Abstract
Atypical teratoid rhabdoid tumor (ATRT) is a rare, highly malignant central nervous system cancer arising in infants and younger children, historically considered to be homogeneous, monogenic, and incurable. Recent use of intensified therapies has modestly improved survival for ATRT; however, a majority of patients will still succumb to their disease. While ATRTs almost universally exhibit loss of SMARCB1 (BAF47/INI1/SNF5), recent whole genome, transcriptome, and epigenomic analyses of large cohorts reveal previously underappreciated molecular heterogeneity. These discoveries provide novel insights into how SMARCB1 loss drives oncogenesis and confer specific therapeutic vulnerabilities, raising exciting prospects for molecularly stratified treatment for patients with ATRT.
Keywords: ATRT , enhancer , epigenomics , rhabdoid tumors , subgroup-specific therapeutics
Rhabdoid tumors comprise a spectrum of poorly understood diseases that can arise in intracranial or extracranial locations, where they are respectively called atypical teratoid rhabdoid tumor (ATRT) or malignant rhabdoid tumor (MRT). ATRTs are central nervous system (CNS) tumors initially recognized based on morphologic resemblance to malignant renal rhabdoid tumors and subsequently defined as a distinct histogenetic entity in the late 1990s. Since 2000, ATRTs have been designated as a formal World Health Organization diagnostic category.1 ATRTs are distinguished by biallelic loss of function mutations in SWItch/sucrose nonfermentable (SWI/SNF) related, matrix associated, actin dependent regulator of chromatin, subfamily B (SMARCB1), a tumor suppressor gene that encodes BAF47 (also called integrase interactor 1 [INI1], hSNF5), a core subunit of the SWI/SNF chromatin remodeling complex. A small percentage of ATRTs with wildtype SMARCB1 harbor biallelic loss of SMARCA4 that encodes SWI/SNF ATPase Brahma/SWI2-related gene 1 (BRG1).2 ATRTs can exhibit substantial histopathologic variation and resemble other embryonal tumors; however, they are distinguished by negative immunostaining for INI1 or BRG1.3,4 Given availability of INI1 and BRG1 immunohistochemistry in routine diagnostic neuropathology, ATRTs are now increasingly identified. ATRTs may arise in any CNS location, and the majority (70–80%) arise in children <3 years of age.5 Up to one-third of children with ATRT have germline SMARCB1 (or SMARCA4) alterations; these patients with rhabdoid tumor predisposition syndrome (RTPS) are at increased risk of developing multiple intra- and/or extracranial rhabdoid tumors at a very young age.6 Although the true incidence of ATRTs is unknown, they are estimated to make up at least 20% of CNS tumors in children <3 years of age7 and are the most common malignant CNS tumor of children age <1 year.5
Large-scale prospective treatment and demographic data on ATRTs are lacking; thus, studies of clinical prognosticators have been mainly derived from small retrospective cohorts. Metastatic disease, seen in 30–40% of ATRT patients at the time of diagnosis,8–11 has been variably correlated with survival,7–9,12–15 while supratentorial tumor location,12,16 complete resection,7,8,11,12 and response to chemotherapy and/or radiation therapy (RT)11,12,15,17 has been associated with improved survival. Older age9,12,17 has also been reported as a positive prognostic factor, though it is unclear whether this reflects a tendency to RT avoidance in younger children or distinct age-related tumor biology.18,19
ATRTs Exhibit Molecular Heterogeneity
Despite heterogeneity in location, treatment response, and disease stage in ATRT patients, whole exome and genome sequencing studies18–22 indicate primary ATRTs have very low mutation rates with only recurrent SMARCB1 alterations. This is in keeping with experimental studies that invoke epigenetic mechanisms as a major driver of cancers resulting from SMARCB1 loss.23 Collective studies from the Hospital for Sick Children (HSC) and German Cancer Research Center (DKFZ) showed that ATRTs comprise 3 main molecular subgroups with distinct epigenomic, transcriptional, clinicopathologic, and therapeutic features.18,19 In a recent consensus publication by Ho et al., global methylation profiling of 388 primary ATRTs demonstrated that Group 1, 2A, and 2B ATRT subtypes reported by Torchia et al largely overlap, respectively, with the sonic hedgehog (SHH), tyrosine (TYR), and myelocytomatosis oncogene (MYC) subgroups reported by DKFZ.24 These findings were further validated by transcriptional analysis of 172 primary ATRTs, including 21 with both methylation and transcription array data for which there was 96% subgroup concordance between platforms.
Although the consensus publication outlines enrichment of age categories and tumor location, there remains significant variation in clinical phenotypes within the 3 major ATRT subgroups. Infra- and supratentorial tumors, as well as metastatic disease, are found across ATRT subtypes. The HSC and DKFZ studies reported an enrichment of SMARCB1 deletions in the MYC ATRT subgroup while Torchia et al also showed up to 20% of ATRTs exhibited other structural alterations and increased frequency of mutational events in the noncoding genome.18 Of note, whole genome sequencing studies of MRTs, which by methylation exhibit similarity to the MYC subgroup, have revealed predominant intergenic mutational events25 and frequent genomic rearrangements mediated by PGDB5,25,26 an embryonic human transposase. How differences in mechanisms of SMARCB1 loss, other genotypic events, and whether insertional mutagenesis events contribute to clinical heterogeneity within and across ATRT subgroups remains to be defined with deeper, comprehensive studies of larger cohorts.
Current Clinical Approaches to ATRTs
Currently, there is no standard treatment approach for ATRTs. Despite improved diagnostic recognition, there have been few ATRT prospective studies, and treatment data have largely been surmised from retrospective and small studies5,7,27 (Table 1). ATRT therapy has generally followed evolution in approaches to infant brain tumors with use of conventional dose chemotherapy regimens in earlier studies and application of high dose chemotherapy (HDC) with stem cell rescue (SCR) in the more recent era as a mechanism of deferring or avoiding RT. Historical pan-infant brain tumor trials reported rapid progression and very poor outcomes for ATRT patients. In North American prospective trials CCG992128 and POG9233/34, most ATRT patients progressed within a year of diagnosis yielding progression-free survival (PFS) of <20%.29 Similarly, patients treated with conventional chemotherapy with or without RT on the German HIT infant CNS tumor protocols had 3-year PFS and overall survival (OS) of only 13% ± 5% and 22% ± 6%, respectively.12 The European Rhabdoid Registry (EU-RHAB)–based protocol demonstrated 6-year respective PFS/OS of 45% ± 9%/46% ± 10% for 31 patients treated using doxorubicin and ifosfamide-based chemotherapy as well as intraventricular and maintenance chemotherapy plus focal or craniospinal irradiation (CSI).17 Though only short-term outcomes are reported, a prospective ATRT study by the Dana Farber Cancer Institute reported 2-year PFS/OS of 53% ± 13%/ 70 ± 10% in 20 evaluable patients using a regimen based on the 3rd Intergroup Rhabdomyosarcoma Study (IRS-III) with triple intrathecal (IT) and focal RT or CSI.11
Table 1.
