Abstract
Background
Multiple immunologic parameters have provided useful prognostic and assessment significance in various cancers, including head-and-neck squamous cell carcinoma (scc). We sought to identify whether pretreatment inflammatory markers could prognosticate recurrence in patients with advanced (stage iii or iv) head-and-neck scc who underwent therapy with curative intent in a tertiary care centre between January 2010 and December 2012.
Methods
In a chart review, we recorded demographics; primary tumour characteristics; p16 status; pretreatment inflammatory markers, including body mass index (bmi), neutrophil-to-lymphocyte ratio (nlr), C-reactive protein (crp), and serum albumin; therapy received; and date of relapse, death, or last follow-up. The main outcome was relapse-free survival (rfs). Overall survival (os) was a secondary outcome.
Results
From among 235 charts reviewed, 118 cases were included: 86 oropharyngeal (50 p16-positive, 18 p16-negative, 17 p16 unavailable, 1 p16 indeterminate), and 32 non-oropharyngeal (7 p16-positive, 19 p16-negative, 6 p16 unavailable). Median follow-up was 2.45 years (25%–75% interquartile range: 1.65–3.3 years). In univariate analysis, p16 status, bmi, modified Glasgow prognostic score, and crp were significant for rfs, but in multivariate analysis, only p16 status, bmi, and crp remained significant. For os, only crp and nlr were significant in both the univariate and multivariate analyses. After adjustment for p16 status, nlr did not remain significant. After adjustment for p16 status, crp remained significant for both rfs and os.
Conclusions
In patients with head-and-neck scc, a stronger prognostic value is associated with human papillomavirus status than with nlr and many other factors, including bmi and albumin. However, even though few of our patients had high crp, serum crp remained significant despite p16-positive status.
Keywords: Head-and-neck cancer, squamous cell carcinoma, C-reactive protein, nlr, hpv
INTRODUCTION
Overall, head-and-neck cancer annually accounts for more than 650,000 cases of malignancy worldwide1. Men are affected significantly more than women, in a ratio ranging from 2:1 to 4:1. Most head-and-neck cancers arise in the mucosa of the upper aerodigestive tract and originate predominantly from squamous cells. Since 2010, the integration of chemotherapy into standard regimens of surgery or radiation therapy (or both) has improved survival and permitted preservation of organ function for many patients with locoregionally advanced head-and-neck cancer2,3.
Multiple immunologic parameters have proved useful for prognostic and assessment significance in cancer. Examples include pretreatment serum albumin4, C-reactive protein (crp), and neutrophil-to-lymphocyte ratio (nlr)5,6. Combinations of clinical and laboratory parameters have been suggested in clinical trials to predict recurrence or mortality, given that the inflammatory response per se is associated with higher mortality trends in large population cohorts7. The modified Glasgow Prognostic Score (mgps) has been studied as a mortality predictor in many cancers8–10, and its prognostic value was found to be improved with the addition of neutrophil and platelet counts and measurement of high-sensitivity crp11. Extensive studies have consistently shown the utility of nlr in the prognosis of head-and-neck cancers12–14.
Since the 1980s, the incidence of oropharyngeal squamous cell carcinoma (scc) related to infection with the human papillomavirus (hpv) has been continually increasing, with a noticeable decrease in non-hpv-related cancers15. A positive hpv status is significantly correlated with improved locoregional tumour control, improved disease-specific survival, and improved overall survival (os)16. So far, only one report has investigated the potential role of the nlr in the prognosis of patients with head-and-neck scc treated with curative intent, when considering p16 status17. A 2017 meta-analysis of nlr as a prognostic factor in head-and-neck cancer noted that a major limitation of previous studies was the lack of inclusion of p16 status in the analysis, both in stratification and in multivariate analysis. Furthermore, the use of nlr as a continuous variable has its own issues6. Our study conforms to those recommendations.
In this retrospective review, we sought to identify whether pretreatment inflammatory markers have prognostic value with respect to recurrence in patients with advanced head-and-neck scc who required systemic therapy in addition to radiation or surgery provided with curative intent.
METHODS
With institutional research ethics board approval, we performed a retrospective review encompassing all cases of advanced-stage scc of the head and neck in which the patient received systemic therapy in combination with radiation therapy or surgery with curative intent between January 2010 and December 2012 at our tertiary care hospital cancer centre.
