Abstract
Objective
In cross-sectional studies, elevated osteopontin (OPN) has been proposed to reflect, and/or precede, progressive organ damage and severity in systemic lupus erythematosus (SLE). We aimed, in a prospective cohort of recent-onset SLE to determine whether raised serum OPN associates with disease activity and/or precedes organ damage.
Methods
We included 345 patients from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort who had 5-years of follow-up data available. All patients fulfilled the 1982 American College of Rheumatology (ACR) criteria. Baseline sera from patients and from age- and sex-matched controls were analyzed for OPN using ELISA. Disease activity and damage were assessed at each annual follow-up visit using the SLE Disease Activity Index 2000 (SLEDAI-2K) and the SLICC/ACR damage index (SDI), respectively.
Results
Compared to controls, baseline OPN was raised fourfold in SLE cases (p<0.0001). A weak correlation was found between baseline OPN and accrual of global damage (r=0.16, p=0.004) at 5 years. OPN levels predicted damage when defined as SDI≥1 (p=0.024), but the damage-predictive value was lost when adjusting damage cut-off to SDI≥2. Baseline OPN correlated with disease activity at inclusion (r=0.27, p<0.0001). Patients with high disease activity (SLEDAI-2K≥5) had raised serum OPN (p<0.0001). Higher OPN levels were also found in patients with persistently raised disease activity (p=0.0005).
Conclusions
Raised OPN at SLE onset was associated with higher disease activity and more severe disease and may as a biomarker help to guide more targeted attempts to control disease activity over time.
Introduction
Systemic lupus erythematosus (SLE) is a multisystemic inflammatory rheumatic disease that often shows periods of flares followed by remissions. Distinguishing ongoing inflammation attributed to SLE from established organ damage caused by SLE, medication or co-morbidities remains a challenge for the clinician. The spectrum of different phenotypes complicates the search for biomarkers that adequately reflect the active disease and/or increasing organ damage.
Osteopontin (OPN), an extracellular matrix protein with multiple functions, has been reported to be involved in inflammation (1). Local production and elevated circulating levels of OPN have been found in several autoimmune diseases, such as multiple sclerosis (2), rheumatoid arthritis (3) and SLE (4, 5). Overexpression of OPN in lupus-prone mice induces B-cell activation and subsequent production of anti-dsDNA antibodies (6, 7), which is a hallmark of SLE. Intracellular OPN has been implicated in numerous cellular processes and its expression is required for TLR-9-dependent production of IFN-α (8), a central cytokine in the SLE pathogenesis (9).
Raised OPN levels have been observed in SLE (4, 5), and associations have been found with disease activity (10), as well as with accumulated organ damage (11). In addition, elevated OPN levels have been suggested to precede the development of organ damage in a study including predominantly pediatric SLE cases (12). We have previously investigated serum OPN in a cross-sectional Swedish SLE cohort where OPN appeared to reflect current global organ damage (4). OPN was also found to associate with lupus nephritis, anti-phospholipid syndrome (APS), as well as with individual clinical and laboratory criteria of APS. In addition, OPN levels showed significant correlations with disease activity, particularly in newly diagnosed cases.
The aims of this study were to determine whether OPN (i) reflects current and/or persistently raised disease activity, and (ii) precedes and predicts future organ damage in a longitudinal international inception cohort of recent-onset SLE.
Methods
The SLICC Inception Cohort
The SLICC group comprises 31 centers from 11 countries in North America, Europe and Asia and the Inception Cohort was recruited from 2000–2011 as previously described (13, 14). Briefly, all clinical data were submitted to the coordinating center at the University of Toronto at enrollment and patients were reviewed annually. Laboratory tests necessary to evaluate disease activity and damage parameters were performed locally. The study was approved by the Institutional Research Ethics Boards of participating centers in accordance with the Declaration of Helsinki’s guidelines.