Summary of prospective trials and retrospective/registry studies of ATRTs
| Protocol Name | Pts <3 years (n=) | HDC | Adjuvant RT | Salvage RT | PFS; OS (%) | Favorable Prognostic Factors | References | |
|---|---|---|---|---|---|---|---|---|
| HDC/SCR | IT | Focal; WB; CSI (n) | Focal; WB; CSI (n) | |||||
| Prospective Clinical Trials | ||||||||
| POG9233/34 (N = 33) | 33 | – | – | – | – | 5y: 0; 0 | – | D. Strother (personal communication) |
| CCG9921 (N = 28) | 28 | – | – | 1; 0; 1 | 9; 0; 0 | 5y: 14; 29 | None significant | 28 |
| Head Start I (N = 7); Head Start II (N = 6) | 9 | CTE | – | – | 2; 0; 2 | 3y: 23 ± 11; 23 ± 11 | GTR | 30 |
| N/A (N = 8) | 4 | ETC | – | – | 5y: 33; 50 | – | 31 | |
| IRS-III-like (N = 20) | 12 | – | Yes | 11; 0; 4 | – | 2y: 53 ± 13; 70 ± 10 | – | 11 |
| N/A (N = 9) | 9 | CTE, CM | No | 0; 0; 1 | 1; 0; 4 | 3y: 0; 53 ± 17 | – | 32 |
| Head Start III (N = 19) | 19 | CTE | – | – | 5;0;0 | 3y: 21 ± 9; 26 ± 10 | – | 33 |
| ACNS0333 (N = 65) | 54 | CT | No | 40; 0; 6 | – | 4y: 37; 43 | – | 39 |
| Retrospective Series | ||||||||
| COG 99703 (N = 8); IRS III-like (N = 7); CCG9921 (N = 6); Others (N = 21) | 30 | Yes (31%) | Yes (38%) | 9; 0; 4 | – | % Not reported | GTR; older age | 7 |
| SJMB96* (N = 7); BB98 (N = 7); PBTC-001 (N = 6); ICE (N = 1); Other (N = 9) | 21 | Yes (26%) | No | – | 1; 0; 3 | 2y: 31 ± 9; 40 ± 10 | RT | 10 |
| Various (N = 17) | 2 | No | Yes (12%) | 0; 2; 15 | – | % Not reported | Early RT | 34 |
| HIT 2000 (N = 18); HIT-SKK-92 (N = 9); HIT-SKK-91 (N = 6); Other (N = 17) | 41 | No | Yes (74%) | 11; 0; 0 | 10; 0; 0 | 3y: 13 ± 5; 22 ± 6 | Age > 1.2 years; Early complete response | 12 |
| AT/RT04 (N = 24); PNET-HR (N = 11); BB-SFOP (N = 9); Palliative (N = 9) | Yes (19%) | No | 10; 0; 0 | – | 1y: 17%; 41% | Age > 2 years; M0 stage | 9 | |
| IRS-III-like, baby brain, or ICE (N = 8); HDC/SCR (N = 23); Palliative (N = 10) | 46 | Yes (45%) | Yes (18%) | 18; 0; 0 | 6; 0; 0 | CC 2y OS: 27 ± 9; HDC/ SCR 2y OS: 48 ± 12 | HDC/SCR | 8 |
| Various (N = 31) | 19 | n/a | No | 19; 0; 18 | – | 4y: 33 ± 10; 53 ± 10 | Early RT | 35 |
| Rhabdoid 2007, EU-RHAB (N = 17) | 14 | Yes (65% at dx, 37% at relapse) | No | 3; 0; 0 | 4; 0; 0 | 2y: 29 ± 11; 50 ± 12 | – | 36 |
| IRS-III-like + HDC/SCR (N = 9) | 5 | CTE | Yes | 5; 0; 0 | – | 5y: 89 ± 11; 100 | – | 14 |
| Rhabdoid 2007 (N = 31) | 23 | Yes (23% at relapse) | Yes | 21; 0; 2 | – | 6y: 45 ± 9; 46 ± 10 | – | 17 |
| Various (N = 28) | 12 | Yes, n = 8 | Yes, n = 3 | 8; 0; 20 | – | % Not reported | Early RT; HDC/SCR | 37 |
HDC/SCR: high-dose chemotherapy/stem cell rescue; RT: radiation therapy; IT: intrathecal or intraventricular; PF: posterior fossa; ST: supratentorial; CSI: craniospinal irradiation; tx: treatment; dx: diagnosis; alt: alternating with; HD MTX: high-dose methotrexate; CT: carboplatin, thiotepa; CECV: cisplatin, etoposide, cyclophosphamide, vincristine; CEIV: carboplatin, etoposide, ifosfamide, vincristine; CTE: carboplatin, thiotepa, etoposide; CM: cyclophosphamide, melphalan; IRS-III: 3rd Intergroup Rhabdomyosarcoma Study; VDC: vincristine, cyclophosphamide, doxorubicin; ICE: ifosfamide, carboplatin, etoposide; TI: trafosfamide, idarubicin; TE: trafosfamide, etoposide; NS: not statistically significant, or n/a: not applicable or not investigated. *Number of patients under 2 years of age instead of 3 years of age.
*Prospective series as footnote.
Similarly, improved survival for a proportion of ATRT patients was reported with HDC/SCR with the North American Head Start and CCG99703 trials representing the first generation of such studies. The Head Start II study reported improved outcome with high-dose methotrexate (HD-MTX)–based induction regimens30; however, the successor Head Start III study31 reported inferior outcomes relative to contemporary studies11,17,35,36 with frequent early progression and numerous toxic deaths. A Korean single institution HDC/SCR ATRT pilot study also reported frequent early progression with 3-year PFS/OS respectively of 0%/53% ± 17% and RT salvage needed for all survivors.32,38 Similarly, an Italian study reported early progression in 6 of 8 patients treated with 4 induction cycles, HDC/SCR, and whole brain RT.31 Interestingly, Slavc et al reported a provocative series with much improved 5-year PFS/OS of 89% ± 11%/100% in 9 patients treated with combined sarcoma-based induction with HDC/SCR, IT chemotherapy, and focal RT. Significantly, results of the Children’s Oncology Group ACNS0333 trial, which represents the largest prospective ATRT study, has validated HDC/SCR as an important modality in ATRT. Using an HD-MTX induction, HDC/SCR consolidation, and age-adapted timing and field of RT, Reddy et al reported a 4-year event-free survival and OS of 37% and 43%, with few events occurring more than 2 years post diagnosis in 65 evaluable patients.39
Based on treatment of more common embryonal CNS tumors, such as medulloblastoma, RT has also been considered an important modality in ATRT therapy. However, use of RT in ATRT patients remains debated due to severe neurocognitive sequelae associated with whole brain RT in very young patients. As a consequence, a wide range of RT timing, dose, and volume has been used in retrospective ATRT studies.8,12,27,40 There are currently no robust data to inform use of RT in this population. A Surveillance, Epidemiology, and End Results study concluded RT improved ATRT survival13 based on examining receipt of RT and survival >6 months post diagnosis in 144 ATRT patients without accounting for impact of other treatment including chemotherapy and surgery. Similarly, a study of 31 ATRT patients treated with heterogeneous chemotherapy regimens suggested RT delays of ≥1 month post-surgery increased risk of disease recurrence.35 However, the prospective ACNS0333 trial indicates timing of RT relative to chemotherapy did not impact survival.39 Similarly, an Austrian study reported excellent outcomes in a series of 9 children treated with a HDC/SCR protocol and focal RT up to 9 months post-diagnosis.14 Interestingly, the Canadian Pediatric Brain Tumor Consortium reported 2-year OS of 48% ± 12% in a retrospective cohort of 18 patients treated with HDC regimens, many of whom received no RT,8 suggesting that a proportion of ATRT patients can be cured without radiation.
