and eliciting new therapeutic targets have become a significant and urgent task.
MicroRNAs (miRNAs) are highly conserved, endogenous non-coding single-stranded small RNAs that are ~22–24 nucleotides long and can bind to target mRNAs with a complementary nucleic acid sequence. In general, miRNAs act post-transcriptionally to inhibit the translation of the target mRNAs or degrade the target mRNA (11). Each miRNA can regulate the expression of multiple target genes, and the same gene can also be regulated by several different miRNAs (12). Currently, >2,000 human miRNAs have been identified, and it is hypothesized that these small molecules regulate about one-third of human genes (13). Therefore, miRNA changes can lead to various diseases. A number of studies have demonstrated that miRNAs serve important biological roles in cell growth, proliferation, differentiation and apoptosis, as well as reconstruction of hematopoietic and damaged tissues (14,15). Therefore, the role of miRNAs in neurological diseases has attracted increasing attention. In the nervous system, miRNAs are closely related to the growth and differentiation of neurons, memory and learning functions, as well as neurological degenerative diseases and mental disorders (16,17). In addition, abnormal miRNA expression is detected in brain tumors, Parkinson's disease, Alzheimer's disease and IS (18–20).
Studies on the identification of target genes for miRNAs associated with cerebral infarction suggest that miRNAs play a key role in the development and progression of cerebral infarction (21,22). Among them, apoptosis is the central link of this process, and related studies have indicated that apoptosis is a highly regulated and controlled death process induced by miRNAs (23). In the present study, bioinformatics analysis was performed to investigate the putative genes and miRNAs involved in the pathogenesis and disease process of IS, and the mechanism was studied at the molecular level, which provided a reference for molecular diagnosis and individualized treatment of IS.
Materials and methods
Microarray data
Two mRNA microarray datasets, GSE22255 and GSE16561, and one miRNA profile, GSE110993, of IS were obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo). GSE22255 is based on the GPL570 (HG-U133_Plus_2) Affymetrix Human Genome U133 Plus 2.0 Array. Gene expression profiling was performed on peripheral blood mononuclear cells (PBMCs) of 20 patients with IS (10 male; 10 female) and 20 sex- and age-matched healthy control (CTL) patients using Affymetrix microarrays. GSE16561 contained a total of 63 samples based on the platform of GPL6883 Illumina HumanRef-8 v3.0 expression beadchip, in which peripheral blood samples were collected in Paxgene Blood RNA tubes from patients who were >18 years of age with MRI diagnosed IS (n=39; 22 females and 17 males) and CTL (n=24; 14 females and 10 males) who were non-stroke neurologically healthy. As for GSE110993, RNA sequencing results were analyzed to study expression changes of circulating miRNAs in a discovery sample of 20 patients (10 males and 10 females) with IS and 20 sex- and age-matched healthy control (CTLs) patients was further applied in independent samples for validation (40 patients with IS and 40 CTL patients) and replication (200 patients with IS, 100 CTLs).
Identification of differentially expressed genes (DEGs)
Background correction and quartile data normalization of the downloaded data were performed using the robust multi-array average algorithm (24). Principal component analysis was subsequently conducted to reduce the dimension of multivariate data to two or three principal components, which can be visualized graphically with the least information loss. We used R software to calculate and visualize the principal component analysis (25). Probes without a corresponding gene symbol were then filtered and the average value of gene symbols with multiple probes was calculated using R software (26). DEGs between two groups were screened by Student's t-test and fold-change (FC) filtering with R software limma package (27). With a threshold of P<0.05 and |FC|>2, volcano plots were constructed to identify the DEGs with statistical significance between two groups by R software ggplot2 package (data not shown) (28). In addition, hierarchical clustering and combined analyses were performed for the DEGs. The GSE16561 dataset was used to validate top 20 of DEGs in the GSE22255 dataset to identify overlapping DEGs in two microarrays.
Protein-Protein Interaction (PPI) network construction and analysis
The PPI network was created by integrating Biological General Repository for Interaction Datasets (BioGRID) and IntAct databases at EMBL-EBI. First, the PPI pairs were used to construct the PPI network. Second, the regulatory relationship between genes was visualized by using Cytoscape (version 3.4.0) (29), and analyzed through a topological property of computing network including the degree distribution of network by using the CentiScape app version 2.2 (30).
Functional enrichment analysis
The Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics resource (http://david.abcc.ncifcrf.gov) was used to conduct functional enrichment analysis to understand the underlying biological processes and pathways of aberrantly expressed intersecting genes.
Gene Ontology (GO) terms for the biological process (BP) were enriched. The most enriched term within a cluster was chosen as the one to represent the cluster. Only terms with P<0.05 and the concentrated gene numbers ≥1.5 were considered meaningful (≥1 gene number exists).
Prediction of miRNA targets
The target genes for differentially expressed (DE) miRNAs in GSE110993 were predicted using three established programs: TargetScan version 7.2.0 (31), miRTarBase version 7.0 (32) and miRDB version 5.03 (33), which are the most popular databases of experimentally validated miRNA interactions. The genes that were predicted by all three programs were chosen as the targets of DE miRNAs and the miRNA-gene network was constructed using miRWalk 2.0 (34). Cytoscape (https://cytoscape.org; version 3.40) was used to visualize the miRNA regulatory network.
