Abstract
Background
Controversy exists as to what may be defined as standard of care (including markers for stratification) for patients with atypical teratoid/rhabdoid tumors (ATRTs). The European Rhabdoid Registry (EU-RHAB) recruits uniformly treated patients and offers standardized genetic and DNA methylation analyses.
Methods
Clinical, genetic, and treatment data of 143 patients from 13 European countries were analyzed (2009–2017). Therapy consisted of surgery, anthracycline-based induction, and either radiotherapy or high dose chemotherapy following a consensus among European experts. Fluorescence in situ hybridization, multiplex ligation-dependent probe amplification, and sequencing were employed for assessment of somatic and germline mutations in SWItch/sucrose nonfermentable related, matrix associated, actin dependent regulator of chromatin, subfamily B (SMARCB1). Molecular subgroups (ATRT-SHH, ATRT-TYR, and ATRT-MYC) were determined using DNA methylation arrays, resulting in profiles of 84 tumors.
Results
Median age at diagnosis of 67 girls and 76 boys was 29.5 months. Five-year overall survival (OS) and event-free survival (EFS) were 34.7 ± 4.5% and 30.5 ± 4.2%, respectively. Tumors displayed allelic partial/whole gene deletions (66%; 122/186 alleles) or single nucleotide variants (34%; 64/186 alleles) of SMARCB1. Germline mutations were detected in 26% of ATRTs (30/117). The patient cohort consisted of 47% ATRT-SHH (39/84), 33% ATRT-TYR (28/84), and 20% ATRT-MYC (17/84). Age <1 year, non-TYR signature (ATRT-SHH or -MYC), metastatic or synchronous tumors, germline mutation, incomplete remission, and omission of radiotherapy were negative prognostic factors in univariate analyses (P < 0.05). An adjusted multivariate model identified age <1 year and a non-TYR signature as independent negative predictors of OS: high risk (<1 y + non-TYR; 5-y OS = 0%), intermediate risk (<1 y + ATRT-TYR or ≥1 y + non-TYR; 5-y OS = 32.5 ± 8.7%), and standard risk (≥1 y + ATRT-TYR, 5-y OS = 71.5 ± 12.2%).
Conclusions
Age and molecular subgroup status are independent risk factors for survival in children with ATRT. Our model warrants validation within future clinical trials.
Keywords: ATRT, DNA methylation profiling, European Rhabdoid Tumor Registry, prognosis, SMARCB1
Key Points.
Non-TYR DNA-methylation signature and age <1 year are independent risk factors in ATRT.
Patients <1 year with a non-TYR signature have a significantly poorer prognosis (5-y OS 0%) compared with those above 1 year and those with a TYR signature.
Patients with an ATRT-TYR signature and age ≥1 year have the best prognosis among the proposed risk groups (5-y OS 71.5 ± 12.2%).
Importance of the Study.
For patients suffering from ATRT, no validated prognostic markers are currently known. Data of 143 uniformly treated patients from 13 countries involved with the EU-RHAB registry suggest that young age (<1 y vs ≥1y) and DNA methylation subgroup status (ATRT-TYR vs non-TYR) are independent predictors of OS. Patients with an ATRT-TYR signature, age ≥1 year, have the best prognosis (5-y OS 71.5 ± 12.2%), while patients with a non-TYR signature and age <1 year have the worst prognosis (5-y OS 0%). All other patients are at intermediate risk (5-y OS 32.5 ± 8.7%). This risk model has potential as a treatment stratification tool in the context of future clinical trials. It deserves further validation in independent cohorts.
Atypical teratoid/rhabdoid tumors (ATRTs) are aggressive malignancies of the central nervous system (CNS) affecting mainly children below 3 years of age. Defining genetic lesions are inactivating mutations of SWItch/sucrose nonfermentable related, matrix associated, actin dependent regulator of chromatin, subfamily B (SMARCB1)1–3 or (rarely) SMARCA4.4 ATRTs exhibit a tendency for large and invasive tumors, metastatic spread, and chemotherapy resistance.5 Reported 5-year overall survival (OS) rates between 15% and 50% remain unsatisfactory, even if improvement has been documented in recent years.6–9 Young age, incomplete resection, metastatic disease, high-dose chemotherapy (HDCT) or radiotherapy (RT), and the presence of SMARCB1/SMARCA4 germline mutations have been suggested to be prognostic.5,10–14 Despite negative prognosticators, patients with prolonged survival times have been reported.6,9,10,15
While recurrent genetic alterations explaining clinical heterogeneity have not been identified,1–3 DNA methylation and expression profiling studies by different research groups have uncovered 3 distinct molecular subgroups: ATRT–sonic hedgehog (SHH), corresponding to Group 1; ATRT-tyrosinase (TYR)/Group 2A; and ATRT-MYC/Group 2B.16,17 These subgroups display not only distinct DNA methylation profiles, gene expression signatures, and differences in SMARCB1 mutation patterns, but also characteristic clinical features, including patients’ age, tumor location, and findings on neuroradiological imaging.16–20
Using data from a well-defined cohort of patients recruited to the EU-RHAB registry, we explored whether clinical or molecular factors may identify high-risk patients.