In aggregate, results of both prospective and retrospective studies utilizing conventional and HDC-based regimens indicate biological heterogeneity may underlie treatment-specific response and survival in ATRT patients.
ATRT Molecular Subgroups Have Varied Therapeutic Vulnerabilities
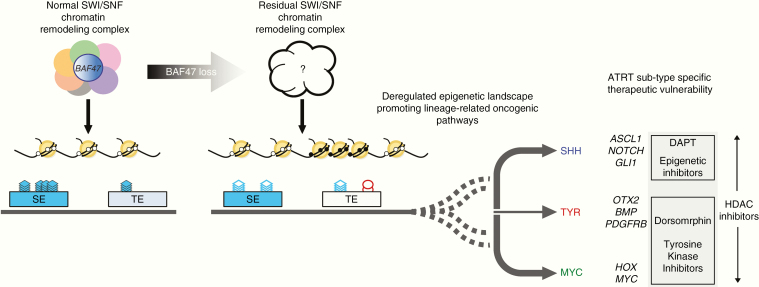
BAF47 is a core component of the polymorphic multi-subunit SWI/SNF chromatin-remodeling complex which controls promoter and enhancer accessibility via nucleosome mobilization, a process normally antagonized by the polycomb repressive complexes (PRCs).41,42 Loss of BAF47 has been shown to increase expression and activity of the PRC2 histone methyl transferase enhancer of zeste homolog 2 (EZH2)43 and a spectrum of associated downstream oncogenic signaling pathways. Recent studies by Wang et al and Nakayama et al indicate BAF47 loss results in a residual SWI/SNF complex with impaired affinity for target promoters and distal enhancers in ATRT and MRT tumor cells.41,44 These findings mirror studies in other SWI/SNF aberrant cancers lacking ARID1A or SMARCA445 and suggest that residual BAF47-deficient SWI/SNF complex may be important in maintaining oncogenic phenotypes in ATRTs and related tumors. Recent work by Erkek et al confirmed binding of residual SWI/SNF protein SMARCA4 at nearly all promoters in ATRT occupied by EZH2 but that lacked repressive H3K27me3 marks.46 SMARCA4 knockdown led to increased H3K27me3 and decreased transcription at these specific sites, supporting the oncogenic potential of SMARCA4, as previously reported.44,47 Together with recent functional epigenomic and modeling studies18,19 defining unique lineage associated enhancer/super-enhancer profiles between ATRT subtypes, these findings suggest the nature of residual SWI/SNF complexes and associated lineage-related oncogenic pathways48 (Fig. 1) may underlie specific therapeutic vulnerability observed in ATRTs subtypes.
Fig. 1.
Function of SWI/SNF chromatin remodeling complex with intact (left) and SMARCB1/BAF47 loss are schematized. Current evidence suggests SWI/SNF complexes mediate functions of typical and lineage associated super-enhancers (left). Loss of BAF47 which impairs SWI/SNF stability (right) is proposed to result in residual SWI/SNF complexes of various composition which mediate aberrant gene regulation at typical and super-enhancers to confer tumor subtype specific therapeutic vulnerabilities (dashed lines). ATRT subgroup specific enrichment patterns and reported vulnerabilities are shown. SE = super enhancer, TE = typical enhancer, blue stacks = H3K27ac marks, red circles = H3K27me3.
Indeed, Torchia et al showed using gamma-secretase (DAPT*) and bone morphogenetic protein 2 (BMP)–pathway (dorsomorphin) inhibitors and gene knockdown experiments that ATRT cell lines subtyped as neurogenic Group 1 (SHH) and mesenchymal Group 2 (TYR/MYC) were distinctly dependent on Notch and BMP signaling which play respective roles in neurogenesis and mesenchymal differentiation.18 They also showed multi–thymidine kinase 1 (TKI) (dasatinib, nilotinib) in vitro and in vivo inhibition of Group 2 (TYR/MYC) ATRT cells correlated with enhancer-mediated upregulation of platelet derived growth factor receptor B (PDGFRB), which has critical roles in mesenchymal differentiation. More recent subgroup classification of cell lines published by Ho et al defined cells susceptible to dasatinib and nilotinib (CHLA266/06, SH, BT16/12) as belonging to the MYC subgroup.24 Thus, further work is required to elucidate the mechanism underlying this susceptibility.
In addition to differences in lineage-related vulnerabilities, Torchia et al also showed ATRT subtype specific cells had different sensitivities to 14 epigenetic inhibitors including the bromodomain and extraterminal domain family (BET/bromodomain) protein, histone methyltransferases and histone deacetylases (HDACs). While HDAC inhibitors LAQ824 and J4 reduced growth of all ATRT cell lines, methyl transferase (UNC0638), EZH2 (UNC1999), and BET/bromodomain (JQ1) inhibitors had greater effects on growth of Group 1/SHH cell lines (CHLA02/04/05).18 A better understanding of the nature of residual SWI/SNF complexes in ATRT molecular subgroups will be important for future development and understanding of how different epigenetic drugs may be tailored to ATRT molecular subtypes.
Although specific targetable activating mutations have not been reported in ATRTs, a number of promising targeted agents have been identified from in vitro and in vivo preclinical studies (Table 2), and some are currently in early phase clinical trials for patients with rhabdoid tumors, including cyclin-dependent kinase (CDK)4/6 inhibitors49 (palbociclib [NCT03065062], abemaciclib [NCT02644460], ribociclib with everolimus [NCT03387020]), an aurora kinase inhibitor50 (alisertib, NCT02114229), and an EZH2 inhibitor50 (tazemetostat [NCT02601937 and NCT03213665]). Whether some or all of these agents will be pan-relevant or restricted to ATRT subtypes remains to be determined. Genomic studies suggest that MRTs may share molecular features with the MYC subclass of ATRTs.24 Indeed, drug screening studies also indicate overlap in drug sensitivity in some ATRT and MRT cell lines.51 The effect of HDAC inhibitor LAQ82 observed by Torchia et al in all ATRT cell lines is also notable, as low-dose panobinostat (LBH589, another HDAC inhibitor) has been shown to induce cellular senescence and promote differentiation in MRT cell lines.52 Studies of transposase-mediated genomic rearrangements also suggest DNA damage pathways may represent common therapeutic vulnerabilities in MRTs and subclasses of ATRT.26 Most recent studies by Leruste et al have demonstrated high CD8+ T cell infiltration in TYR and MYC ATRTs. Using allografts derived from an inducible Smarcb1flox/flox;Rosa26-CreERT2 ATRT model, they also demonstrated efficacy of immune checkpoint blockade indicating that immunotherapy may treat a proportion of ATRT subtypes.51
Table 2.