Patients and controls
SLE cases were enrolled within 15 months (mean 6 months, range 0–15) of SLE diagnosis, which was based on the fulfillment of at least 4 of the American College of Rheumatology 1982 (ACR-82) criteria (15). Herein, we selected patients from the Inception Cohort who had baseline serum available and for which there were 5 years of annual follow-up data completed. In addition, absence of organ damage at baseline was a requirement. Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) (16) and SLICC/ACR damage index (SDI) (17) were assessed at each visit. At baseline, peripheral venous blood was drawn from each individual. Sera were prepared and stored at −70°C until analyzed.
Sera from population-based controls matched 1:1 according to sex and age included in the EIRA cohort (acronym for the Swedish Epidemiological Investigation of Rheumatoid Arthritis) served as controls for the OPN analyses (18).
All patients and controls provided written informed consent.
OPN immunoassay
A serum- and plasma-validated enzyme-linked immunosorbent assay (ELISA) kit was used to analyze OPN levels in SLE and control sera (Quantikine®, R&D Systems, MN, USA). Analyses were performed according to the manufacturers’ instructions. Briefly, serum (diluted 1:25) was added to microwells pre-coated with monoclonal antibodies directed against human OPN. After incubation and washing, a horseradish-peroxide conjugated polyclonal anti-OPN antibody was added and the plate incubated, followed by washing and addition of tetramethylbenzidine substrate. The enzymatic reaction was stopped by adding 2 N sulfuric acid and read at 450 nm (plate reader Sunrise, Tecan, Männedorf, Switzerland; software Magellan version 7.1, Tecan).
Statistics
Independent samples t-test was used to evaluate differences in OPN levels between SLE patients and controls.
Pearson correlation analyses between OPN and disease activity variables were performed, and significant associations were further analyzed in a univariate general linear model with adjustment for age, sex, ethnicity and daily glucocorticoid dose at baseline.
Binary logistic regression was used to predict damage accrual and persistently raised disease activity, with adjustment for age, sex, ethnicity and glucocorticoid therapy.
P-values below 0.05 were considered significant. Statistical analyses were performed with SPSS Statistics 22 (IBM, Armonk, NY, USA) or GraphPad Prism, version 5.04 (GraphPad Software, La Jolla, CA, USA).
Results
There were a total of 345 SLE cases (316 women and 29 men; mean age 34.0 years, range 12–73) included in the study. The majority of patients (n=200, 58.0%) were of Caucasian ethnicity. Detailed characteristics of the study population are found in Table 1. Of the 345 controls (316 women and 29 men; mean age 34.4 years, range 15–73), 328 (95.1%) were of Caucasian ethnicity.
Table 1.
Characteristics at baseline of the 345 SLE patients
| Mean (range) or number (percentage) | |
|---|---|
| Age | 34.0 (12–73) |
| Females | 316 (91.6%) |
| Glucocorticoid (prednisone) daily dose at baseline (mg/day) | 14 (0–90) |
| Ethnicities | |
| Caucasian | 200 (58.0%) |
| African descendants | 52 (15.1%) |
| Asian | 65 (18.8%) |
| Other | 28 (8.1%) |
| SLEDAI-2K (score) | 5.0 (0–30) |
SLE = systemic lupus erythematosus, SLEDAI-2K = SLE disease activity index 2000
Baseline OPN levels are increased in SLE
Circulating levels of OPN were markedly higher among cases with SLE (mean 45.5 ng/ml) compared to the controls (mean 11.8 ng/ml, p<0.0001; Figure 1). No differences were observed between men and women among the controls. However, among the SLE patients, men displayed higher OPN levels (mean 79.5 ng/ml) compared to women (mean 42.4 ng/ml, p<0.0001). Differences were also identified between patients of Caucasian ethnicity (mean 38.2 ng/ml) compared to non-Caucasians (mean 55.6 ng/ml, p<0.0001). Such difference was not found among the controls (p=0.87).
Figure 1. Serum osteopontin (OPN) levels in 345 population-based controls and in 345 cases with systemic lupus erythematosus (SLE).