Results
Identification of differentially expressed genes in female and male IS patients
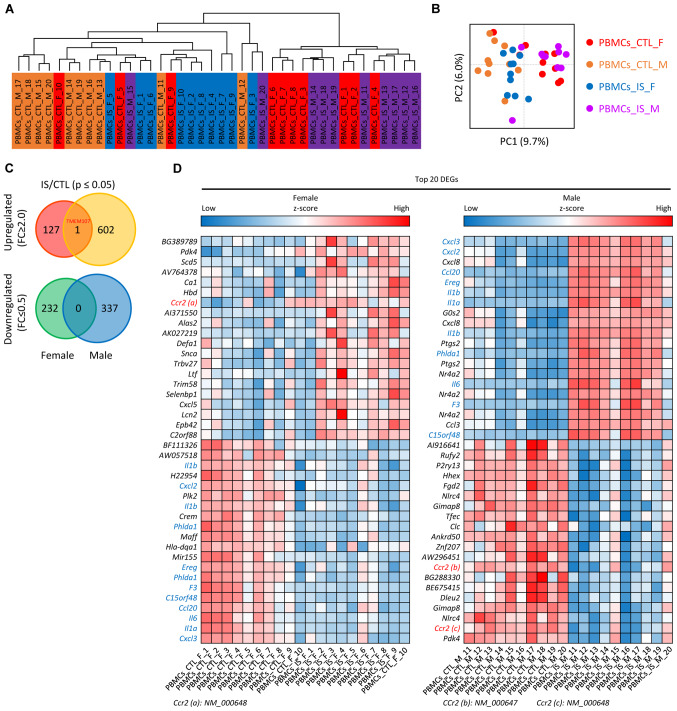
A total of 10 female and 10 male patients with IS, and 20 sex- and age-matched respective CTLs from the data in the GEO database (GSE22255) were clustered and divided into four groups (Fig. 1A): i) PBMCs_IS_M group, ii) PBMCs_IS_F group, iii) PBMCs_CTL_M group and iv) PBMCs_CTL_F group, as depicted in Fig. 1A. Principle component analysis of genes in patients with IS and CTL is presented in Fig. 1B. Following data preprocessing, the unique genes in the four groups of samples were analyzed and it was determined that a total of 1,300 genes were differentially expressed in the IS samples compared with the CTL samples. Among these DEGs, 128 genes were upregulated in females, 603 genes were upregulated in males, 232 were genes downregulated in females and 337 genes were downregulated in males. For instance, it was found that the chemokines C-X-C motif chemokine (CXCL)3, CXCL2 and CXCL8 were significantly upregulated in male IS patients. However, TMEM107 was the only overlapped upregulated DEG between the female and male group, which is displayed in a Venn diagram (Fig. 1C). A heat map of the top 20 up- and downregulated DEGs in male and female groups is shown in Fig. 1D.
Figure 1.
Identification of DEGs in female and male patients with IS. (A) Bidirectional hierarchical clustering and (B) principal component analysis of samples based on differentially expressed mRNAs. (C) Venn diagram of differentially expressed mRNAs from IS/CTL group. (D) Heatmap showing the expression levels of the top 20 mRNAs in the female (left) and male (right) dataset. The expression levels are from blue (low expression) to red (high expression). CTL, control; DEG, differentially expressed genes; F, female; FC, fold-change; IS, ischemic stroke; M, male; PBMC, peripheral blood mononuclear cells.
Functional and pathway enrichment analyses
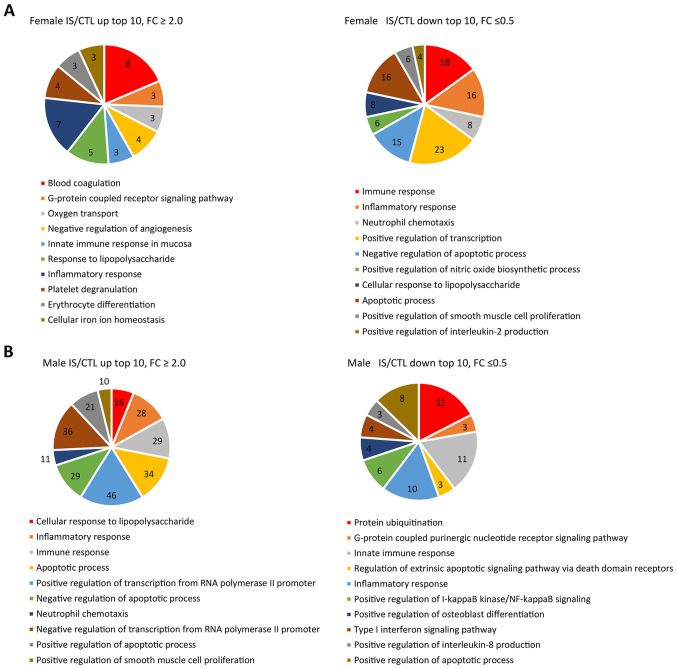
DAVID was used to perform GO term enrichment analyses to explore the function and pathways of 1,300 identified DEGs (Fig. 2). GO-BP term enrichment analysis demonstrated that the upregulated genes were significantly enriched in ‘blood coagulation’ and ‘inflammatory response’ in the female IS compared with the CTL group. In addition, ‘positive regulation of transcription from RNA polymerase II promoter’ and ‘neutrophil chemotaxis’ were enriched in the male IS compared with the CTL group (Fig. 2A). Downregulated genes were mainly involved in ‘positive regulation of transcription’ and ‘immune response’ in the female IS group (Fig. 2B). It was also revealed that most of the downregulated genes were involved in ‘inflammatory response’ and ‘protein ubiquitination’ in the male IS group, respectively. Of note, in both the male and female groups, the pathways of differential expression were mainly involved in ‘immune response’, ‘inflammatory response’, ‘apoptotic process’ and ‘neutrophil chemotaxis’.
Figure 2.
Gene Ontology biological function term enrichment analyses from IS compared with the CTL group in the (A) female and (B) male patients. The numbers in the pie slices represent the number of differentially expressed genes involved in the function. CTL, control; IS, ischemic stroke.