Materials and Methods
The EU-RHAB Registry
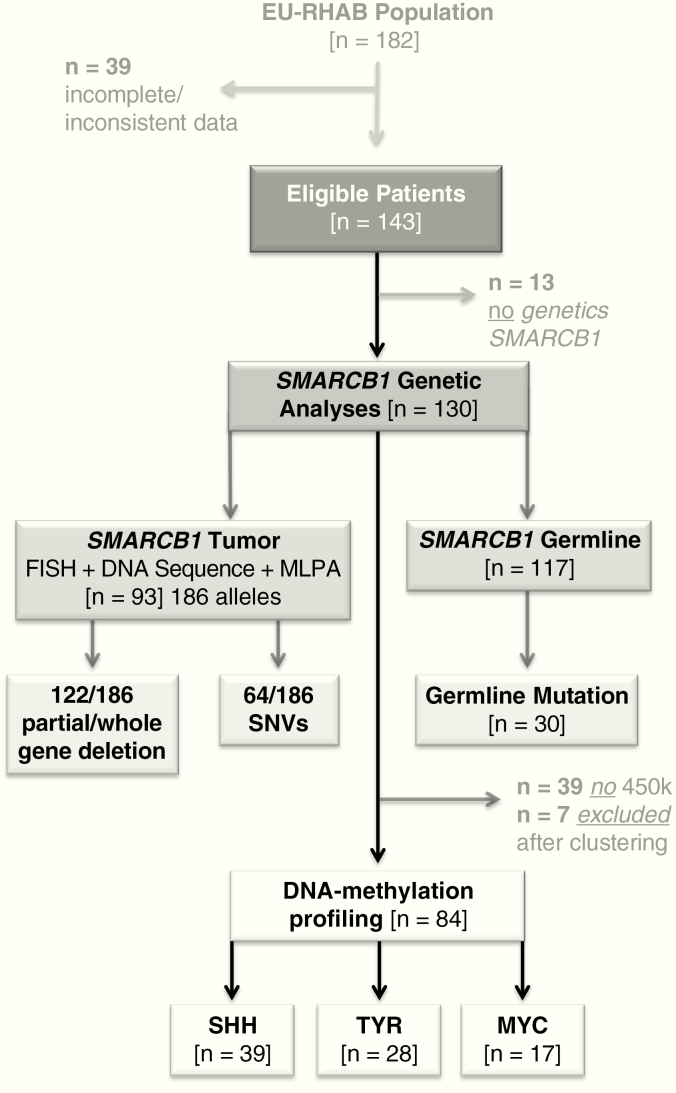
The European Rhabdoid Registry (EU-RHAB) was designed as a clinical registry including an expert consensus therapy recommendation (http://www.rhabdoid.de/downloads.html). EU-RHAB prospectively collects data on uniformly treated patients with rhabdoid tumors of all anatomic locations across participating European countries. A system of high-quality reference diagnostics and expert counseling for diagnostics and therapy (Supplementary Figure 1) is provided. Inclusion criteria are (i) diagnosis of ATRT according to World Health Organization (WHO) criteria confirmed by central neuropathology review, (ii) age below 18 years, and (iii) informed consent. EU-RHAB has received continuous approval by the ethics committee of the University of Münster (ID 2009–532-f-S, latest amendment 12/2016). Informed consent was obtained from all participating patients. Data collection follows a Case Report Form (CRF)–based approach including queries and collection of reference reports. Between June 2009 and July 2017 EU-RHAB contained 329 patient files. Out of 201 ATRTs, 19 had not been treated according to recommendations and 39 demonstrated either incomplete and/or inconsistent datasets or were still on treatment. A total of 143 patients were eligible for analyses (Fig. 1). For all 143 patients, completed and validated CRFs were available. Whenever inconsistencies were noted, source file data were requested and/or treating physicians contacted. Once inconsistencies could not be resolved, patients were excluded from analyses. The only SMARCA4-mutated case of the cohort was excluded from statistical analysis.
Fig. 1.
The ATRT cohort of the EU-RHAB registry. A total of 143 ATRTs were analyzed. In 130 cases, enough DNA was available for SMARCB1 mutation analyses. In 93 tumors (= 186 alleles), enough material was present for analyses by FISH; sequencing and MLPA germline information was obtained in 117 patients. A total of 84 samples could be subclassified by 450k DNA methylation arrays.
Validation Cohort
Data on an independent cohort of 69 patients (all with confirmed ATRT) with information on DNA methylation subgroup (classifier score >0.9), age at diagnosis, and OS (but incomplete information on treatment modalities) were retrieved from the archives of the Institute of Neuropathology, University Hospital Münster and the Department of Neuropathology, NN Burdenko Neurosurgical Institute Moscow (see Supplementary Table 1 and Supplementary Figure 2).
Diagnostic Measures
The central Neuropathology Reference Center in Münster, Germany (M.H., W.P.,) reviewed all tumors according to WHO criteria and routinely included immunohistochemistry for SMARCB1/integrase interactor 121 and SMARCA4/Brahma/SWI2-related gene 1 (BRG1). Neuroradiological imaging studies were reviewed centrally according to criteria of the German National Reference Center for Neuroradiology (M.W-M.).22 Fluorescence in situ hybridization (FISH), multiplex ligation-dependent probe amplification (MLPA), and sequencing of SMARCB1/SMARCA4 were performed at reference institutions in Kiel and Ulm (R.Si., until/after 2016) and Hamburg, Germany (R.Sch., U.K.) according to standard protocols (Supplementary Methods 1).23,24
Toxicity
Toxicity was assessed following the Common Terminology Criteria for Adverse Events v3.0. Reporting of serious adverse events was requested but not monitored.
DNA Methylation Subgrouping
According to an analysis including subgroup data from Heidelberg, Toronto, Newcastle, and Paris, ATRT-SHH tumors of the Heidelberg cohort correspond to Group 1 tumors of the Toronto group, while ATRT-TYR tumors match with Group 2A and ATRT-MYC with Group 2B (https://doi.org/10.1093/neuonc/noy059.010). Within this study we chose to employ the internationally acknowledged terms ATRT-SHH, ATRT-TYR, and ATRT-MYC. Molecular subgrouping was performed at the Institute of Neuropathology, University Hospital Münster in cooperation with Life & Brain (Bonn, Germany) or at the German Cancer Research Center Genomics and Proteomics Core Facility. For allocation of samples to the respective subgroups we used a stepwise procedure: We first considered the calibrated score as obtained from the recently published “Neuropath Classifier.” 25 To review the accuracy of subgrouping and to validate the consistency with the classifier predictions, we clustered the samples using confirmatory t-distributed stochastic neighbor embedding (t-SNE). Only samples with an unequivocal subgroup allocation in both methods were considered. The main output of these analyses was a classification score allowing for assignment to one of the subclasses ATRT-TYR, -SHH, and -MYC (for details, see Supplementary Methods 2).
Statistical Analyses
OS and event-free survival (EFS) were determined according to Kaplan–Meier estimates. OS was defined as the time from diagnosis until death of any cause or last visit. EFS was defined as the time from diagnosis until first progression, relapse, death of any cause, or last contact. Analysis of factors influencing OS and EFS was as follows: Kaplan–Meier analyses were performed for age, metastases, tumor location, extent of resection, germline mutation (GLM), HDCT, RT, maintenance therapy, achievement of a complete remission (CR), and relapse or progression. Time-dependent factors including RT, CR, and maintenance therapy were evaluated using Cox regression for time-dependent covariates. Multivariate Cox regression identified independent prognostic factors of OS. After model building and variable selection, the final model and corresponding results were confirmed in an independent validation cohort. P-values were regarded as significant for P ≤ 0.05. To evaluate the impact of DNA methylation subgroup on survival, a stepwise approach was taken. Within the multivariate model, individual groups were tested against each other and eventually also in an approach of one individual group versus all other groups combined.