Inclusion Criteria
To be included, patients had to meet these criteria:
■ Histologic evidence of scc of the head and neck
■ Stage iii or iv disease, based on the TNM staging system in the 7th edition of the American Joint Committee on Cancer’s cancer staging manual (2010)
■ Receipt of chemotherapy together with either radiation or surgery with curative intent
Exclusion Criteria
These exclusion criteria were applied:
■ No histologic evidence of scc of the head and neck
■ No receipt of systemic therapy of any type as part of the curative approach
■ Disease other than stage III or IV, based on the TNM staging system in the 7th edition of the American Joint Committee on Cancer’s cancer staging manual (2010)
Outcomes of Interest
The primary outcome of interest was relapse-free survival (rfs), defined as the time from completion of curative treatment until evidence of relapse by imaging or physical examination findings, or death from any cause. The secondary outcome was os, defined as the time from completion of therapy with curative intent until death from any cause.
Data Collection
All cases were assigned a study number, and no personal identifying information about the patient was used during data analysis. We examined patient demographics, anatomic site of primary tumour, cancer stage, performance status before treatment, histologic testing for p16, all therapeutic interventions, date of relapse, and date of death or last follow-up.
Because of the relevance of hpv in the prognosis of some head-and-neck cancers, we searched the records for hpv testing. We also endeavored to obtain p16 test results for all available samples, p16 being a well-recognized surrogate marker of hpv status18,19.
All inf lammatory markers measured within the 3 weeks before initiation of the curative interventions were documented, including body mass index (bmi), albumin level, crp, and neutrophil and lymphocyte counts.
We defined advanced-stage disease as that classified stage iii or higher by the TNM staging system in the 7th edition of the American Joint Committee on Cancer’s cancer staging manual (2010). Induction therapy is the administration of early chemotherapy treatment to shrink a tumour before surgery or radiotherapy. Chemoradiotherapy is systemic therapy given synchronously with radiation therapy3. “Sequential therapy” refers to the administration of induction chemotherapy followed by concurrent chemoradiotherapy.
Statistical Analysis
Proportional data are presented as percentages. Parametric variables are reported as medians with 25%–75% interquartile range (iqr), and the Student t-test was used for comparisons. Univariate Cox regression was performed for each variable of interest with respect to both rfs and os. Stepwise multivariate Cox regression was also performed, adjusting for age, sex, smoking status, and cancer stage. No adjustments were made for further comorbidities; no patient had major comorbidities that would preclude the receipt of aggressive curative therapy. Additional adjustment for p16 status was performed when multivariate results were found to be significant. Kaplan–Meier curves adjusted for p16 status were generated. For the rfs analysis, an event was defined as the clinical identification of relapse (imaging, physical exam, biopsy report) or death. Patients without an event occurrence were censored at the date of last follow-up.
RESULTS
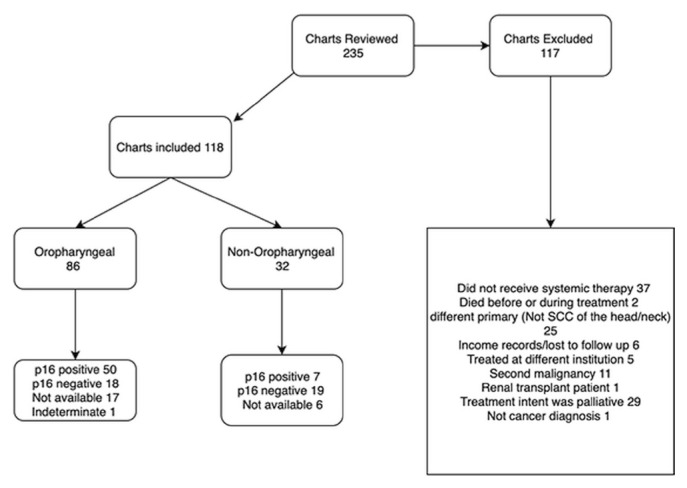
Of 235 patients reviewed in the inclusion and exclusion processes (Figure 1), 117 were excluded: 37 because systemic therapy was not used, 2 because of death before or during treatment, 25 because they had scc of other than the head and neck, 6 because of incomplete data, 5 because treatment occurred at a different institution, 11 because they had a second malignancy, 1 because of renal transplantation, 1 because of a non-cancer diagnosis, and 29 because treatment intent was palliative. The final analysis included the remaining 118 patients. The primary tumour was oropharyngeal in 86 cases (72.9%) and non-oropharyngeal in 32 (27.1%, Figure 1).
FIGURE 1.
Flow diagram of case inclusion process. SCC = squamous-cell carcinoma.
Baseline Characteristics
Median age at diagnosis was 58 years (iqr: 52–64 years). The population consisted predominantly of men (80.5%). With respect to smoking, 41 patients were never-smokers (34.8%), and 77 (65.2%) were either ex-smokers or current smokers (16 current smokers, 41 ex-smokers, and 20 who were known to have smoked, but who had an unclear quit status). Table I shows baseline clinical characteristics for the study cohort.
TABLE I.