Construction of PPI network and module analysis
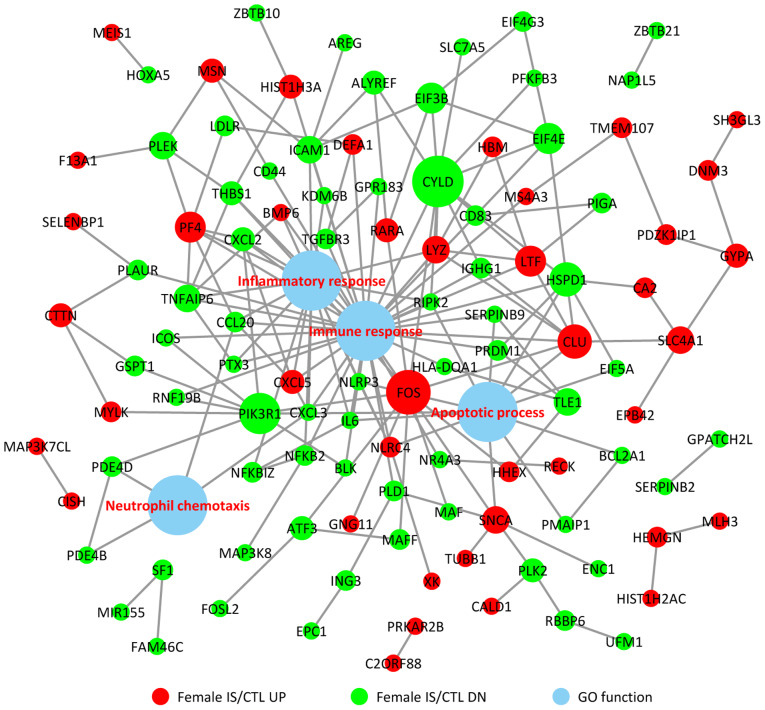
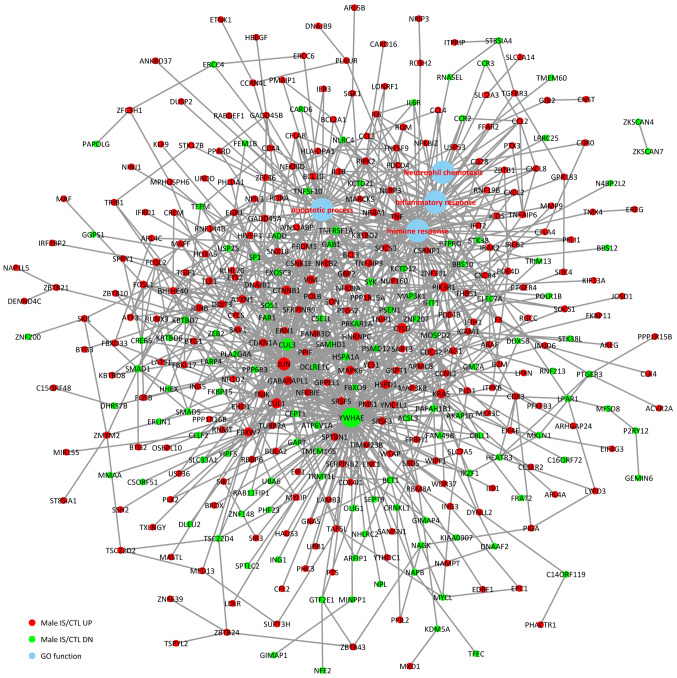
To investigate the associations between the DEGs and BPs, a comprehensive analysis of protein interactions of the top up- and downregulated DEGs was conducted using BioGRID, IntAct database and Cytoscape software (Fig. 3). Direct or indirect interactions were identified, and the highest-scoring nodes were screened as central genes in the female IS/CTL group, including FOS, CLU, LTF, PF4, CXCL5 and CYLD (Fig. 3). In the male IS/CTL group, the top 3 genes with the most significant expression were CUL3, YWHAE and JUN (Fig. 4). The associations between BP and gene expression were categorized into 4 processes, namely inflammatory response, immune response, apoptotic process and neutrophil chemotaxis.
Figure 3.
Protein-protein interaction of the upregulated and downregulated genes in female patients with IS and their association with immune response, inflammatory response, apoptotic process and neutrophil chemotaxis. CTL, control; DN, downregulated; GO, Gene Ontology; IS, ischemic stroke; UP, upregulated.
Figure 4.
Protein-protein interaction of the upregulated and downregulated genes in male patients with IS and their association with immune response, inflammatory response, apoptotic process and neutrophil chemotaxis. CTL, control; DN, downregulated; GO, Gene Ontology; IS, ischemic stroke; UP, upregulated.