Results
The EU-RHAB Cohort
The EU-RHAB cohort comprised 143 ATRT patients (76 boys and 67 girls) (Fig. 1). For all patients, diagnostic and therapeutic measures followed a specific protocol. Details can be found in detail at http://www.rhabdoid.de. At diagnosis, 35% (n = 50) of patients were younger than 1 year; 51% (n = 73) between 1 and 3 years; and 14% (n = 20) over 3 years. Tumors were located infratentorially in 60% of patients (n = 86), supratentorially in 37% (n = 53), and spinally in 2% (n = 3). One patient harbored a large tumor extending to both supra- and infratentorial regions. Metastatic disease at diagnosis was detected in 30% (n = 43) of patients. In 34% (n = 49) of children, a gross total resection (GTR) was achieved (Tables 1 and 2).
Table 1.
Clinical characteristics of 143 eligible patients with ATRT
| Total | % | |
|---|---|---|
| Median age, mo (range) | 29.5 (0–231) | |
| Age, y, at diagnosis | ||
| <12 | 50 | 35 |
| 12–36 | 73 | 51 |
| >36 | 20 | 14 |
| Origin | ||
| Germany | 110 | 77 |
| Other countries | 33 | 23 |
| Sex | ||
| Female | 67 | 47 |
| Male | 76 | 53 |
| Localization | ||
| Infratentorial | 86 | 60 |
| Cerebellum | 54 | |
| IVth ventricle | 18 | |
| Cerebellopontine angle | 2 | |
| Brainstem | 1 | |
| Mesencephalon | 5 | |
| Tectum mesencephalii | 2 | |
| Medulla oblongata | 4 | |
| Supratentorial | 53 | 37 |
| Hemisphere | 32 | |
| Lateral ventricle | 6 | |
| Basal ganglia | 4 | |
| Pineal gland | 5 | |
| Suprasellar area | 2 | |
| Thalamus | 1 | |
| Ist–IIIrd ventricle | 2 | |
| Hypothalamus | 1 | |
| Infra + supratentorial | 1 | 1 |
| IVth ventricle + lateral ventricle, IIIrd ventricle | 1 | |
| Spinal | 3 | 2 |
| Synchronous tumors | 9 | |
| eMRT | 5 | 56 |
| RTK | 3 | 33 |
| eMRT + RTK | 1 | 11 |
| Stage | ||
| M0 | 100 | 70 |
| M1 | 7 | 5 |
| M2 | 7 | 5 |
| M3 | 24 | 17 |
| M4 | 5 | 3 |
Abbreviations: eMRT, extracranial/extrarenal malignant rhabdoid tumor; RTK, rhabdoid tumor of the kidney.
Table 2.
Treatment details of 143 eligibile patients with ATRT
| Total | % | |
|---|---|---|
| Extent of surgical resection | ||
| Complete | 49 | 34 |
| Incomplete | 94 | 66 |
| HDCT | ||
| Yes | 34 | 24 |
| No | 109 | 76 |
| Completed chemotherapy according to EU-RHAB | ||
| Yes | 107 | 75 |
| No | 36 | 25 |
| Radiotherapy# | ||
| Yes | 81 | 87 |
| No | 12 | 13 |
| Complete remission | ||
| Yes | 76 | 53 |
| After surgery | 23 | |
| After chemotherapy | 53 | |
| No | 67 | 47 |
| Progression | ||
| No | 52 | 36.5 |
| PD on CT* | 49 | 34 |
| PD after CT** | 42 | 29.5 |
| SAE | n = 20 | |
| VOD§ | 11 | |
| CNS toxicities& | 5 | |
| Severe infection (pneumonia) | 1 | |
| AML*** | 3 | |
| Present status | ||
| CR | 44 | 31 |
| Stable disease | 10 | 7 |
| Progressive disease | 7 | 5 |
| Death | 82 | 57 |
Abbreviations: CR, complete remission; CT, chemotherapy; HDCT, high dose chemotherapy; PD, progressive disease; SAE, serious adverse event; VOD, veno-occlusive disease; AML, acute myeloid leukemia.
#Only patients >12 months at diagnosis (n = 93) were analyzed.
*During CT, analyzed within 4 months from diagnosis.
**After CT, <1 year from diagnosis.
§All VOD resolved.
&2 infections, 2 leukoencephalopathies, 1 central apnea.
***32, 23, and 53 months from diagnosis. Two of them died due to AML (one with SD of the ATRT and no GLM, the other with a GLM in SMARCB1 in CR of the ATRT). The third patient continues to be in CR following GTR for ATRT and is in first CR following chemotherapy for AML which was diagnosed 4 years after the diagnosis of ATRT.
110 patients were from German speaking countries (Germany, Austria, and Switzerland), the remainder (n = 33) from the Czech Republic, Denmark, Norway, Sweden, Hungary, Ireland, Italy, the Netherlands, Poland, Portugal, and Spain.
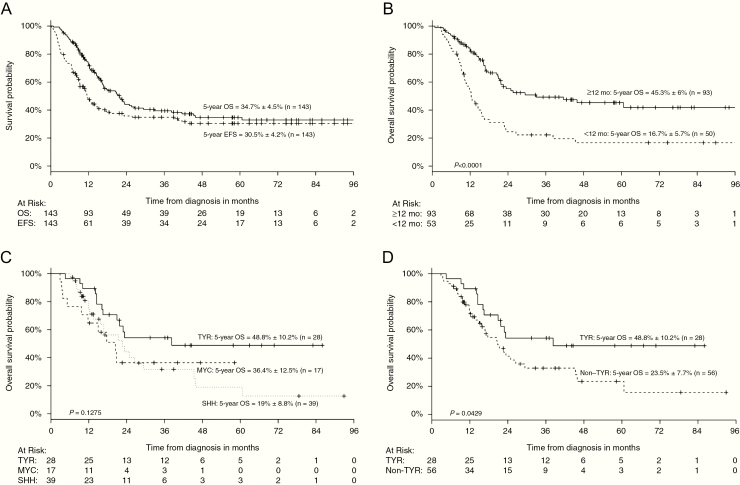
The OS and EFS estimates at 5 years were 34.7 ± 4.5% and 30.5 ± 4.2%, respectively (Fig. 2A). The median follow-up was 49.9 months (range, 4–104 mo). At the time of analyses, 57% (82/143) of patients had died. In total 64% (n = 91) of patients suffered from relapse or progression. In 75% (68/91), relapse occurred locally (21 relapses; 47 progressions), in 13% (n = 12) it was combined, and in 12% (n = 11) distant only (n = 1 extracerebral in lung and liver, all others within the CNS). No patient died due to toxicity. Essentially all evaluable patients (n = 109) demonstrated grade 3 or 4 hematologic toxicity at any time during therapy. A total of 20 severe adverse events (SAEs) were specified. Eleven of these were associated with veno-occlusive disease (VOD) (all of which resolved), and 5 were CNS toxicities (2 infections, 2 leukoencephalopathies, and 1 case of central apnea). Three cases of secondary acute myeloid leukemia (AML) were reported (23, 32, and 53 mo following diagnosis of ATRT). Two of these died due to the AML. One patient demonstrated a stable ATRT residue and no GLM, the other exhibited a GLM in SMARCB1 and died in CR of the ATRT. The third patient continues to be in CR for ATRT and AML following chemotherapy for both. All clinical, toxicity, and treatment variables are summarized in Tables 1 and 2 and Supplementary Table 2.