Demographic characteristics of the 118 study patients
| Characteristic | Value |
|---|---|
| Age (years) | |
| Median | 58 |
| IQR | 52–65 |
|
| |
| Sex (n) | |
| Women | 23 |
| Men | 95 |
|
| |
| Sex ratio | 1:4.13 |
|
| |
| Smoking status [n (%)] | |
| Nonsmoker | 41 (34.75) |
| Smoker (current or ex) | 77 (65.25) |
|
| |
| Primary site [n (%)] | |
| Oropharyngeal | 86 (72.90) |
| Non-oropharyngeal | 32 (27.10) |
|
| |
| Stage [n (%)] | |
| III | 18 (15.25) |
| IVA | 92 (77.97) |
| IVB | 8 (6.78) |
|
| |
| Intervention [n (%)] | |
| Sequential therapy | 7 (5.93) |
| Induction systemic therapy followed by RT | 1 (0.85) |
| Concurrent CRTx | 109 (92.37) |
| Surgery followed by CRTx | 1 (0.85) |
|
| |
| p16 Status [n (%)] | |
| Positive | 57 (49.15) |
| Negative | 37 (17.80) |
| Not available or indeterminate | 24 (19.49) |
|
| |
| Inflammatory markers | |
| BMI (kg/m2) | |
| Median | 27.10 |
| IQR | 23.24–29.68 |
| C-Reactive protein (mg/L) | |
| Median | 3.45 |
| IQRa | 1.00–8.00 |
| Neutrophil-to-lymphocyte ratio | |
| Overall | |
| Median | 3.03 |
| IQR | 2.33–4.38 |
| Oropharyngeal | |
| Median | 2.91 |
| IQR | 2.20–4.42 |
| Non-oropharyngeal | |
| Median | 3.47 |
| IQR | 2.62–4.10 |
|
| |
| Modified Glasgow score group [n (%)]b | |
| 0 | 44 (37.29) |
| 1 | 14 (11.86) |
| 2 | 3 (2.54) |
| Not available | 57 (48.31) |
Measured in only 54 patients before therapy.
Information sufficient to determine the score was available for only 51 patients.
IQR = 25%–75% interquartile range; RT = radiation therapy; CRTx = chemoradiation; BMI = body mass index.
Stage iii disease was diagnosed in 18 patients (15.25%), and stage iv disease in 100 (84.75%). Median follow-up for all patients in the study was 2.45 years (iqr: 1.65–3.3 years), with the 20% of patients who were followed until death having a median follow-up duration of 1.2 years (iqr: 1–1.7 years). The remaining patients had a median follow-up duration of 2.7 years (iqr: 1.9–3.4 years).
All registered hpv testing was reported by p16 assay. Of the 86 patients with oropharyngeal cancer, 50 (58.1%) were p16-positive, 18 (21.0%) were p16-negative, and 1 had an indeterminate finding. For the remaining 17 patients (19.8%), p16 testing was not available, mainly because of a lack of sufficient tissue. Of the 32 patients with non-oropharyngeal cancer, 7 (21.9%) were p16-positive, and 19 (59.4%) were p16-negative. For the remaining 6 patients (18.7%), p16 status was not available and could not be tested.
Baseline Inflammatory Markers
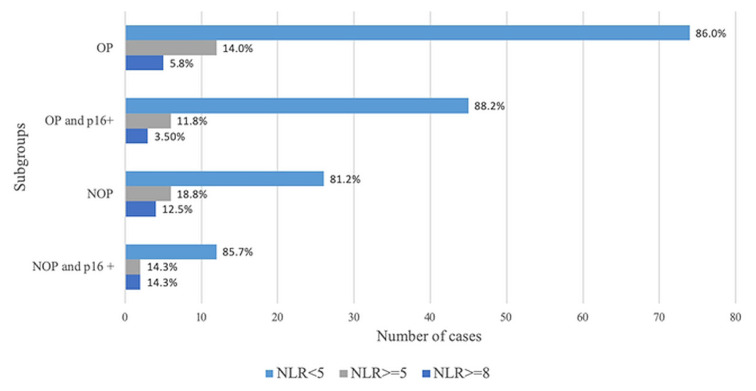
Calculation of nlr and bmi was possible in all patients. Median nlr was 3.03 (iqr: 2.33–4.38), median bmi was 27.10 (iqr: 23.24–29.68). The patients who were p16-positive had a median nlr of 2.91 (iqr: 2.25–4.20); those who were p16-negative had a median nlr of 3.22 (iqr: 2.36–4.25). Figure 2 illustrates the proportions of patients by baseline p16 and nlr results. Serum crp was available for 54 patients, 15 of whom (27.8%) relapsed, and 39 of whom (72.2%) did not relapse (p = 0.84). In those 54 patients, median crp was 3.45 mg/L (iqr: 1.00 mg/L–8.00 mg/L). Data to determine a mgps were sufficient for only 61 patients, 17 of whom (33.33%) relapsed, and 44 of whom (86.27%) did not.