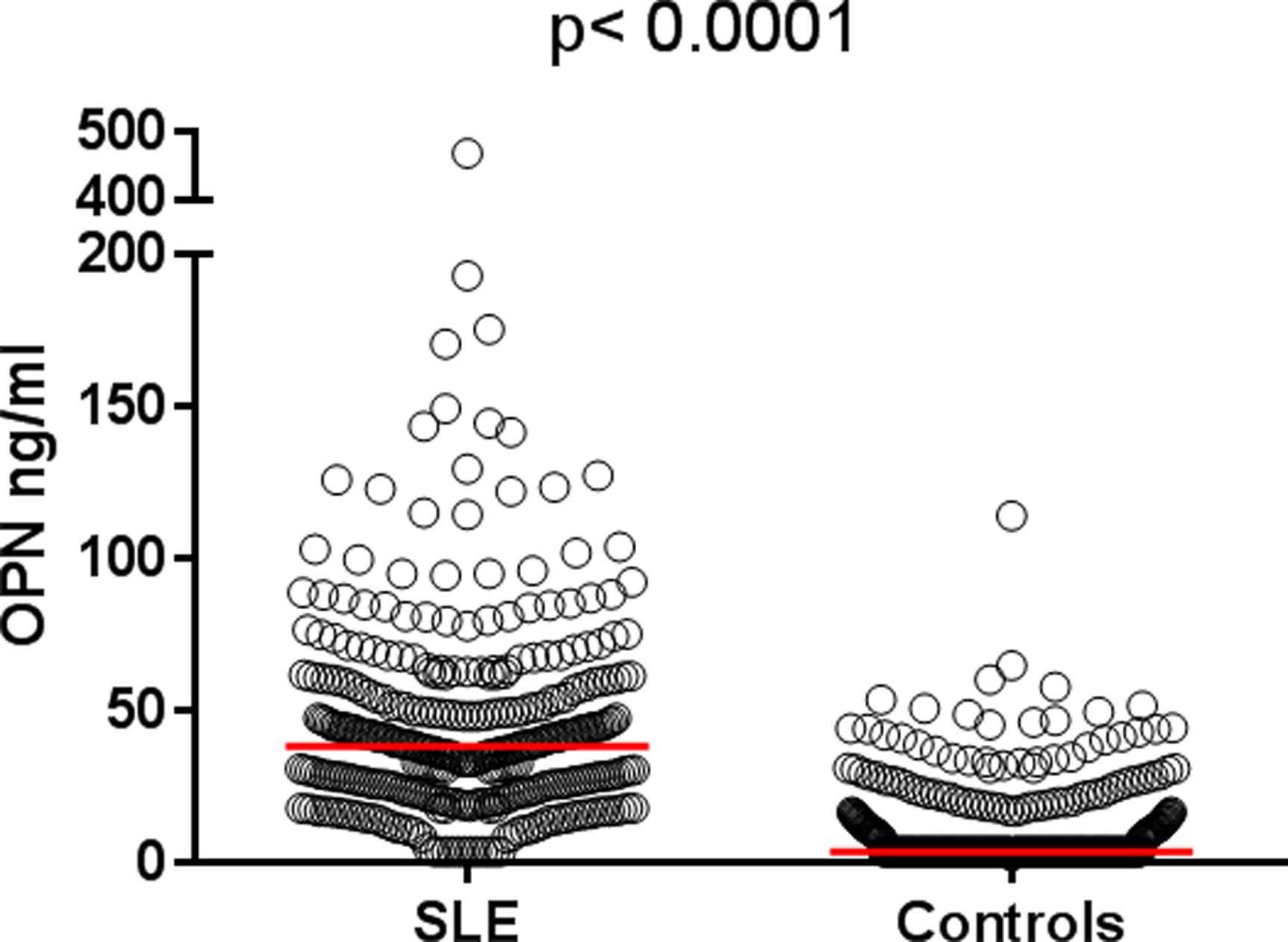
Baseline levels of OPN were significantly higher among patients with SLE (mean 45.5 ng/ml) compared to controls (mean 11.8 ng/ml).
OPN levels correlated inversely with age, both among the patients (r=−0.23, p<0.0001) and controls (r=−0.27, p<0.0001).