Fig. 2.
(A) Five-year survival (OS) of 143 consecutive patients treated according to the EU-RHAB consensus therapy. The 5-year overall survival (5y-OS) of the EU-RHAB cohort of 143 patients with ATRT was 34.7 ± 4.5% while the 5-year event-free survival (5y-EFS) of the same cohort was 30.5 ± 4.2%. OS was defined as the time from diagnosis until death of any cause or last visit. EFS was defined as the time from diagnosis until first progression, relapse, death of any cause, or last contact. (B) Age <1 year at diagnosis as an independent negative prognostic factor. The 5-year OS was 45.3 ± 6% for patients diagnosed after age 1 and 16.7 ± 5.7% for those <1 year at diagnosis. (C) Patients of the ATRT-TYR group demonstrate superior outcome compared with those of the ATRT-SHH and ATRT-MYC groups. The 5-year OS was superior in patients of the ATRT-TYR subgroup (48.8 ± 10.2%) versus 19 ± 8.8% for ATRT-SHH and final level not reached for the ATRT-MYC DNA-methylation subgroup (36.4 ± 12.5%). (D) Patients of the ATRT-TYR DNA-methylation subgroup have a significantly better prognosis compared with those of the non-TYR group. The 5-year OS was superior in patients of the ATRT-TYR subgroup (48.8 ± 10.2%) compared with those of the non-TYR subgroup (23.5 ± 7.7%).
Association of clinical factors with outcome
As radiotherapy was not recommended in patients below 1 year of age, we employed a rough distinction into 3 age groups: <1 year, 2–3 years, and >3 years. Age represented the most significant determinant of survival, with a 5-year OS of only 16.7 ± 5.7% for patients <1 year (n = 50) at diagnosis (Fig. 2B; 5-y OS above 12 mo 45.3 ± 6%, n = 93). Metastatic disease was also a significant prognostic factor, with only 16.9 ± 6.1% of M+ patients surviving 5 years or longer (M0, 5-y OS = 43 ± 5.7%, n = 100). Synchronicity of lesions, most commonly involving the CNS and the kidney, was associated with an inferior prognosis, with none of the 9 patients with synchronous tumors surviving (P < 0.05).
Influence of treatment modalities on outcome
A total of 75% (107/143) of patients completed chemotherapy and demonstrated a 5-year OS of 43.5 ± 5.5% versus 8.8 ± 5.4% for those who did not, mostly due to progressions (Table 2). Radiotherapy had a significant impact on OS (hazard ratio [HR] = 0.2, 95% CI: 0.06–0.6). These data have to be interpreted with caution, as age is a potential confounder despite the fact that we analyzed patients >12 months of age at RT only. Median age at RT was 40 months (range, 12–163 mo). No improvement in survival was seen for patients treated by HDCT. HDCT had no significant prognostic importance for OS (HR = 0.8, 95% CI: 0.5–1.4). Complete response to multimodal treatment had a significant influence on OS (HR = 0.3, 95% CI: 0.2–0.5). Finally, progressive disease on therapy and relapse were additional poor prognostic factors. Only 14% (7/49) of children with a lack of response or progression were alive 5 years following diagnosis (HR vs patients without progression = 242, 95% CI: 33–1800, P < 0.05). Only 5% (2/42) of patients with early relapse were alive 5 years ensuing diagnosis (HR vs patients without progression = 489, 95% CI: 64–3722, P < 0.05) compared with 98% (51/52) of patients without progression.
Spectrum of somatic tumor and germline SMARCB1/SMARCA4 mutations
Analyses of genetic alterations in SMARCB1 were available for 91% (130/143) (tumor and/or blood; Fig. 1). A total of 66% (122/186 alleles in 93 tumors with complete genetic information) of SMARCB1 alterations were structural variants (partial or whole gene deletions) and 34% (64/186 alleles) were single nucleotide variants (nonsense and frameshift mutations). With the exception of a single missense mutation, all alterations were truncating. SMARCB1 GLMs were detected in 26% (30/117) (Supplementary Table 3). A single tumor demonstrated loss of SMARCA4/BRG1. Only 13% (4/30) of patients with a GLM lived longer than 5 years (P < 0.05). In a multivariate model the factor GLM had no significant impact on outcome. No other significant associations between genetic alterations and clinical factors were detected.
DNA methylation subgroup status and outcome
DNA methylation profiling in 58% (84/143) of cases clearly categorized ATRT into one of the 3 described molecular subgroups, ie, 47% (n = 39) ATRT-SHH, 33% (n = 28) ATRT-TYR, and 20% (n = 17) ATRT-MYC. On t-SNE analysis, DNA methylation profiles formed 3 independent clusters (Supplementary Figure 2A). Patients with available DNA methylation profiling data did not differ significantly from the whole study population except for a higher percentage of patients with GTR (43% vs 22%). This, however, is an unavoidable bias as DNA methylation profiling can only be performed if there is sufficient material (ideally from a GTR). Supplementary Table 4 summarizes the clinical characteristics of patients according to molecular subgroup.
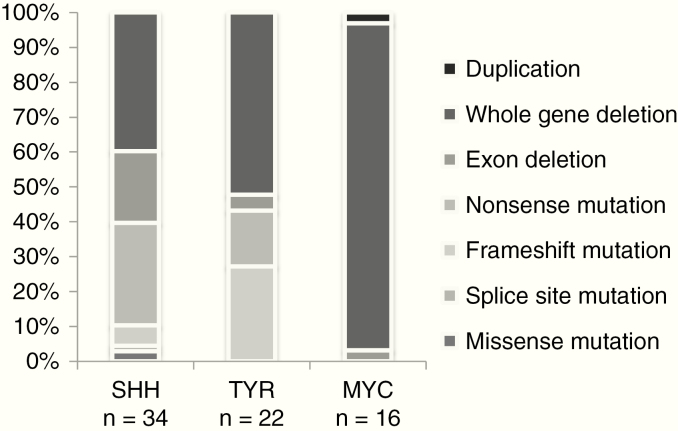
Patients with a germline mutation were more commonly detected in the DNA methylation subgroups ATRT-SHH (41%; 14/34) and ATRT-TYR (27%; 7/26). Only one patient of the ATRT-MYC subgroup demonstrated a germline mutation (7%; 1/15; ATRT-SHH vs ATRT-MYC: P < 0.05). As shown in Fig. 3, somatic mutations in SMARCB1 alleles differed among DNA methylation groups: somatic whole gene deletions were common in the ATRT-MYC subgroup and nonsense mutation in the ATRT-SHH subgroup (P < 0.05). Frameshift mutations were rare in the ATRT-SHH group and absent in ATRT-MYC. Interestingly, frameshift mutations represented 27% of alterations in the ATRT-TYR cohort (P < 0.05) (data derived from 72 tumor samples for which 450k and SMARCB1 DNA sequence data were available).