FIGURE 2.
Bar graph demonstrating the relationship between neutrophil-to-lymphocyte ratio and p16 positivity (marker of human papillomavirus) in patients with oropharyngeal (OP) and non-oropharyngeal (NOP) tumours.
Therapeutic Interventions
Induction chemotherapy was given in 8 patients (6.7%), 7 of whom were subsequently treated with concurrent chemoradiation, and 1, with radiation alone. The induction chemotherapy regimens were docetaxel–cisplatin–fluorouracil (6 cases, 75%), cisplatin–fluorouracil (1 case, 12.5%), and carboplatin–paclitaxel (1 case, 12.5%). The curative intervention in 117 patients was concurrent chemoradiation; in 1 case, it was surgery.
Chemoradiation consisted of a median total radiation dose of 70 Gy (iqr: 70 Gy–70 Gy) given simultaneously with either a platinum compound as a single agent (cisplatin 65.8%, carboplatin 5.1%), an epidermal growth factor inhibitor as a single agent (cetuximab or panitumumab, 23%), docetaxel–cisplatin–fluorouracil (0.9%), or a platinum compound (carboplatin or cisplatin) in combination with fluorouracil, paclitaxel, or an epidermal growth factor inhibitor (4.3%).
Prognostic Value of Inflammatory Markers for RFS
Of the 35 patients reported to experience relapse (30%), 22 (63%) were followed until death. Another 2 patients with no report of relapse were also followed until death. At 1, 2, and 3 years from diagnosis, the rfs was 90%, 74%, and 66%.
On univariate analysis, variables found to have prognostic relevance with respect to risk of relapse were p16-negative status, bmi 35 or greater, mgps 2 compared with 1 (2vs1), and crp 6 mg/L or greater. The hazard ratio (hr) for p16 negativity was 2.878 [95% confidence interval (ci): 1.4828 to 5.802; p = 0.003]; for crp 6 mg/L or greater, it was 3.468 (95% ci: 1.229 to 9.789); and for bmi 35 or greater, it was 4.171 (95% ci: 0.819 to 6.610; p = 0.019). On multivariate analysis, each of those factors remained significant.
On further examination of the relapsed and non-relapsed groups with respect to nlr, we found no statistical differences in median nlr or neutrophil or lymphocyte count. Patients with nlr values of 5 or greater were more likely to have both a higher neutrophil count (median: 8.6×109/L; iqr: 6.70–9.88×109/L) and a lower lymphocyte count (1.15×109/L; iqr: 0.78–1.42×109/L) than did patients with a lower nlr [median neutrophils: 4.9×109/L (iqr: 4.00–5.98×109/L), p < 0.001; median lymphocytes: 1.7×109/L (iqr: 1.40–2.30×109/L), p = 0.001).
Prognostic Value of Inflammatory Markers for OS
The os rates at 1, 2, and 3 years from diagnosis were 94%, 83%, and 79% respectively.
On univariate analysis, relevant variables were nlr 5 or greater and crp 6 mg/L or greater, which were significantly associated with shorter survival at unadjusted hrs of 2.997 (95% ci: 1.280 to 7.017; p = 0.011) and 9.715 (95% ci: 1.133 to 83.299; p = 0.038) respectively. At a cut-off value of 3, nlr was nonsignificant on univariate analysis. The hr for p16 negativity was 1.920 (95% ci: 0.844 to 4.371; p = 0.120), but that variable did not remain significant for os. In multivariate analysis, nlr remained significant for both rfs and os. The adjusted hr for nlr 5 or greater was 3.342 (95% ci: 1.398 to 7.987; p = 0.007); for crp 6 mg/L or greater, it was 16.727 (95% ci: 1.562 to 177.14; p = 0.020).