An overview of drugs, drug-like compounds, and chemical compounds tested on ATRT cell lines, animal models, and clinical trials*
| Target/Mode of Action/Class | Phase of testing at which found to be effective or used | Refs | ||
|---|---|---|---|---|
| Preclinical Studies | Early Phase Clinical Trials | |||
| Classic Chemotherapy and DNA Damaging Agents | Alkylating agents | Carmustine,† thiotepa,† ifosfamide† | Carmustine, ifosfamide, temozolomide (nct00946335, nct01076530) | 14,31, 54–59 |
| Antimetabolite | Intraventricular methotrexate (NCT01737671, NCT02684071) | 60 | ||
| Guanosine analogs | Ribavarin†‡ | 61 | ||
| Intercalating agents | Actinomycin D,† idarubicin,† mitoxantrone,† doxorubicin†‡ | 51,54, 62 | ||
| Platinum compounds | Oxaliplatin† | Oxaliplatin (NCT00047177) | 54,63, 64 | |
| Topoisomerase inhibitors | Irinotecan,† etoposide† | Irinotecan (NCT00138216), Etoposide (NCT00392886) | 62,65, 66 | |
| Vinca alkaloid | Vinorelbine,† vincristine†‡ | 51,54 | ||
| Kinase Inhibitors | AKT | MK-2206† | 67 | |
| ALK, TGFbeta | SB431542† | 68 | ||
| Aurora A | Alisertib (MLN8237)† | Alisertib (NCT02114229) | 69–72 | |
| EGFR-HER2 | Lapatinib*†‡ | 73 | ||
| IGF-1R | NVP-AEW451† | 67 | ||
| MEK | Selumetinib† | 74 | ||
| mTOR | Rapamycin*† | Rapamycin* (NCT03387020, NCT01331135) | 54 | |
| mTORC1/2 | TAK228 (sapanisertib)†‡ | 75 | ||
| Multi-TKI | Dasatinib,*†‡ imatinib,*† kw-2449,† nilotinib*,† r-1530,*† sorafenib,*† sunitinib,*† lenvatinib*†‡ | 18,51, 54,65, 72,76 | ||
| PDGFR/FGFR | Ponatinib*† | 77 | ||
| PLK1 | Volasertib (BI6727)*†‡ | 78 | ||
| PLK4 | CFI-400945,†‡ CFI-400437,† centrinone,† centrinone-B† | 72,79, 80 | ||
| PTK7 | Vatalanib† | 81 | ||
| VEGF | Axitinib,*† cabozantinib,*†‡ pazopanib,*†‡ | Cediranib (NCT00326664) | 4,72 | |
| Cell Cycle Targets | CDK2 inhibitors | Roscovitine† | 54 | |
| CDK4/6 inhibitors | Palbociclib*†‡ | Ribociclib* (NCT03387020), Abemaciclib* (NCT02644460) | 49,82 | |
| Epigenetic Targeting Compounds | Bromo/BET | JQ1†‡ | 18,83, 84 | |
| BRD9 | BI-9564,† I-BRD9† | 85 | ||
| Demethylating agent | 5-AZA-2′-deoxycytidine (decitabine)*† | 62 | ||
| EZH2 | 3-Deazaneplanocin A (DZNep),† UNC1999,† tazemetostat*†‡ | Tazemetostat* (NCT02601937, NCT02875548, NCT02601950), Pediatric MATCH (NCT03213665) | 18,62, 86,87 | |
| G9a lysine methyltransferase | UNC0638† | 18 | ||
| Histone deacetylase inhibitors (HDACi) | LAQ824 (Dacinostat),† vorinostat (SAHA),*† valproic acid,*† SNDX-275 (entinostat),*† trichostatin A*† | Vorinostat* (SAHA), (NCT01076530, NCT00217412), valproic acid* | 18,62, 88–92 | |
| Pathway/Lineage Specific Compounds | BMP | Dorsomorphin*† | 18 | |
| Notch | DAPT*† | RO4929097 (NCT01088763) | 18 | |
| WNT inhibitor | Casin,*† niclosamide,† pyrvinium,† WNT-c59† | 93 | ||
| Antibody | 131-I-labeled monoclonal Ab: 8H9 (NCT00089245), 3F8 (NCT00445965) | |||
| Ornithine decarboxylase | DFMO (NCT03581240) | |||
| Oncolytic virus | Measles virus (MV)*†‡ | Modified measles virus (MV-NIS, NCT02962167) | 94 | |
| Other compounds | ALDH inhibitor | Disulfiram*†‡ | 55 | |
| LOX inhibitor | BAPN†‡ | 95 | ||
| Diferuoylmethane | Curcumin*† | 54 | ||
| Flavonoid | Apigenin*† | 54 | ||
| PPARg agonist | Ciglitazone† | 54 | ||
| Exosome release inhibitor | GW4869† | 96 | ||
| MDM2, MDM4, MDMX | Idasanutlin,*† ATSP-7041† | ALRN-6924 (NCT03654716) | 97 |
*In vitro and in vivo studies are denoted with a dagger (†) or double dagger (‡), respectively. Agents without FDA approval are denoted with an asterisk (*). FDA approved targeted agents with preclinical or clinical data suggesting favorable blood‒brain barrier penetration are bolded. Many of agents are FDA approved, and therefore with further preclinical testing (namely in vivo testing on transgenic mouse models and/or xenografts) may be promising agents to quickly translate into clinical use. Citations include only compounds deemed by the authors to be effective in their study.