miRNA target regulatory network analysis
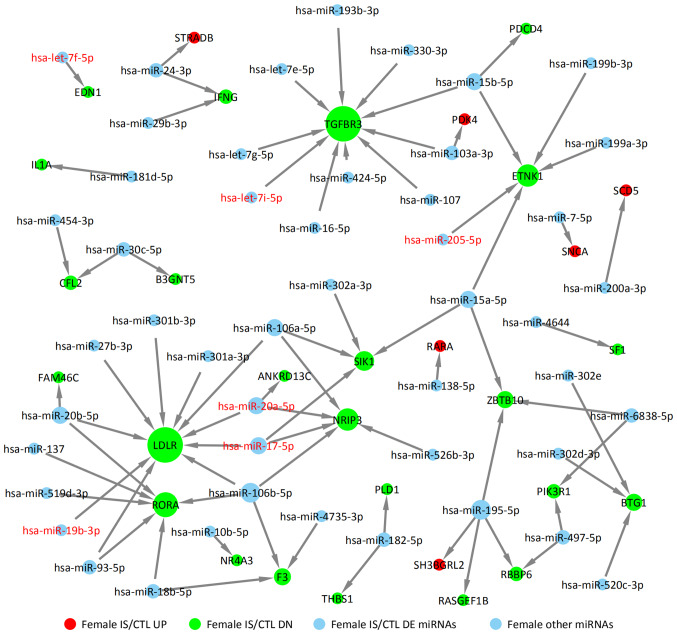
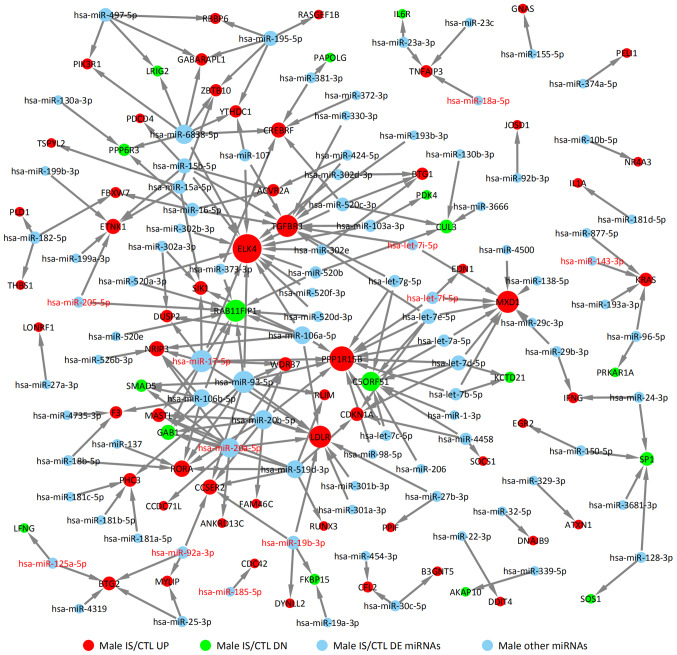
DE miRNAs obtained from GSE110993 database were analyzed. For the female IS/CTL group, six DE miRNAs were identified, including let-7f-5p, let-7i-5p, miR-17-5p, miR-20a-5p, miR-19b-3p and miR-205-5p (Fig. 5). In the male IS/CTL group, there were 11 DE miRNA identified, such as miR-205-5p, miR-17-5p, miR-20a-5p, miR-125a-5p, miR-92a-3p, miR-18a-5p and miR-143-3p (Fig. 6). Their target genes were predicted using TargetScan, miRTarBase and miRDB. The upregulated targets were STRADB, PDK4, SCD5, SNCA, RARA and SHBGRL2 in the female IS group (solid red circles in Fig. 5); the downregulated target genes are also indicated, including TGFBR3, ETNK1, LDLR, RORA and RBBP6 (solid green circles in Fig. 5). The targets of miRNAs for the male IS/CTL group are shown in Fig. 6 (solid green circles represent downregulated target genes, such as RAB11FIP1, C5ORF51, GAB1 and SMAD5, and red circles represented upregulated target genes such as PPP1R15B, ELK4, MXD1 and LDLR).
Figure 5.
miRNA target network of the upregulated and downregulated genes of female patients with IS with differentially expressed miRNAs. CTL, control; diffexp, differentially expressed; DN, downregulated; IS, ischemic stroke; miRNA, microRNA; UP, upregulated.
Figure 6.
miRNA target network of the upregulated and downregulated genes of male patients with IS with differentially expressed miRNAs. CTL, control; diffexp, differentially expressed; DN, downregulated; IS, ischemic stroke; miRNA, microRNA; UP, upregulated.
Validation of DEGs in IS
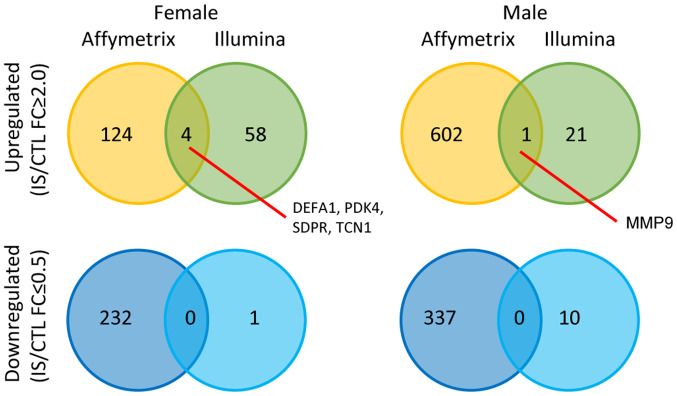
Crosscheck validation analysis of female and male IS patient DEGs from GSE22255 and another set of Illumina HumanRef-8 v3.0 expression beadchip (GSE16561) are shown in Fig. 7. There were 4 genes that are upregulated in both datasets in the female group including defensin α 1 (DEFA1), pyruvate dehydrogenase kinase 4 (PDK4), caveolae associated protein 2 (SDPR) and transcobalamin 1 (TCN1) and only one shared upregulated gene in the male group, matrix metalloproteinase 9 (MMP9). These findings suggested that the differential expression targets selected had certain repeatability. Although numerous studies have been published regarding DEGs as candidate biomarkers for IS (35–37), the reliability of these findings remains uncertain, as they were generated from the studies without sufficient evidence of reproducibility and independent clinical validation.
Figure 7.
Overlapping upregulated and downregulated genes from two different beadchips (Affymetrix Human Genome U133 Plus 2.0 array and Illumina HumanRef-8 v3.0 expression beadchip) in female and male patients. CTL, control; FC, fold-change; IS, ischemic stroke; DEFA1, defensin alpha 1; PDK4, pyruvate dehydrogenase kinase 4; SDPR, caveolae associated protein 2; TCN1, transcobalamin 1; MMP9, matrix metalloproteinase 9.
Discussion
As a high-throughput genomics research technology, gene chips serve an important role in datamining of potential biological information and exploring the structure and function of the genome (38). Gene expression profiling experiments are mainly used to determine DEGs under different physiological or pathological conditions (39). Such analysis will facilitate the exploration of the relationship between health and disease at the molecular level and the potential mechanism through network regulation. However, the etiology and physiological pathology of IS are still not fully understood. Therefore, further clarifying the pathogenesis of IS, determining its risk factors and developing effective therapeutic strategies will significantly improve the clinical outcome of stroke treatment.