Prognostic Value of Inflammatory Markers for RFS and OS Adjusted for p16 Status
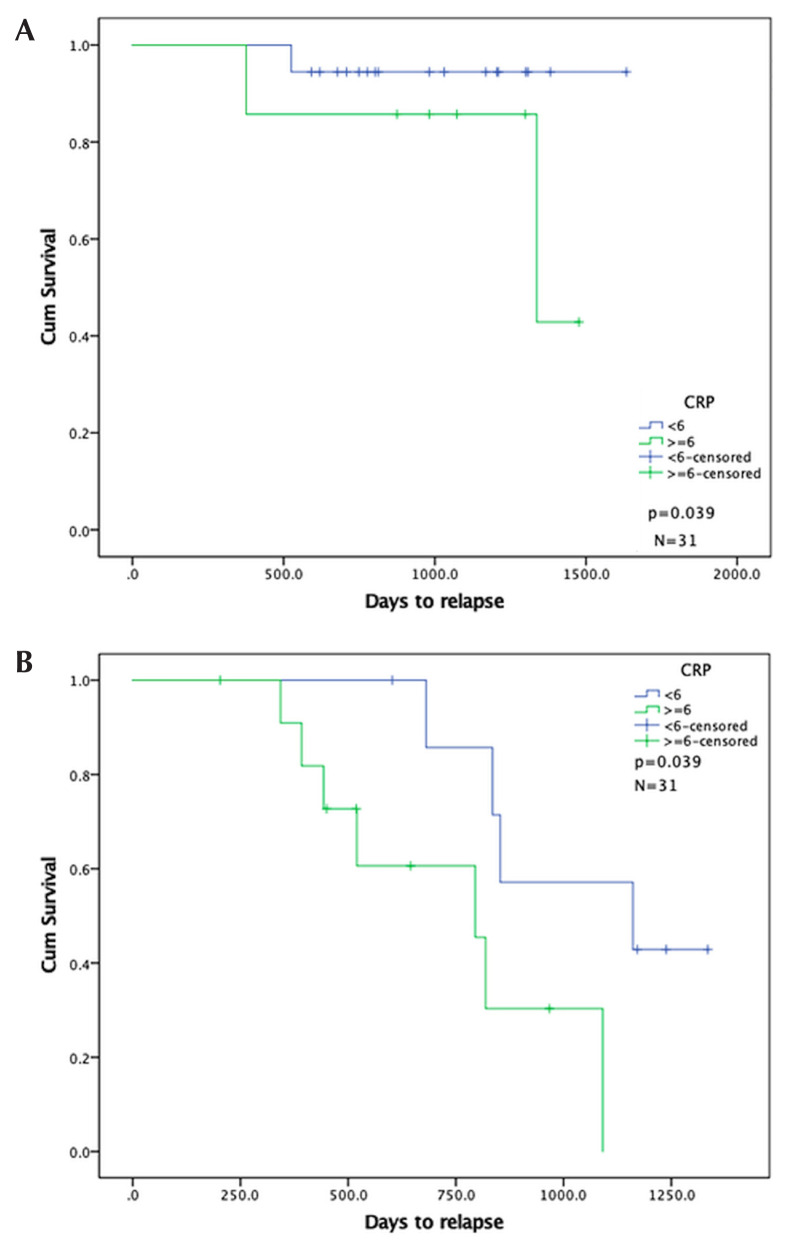
After adjusting for p16 status in the multivariate analysis for rfs, we found that bmi 35 or greater did not remain significant (p = 0.166). However, crp 6 mg/L or greater did remain significant after the adjustment (p = 0.039).
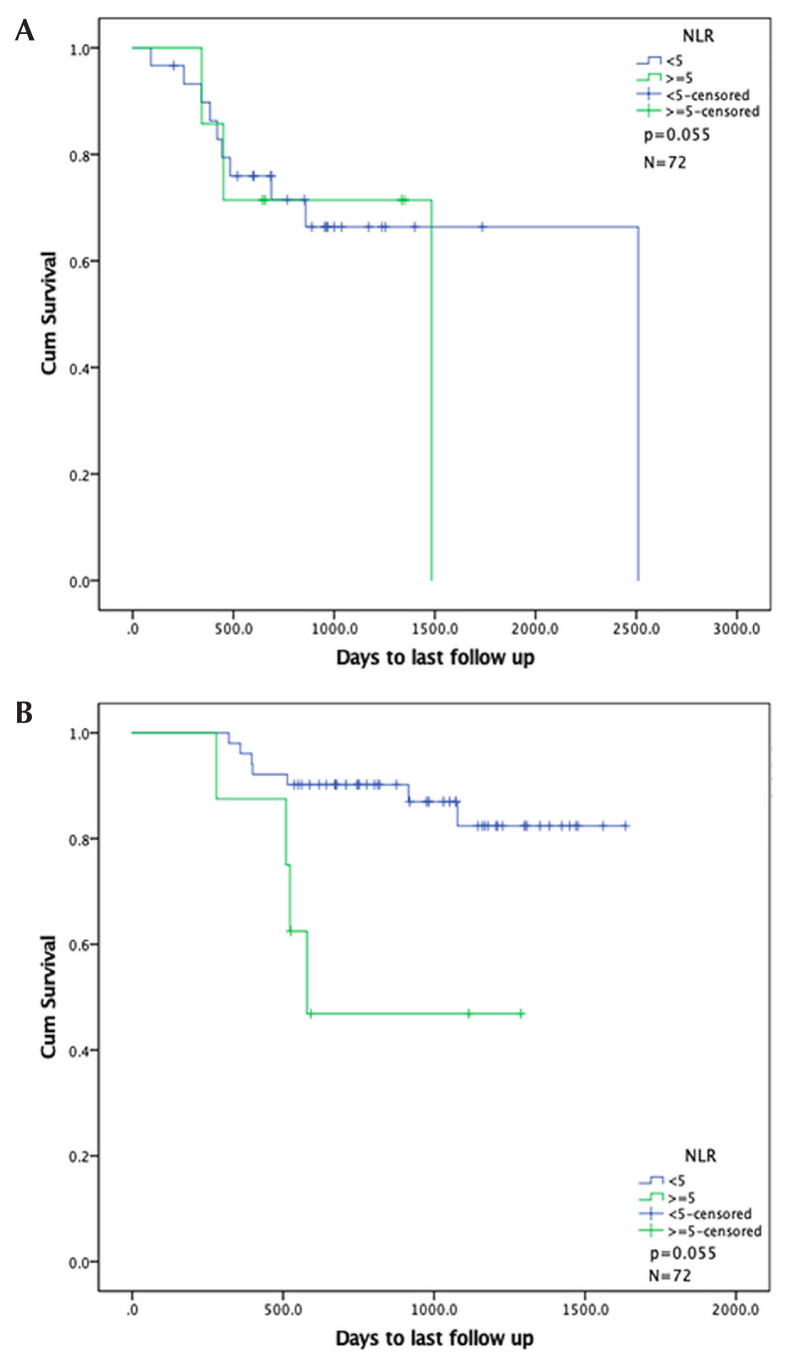
After adjusting for p16 status in the multivariate analysis for os, crp 6 mg/L or greater remained significant (p = 0.028), but nlr 5 or greater did not (p = 0.055, Table II). Figures 3 and 4 show Kaplan–Meier curves for nlr 5 or greater and crp 6 mg/L or greater after adjustment for p16 status.
TABLE II.
Multivariate analysis adjusted for p16 statusa
| Variable | Adjusted HR | Adjusted 95% CL | p Value | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| Relapse-free survival | ||||
|
| ||||
| BMI | 0.927 | 0.863 | 0.997 | 0.041 |
|
| ||||
| BMI≥35 | 2.674 | 0.665 | 10.748 | 0.166 |
|
| ||||
| CRP | 1.023 | 0.993 | 1.055 | 0.137 |
|
| ||||
| CRP≥6 mg/L | 4.974 | 1.087 | 22.755 | 0.039 |
| Overall survival | ||||
|
| ||||
| NLR≥5 | 2.995 | 1.174 | 7.641 | 0.055 |
| CRP≥6 mg/L | 20.409 | 1.380 | 301.82 | 0.028 |
Results are shown only for variables that remained significant in multivariate analysis.
HR = hazard ratio; CL = confidence limits; BMI = body mass index; CRP = C-reactive protein; NLR = neutrophil-to-lymphocyte ratio.