Future studies will need to examine how lineage-specific signaling inhibitors, multi-kinase or newer generations of PDGFRB inhibitors,98 and other biologics can be combined with epigenetic inhibitors, immune-therapeutics, as well as conventional chemotherapy and/or RT to improve survival for ATRT patients.49,82
Refining Therapeutic Approaches to ATRTs
Some clinical factors that have most consistently been linked to poorer outcome in ATRT patients include young age, infratentorial location, germline SMARCB1 mutations, and metastases. However, neither germline SMARCB1 mutation nor metastases portended worse outcome in ACNS0333, the largest prospective trial. With the exception of spinal location (almost exclusively MYC subgroup), there is no consistent correlation of any other clinical prognostic factors with ATRT molecular subgroups, thus an integrated risk model may most precisely inform therapeutic approaches to ATRTs. Based on their initial small cohort study, Torchia et al proposed that neurogenic signatures indicated by high expression of achaete-scute homolog 1 and correlated with Group1/SHH ATRTs may represent a favorable prognostic factor and proposed a risk-stratification model for ATRTs which integrates clinical and molecular prognostic factors.16 Notably, their data suggest that RT may be spared for a subset of patients with lower risk disease. As much of their outcome data were collected over a period of change in treatment approaches from conventional chemotherapy to HDC regimens, the impact of ATRT subtypes on therapeutic outcomes needs to be validated in uniformly treated prospective cohorts. In contrast, Fruhwald et al recently reported biological subclassification of tumors from 84 patients who, while not treated on a prospective clinical trial, were treated more uniformly as per the EU-RHAB registry protocol and in multivariate analyses defined age <1 year and SHH or MYC subgroup designation as independently negative prognosticators of OS.15 Such discrepancies highlight the need for international cooperation to collate prospective obtained clinical and biological data to develop an integrative model to stratify choice of chemotherapy and/or RT in addition to selecting appropriate subtyped tailored biologic agents.
Ultimately, it will be critical to incorporate rapid, reproducible, clinically certified molecular subgrouping tools, similar to those now implemented for medulloblastoma,99 in future trials to fully evaluate the impact of disease biology on clinical outcomes. Global platforms such as RNASeq and DNA methylation arrays robustly demarcate molecular differences but may not be readily available in the clinical setting in most institutions. Hence, development of alternate, clinically certified, cost-effective methods, such as RNA-based NanoString and/or immunohistochemistry, is imperative for developing global clinical trials. Similarly, comparison and harmonization of preclinical reagents and models, and reevaluation of previously tested ATRT drugs in subtype-specific models will be important to inform future trial design. Validation of subtyping tools and studies of molecular subgroup correlation with clinical prognostic factors in a prospective clinical cohort will also be a critical next step. The recent establishment of a consensus on molecular grouping and nomenclature represents a first important step for advancing medical and scientific discussion and integration of molecular subgrouping into treatment planning for ATRTs.
Discovery of biological heterogeneity has tremendous potential to shape future risk and treatment stratification for patients with ATRT. However, the promise of biology-based therapies for patients with ATRT is tempered by the present lack of consensus regarding the best therapeutic backbone. Conflicting data surrounding treatment modalities, including HDC/SCR, RT, and IT chemotherapy, have yielded widely varied therapeutic practices, which has proven challenging for global collaborations for the next generation of ATRT trials. How chemotherapy backbone and/or tumor biology influences the requirement, timing, dose, and field of RT in ATRT therapy will be important to address in future prospective studies. Ultimately for this primarily infant disease, identification of patients for whom a combination of biologically targeted agents can delay, reduce, or completely abolish the need for intensive chemotherapy and RT is essential for not only advancing cure but also improving quality of survival for these very young patients. Treatment modalities will have an impact on risk of second cancers100 and will inform surveillance protocols for survivors with RTPS.101 As long-term ATRT survivors have been a relatively recent phenomenon, neurocognitive outcomes for only a small number of ATRT patients27 have been reported. Thus, measures of quality of life, neurocognitive, and functional outcomes of ATRT survivors will be important to incorporate in future clinical trials to evaluate the contribution of various treatment modalities to cognitive outcome in these very young survivors.
Summary
Currently, ATRT remains a highly lethal disease where maximum intensity chemoradiotherapeutic regimens have produced promising albeit modest gains in survival. The discovery of molecular heterogeneity in ATRTs has provided a much-needed advance. Together with integrated risk stratification, development of sensitive prognostic biomarkers and a wider spectrum of genetic therapeutic models will be needed to enable precise titration of treatment toxicity and efficacy for the very young ATRT population. Additionally, greater elucidation of the relevance of germline predisposition, a risk that should prompt genetic counseling for all children with ATRT, and genotype-phenotype correlations in those with RTPS will be important.
The field and interest in clinical and basic science studies of ATRT have grown substantially for this once neglected orphan disease. This has been underscored by the scope of a 2018 international ATRT meeting which also enabled harmonization of nomenclature and cross-fertilized clinical and scientific interest in this disease and bodes well for the future of ATRT patients.
Funding
This project was supported by a Canadian Cancer Society Research Institute Impact grant (#705056) to AH. Authors report no conflicts of interest.
Footnotes
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Schneppenheim R, Frühwald MC, Gesk S, et al. . Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet. 2010;86(2):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haberler C, Laggner U, Slavc I, et al. . Immunohistochemical analysis of INI1 protein in malignant pediatric CNS tumors: lack of INI1 in atypical teratoid/rhabdoid tumors and in a fraction of primitive neuroectodermal tumors without rhabdoid phenotype. Am J Surg Pathol. 2006;30(11):1462–1468. [DOI] [PubMed] [Google Scholar]
- 4. Hasselblatt M, Nagel I, Oyen F, et al. . SMARCA4-mutated atypical teratoid/rhabdoid tumors are associated with inherited germline alterations and poor prognosis. Acta Neuropathol. 2014;128(3):453–456. [DOI] [PubMed] [Google Scholar]
- 5. Woehrer A, Slavc I, Waldhoer T, et al. . Incidence of atypical teratoid/rhabdoid tumors in children: a population-based study by the Austrian Brain Tumor Registry, 1996–2006. Cancer. 2010;116(24):5725–5732. [DOI] [PubMed] [Google Scholar]