Fig. 3.
The genetic heterogeneity of SMARCB1 mutations in ATRT. The spectrum of SMARCB1 mutations in ATRT DNA-methylation subgroups among 72 patients is presented. Each column represents a DNA methylation subgroup as defined by a DNA methylation classifier. The x-axis gives the percentage of mutations detected in alleles in each subgroup. Whole gene deletions were rather common, followed in frequency by exon deletions and nonsense single nucleotide variations.
Patients whose tumors exhibited an ATRT-MYC signature were significantly older (median age 25.0, 7–136 mo, vs 12.5, 1–84 mo in ATRT-TYR and 16.0, 0–72 mo in ATRT-SHH; Supplementary Table 4) and tumors were more commonly located in the supratentorial compartment (ATRT-MYC: 82%, 14/17 vs ATRT-TYR: 11%, 3/28 and ATRT-SHH: 36%, 14/39; P < 0.05).
Progression on chemotherapy or relapse occurred frequently (34% and 30% responses out of 143 patients) without significant differences between the 3 subgroups (ATRT-MYC: 65%, ATRT-SHH: 67%, and ATRT-TYR: 57%). ATRT-TYR patients achieved a CR in 71% (Supplementary Table 4). Consistently, the 5-year OS was superior in the ATRT-TYR group (48.8 ± 10.2% vs 19 ± 8.8% ATRT-SHH and final level not reached for ATRT-MYC; Fig. 2C).
As the ATRT-TYR group appeared to be distinct from the other 2 groups in terms of survival, we summarized data for 2 strata (ATRT-TYR vs ATRT non-TYR = ATRT-SHH + ATRT-MYC). Median follow-up in the ATRT-TYR group was 44.6 months and 35.1 months in the non-TYR-group. Median OS in the non-TYR group was 20.8 months and 38.3 in the ATRT-TYR DNA-methylation subgroup (P < 0.05; Fig. 2D).
A Combined Clinical and Genetic Risk Model for the Stratification of ATRT
Clinical and genetic factors were included in a multivariate Cox regression model. The following candidate prognostic factors were considered: age at diagnosis <1 year versus ≥1 year, tumor location infratentorial (it)/supratentorial (st)/st + it/spinal, synchronous tumor (yes/no), metastases (yes/no), GTR, GLM, ATRT‐MYC versus -TYR versus -SHH, ATRT‐MYC versus non‐MYC, ATRT‐TYR versus non‐TYR, and ATRT‐SHH versus non‐SHH. All of these factors were included in a stepwise selection procedure. The procedure resulted in a final multivariate model with the prognostic factors being age at diagnosis (<1 y vs ≥1 y) and ATRT‐TYR (vs non‐TYR) significantly impacting OS. Thus, age below <1 year and classification into the non-TYR DNA-methylation subgroup (ATRT-SHH or ATRT-MYC) predicted negative outcome better than any other risk factor (P-values in Table 3). Even when excluding all patients with M+ disease from analysis, significant differences remained.
Table 3.
Significant prognostic factors in ATRT
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| P | RR (95% CI) | P | ||
| Independent | Age <1 y vs ≥1 y | <0.0001 | 4.0 (2.2−7.4) | <0.0001 |
| TYR vs non-TYR | 0.04 | 0.4 (0.2−0.7) | 0.004 | |
| Not independent | Synchronous tumor yes vs no | <0.0001 | ||
| Metastases yes vs no | <0.0001 | |||
| Radiotherapy yes vs no* | 0.006 | |||
| CR yes vs no | <0.0001 | |||
| GLM yes vs no | 0.0002 |
Note. Age, localization, synchronous tumors, M+ status, GTR, conventional chemotherapy according to EU-RHAB, radiotherapy, HDCT, maintenance therapy, CR, progress or early relapse, GLM, and genetic subgroups were analyzed. Factors with significance on a univariate and multivariate level are listed (see also Supplementary Table 5).
Abbreviations: RR, relative risk; n.a., not applicable.
*Only patients irradiated with a curative intent (n = 93) were analyzed.
Then, we employed the independent risk factors of our multivariate analysis to construct a model for potential stratification:
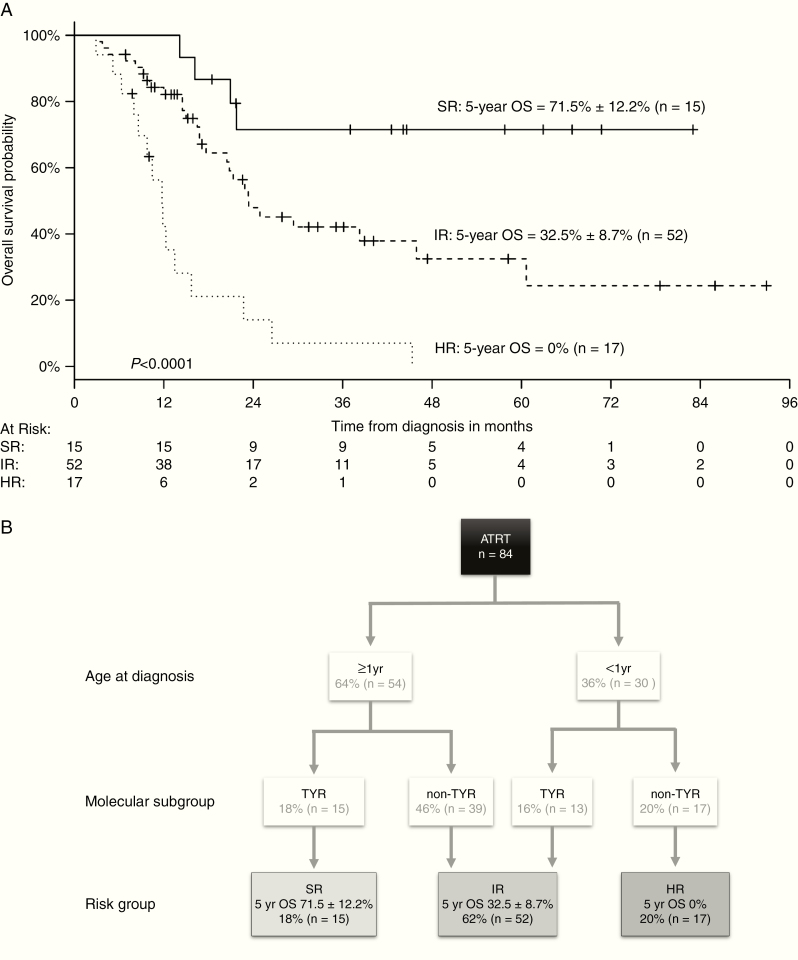
Patients at high risk demonstrated a significantly inferior 5-year OS (<1 y + non-TYR; 5-y OS 0%) compared with those with at intermediate risk (<1 y + ATRT-TYR or ≥1 y + non-TYR; 5-y OS 32.5 ± 8.7%) and a standard risk group (≥1 y + ATRT-TYR; 5-y OS 71.5 ± 12.2%, P < 0.05; Fig. 4A, B). Within the intermediate risk cohort (≥1 y), non-TYR patients accounted for 75% (39/52), 64% (25/39) of whom were ATRT-SHH. There was no significant difference between the number of ATRT-SHH and -MYC patients in this cohort.