The present study used bioinformatics tools and methods to screen the key DEGs and their functions in stroke. A total of 128 upregulated genes and 231 downregulated genes were found in female patients with IS, and 604 upregulated genes and 337 downregulated genes in male patients with IS. These DEGs were mainly associated with inflammatory response and apoptosis, and other biological pathways. Combining PPI and GO term enrichment results, it was also found that the genes involved in the GO analysis pathway were almost identical to the PPI network nodule-related genes, especially the CXCL family; tumor necrosis factor and PDK were most closely related to these pathways, which was consistent with the reported results.
Following a stroke, the damaged brain tissue releases proinflammatory chemokines, which trigger the inflammatory pathway in immune cells (40). Depending on the sequence of N-terminal residues, chemokines can be divided into several different types, including CXC, CC, XC and CX3C, where C is an N-terminal cysteine residue and X represents any amino acid residue (41). The chemokine ligands and receptors are emerging as potential therapeutic targets for cardiovascular diseases and have become increasingly studied in recent years. Brait et al (42) found that the expression of CXCR2 and its ligands CXCL1 and CXCL2 is significantly upregulated and peaks 1–3 days after injury in a middle cerebral artery occlusion (MCAO) rat model. Villa et al (43) inhibited CXCR1 with interleukin (IL)-8/CXCL8 receptor inhibitors in MCAO model rats and demonstrated that CXCR2, myeloperoxidase and IL-1A expression levels decreased, which reduced the symptoms of neurological deficits. In the present study, it was found that the chemokine receptors CXCL3, CXCL2 and CXCL8 were significantly upregulated in male patients with IS, and this was consistent with the previous reports.
PDK4 is a key enzyme that regulates pyruvate dehydrogenase complex (PDC) activity and is a key regulator of pyruvate oxidation and glucose maintenance in vivo (44). In calcified vascular smooth muscle cells and calcified vessels of patients with atherosclerosis, PDK4 expression is found to be upregulated alongside increased PDC phosphorylation, suggesting that PDK4 serves an important role in vascular calcification (45). In phosphate-treated vascular smooth muscle cells, PDK4 expression is upregulated, inducing mitochondrial dysfunction and, subsequently, apoptosis. Atherosclerosis is the result of cholesterol and lipid deposition on the arterial wall, often related to calcification, which is formed by the deposition of inorganic calcium on the arterial wall. Thus, the upregulation of PDK4 increased osteogenic markers and enhanced vascular calcification without significant effects on bone formation (46). This indicated that PDK4 may be a new target for the treatment of vascular calcification (47,48).
In the pathogenesis of various injuries, the immune inflammatory signaling pathway caused by ischemia serves a major role, whereas ischemia itself serves a secondary role (49). In IS the key role of the immune inflammatory response has been widely recognized, but whether it plays a role in brain damage or brain protection may depend on the degree and stage of ischemia. Secondary injuries are caused by the inflammatory cascade following ischemia. Various inflammatory mediators, including cytokines, chemokines and cell adhesion molecules, aggravate ischemic brain damage (27,28). In the early stage of ischemia, the inflammatory response exacerbates brain damage, and in the late stage of ischemia, it serves an important role in the recovery of nerve function and tissue repair. In addition, patients with IS are prone to a hypercoagulable state and advanced fibrinolysis. Most patients have coagulation changes in the clinic, which may lead to adverse events such as thrombosis and infarction, which affects the prognosis (50).
miRNAs are important post-transcriptional regulators that coordinate the regulation of target genes with multi-target mRNA. Risk factors involved in IS include hypertension, atherosclerosis, atrial fibrillation, diabetes and dyslipidemia. The role of miRNAs in the pathophysiology of stroke has become a focus of interest in recent research (51). A previous study reported that circulating miRNA expression in peripheral blood is relatively stable (52), suggesting that differential expression of specific miRNAs following stroke is expected to be a potential biomarker for diagnosis and prognosis, providing an effective theoretical basis for elucidating the pathogenesis of IS (53).
In the present study, the GSE110993 dataset was analyzed and 17 differentially expressed miRNAs (6 miRNAs in females and 11 miRNAs in males) were screened. According to previous studies, there are differences in the expression patterns of miRNAs in IS and in normal healthy people (54). For example, the abnormal expression of miR-221 is related to metabolic syndrome, whereas the expression of miR-221 in healthy people was significantly higher compared with expression levels in patients with IS (55). Studies have demonstrated that the abnormal expression level of miR-221 is significant for distinguishing healthy individual and those with cardiovascular and cerebrovascular diseases, tumor populations and endocrine populations (56,57). Other previous studies have shown that miR-143/145 (57) and miR-125-5p (58) can also be used as markers for the risk of IS, which is consistent with the findings of the present study. miR-143/145 is most abundant in normal vascular tissues compared with other miRNAs and is selective in vascular smooth muscle cells expression. miR-125-5p has been found to be significantly upregulated in atherosclerosis (58). Inhibition of miR-125-5p expression significantly increases the uptake of lipids and enhances the clearance of human expression and secretion of inflammatory cell molecules (59). Some of these inflammatory cell molecules (60) may be involved in the inflammatory response of ox-LDL-stimulated monocytes, whereas ox-LDL may be taken up by macrophages, converted into foam cells that secrete pro-inflammatory factors and leads to cell necrosis and plaque formation. This series of chronic inflammatory reactions is atherosclerosis.
There are several limitations to the present study. First, it is notable that only one upregulated gene overlapped and none of downregulated genes overlapped between male and female groups. This may be due to differences in the effects of different types of sex hormones on platelet function. Androgens increase platelet activity, whereas estrogen inhibits its function (61). This may have an effect on the relative expression levels of mRNA and miRNA in the blood, suggesting that doctors might consider different medications for stroke patients of different sexes (62). Secondly, the data in Fig. 7 do not give confidence in the high-throughput genomics analyses because there are only 4 genes that are upregulated in both dataset in the female group and only one shared gene upregulated in the male group. This phenomenon may be because GSE22255 is based on GPL570 Affymetrix Human Genome U133 Plus 2.0 Array, whereas GSE16561 contained a total of 63 samples based on the platform of GPL6883 Illumina HumanRef-8 v3.0 expression beadchip. On the one hand, these two different gene platforms contain different numbers and different types of genes; on the other hand, there may be detection errors in chips from Illumina and Affymetrix.