FIGURE 3.
Kaplan–Meier curves for overall survival in patients by neutrophil-to-lymphocyte ratio (NLR), stratified by p16 status. (A) p16-Positive group. (B) p16-Negative group.
FIGURE 4.
Kaplan–Meier curves for relapse-free survival in patients by serum C-reactive protein level, stratified by p16 status. (A) p16-Positive group. (B) p16-Negative group.
Non-oropharyngeal Cancers
No significant prognostic value for inflammatory markers or p16 status with respect to rfs or os was observed during the follow-up period.
DISCUSSION
We report on 118 cases of head-and-neck scc (72.9% oropharyngeal, 27.1% non-oropharyngeal), with an significant proportion of confirmed p16-positive cases overall (58.1% of oropharyngeal primaries, 21.9% of non-oropharyngeal primaries, with 20% overall not able to be tested).
With respect to rfs, markers significantly predicting a greater chance of relapse included p16 negativity, crp 6 mg/L or greater, bmi 35 or greater, and mgps 2vs1. All factors except mgps 2vs1 remained significant in multivariate analysis. However, when adjusted for p16 status, bmi 35 or greater did not remain a significant predictor for relapse.
With respect to os, we found that nlr 5 or greater and crp 6 mg/L or greater were significant on both univariate and multivariate analysis. However, only crp 6 mg/L or greater remained significant after adjustment for p16 status; nlr 5 or greater did not remain significant.