Fig. 4.
A combined clinical and genetic risk model for stratification in ATRT
Kaplan–Meier analyses (A). Patients with the risk factors age < or ≥1 year and features of the ATRT-TYR or non-TYR DNA-methylation subgroups were analyzed for their 5-year OS. Three risk strata were delineated: high risk (<1 y + non-TYR; 5-y OS 0%), intermediate risk (<1 y + TYR or ≥1 y + non-TYR; 5-y OS 32.5 ± 8.7%), and standard risk (≥1 y + ATRT-TYR; 5-y OS 71.5 ± 12.2). Potential risk model for the stratification of ATRT. (B) Age at diagnosis (<1 y vs ≥1 y) and DNA methylation subgroup (non-TYR vs TYR) may predict the potential risk of patients affected by ATRT independently of any other clinical or known genetic factor.
Independent validation cohort
The risk model was corroborated in an independent validation cohort (see below and Supplementary Figure 3A, B). Median age of the 69 patients of this cohort was 1.4 years (0–28 y) (Supplementary Table 2). The 5-year OS was 28.6 ± 9.0%. On confirmatory t-SNE analysis, DNA methylation profiles formed 3 independent clusters (Supplementary Figure 2B). The OS of ATRT-TYR patients was longer compared with ATRT-SHH and ATRT-MYC (P < 0.05; Supplementary Figure 3A). Furthermore, OS of standard risk patients (≥1 y + ATRT-TYR) was significantly longer compared with intermediate and high risk patients (Supplementary Figure 3B).
Discussion
Analyzing 143 patients with ATRT we identified superior outcome for children older than 1 year of age, those achieving a CR, and patients displaying a constitutional wild-type gene SMARCB1.12,18,26,27 Patients whose tumors were classified by DNA methylation as ATRT-TYR had a significantly better outcome than those of the ATRT-MYC or ATRT-SHH groups.
Definition of Robust Clinical and Genetic Markers for Stratification
Prognostic markers for ATRT have been studied widely in the literature. In a multivariate analysis of prognostic factors for ATRT, age below 1 year, M+ disease, HDCT, adjuvant radiotherapy, and intraventricular chemotherapy prevailed as prognostic factors for OS.9 Furthermore, Fischer-Valuck et al demonstrated age below 2 years, M+ disease, trimodal therapy, and the “era” of diagnosis (2004–2008 vs 2009–2012) as prognostic.28 In an analysis of 56 patients recruited to the different HIT (HIrn-Tumor) cohorts cohorts (1998–2004, HIT 2000, HIT SKK92, -97, or HIT-91), age at diagnosis and M+ status were the only independent prognostic factors. Employing univariate analyses, significant factors were location (supratentorial vs infratentorial) and achievement of a CR (P < 0.05).29 In the 20 patients reported by Chi et al, surgery leading to a CR and tumor site were the only prognostic factors withstanding multivariate analysis.7 Lafay-Cousin and colleagues suggested reduced survival in infants (<1 y) with less than a GTR.12 Interestingly, our own pilot cohort of 31 patients indicated only age above 3 years, surgical achievement of a CR, and radiotherapy as significant positive prognosticators.6
Results concerning post-baseline factors need to be interpreted with caution—i.e., the improved prognosis of irradiated patients may be attributed to a beneficial effect of radiotherapy or to a confounder, as it may actually result from the patient’s positive general condition before radiotherapy influencing the decision to proceed even in younger children.
Our multivariate model, supported by an independent validation cohort, demonstrated age <1 year and the molecular subgroup “non-TYR” as the only independent negative prognostic factors. Dependent prognostic factors were radiotherapy, achievement of a CR, presence of a GLM, synchronous tumors, and metastatic disease. Presence of a GLM proved to be a robust marker corroborated in 93 patients. Patients with a GLM did very poorly, with 5-year survival rates in the range of only 9.0 ± 6.0%. When adjusting for age (≥1 and <1 y of age), significance was maintained (GLM was rarer among older patients), making this an important high risk marker.
Apart from clinical data, analyses for mutations in SMARCB1 and SMARCA4 should be routine in the diagnostic process for ATRT to exclude tumor predisposition such as in rhabdoid tumor predisposition syndrome 1 and 2, which will trigger a screening program.
A Novel Risk Model Integrating Clinical and Molecular Risk Factors
Very recently DNA methylation analyses have taken center stage as an asset for the diagnosis of CNS tumors.25 The same tool has been applied to subgroup ATRT into at least 3 strata.16,17 Integrating subgroup specification with clinical and genetic information in a large series of pro- and retrospectively collected series of ATRT, we have been able to construct a stratification matrix. Even though the resulting risk stratification model provides exploratory rather than confirmatory scientific evidence, it deserves further validation in independent cohorts. The brain tumor group of SIOPE (International Society of Pediatric Oncology–Europe) will launch a multinational European trial investigating the non-inferiority of HDCT versus conventional chemotherapy plus radiotherapy in children 12–35 months of age. The statistical design projects matching strata of children <1 year and those with non-TYR signature among the 2 randomized arms.