In summary, all of the miRNAs described above have a certain correlation with the pathogenesis and development of IS, which is consistent with the findings of stroke researchers worldwide in recent years. The correlation between some miRNAs selected in this study have not been reported previously in the literature to the best of our knowledge. The possibility that these miRNAs are novel biomarkers for IS and its biological significance still needs further study. Further studies on the role of miRNAs will provide a theoretical basis for understanding the molecular mechanism of the pathogenesis and development of IS, as well as new research programs for prevention and treatment.
Acknowledgements
Not applicable.
Funding
This work was funded by HeBei Natural Science Foundations (grant no. H2017307016).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LL designed the experiments; HW and SS performed the experiments; LD and JC conducted the statistical analysis of the data and drafted the manuscript; CZ and CB conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ishak B, Abdul-Jabbar A, Singla A, Yilmaz E, von Glinski A, Ramey WL, Blecher R, Tymchak Z, Oskouian R, Chapman JR. Intraoperative ischemic stroke in elective spine surgery: A retrospective study of incidence and risk. Spine (Phila Pa 1976) 2020;45:109–115. doi: 10.1097/BRS.0000000000003184. [DOI] [PubMed] [Google Scholar]
- 2.Weiss HR, Mellender SJ, Kiss GK, Liu X, Chi OZ. Improvement in microregional oxygen supply/consumption balance and infarct size after cerebral ischemia-reperfusion with inhibition of p70 ribosomal S6 kinase (S6K1) J Stroke Cerebrovasc Dis. 2019;28:104276. doi: 10.1016/j.jstrokecerebrovasdis.2019.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Weisberg LA. ‘Stroke and struck’: Protecting the brain from cerebrovascular disease. South Med J. 2003;96:331. doi: 10.1097/01.SMJ.0000063472.85323.DB. [DOI] [PubMed] [Google Scholar]
- 4.Singh RB, Suh IL, Singh VP, Chaithiraphan S, Laothavorn P, Sy RG, Babilonia NA, Rahman AR, Sheikh S, Tomlinson B, Sarraf-Zadigan N. Hypertension and stroke in Asia: Prevalence, control and strategies in developing countries for prevention. J Hum Hypertens. 2000;14:749–763. doi: 10.1038/sj.jhh.1001057. [DOI] [PubMed] [Google Scholar]
- 5.West R, Hill K, Hewison J, Knapp P, House A. Psychological disorders after stroke are an important influence on functional outcomes: A prospective cohort study. Stroke. 2010;41:1723–1727. doi: 10.1161/STROKEAHA.110.583351. [DOI] [PubMed] [Google Scholar]
- 6.Ickenstein GW, Horn M, Schenkel J, Vatankhah B, Bogdahn U, Haberl R, Audebert HJ. The use of telemedicine in combination with a new stroke-code-box significantly increases t-PA use in rural communities. Neurocrit Care. 2005;3:27–32. doi: 10.1385/NCC:3:1:027. [DOI] [PubMed] [Google Scholar]
- 7.Keller L, Hobohm C, Zeynalova S, Classen J, Baum P. Does treatment with t-PA increase the risk of developing epilepsy after stroke? J Neurol. 2015;262:2364–2372. doi: 10.1007/s00415-015-7850-0. [DOI] [PubMed] [Google Scholar]
- 8.Rudnick SI, Swaminathan J, Sumaroka M, Liebhaber S, Gewirtz AM. Effects of local mRNA structure on posttranscriptional gene silencing. Proc Natl Acad Sci USA. 2008;105:13787–13792. doi: 10.1073/pnas.0805781105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Ahn JH, Song M, Kim H, Park CW, Park YE, Lee TK, Lee JC, Kim DW, Lee CH, et al. A 2-min transient ischemia confers cerebral ischemic tolerance in non-obese gerbils, but results in neuronal death in obese gerbils by increasing abnormal mTOR activation-mediated oxidative stress and neuroinflammation. Cell. 2019;8:1126. doi: 10.3390/cells8101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Jin R, Xiao AY, Zhong W, Li G. Inhibition of CD147 improves oligodendrogenesis and promotes white matter integrity and functional recovery in mice after ischemic stroke. Brain Behav Immun. 2019;82:13–24. doi: 10.1016/j.bbi.2019.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feschotte C, Jiang N, Wessler SR. Plant transposable elements: Where genetics meets genomics. Nat Rev Genet. 2002;3:329–341. doi: 10.1038/nrg793. [DOI] [PubMed] [Google Scholar]
- 12.Sunkar R, Zhu JK. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell. 2004;16:2001–2019. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crippa S, Cassano M, Sampaolesi M. Role of miRNAs in muscle stem cell biology: Proliferation, differentiation and death. Curr Pharm Des. 2012;18:1718–1729. doi: 10.2174/138161212799859620. [DOI] [PubMed] [Google Scholar]
- 15.Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 16.Mehler MF, Mattick JS. Non-coding RNAs in the nervous system. J Physiol. 2006;575:333–341. doi: 10.1113/jphysiol.2006.113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung HL, Choi J, Hwang YK, Kim DH, Yoo KH, Sung KW, Koo HH. Inhibition of survivin expression by small interfering RNA (siRNA) in brain tumor cell lines. Cancer Res. 2005;65 [Google Scholar]
- 18.Pantano L, Friedländer MR, Escaramís G, Lizano E, Pallarès-Albanell J, Ferrer I, Estivill X, Martí E. Specific small-RNA signatures in the amygdala at premotor and motor stages of Parkinson's disease revealed by deep sequencing analysis. Bioinformatics. 2016;32:673–681. doi: 10.1093/bioinformatics/btv632. [DOI] [PubMed] [Google Scholar]