In 2017, Rosculet et al.17 reported a retrospective evaluation of nlr, platelet-to-lymphocyte ratio, and neutrophil and monocyte counts in a multivariate Cox regression analysis of data for 123 patients with head-and-neck scc treated with primary chemoradiotherapy. In their study, p16 status was not known in 41.5% of cases, and it was not clear whether p16 status was determined in non-oropharyngeal tumours. Those authors reported a median nlr of 2.7 as the cut-off for reference and found that nlr was an indicator of both rfs and os on univariate analysis. However, nlr lost all relevance when p16 status was considered in the multivariate analysis (hr: 2.42; p = 0.153). At a median nlr of 3.0, no significant association with rfs or os was observed in our cohort; however, we observed a trend toward significance at a value of 5.0 or greater.
Two meta-analyses underscore the prognostic effect of nlr in scc of the head and neck. The most recent, by Tham et al.14 could not comment about the predictive value of nlr with respect to disease-free survival or rfs. Takenaka et al.6 could not address that question either. Our report adds to the report by Rosculet et al.17 in that regard.
The average demographics of our patient sample accords with those of patients with advanced head-and-neck cancer in general: median age 56.1 years (range: 38–70 years), 88.5% men, and a predominance of oropharyngeal cancer3,20. Our patient sample showed a high proportion of p16-positive cases—73.13% of the cases of oropharyngeal cancer and 26.92% of the cases of non-oropharyngeal cancer compared with the proportions reported in other studies—45.8% and 46.3% respectively21,22. Not surprisingly, our relapse rate of 26% at 2 years is lower than those found in other reports23.
Serum crp and mgps (largely based on serum crp and albumin) were associated with an increased risk of relapse, but on multivariate analysis, mgps 2vs1 was not associated with an increased risk of death. The mgps has previously been shown to prognosticate treatment tolerance, toxicity, and survival in patients with head-and-neck cancers24. In the present study, serum crp was significant on multivariate analysis and remained significant even after adjustment for p16 status. An increased risk of relapse with high crp (6 mg/L or greater in our sample) accords with observations in other cancers25 and in various treatment strategies, including rehabilitation and palliative care26. Cut-off values above 10 mg/L have historically been associated with acute inflammation, but in some studies in oral scc, levels above 5 mg/L have been associated with poor outcome27. However, the patient subgroup for which we had crp data was quite small. Our confidence intervals are therefore large, and caution is needed when interpreting the results.
With respect to non-oropharyngeal carcinomas, it is clear that some can be associated with hpv, but the prognostic and predictive value of hpv positivity remains uncertain28. We found no meaningful association of p16 positivity with risk of relapse or death in our small group of patients with nonoropharyngeal cancer.
Limitations
Our study is limited by several factors. First, we were surprised to find that, in this high-risk population, only a relatively small proportion of patients had a high nlr, which is probably explained by the unexpectedly high proportion of p16 positivity. The number of patients for whom we had crp data was small, but in our opinion, quite prognostically telling. Unavoidably, the ability to determine a mgps score was also affected, because crp is an integral part of that tool. And unfortunately, follow-up could not continue beyond the durations reported here.
CONCLUSIONS
Our findings suggest that the prognostic value of hpv status is stronger than that for many other inflammatory markers, including nlr and albumin, in patients with head-and-neck scc. However, despite the small number of patients for whom data were available, crp remained significant after adjustment for p16 status—a result that has to be interpreted in the context of the small sample size.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Glenny AM, Furness S, Worthington HV, et al. on behalf of the csroc Expert Panel. Interventions for the treatment of oral cavity and oropharyngeal cancer: radiotherapy. Cochrane Database Syst Rev. 2010:CD006387. doi: 10.1002/14651858.CD006387.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madera Anaya M, Franco JVA, Ballesteros M, Solà I, Urrútia Cuchí G, Bonfill Cosp X. Evidence mapping and quality assessment of systematic reviews on therapeutic interventions for oral cancer. Cancer Manag Res. 2018;11:117–30. doi: 10.2147/CMAR.S186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salas S, Deville JL, Giorgi R, et al. Nutritional factors as predictors of response to radio-chemotherapy and survival in unresectable squamous head and neck carcinoma. Radiother Oncol. 2008;87:195–200. doi: 10.1016/j.radonc.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Takenaka Y, Oya R, Kitamiura T, et al. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: a meta-analysis. Head Neck. 2018;40:647–55. doi: 10.1002/hed.24986. [DOI] [PubMed] [Google Scholar]