Other researchers have contributed significantly to the definition of potential molecularly defined risk groups. Torchia et al had previously identified 2 distinct subgroups of ATRT18 (Groups 1 and 2) with an impact on survival. As there was no unified treatment approach at the time of analysis, a conclusion as to the significance of individual factors was hard to achieve at that point. Nevertheless, the authors identified achaete-scute homolog 1 (ASCL1) expression (by mRNA expression array and immunohistochemistry) as a potential positive predictor of survival. ASCL1 was present on average at higher levels in Group1/ATRT-SHH versus Group 2/ATRT-TYR/-MYC (see Supplemental data in Torchia et al, Fig. S718). The significance of this factor has until now not been validated in an independent cohort.
In line with the findings of Torchia et al, we identify the significance of a molecular risk factor, a non-TYR signature, as an independent poor prognostic factor in both our test and validation cohorts. Of note, a sizable number of patients in the ATRT-MYC group had received radiotherapy, adding a potential survival benefit. Nevertheless, this potential positive prognosticator did not improve survival.
A potential explanation for the potential discrepancy between the results of Torchia et al and our series is of technical origin. It has been demonstrated that methylation patterns may be better suited at distinguishing subgroups than expression analyses.25 Prospective analyses will have to reconcile the fact that as opposed to Torchia et al, we detect a TYR signature as a positive predictor of survival.
The side-by-side comparison of all currently available subgroup data clearly warrants the urgent need for a consensus agreement on molecular subgroups in ATRT.16–18 Our results suggest that (epi)genetic data should be an integral part of the diagnostic workup of any child with ATRT.
EU-RHAB Provides a Large and Clinically Well-Annotated Cohort of Patients with ATRT
Our analysis comprises the currently largest reported cohort of ATRT treated according to the same therapeutic framework.5,11,18,28 Reported survival rates for ATRT vary widely among reported cohorts. Dufour et al had reported a median OS of 9 months in 58 non-uniformly treated patients (1998–2008).8 Chi et al demonstrated a 2-year OS of 70% in 20 children in the Dana-Farber Cancer Institute (DFCI) protocol.7 Five- and 6-year OS rates, however, are in the range of 45–50% (S. Chi, personal communication). We recently reported comparable 6-year OS and EFS rates of 46% and 45%, respectively, on the pilot consensus regimen of the registry trial Rhabdoid 2007 (n = 31).6 The reason why OS and EFS of the current cohort are inferior to these series might be related to the fact that high risk patients represented a larger proportion of patients compared with the Rhabdoid 2007 and DFCI cohorts.6,7 For example, metastases were present in only 19% of patients in Rhabdoid 2007 but in 34.5% of patients in the EU-RHAB cohort. Furthermore, 37% of the patients of EU-RHAB were below 1 year at diagnosis, while in the DFCI trial only 20% were in this age group. Only 35% of patients in the EU-RHAB cohort had a GTR compared with 50% in the DFCI series. The lower percentage of GTR in our series might well be related to the large number of very young patients often presenting with large, difficult-to-resect tumors. We suggest that our cohort is truly representative of ATRT in Europe as we find a near 100% correlation of patients with the population-based ATRT cohort of the German Childhood Cancer Registry.
A Pledge for an International Controlled Clinical Trial Framework in ATRT
Presuming our dataset can be validated in an independent cohort, we suggest that patients in the high-risk group should be preferentially treated in the frame of international phase I/II trials, while the standard-risk group may be treated according to standard reduced-toxicity regimens within phase III trials. The intermediate-risk group, and thus the largest cohort, deserves increased attention as it contains patients with a rather mixed prognosis not explained by any clinical or currently specified molecular factor. More in-depth analysis is urgently required to better understand the molecular structure of the different subgroups. This is especially true as Johann et al detected potential additional clusters especially among the ATRT-SHH group suggestive of additional heterogeneity in their profiling analyses of 150 ATRTs.
As early as 2002, reporting the results of an international workshop on ATRT, Packer and colleagues demanded that “given the rarity of these tumors,” unified protocols specifically designed for this disease should be “multi-institutional and preferably . . . multinational.” 27 This statement holds true 16 years later.
Funding
EU-RHAB is supported by grants to M.C.F. by the “Deutsche Kinderkrebsstiftung” DKKS 2010.03, the German Research Foundation (DFG) (FR 1516/4–1), and the parent organization Lichtblicke. M.H. is supported by the DFG (HA 3060/8–1) and the IZKF Münster (Ha3/017/20). R.Sch.’s and R.S.’s research is supported by the Fördergemeinschaft Kinderkrebszentrum Hamburg e.V. R.Si.’s research on pediatric tumors is supported by the KinderKrebsInitiative Buchholz/Holm-Seppensen. M.C.F., M.K., and K.K. are supported by grant 111537 of the Deutsche Krebshilfe. M.C.F. is supported by grant 1516/4–1 of the DFG.
Supplementary Material
Acknowledgments
We thank the following scientists for providing biological material and patient data: Andrey Korshunov, CCU Neuropathology; German Cancer Research Center and Department of Neuropathology, Heidelberg University Hospital, Heidelberg, Germany; Marina Ryzhova, Department of Neuropathology, NN Burdenko Neurosurgical, Institute, Moscow, Russia; Olga Zheludkova, Department of Neuro-Oncology, Russian Scientific Center of Radiology, Moscow, Russia; Ella Kumirova, Department of Pediatric Neurooncology, Dmitry Rogachev Federal Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russia. The support of the technical staff of the molecular cytogenetics laboratory of the Institute of Human Genetics of Ulm University is gratefully acknowledged.
Conflict of interest statement. The authors have no conflict of interest to declare.
Authorship statement. MCF and NG led the study and provided supervision of the project. MCF, MH, KN, MS, RS, and MK prepared all figures and wrote the final manuscript. MCF, MH, SB, PDJ, KK, PH, EQ, PSP, VB, MJGDC, MP, MvDW, DS, JP, NS, SH, HH, NUG, MG, ME, ST, WP, RF, PHD, HR, SR, PGS, IS, RDK, BT, MW, UK, KN, RSch, RS, MK, and NG gathered samples and clinical information. JG and MH performed all biostatistical analyses. MCF and NG designed and wrote the registry document. MCF is the principal investigator of the project. All authors had substantial input to the conception of the work and revised and approved the final manuscript. The authors know that they are accountable for all aspects of the work ensuring that questions regarding the accuracy and integrity of any part are appropriately investigated and resolved. Parts of the current study were presented at ISPNO2018, the 18th International Symposium on Pediatric Neuro-Oncology in Denver, CO.