OPN is a weak predictor of damage accrual
At the 3-year follow-up visit, 64 (19%) patients had developed any damage (i.e. SDI≥1), and 99 (29%) had damage after 5 years. Since only 19% had an SDI score of ≥1 three years after inclusion, we focused mainly on the 5-year data. Weak correlations were found between baseline OPN and accrual of damage after 3 years (r=0.11, p=0.037) and after 5 years (r=0.16, p=0.004). Binary logistic regression was used to predict damage after 5 years, with adjustment for age, sex, ethnicity and glucocorticoid therapy. OPN levels had a modest predictive value for future damage when defined as SDI≥1 (p=0.024; Table 2), with a ROC area under curve (AUC) of 0.65. However, when the cut-off for damage was changed to SDI≥2 (n=33), the damage predictive value did no longer reach statistical significance.
Table 2.
Binary logistic regression for the outcome of organ damage (SLICC/ACR damage index; yes or no) at 5 years.
| Variable | p value | OR (95% CI) |
|---|---|---|
| OPN at baseline | 0.064 | 1.008 (1.000–1.017) |
| Age at baseline | 0.004 | 1.029 (1.009–1.050) |
| Sex | 0.111 | 0.499 (0.212–1.174) |
| Caucasian ethnicity | 0.057 | 0.599 (0.353–1.016) |
| Daily glucocorticoid dose at baseline | 0.119 | 0.986 (0.968–1.004) |
| SLEDAI-2K at baseline | 0.026 | 1.061 (1.007–1.118) |
We identified no significant differences in baseline OPN levels when separating patients’ SDI after 5 years into ‘no damage’ (i.e. SDI=0, mean 37.4 ng/ml), ‘moderate damage’ (SDI 1–2, mean 41.3) and ‘extensive damage’ (SDI≥3, mean 63.4 ng/ml).