- 6. Bourdeaut F, Lequin D, Brugières L, et al. . Frequent hSNF5/INI1 germline mutations in patients with rhabdoid tumor. Clin Cancer Res. 2011;17(1):31–38. [DOI] [PubMed] [Google Scholar]
- 7. Hilden JM, Meerbaum S, Burger P, et al. . Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol. 2004;22(14):2877–2884. [DOI] [PubMed] [Google Scholar]
- 8. Lafay-Cousin L, Hawkins C, Carret AS, et al. . Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer. 2012;48(3):353–359. [DOI] [PubMed] [Google Scholar]
- 9. Dufour C, Beaugrand A, Le Deley MC, et al. . Clinicopathologic prognostic factors in childhood atypical teratoid and rhabdoid tumor of the central nervous system: a multicenter study. Cancer. 2012;118(15):3812–3821. [DOI] [PubMed] [Google Scholar]
- 10. Tekautz TM, Fuller CE, Blaney S, et al. . Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol. 2005;23(7):1491–1499. [DOI] [PubMed] [Google Scholar]
- 11. Chi SN, Zimmerman MA, Yao X, et al. . Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27(3):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoff von K, Hinkes B, Dannenmann-Stern E, et al. . Frequency, risk-factors and survival of children with atypical teratoid rhabdoid tumors (AT/RT) of the CNS diagnosed between 1988 and 2004, and registered to the German HIT database. Pediatr Blood Cancer. 2011;57(6):978–985. [DOI] [PubMed] [Google Scholar]
- 13. Buscariollo DL, Park HS, Roberts KB, Yu JB. Survival outcomes in atypical teratoid rhabdoid tumor for patients undergoing radiotherapy in a Surveillance, Epidemiology, and End Results analysis. Cancer. 2012;118(17):4212–4219. [DOI] [PubMed] [Google Scholar]
- 14. Slavc I, Chocholous M, Leiss U, et al. . Atypical teratoid rhabdoid tumor: improved long-term survival with an intensive multimodal therapy and delayed radiotherapy. The Medical University of Vienna Experience 1992–2012. Cancer Med .2014;3(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frühwald MC, Hasselblatt M, Nemes K, et al. . Age and DNA-methylation subgroup as potential independent risk factors for treatment stratification in children with atypical teratoid/rhabdoid tumors. Neuro Oncol. 2020;22(7):1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Torchia J, Picard D, Lafay-Cousin L, et al. . Molecular subgroups of atypical teratoid rhabdoid tumours in children: an integrated genomic and clinicopathological analysis. Lancet Oncol. 2015;16(5):569–582. [DOI] [PubMed] [Google Scholar]
- 17. Bartelheim K, Nemes K, Seeringer A, et al. . Improved 6-year overall survival in AT/RT—results of the registry study Rhabdoid 2007. Cancer Med. 2016;5(8):1765–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torchia J, Golbourn B, Feng S, et al. . Integrated (epi)-genomic analyses identify subgroup-specific therapeutic targets in CNS rhabdoid tumors. Cancer Cell. 2016;30(6):891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johann PD, Erkek S, Zapatka M, et al. . Atypical teratoid/rhabdoid tumors are comprised of three epigenetic subgroups with distinct enhancer landscapes. Cancer Cell. 2016;29(3):379–393. [DOI] [PubMed] [Google Scholar]
- 20. Kieran MW, Roberts CW, Chi SN, et al. . Absence of oncogenic canonical pathway mutations in aggressive pediatric rhabdoid tumors. Pediatr Blood Cancer. 2012;59(7):1155–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee RS, Stewart C, Carter SL, et al. . A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest. 2012;122(8):2983–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasselblatt M, Isken S, Linge A, et al. . High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes Chromosomes Cancer. 2013;52(2):185–190. [DOI] [PubMed] [Google Scholar]
- 23. Tegeder I, Thiel K, Erkek S, et al. . Functional relevance of genes predicted to be affected by epigenetic alterations in atypical teratoid/rhabdoid tumors. J Neurooncol. 2019;141(1):43–55. [DOI] [PubMed] [Google Scholar]
- 24. Ho B, Johann PD, Grabovska Y, et al. . Molecular subgrouping of atypical teratoid/rhabdoid tumors—a reinvestigation and current consensus. Neuro Oncol. 2020;22(5):613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henssen AG, Koche R, Zhuang J, et al. . PGBD5 promotes site-specific oncogenic mutations in human tumors. Nat Genet. 2017;49(7):1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henssen AG, Reed C, Jiang E, et al. . Therapeutic targeting of PGBD5-induced DNA repair dependency in pediatric solid tumors. Sci Transl Med. 2017;9(414). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lafay-Cousin L, Fay-McClymont T, Johnston D, et al. . Neurocognitive evaluation of long term survivors of atypical teratoid rhabdoid tumors (ATRT): the Canadian registry experience. Pediatr Blood Cancer. 2015;62(7):1265–1269. [DOI] [PubMed] [Google Scholar]
- 28. Geyer JR, Sposto R, Jennings M, et al. ; Children’s Cancer Group Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol. 2005;23(30):7621–7631. [DOI] [PubMed] [Google Scholar]
- 29. Ginn KF, Gajjar A. Atypical teratoid rhabdoid tumor: current therapy and future directions. Front Oncol. 2012;2:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gardner SL, Asgharzadeh S, Green A, Horn B, McCowage G, Finlay J. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008;51(2):235–240. [DOI] [PubMed] [Google Scholar]
- 31. Fidani P, De Ioris MA, Serra A, et al. . A multimodal strategy based on surgery, radiotherapy, ICE regimen and high dose chemotherapy in atypical teratoid/rhabdoid tumours: a single institution experience. J Neurooncol. 2009;92(2):177–183. [DOI] [PubMed] [Google Scholar]
- 32. Park ES, Sung KW, Baek HJ, et al. . Tandem high-dose chemotherapy and autologous stem cell transplantation in young children with atypical teratoid/rhabdoid tumor of the central nervous system. J Korean Med Sci. 2012;27(2):135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zaky W, Dhall G, Ji L, et al. . Intensive induction chemotherapy followed by myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for young children newly-diagnosed with central nervous system atypical teratoid/rhabdoid tumors: the Head Start III experience. Pediatr Blood Cancer. 2014;61(1):95–101. [DOI] [PubMed] [Google Scholar]
- 34. Chen YW, Wong TT, Ho DM, et al. . Impact of radiotherapy for pediatric CNS atypical teratoid/rhabdoid tumor (single institute experience). Int J Radiat Oncol Biol Phys. 2006;64(4):1038–1043. [DOI] [PubMed] [Google Scholar]
- 35. Pai Panandiker AS, Merchant TE, Beltran C, et al. . Sequencing of local therapy affects the pattern of treatment failure and survival in children with atypical teratoid rhabdoid tumors of the central nervous system. Int J Radiat Oncol Biol Phys. 2012;82(5):1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benesch M, Bartelheim K, Fleischhack G, et al. . High-dose chemotherapy (HDCT) with auto-SCT in children with atypical teratoid/rhabdoid tumors (AT/RT): a report from the European Rhabdoid Registry (EU-RHAB). Bone Marrow Transplant. 2014;49(3):370–375. [DOI] [PubMed] [Google Scholar]
- 37. Yang WC, Yen HJ, Liang ML, et al. . Role of early and aggressive post-operative radiation therapy in improving outcome for pediatric central nervous system atypical teratoid/rhabdoid tumor. Childs Nerv Syst. 2019;35(6):1013–1020. [DOI] [PubMed] [Google Scholar]