- 7.Proctor MJ, Talwar D, Balmar SM, et al. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer. 2010;103:870–6. doi: 10.1038/sj.bjc.6605855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafique K, Proctor MJ, McMillan DC, et al. The modified Glasgow prognostic score in prostate cancer: results from a retrospective clinical series of 744 patients. BMC Cancer. 2013;13:292. doi: 10.1186/1471-2407-13-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishiki T, Masaki T, Matsuoka H, et al. Modified Glasgow prognostic score in patients with incurable stage iv colorectal cancer. Am J Surg. 2013;206:234–40. doi: 10.1016/j.amjsurg.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Hirashima K, Watanabe M, Shigaki H, et al. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014;49:1040–6. doi: 10.1007/s00535-013-0855-5. [DOI] [PubMed] [Google Scholar]
- 11.Proctor MJ, Horgan PG, Talwar D, Fletcher CD, Morrison DS, McMillan DC. Optimization of the systemic inf lammation-based Glasgow prognostic score. Cancer. 2013;119:2325–32. doi: 10.1002/cncr.28018. [DOI] [PubMed] [Google Scholar]
- 12.Cho Y, Kim JW, Yoon HI, Lee CG, Keum KC, Lee IJ. The prognostic significance of neutrophil-to-lymphocyte ratio in head and neck cancer patients treated with radiotherapy. J Clin Med. 2018;7:E512. doi: 10.3390/jcm7120512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, Wang H, Yan A, et al. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: a meta-analysis. BMC Cancer. 2018;18:383. doi: 10.1186/s12885-018-4230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tham T, Bardash Y, Herman SW, Costantino PD. Neutrophil-to-lymphocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis. Head Neck. 2018;40:2546–57. doi: 10.1002/hed.25324. [DOI] [PubMed] [Google Scholar]
- 15.Lindquist D, Romanitan M, Hammarstedt L, et al. Human papillomavirus is a favourable prognostic factor in tonsillar cancer and its oncogenic role is supported by the expression of E6 and E7. Mol Oncol. 2007;1:350–5. doi: 10.1016/j.molonc.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of hpv-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–8. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 17.Rosculet N, Zhou XC, Ha P, et al. Neutrophil-to-lymphocyte ratio: prognostic indicator for head and neck squamous cell carcinoma. Head Neck. 2017;39:662–7. doi: 10.1002/hed.24658. [DOI] [PubMed] [Google Scholar]
- 18.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer hpv status determination in US Cooperative Group trials. Am J Surg Pathol. 2012;36:945–54. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantley RL, Gabrielli E, Montebelli F, Cimbaluk D, Gattuso P, Petruzzelli G. Ancillary studies in determining human papillomavirus status of squamous cell carcinoma of the oropharynx: a review. Patholog Res Int. 2011;2011 doi: 10.4061/2011/138469. 138469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann M, Tribius S, Quabius ES, et al. hpv dna, E6*I–mrna expression and p16INK4A immunohistochemistry in head and neck cancer—how valid is p16INK4A as surrogate marker? Cancer Lett. 2012;323:88–96. doi: 10.1016/j.canlet.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 21.Chen HH, Chen IH, Liao CT, Wei FC, Lee LY, Huang SF. Preoperative circulating C-reactive protein levels predict pathological aggressiveness in oral squamous cell carcinoma: a retrospective clinical study. Clin Otolaryngol. 2011;36:147–53. doi: 10.1111/j.1749-4486.2011.02274.x. [DOI] [PubMed] [Google Scholar]
- 22.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90:215–19. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 23.Chasen MR, Feldstain A, Gravelle D, MacDonald N, Pereira J. An interprofessional palliative care oncology rehabilitation program: effects on function and predictors of program completion. Curr Oncol. 2013;20:301–9. doi: 10.3747/co.20.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang PH, Yeh KY, Wang CH, et al. Impact of the pretreatment Glasgow prognostic score on treatment tolerance, toxicities, and survival in patients with advanced head and neck cancer undergoing concurrent chemoradiotherapy. Head Neck. 2017;39:1990–6. doi: 10.1002/hed.24853. [DOI] [PubMed] [Google Scholar]
- 25.Scirica BM, Cannon CP, Sabatine MS, et al. on behalf of the prove it-timi 22 investigators. Concentrations of C-reactive protein and B-type natriuretic peptide 30 days after acute coronary syndromes independently predict hospitalization for heart failure and cardiovascular death. Clin Chem. 2009;55:265–73. doi: 10.1373/clinchem.2008.117192. [DOI] [PubMed] [Google Scholar]
- 26.Morrow DA, Rifai N, Antman EM, et al. C-Reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a timi 11A substudy. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1998;31:1460–5. doi: 10.1016/S0735-1097(98)00136-3. [DOI] [PubMed] [Google Scholar]
- 27.Milazzo D, Biasucci LM, Luciani N, et al. Elevated levels of C-reactive protein before coronary artery bypass grafting predict recurrence of ischemic events. Am J Cardiol. 1999;84:459–61. doi: 10.1016/S0002-9149(99)00333-1. [DOI] [PubMed] [Google Scholar]
- 28.Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6(suppl 1):S104–20. doi: 10.1007/s12105-012-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]