Examining the components of the SDI individually revealed a significant positive impact on OPN levels regarding the musculoskeletal domain (n=23, p=0.027, AUC=0.68).
OPN reflects disease activity and renal involvement
Baseline OPN correlated with disease activity at inclusion (r=0.27, p<0.0001). Patients with a SLEDAI-2K score of greater than 5 had higher levels of OPN (mean 56.8 ng/ml) than patients with SLEDAI-2K<5 (mean 38.5 ng/ml, p<0.0001; Figure 2). The erythrocyte sedimentation rate, indicative of disease activity, correlated with OPN (r=0.38, p<0.0001). The above-mentioned associations remained significant after adjustments for age, sex, ethnicity and glucocorticoid therapy in a univariate general linear model analysis.
Figure 2. Serum osteopontin (OPN) levels in patients with raised versus low/no disease activity.
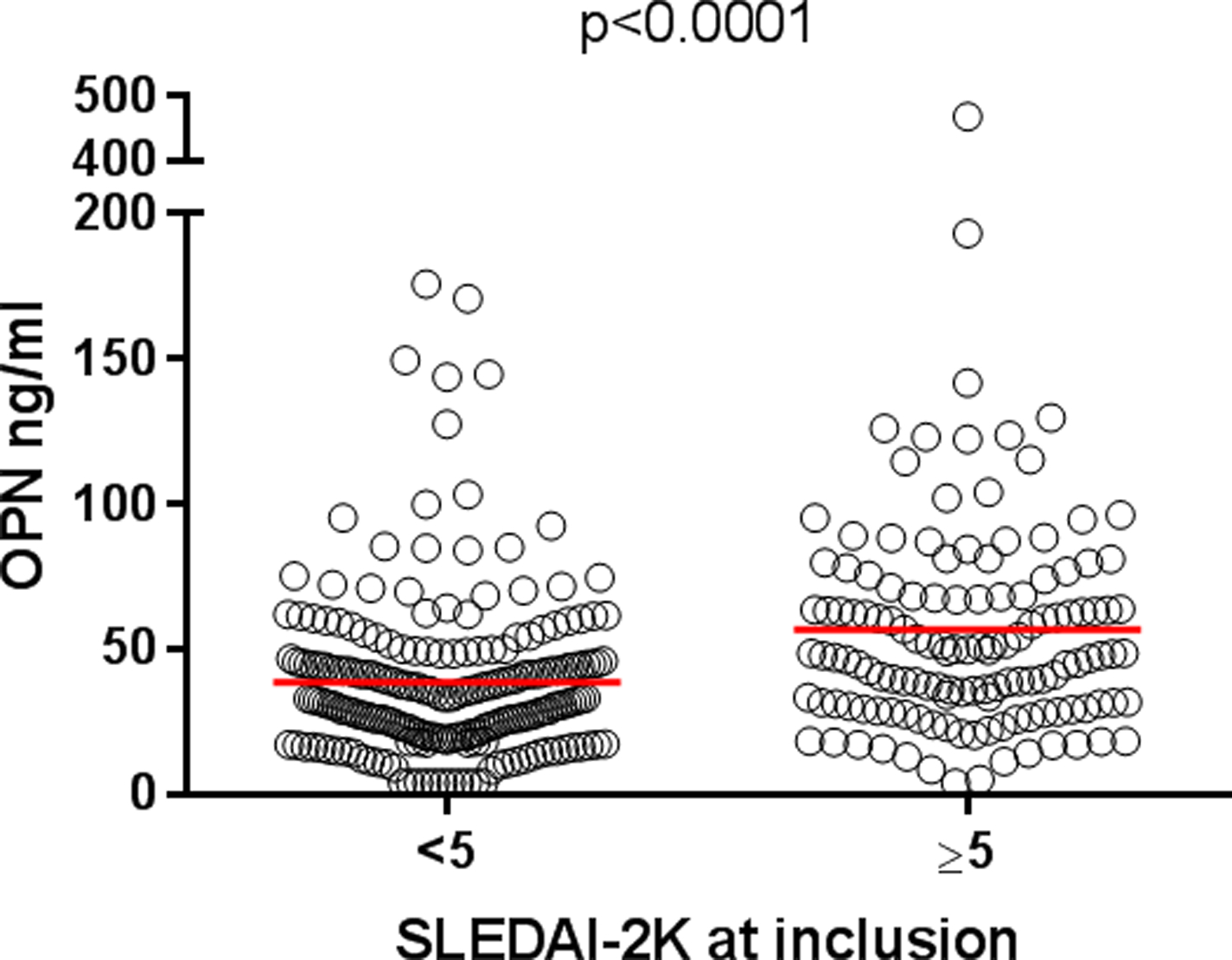
Patients with a SLEDAI-2K score of less than 5 had higher baseline levels of OPN (mean 56.8 ng/ml, n=132) than patients with SLEDAI-2K<5 (mean 38.5 ng/ml, n=213).
We further evaluated associations with different disease phenotypes (i.e. fulfilled ACR criteria). Patients meeting the renal disorder criterion (ACR-7) had higher levels of OPN (mean 63.9 ng/ml, n=76) compared to those without renal involvement (mean 40.3 ng/ml, n=269, p<0.0001; Figure 3). The total number of fulfilled ACR criteria associated significantly with OPN levels (r=0.17, p<0.03). The associations with nephritis and ACR criteria remained significant after adjustments for age, sex, ethnicity and glucocorticoid therapy in a univariate general linear model analysis.
Figure 3. Serum osteopontin (OPN) levels in SLE cases with or without renal involvement.