- 19.Roy J, Sarkar A, Parida S, Ghosh Z, Mallick B. Small RNA sequencing revealed dysregulated piRNAs in Alzheimer's disease and their probable role in pathogenesis. Mol Biosyst. 2017;13:565–576. doi: 10.1039/C6MB00699J. [DOI] [PubMed] [Google Scholar]
- 20.Bam M, Yang X, Sen S, Zumbrun EE, Dennis L, Zhang J, Nagarkatti PS, Nagarkatti M. Characterization of dysregulated miRNA in peripheral blood mononuclear cells from ischemic stroke patients. Mol Neurobiol. 2018;55:1419–1429. doi: 10.1007/s12035-016-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Q, Zhao H, Tao Z, Wang R, Liu P, Han Z, Ma S, Luo Y, Jia J. MicroRNA-181c exacerbates brain injury in acute ischemic stroke. Aging Dis. 2016;7:705–714. doi: 10.14336/AD.2016.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson SJ, Akil H. Gene chips and arrays revealed: A primer on their power and their uses. Biol Psychiatry. 1999;45:533–543. doi: 10.1016/S0006-3223(98)00339-4. [DOI] [PubMed] [Google Scholar]
- 23.Johnston M. Gene chips: Array of hope for understanding gene regulation. Curr Biol. 1998;8:R171–R174. doi: 10.1016/S0960-9822(98)70103-4. [DOI] [PubMed] [Google Scholar]
- 24.Hochreiter S, Clevert DA, Obermayer K. A new summarization method for Affymetrix probe level data. Bioinformatics. 2006;22:943–949. doi: 10.1093/bioinformatics/btl033. [DOI] [PubMed] [Google Scholar]
- 25.Teng L, Wang K, Liu Y, Ma Y, Chen W, Bi L. Based on integrated bioinformatics analysis identification of biomarkers in hepatocellular carcinoma patients from different regions. Biomed Res Int. 2019;2019:1742341. doi: 10.1155/2019/1742341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelaw YA, Williams G, Assefa Y, Asressie M, Soares Magalhães RJ. Sociodemographic profiling of tuberculosis hotspots in Ethiopia, 2014–2017. Trans R Soc Trop Med Hyg. 2019;113:379–391. doi: 10.1093/trstmh/trz017. [DOI] [PubMed] [Google Scholar]
- 27.Thodberg M, Thieffry A, Vitting-Seerup K, Andersson R, Sandelin A. CAGEfightR: Analysis of 5′-end data using R/Bioconductor. 2019;20:487. doi: 10.1186/s12859-019-3029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postma M, Goedhart J. PlotsOfData-A web app for visualizing data together with their summaries. PLoS Biol. 2019;17:e3000202. doi: 10.1371/journal.pbio.3000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doncheva NT, Morris JH, Gorodkin J, Jensen LJ. Cytoscape StringApp: Network analysis and visualization of proteomics data. J Proteome Res. 2019;18:623–632. doi: 10.1021/acs.jproteome.8b00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scardoni G, Tosadori G, Faizan M, Spoto F, Fabbri F, Laudanna C. Biological network analysis with CentiScaPe: Centralities and experimental dataset integration. F1000Res. 2014;3:139. doi: 10.12688/f1000research.4477.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalhori MR, Arefian E, Fallah Atanaki F, Kavousi K, Soleimani M. miR-548× and miR-4698 controlled cell proliferation by affecting the PI3K/AKT signaling pathway in Glioblastoma cell lines. Sci Rep. 2020;10:1558. doi: 10.1038/s41598-020-57588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang D, Zhao X, Zhang L, Wang C. Comprehensive analysis of pseudogene HSPB1P1 and its potential roles in hepatocellular carcinoma. J Cell Physiol. 2020 Jan 27; doi: 10.1002/jcp.29459. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 33.Jiang W, Zheng L, Yan Q, Chen L, Wang X. miR-532-3p inhibits metastasis and proliferation of non-small cell lung cancer by targeting FOXP3. J BUON. 2019;24:2287–2293. [PubMed] [Google Scholar]
- 34.Sticht C, De La Torre C, Parveen A, Gretz N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS One. 2018;13:e0206239. doi: 10.1371/journal.pone.0206239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monbailliu T, Goossens J, Hachimi-Idrissi S. Blood protein biomarkers as diagnostic tool for ischemic stroke: A systematic review. Biomark Med. 2017;11:503–512. doi: 10.2217/bmm-2016-0232. [DOI] [PubMed] [Google Scholar]
- 36.Li P, Teng F, Gao F, Zhang M, Wu J, Zhang C. Identification of circulating microRNAs as potential biomarkers for detecting acute ischemic stroke. Cell Mol Neurobiol. 2015;35:433–447. doi: 10.1007/s10571-014-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolmans LS, Rutten FH, Koenen NCT, Bartelink MEL, Reitsma JB, Kappelle LJ, Hoes AW. Candidate biomarkers for the diagnosis of transient ischemic attack: A systematic review. Cerebrovasc Dis. 2019;47:207–216. doi: 10.1159/000502449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller MC, Mayo KH. Chemokines from a structural perspective. Int J Mol Sci. 2017;18(pii):E2088. doi: 10.3390/ijms18102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirabelli-Badenier M, Braunersreuther V, Viviani GL, Dallegri F, Quercioli A, Veneselli E, Mach F, Montecucco F. CC and CXC chemokines are pivotal mediators of cerebral injury in ischaemic stroke. Thromb Haemost. 2011;105:409–420. doi: 10.1160/TH10-10-0662. [DOI] [PubMed] [Google Scholar]