References
- 1. Hasselblatt M, Isken S, Linge A, et al. High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes Chromosomes Cancer. 2013;52(2):185–190. [DOI] [PubMed] [Google Scholar]
- 2. Kieran MW, Roberts CW, Chi SN, et al. Absence of oncogenic canonical pathway mutations in aggressive pediatric rhabdoid tumors. Pediatr Blood Cancer. 2012;59(7):1155–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee RS, Stewart C, Carter SL, et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest. 2012;122(8):2983–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hasselblatt M, Nagel I, Oyen F, et al. SMARCA4-mutated atypical teratoid/rhabdoid tumors are associated with inherited germline alterations and poor prognosis. Acta Neuropathol. 2014;128(3): 453–456. [DOI] [PubMed] [Google Scholar]
- 5. Frühwald MC, Biegel JA, Bourdeaut F, Roberts CW, Chi SN. Atypical teratoid/rhabdoid tumors—current concepts, advances in biology, and potential future therapies. Neuro Oncol. 2016;18(6):764–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartelheim K, Nemes K, Seeringer A, et al. Improved 6-year overall survival in AT/RT—results of the registry study Rhabdoid 2007. Cancer Med. 2016;5(8):1765–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chi SN, Zimmerman MA, Yao X, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27(3):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dufour C, Beaugrand A, Le Deley MC, et al. Clinicopathologic prognostic factors in childhood atypical teratoid and rhabdoid tumor of the central nervous system: a multicenter study. Cancer. 2012;118(15):3812–3821. [DOI] [PubMed] [Google Scholar]
- 9. Fossey M, Li H, Afzal S, et al. Atypical teratoid rhabdoid tumor in the first year of life: the Canadian ATRT registry experience and review of the literature. J Neurooncol. 2017;132(1):155–162. [DOI] [PubMed] [Google Scholar]
- 10. Bartelheim K, Sumerauer D, Behrends U, et al. Clinical and genetic features of rhabdoid tumors of the heart registered with the European Rhabdoid Registry (EU-RHAB). Cancer Genet. 2014;207(9):379–383. [DOI] [PubMed] [Google Scholar]
- 11. Athale UH, Duckworth J, Odame I, Barr R. Childhood atypical teratoid rhabdoid tumor of the central nervous system: a meta-analysis of observational studies. J Pediatr Hematol Oncol. 2009;31(9):651–663. [DOI] [PubMed] [Google Scholar]
- 12. Lafay-Cousin L, Hawkins C, Carret AS, et al. Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer. 2012;48(3):353–359. [DOI] [PubMed] [Google Scholar]
- 13. Schrey D, Carceller Lechón F, Malietzis G, et al. Multimodal therapy in children and adolescents with newly diagnosed atypical teratoid rhabdoid tumor: individual pooled data analysis and review of the literature. J Neurooncol. 2016;126(1):81–90. [DOI] [PubMed] [Google Scholar]
- 14. Hasselblatt M, Gesk S, Oyen F, et al. Nonsense mutation and inactivation of SMARCA4 (BRG1) in an atypical teratoid/rhabdoid tumor showing retained SMARCB1 (INI1) expression. Am J Surg Pathol. 2011;35(6):933–935. [DOI] [PubMed] [Google Scholar]
- 15. Seeringer A, Reinhard H, Hasselblatt M, et al. Synchronous congenital malignant rhabdoid tumor of the orbit and atypical teratoid/rhabdoid tumor—feasibility and efficacy of multimodal therapy in a long-term survivor. Cancer Genet. 2014;207(9):429–433. [DOI] [PubMed] [Google Scholar]
- 16. Johann PD, Erkek S, Zapatka M, et al. Atypical teratoid/rhabdoid tumors are comprised of three epigenetic subgroups with distinct enhancer landscapes. Cancer Cell. 2016;29(3):379–393. [DOI] [PubMed] [Google Scholar]
- 17. Torchia J, Golbourn B, Feng S, et al. Integrated (epi)-genomic analyses identify subgroup-specific therapeutic targets in CNS rhabdoid tumors. Cancer Cell. 2016;30(6):891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torchia J, Picard D, Lafay-Cousin L, et al. Molecular subgroups of atypical teratoid rhabdoid tumours in children: an integrated genomic and clinicopathological analysis. Lancet Oncol. 2015;16(5):569–582. [DOI] [PubMed] [Google Scholar]
- 19. Birks DK, Donson AM, Patel PR, et al. Pediatric rhabdoid tumors of kidney and brain show many differences in gene expression but share dysregulation of cell cycle and epigenetic effector genes. Pediatr Blood Cancer. 2013;60(7):1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nowak J, Nemes K, Hohm A, et al. Magnetic resonance imaging surrogates of molecular subgroups in atypical teratoid/rhabdoid tumor. Neuro Oncol. 2018;20(12):1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Judkins AR, Mauger J, Ht A, Rorke LB, Biegel JA. Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol. 2004;28(5):644–650. [DOI] [PubMed] [Google Scholar]
- 22. Warmuth-Metz M, Bison B, Leykamm S. Neuroradiologic review in pediatric brain tumor studies. Klin Neuroradiol. 2009;19(4):263–273. [DOI] [PubMed] [Google Scholar]
- 23. Kordes U, Gesk S, Frühwald MC, et al. Clinical and molecular features in patients with atypical teratoid rhabdoid tumor or malignant rhabdoid tumor. Genes Chromosomes Cancer. 2010;49(2):176–181. [DOI] [PubMed] [Google Scholar]
- 24. Kordes U, Bartelheim K, Modena P, et al. Favorable outcome of patients affected by rhabdoid tumors due to rhabdoid tumor predisposition syndrome (RTPS). Pediatr Blood Cancer. 2014;61(5):919–921. [DOI] [PubMed] [Google Scholar]
- 25. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hilden JM, Watterson J, Longee DC, et al. Central nervous system atypical teratoid tumor/rhabdoid tumor: response to intensive therapy and review of the literature. J Neurooncol. 1998;40(3):265–275. [DOI] [PubMed] [Google Scholar]
- 27. Packer RJ, Biegel JA, Blaney S, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: report on workshop. J Pediatr Hematol Oncol. 2002;24(5):337–342. [DOI] [PubMed] [Google Scholar]
- 28. Fischer-Valuck BW, Chen I, Srivastava AJ, et al. Assessment of the treatment approach and survival outcomes in a modern cohort of patients with atypical teratoid rhabdoid tumors using the National Cancer Database. Cancer. 2017;123(4):682–687. [DOI] [PubMed] [Google Scholar]
- 29. von Hoff K, Hinkes B, Dannenmann-Stern E, et al. Frequency, risk-factors and survival of children with atypical teratoid rhabdoid tumors (AT/RT) of the CNS diagnosed between 1988 and 2004, and registered to the German HIT database. Pediatr Blood Cancer. 2011;57(6):978–985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.