- 38. Sung KW, Lim DH, Yi ES, et al. . Tandem high-dose chemotherapy and autologous stem cell transplantation for atypical teratoid/rhabdoid tumor. Cancer Res Treat. 2016;48(4):1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reddy AT, Strother DR, Judkins AR, et al. . Efficacy of high-dose chemotherapy and three-dimensional conformal radiation for atypical teratoid/rhabdoid tumor: a report from the Children’s Oncology Group Trial ACNS0333. J Clin Oncol. 2020;38(11):1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Athale UH, Duckworth J, Odame I, Barr R. Childhood atypical teratoid rhabdoid tumor of the central nervous system: a meta-analysis of observational studies. J Pediatr Hematol Oncol. 2009;31(9):651–663. [DOI] [PubMed] [Google Scholar]
- 41. Nakayama RT, Pulice JL, Valencia AM, et al. . SMARCB1 is required for widespread BAF complex-mediated activation of enhancers and bivalent promoters. Nat Genet. 2017;49(11):1613–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim KH, Roberts CW. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genet. 2014;207(9):365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wilson BG, Wang X, Shen X, et al. . Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell. 2010;18(4):316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang X, Lee RS, Alver BH, et al. . SMARCB1-mediated SWI/SNF complex function is essential for enhancer regulation. Nat Genet. 2017;49(2):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463(7280):474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Erkek S, Johann PD, Finetti MA, et al. . Comprehensive analysis of chromatin states in atypical teratoid/rhabdoid tumor identifies diverging roles for SWI/SNF and polycomb in gene regulation. Cancer Cell. 2019;35(1):95–110.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang X, Sansam CG, Thom CS, et al. . Oncogenesis caused by loss of the SNF5 tumor suppressor is dependent on activity of BRG1, the ATPase of the SWI/SNF chromatin remodeling complex. Cancer Res. 2009;69(20):8094–8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Helming KC, Wang X, Roberts CWM. Vulnerabilities of mutant SWI/SNF complexes in cancer. Cancer Cell. 2014;26(3):309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hashizume R, Zhang A, Mueller S, et al. . Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol. 2016;18(11):1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Frühwald MC, Biegel JA, Bourdeaut F, Roberts CW, Chi SN. Atypical teratoid/rhabdoid tumors-current concepts, advances in biology, and potential future therapies. Neuro Oncol. 2016;18(6):764–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oberlick EM, Rees MG, Seashore-Ludlow B, et al. . Small-Molecule and CRISPR screening converge to reveal receptor tyrosine kinase dependencies in pediatric rhabdoid tumors. Cell Rep. 2019;28(9):2331–2344.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Muscat A, Popovski D, Jayasekara WS, et al. . Low-dose histone deacetylase inhibitor treatment leads to tumor growth arrest and multi-lineage differentiation of malignant rhabdoid tumors. Clin Cancer Res. 2016;22(14):3560–3570. [DOI] [PubMed] [Google Scholar]
- 53. Leruste A, Tosello J, Ramos RN, et al. . Clonally expanded T cells reveal immunogenicity of rhabdoid tumors. Cancer Cell. 2019;36:597–612.e8. [DOI] [PubMed] [Google Scholar]
- 54. Lünenbürger H, Lanvers-Kaminsky C, Lechtape B, Frühwald MC. Systematic analysis of the antiproliferative effects of novel and standard anticancer agents in rhabdoid tumor cell lines. Anticancer Drugs. 2010;21(5):514–522. [DOI] [PubMed] [Google Scholar]
- 55. Choi SA, Choi JW, Wang KC, et al. . Disulfiram modulates stemness and metabolism of brain tumor initiating cells in atypical teratoid/rhabdoid tumors. Neuro Oncol. 2015;17(6):810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gilman AL, Jacobsen C, Bunin N, et al. . Phase I study of tandem high-dose chemotherapy with autologous peripheral blood stem cell rescue for children with recurrent brain tumors: a Pediatric Blood and MarrowTransplant Consortium study. Pediatr Blood Cancer. 2011;57(3):506–513. [DOI] [PubMed] [Google Scholar]
- 57. Biswas A, Julka PK, Bakhshi S, Suri A, Rath GK. Intracranial atypical teratoid rhabdoid tumor: current management and a single institute experience of 15 patients from north India. Acta Neurochir (Wien). 2015;157(4):589–596. [DOI] [PubMed] [Google Scholar]
- 58. Akyüz C, Demir HA, Varan A, Yalçin B, Kutluk T, Büyükpamukçu M. Temozolomide in relapsed pediatric brain tumors: 14 cases from a single center. Childs Nerv Syst. 2012;28(1):111–115. [DOI] [PubMed] [Google Scholar]
- 59. Wang CH, Hsu TR, Wong TT, Chang KP. Efficacy of temozolomide for recurrent embryonal brain tumors in children. Childs Nerv Syst. 2009;25(5):535–541. [DOI] [PubMed] [Google Scholar]
- 60. Sandberg DI, Rytting M, Zaky W, et al. . Methotrexate administration directly into the fourth ventricle in children with malignant fourth ventricular brain tumors: a pilot clinical trial. J Neurooncol. 2015;125(1):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Casaos J, Huq S, Lott T, et al. . Ribavirin as a potential therapeutic for atypical teratoid/rhabdoid tumors. Oncotarget. 2018;9(8):8054–8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Unland R, Borchardt C, Clemens D, Kool M, Dirksen U, Frühwald MC. Analysis of the antiproliferative effects of 3-deazaneoplanocin A in combination with standard anticancer agents in rhabdoid tumor cell lines. Anticancer Drugs. 2015;26(3):301–311. [DOI] [PubMed] [Google Scholar]
- 63. McGregor LM, Spunt SL, Santana VM, et al. . Phase 1 study of an oxaliplatin and etoposide regimen in pediatric patients with recurrent solid tumors. Cancer. 2009;115(3):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fouladi M, Blaney SM, Poussaint TY, et al. . Phase II study of oxaliplatin in children with recurrent or refractory medulloblastoma, supratentorial primitive neuroectodermal tumors, and atypical teratoid rhabdoid tumors: a pediatric brain tumor consortium study. Cancer. 2006;107(9):2291–2297. [DOI] [PubMed] [Google Scholar]
- 65. Jayanthan A, Bernoux D, Bose P, Riabowol K, Narendran A. Multi-tyrosine kinase inhibitors in preclinical studies for pediatric CNS AT/RT: evidence for synergy with topoisomerase-I inhibition. Cancer Cell Int. 2011;11(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Blaney S, Berg SL, Pratt C, et al. . A phase I study of irinotecan in pediatric patients: a pediatric oncology group study. Clin Cancer Res. 2001;7(1):32–37. [PubMed] [Google Scholar]
- 67. Li T, Wang J, Liu P, et al. . Insulin-like growth factor 2 axis supports the serum-independent growth of malignant rhabdoid tumor and is activated by microenvironment stress. Oncotarget. 2017;8(29):47269–47283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jeibmann A, Schulz J, Eikmeier K, et al. . SMAD dependent signaling plays a detrimental role in a fly model of SMARCB1-deficiency and the biology of atypical teratoid/rhabdoid tumors. J Neurooncol. 2017;131(3):477–484. [DOI] [PubMed] [Google Scholar]