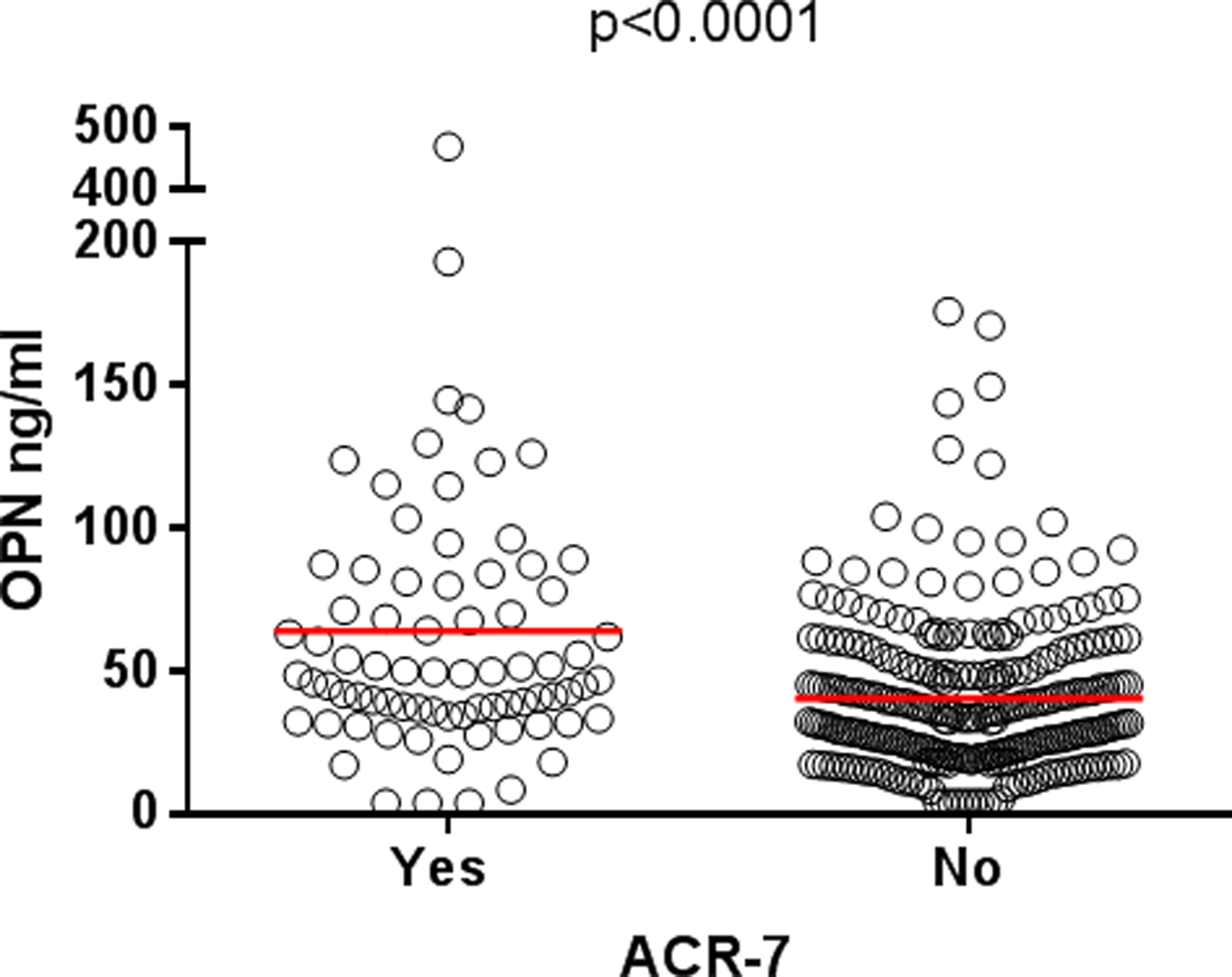
Patients meeting the renal disorder criterion (ACR-7) had higher baseline levels of OPN (mean 63.9 ng/ml, n=76) compared to those without renal involvement (mean 40.3 ng/ml, n=269).
OPN predicts persistently raised disease activity
To further analyze the association between OPN and disease activity, we separated patients based on ‘persistently raised disease activity’ which was defined as a SLEDAI-2K score of ≥5 at ≥3 separate occasions during the 5-year follow-up from baseline. Higher levels of OPN were found among the 52 patients (15%) with persistently raised disease activity (mean 53.7 ng/ml) compared to those without (mean 38.5 ng/ml, p=0.0005; Figure 4A). To evaluate the possible impact of organ damage on OPN levels in cases with persistently raised disease activity, patients who had developed any damage (i.e. SDI≥1) after 5 years were compared to those without any damage. No statistical significance in OPN levels was observed (Figure 4B).