- 41.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 42.Brait VH, Rivera J, Broughton BR, Lee S, Drummond GR, Sobey CG. Chemokine-related gene expression in the brain following ischemic stroke: No role for CXCR2 in outcome. Brain Res. 2011;1372:169–179. doi: 10.1016/j.brainres.2010.11.087. [DOI] [PubMed] [Google Scholar]
- 43.Villa P, Triulzi S, Cavalieri B, Di Bitondo R, Bertini R, Barbera S, Bigini P, Mennini T, Gelosa P, Tremoli E, et al. The interleukin-8 (IL-8/CXCL8) receptor inhibitor reparixin improves neurological deficits and reduces long-term inflammation in permanent and transient cerebral ischemia in rats. Mol Med. 2007;13:125–133. doi: 10.2119/2007-00008.Villa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris RA, Bowker-Kinley MM, Huang B, Wu PJ. Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzyme Regul. 2002;42:249–259. doi: 10.1016/S0065-2571(01)00061-9. [DOI] [PubMed] [Google Scholar]
- 45.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003;31:1143–1151. doi: 10.1042/bst0311143. [DOI] [PubMed] [Google Scholar]
- 46.Cohoon KP, Criqui MH, Budoff MJ, Lima JA, Blaha MJ, Decker PA, Durazo R, Liu K, Kramer H. Relationship of aortic wall distensibility to mitral and aortic valve calcification: The multi-ethnic study of atherosclerosis. Angiology. 2018;69:443–448. doi: 10.1177/0003319717730636. [DOI] [PubMed] [Google Scholar]
- 47.Yang R, Zhu Y, Wang Y, Ma W, Han X, Wang X, Liu N. HIF-1α/PDK4/autophagy pathway protects against advanced glycation end-products induced vascular smooth muscle cell calcification. Biochem Biophys Res Commun. 2019;517:470–476. doi: 10.1016/j.bbrc.2019.07.102. [DOI] [PubMed] [Google Scholar]
- 48.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284:E855–E862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 49.Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao Q, Wu J, Wang X, Song C. Noncoding RNAs in vascular aging. Oxid Med Cell Longev. 2020;2020:7914957. doi: 10.1155/2020/7914957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta MK, Halley C, Duan ZH, Lappe J, Viterna J, Jana S, Augoff K, Mohan ML, Vasudevan NT, Na J, et al. miRNA-548c: A specific signature in circulating PBMCs from dilated cardiomyopathy patients. J Mol Cell Cardiol. 2013;62:131–141. doi: 10.1016/j.yjmcc.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho EY, Lee SJ, Nam KW, Shin J, Oh KB, Kim KH, Mar W. Amelioration of oxygen and glucose deprivation-induced neuronal death by chloroform fraction of bay leaves (Laurus nobilis) Biosci Biotechnol Biochem. 2010;74:2029–2035. doi: 10.1271/bbb.100301. [DOI] [PubMed] [Google Scholar]
- 53.He W, Chen S, Chen X, Li S, Chen W. Bioinformatic analysis of potential microRNAs in ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25:1753–1759. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 54.Sørensen SS, Nygaard AB, Nielsen MY, Jensen K, Christensen T. miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl Stroke Res. 2014;5:711–718. doi: 10.1007/s12975-014-0364-8. [DOI] [PubMed] [Google Scholar]
- 55.Huang D, Chen Z, Wang J, Chen Y, Liu D, Lin K. MicroRNA-221 is a potential biomarker of myocardial hypertrophy and fibrosis in hypertrophic obstructive cardiomyopathy. Biosci Rep. 2020;40(pii):BSR20191234. doi: 10.1042/BSR20191234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Martino MT, Arbitrio M, Fonsi M, Erratico CA, Scionti F, Caracciolo D, Tagliaferri P, Tassone P. Allometric scaling approaches for predicting human pharmacokinetic of a locked nucleic acid oligonucleotide targeting cancer-associated miR-221. Cancers (Basel) 2019;12(pii):E27. doi: 10.3390/cancers12010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei YS, Xiang Y, Liao PH, Wang JL, Peng YF. An rs4705342 T>C polymorphism in the promoter of miR-143/145 is associated with a decreased risk of ischemic stroke. Sci Rep. 2016;6:34620. doi: 10.1038/srep34620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang Y, Xu J, Wang Y, Tang JY, Yang SL, Xiang HG, Wu SX, Li XJ. Inhibition of miRNA-125b decreases cerebral ischemia/reperfusion injury by targeting CK2α/NADPH oxidase signaling. Cell Physiol Biochem. 2018;45:1818–1826. doi: 10.1159/000487873. [DOI] [PubMed] [Google Scholar]
- 59.Li JQ, Hu SY, Wang ZY, Lin J, Jian S, Dong YC, Wu XF, Lan D, Cao LJ. MicroRNA-125-5p targeted CXCL13: A potential biomarker associated with immune thrombocytopenia. Am J Transl Res. 2015;7:772–780. [PMC free article] [PubMed] [Google Scholar]
- 60.Chen T, Huang Z, Wang L, Wang Y, Wu F, Meng S, Wang C. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res. 2009;83:131–139. doi: 10.1093/cvr/cvp121. [DOI] [PubMed] [Google Scholar]
- 61.Shi C, Na N, Zhu X, Xu J. Estrogenic effect of ginsenoside Rg1 on APP processing in post-menopausal platelets. Platelets. 2013;24:51–62. doi: 10.3109/09537104.2012.654839. [DOI] [PubMed] [Google Scholar]
- 62.Giustino G, Overbey J, Taylor D, Ailawadi G, Kirkwood K, DeRose J, Gillinov MA, Dagenais F, Mayer ML, Moskowitz A, et al. Sex-based differences in outcomes after mitral valve surgery for severe ischemic mitral regurgitation: From the cardiothoracic surgical trials network. JACC Heart Fail. 2019;7:481–490. doi: 10.1016/j.jchf.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.