- 69. Venkataraman S, Alimova I, Tello T, et al. . Targeting aurora kinase A enhances radiation sensitivity of atypical teratoid rhabdoid tumor cells. J Neurooncol. 2012;107(3):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maris JM, Morton CL, Gorlick R, et al. . Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr Blood Cancer. 2010;55(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wetmore C, Boyett J, Li S, et al. . Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol. 2015;17(6):882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Suri A, Bailey AW, Tavares MT, et al. . Evaluation of protein kinase inhibitors with PLK4 cross-over potential in a pre-clinical model of cancer. Int J Mol Sci. 2019;20(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Singh A, Lun X, Jayanthan A, et al. . Profiling pathway-specific novel therapeutics in preclinical assessment for central nervous system atypical teratoid rhabdoid tumors (CNS ATRT): favorable activity of targeting EGFR- ErbB2 signaling with lapatinib. Mol Oncol. 2013;7(3):497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Weingart MF, Roth JJ, Hutt-Cabezas M, et al. . Disrupting LIN28 in atypical teratoid rhabdoid tumors reveals the importance of the mitogen activated protein kinase pathway as a therapeutic target. Oncotarget. 2015;6(5):3165–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rubens JA, Wang SZ, Price A, et al. . The TORC1/2 inhibitor TAK228 sensitizes atypical teratoid rhabdoid tumors to cisplatin-induced cytotoxicity. Neuro Oncol. 2017;19(10):1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kolb EA, Gorlick R, Houghton PJ, et al. . Initial testing of dasatinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(6):1198–1206. [DOI] [PubMed] [Google Scholar]
- 77. Wong JP, Todd JR, Finetti MA, et al. . Dual targeting of PDGFRα and FGFR1 displays synergistic efficacy in malignant rhabdoid tumors. Cell Rep. 2016;17(5):1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Alimova I, Pierce AM, Harris P, et al. . Targeting polo-like kinase 1 in SMARCB1 deleted atypical teratoid rhabdoid tumor. Oncotarget. 2017;8(57):97290–97303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sredni ST, Suzuki M, Yang JP, et al. . A functional screening of the kinome identifies the polo-like kinase 4 as a potential therapeutic target for malignant rhabdoid tumors, and possibly, other embryonal tumors of the brain. Pediatr Blood Cancer. 2017;64(11). [DOI] [PubMed] [Google Scholar]
- 80. Sredni ST, Bailey AW, Suri A, et al. . Inhibition of polo-like kinase 4 (PLK4): a new therapeutic option for rhabdoid tumors and pediatric medulloblastoma. Oncotarget. 2017;8(67):111190–111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Messerli SM, Hoffman MM, Gnimpieba EZ, Bhardwaj RD. Therapeutic targeting of PTK7 is cytotoxic in atypical teratoid rhabdoid tumors. Mol Cancer Res. 2017;15(8):973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Geoerger B, Bourdeaut F, DuBois SG, et al. . A phase I study of the CDK4/6 inhibitor ribociclib (LEE011) in pediatric patients with malignant rhabdoid tumors, neuroblastoma, and other solid tumors. Clin Cancer Res. 2017;23(10):2433–2441. [DOI] [PubMed] [Google Scholar]
- 83. Tang Y, Gholamin S, Schubert S, et al. . Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nat Med. 2014;20(7):732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alimova I, Pierce A, Danis E, et al. . Inhibition of MYC attenuates tumor cell self-renewal and promotes senescence in SMARCB1-deficient Group 2 atypical teratoid rhabdoid tumors to suppress tumor growth in vivo. Int J Cancer. 2019;144(8):1983–1995. [DOI] [PubMed] [Google Scholar]
- 85. Kramer KF, Moreno N, Fruhwald MC, Kerl K. BRD9 Inhibition, alone or in combination with cytostatic compounds as a therapeutic approach in rhabdoid tumors. Int J Mol Sci. 2017;18(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Alimova I, Birks DK, Harris PS, et al. . Inhibition of EZH2 suppresses self-renewal and induces radiation sensitivity in atypical rhabdoid teratoid tumor cells. Neuro Oncol. 2013;15(2):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kurmasheva RT, Sammons M, Favours E, et al. . Initial testing (stage 1) of tazemetostat (EPZ-6438), a novel EZH2 inhibitor, by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2017;64(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Knipstein JA, Birks DK, Donson AM, Alimova I, Foreman NK, Vibhakar R. Histone deacetylase inhibition decreases proliferation and potentiates the effect of ionizing radiation in atypical teratoid/rhabdoid tumor cells. Neuro Oncol. 2012;14(2):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fouladi M, Park JR, Stewart CF, et al. . Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children’s Oncology Group phase I consortium report. J Clin Oncol. 2010;28(22):3623–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kerl K, Ries D, Unland R, et al. . The histone deacetylase inhibitor SAHA acts in synergism with fenretinide and doxorubicin to control growth of rhabdoid tumor cells. BMC Cancer. 2013;13:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Furchert SE, Lanvers-Kaminsky C, Juürgens H, Jung M, Loidl A, Frühwald MC. Inhibitors of histone deacetylases as potential therapeutic tools for high-risk embryonal tumors of the nervous system of childhood. Int J Cancer. 2007;120(8):1787–1794. [DOI] [PubMed] [Google Scholar]
- 92. Su JM, Li XN, Thompson P, et al. . Phase 1 study of valproic acid in pediatric patients with refractory solid or CNS tumors: a Children’s Oncology Group report. Clin Cancer Res. 2011;17(3):589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chakravadhanula M, Hampton CN, Chodavadia P, et al. . Wnt pathway in atypical teratoid rhabdoid tumors. Neuro Oncol. 2015;17(4): 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Studebaker AW, Hutzen B, Pierson CR, Shaffer TA, Raffel C, Jackson EM. Oncolytic measles virus efficacy in murine xenograft models of atypical teratoid rhabdoid tumors. Neuro Oncol. 2015;17(12):1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Golan H, Shukrun R, Caspi R, et al. . In vivo expansion of cancer stemness affords novel cancer stem cell targets: malignant rhabdoid tumor as an example. Stem Cell Reports. 2018;11(3):795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yang YP, Nguyen PNN, Ma HI, et al. . Tumor mesenchymal stromal cells regulate cell migration of atypical teratoid rhabdoid tumor through exosome-mediated miR155/SMARCA4 pathway. Cancers (Basel). 2019;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Howard TP, Arnoff TE, Song MR, et al. . MDM2 and MDM4 are therapeutic vulnerabilities in malignant rhabdoid tumors. Cancer Res. 2019;79(9):2404–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chauvin C, Leruste A, Tauziede-Espariat A, et al. . High-throughput drug screening identifies pazopanib and clofilium tosylate as promising treatments for malignant rhabdoid tumors. Cell Rep. 2017;21(7):1737–1745. [DOI] [PubMed] [Google Scholar]
- 99. Northcott PA, Shih DJ, Remke M, et al. . Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bhatt MD, Al-Karmi S, Druker H, et al. . Second rhabdoid tumor 8 years after treatment of atypical teratoid/rhabdoid tumor in a child with germline SMARCB1 mutation. Pediatr Blood Cancer. 2019;66(3):e27546. [DOI] [PubMed] [Google Scholar]
- 101. Foulkes WD, Kamihara J, Evans DGR, et al. . Cancer surveillance in gorlin syndrome and rhabdoid tumor predisposition syndrome. Clin Cancer Res. 2017;23(12):e62–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]