Figure 4. Baseline osteopontin (OPN) levels in patients with persistently raised disease activity.
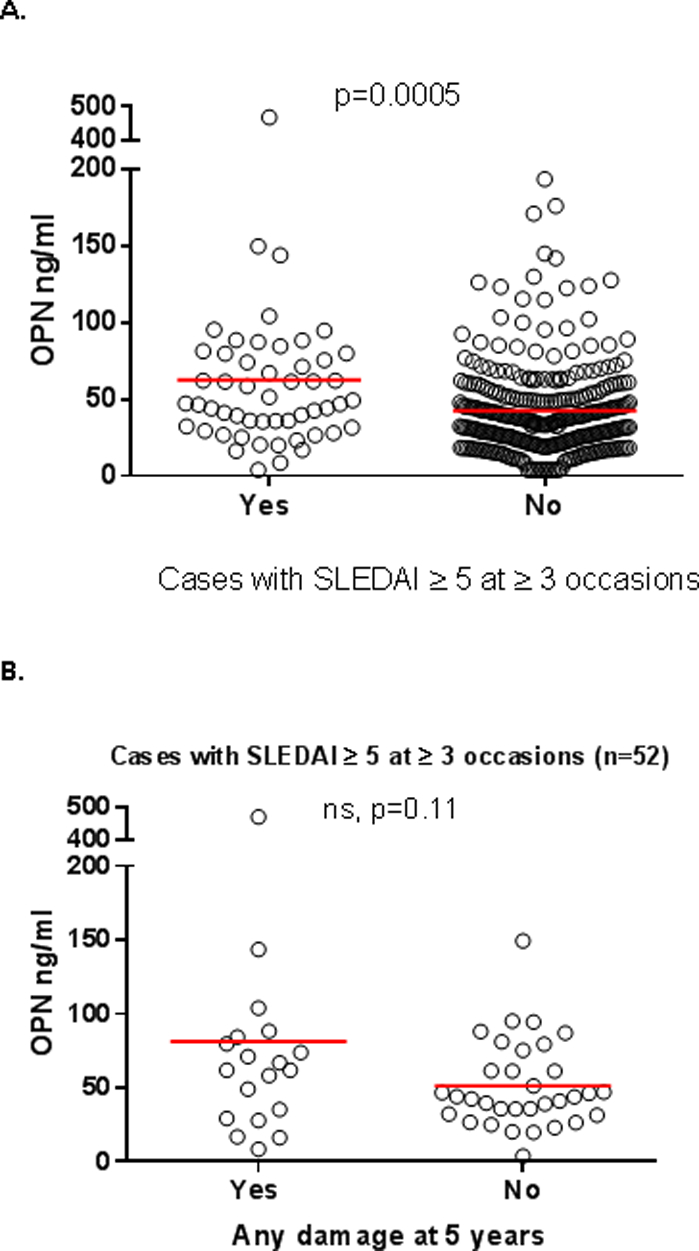
(A) Higher levels of OPN were found in the 52 patients with persistently raised disease activity (mean 53.7 ng/ml) compared to those without (mean 38.5 ng/ml, n=293). (B) To investigate the possible impact of damage on OPN levels in cases with persistently raised disease activity, we compared patients who had developed any damage (i.e. SDI≥1) after 5 years to those without any damage. No significant difference in OPN levels was observed between patients with any damage (mean 81.4 ng/ml, n=19) compared with those without (mean 51.4 ng/ml, n=33).
A binary logistic regression with adjustments for age, sex, ethnicity, and glucocorticoids was performed to evaluate whether OPN could predict persistently raised disease activity. OPN levels had a significant impact on disease activity outcome (p=0.010, AUC=0.67), and this impact remained significant after addition of SDI into the model (p=0.014, AUC=0.68; Table 3).
Table 3.
Binary logistic regression for the outcome of persistent disease activity (yes or no) for 5 years
| Variable | p value | OR (95% CI) |
|---|---|---|
| OPN at baseline | 0.014 | 1.011 (1.002–1.020) |
| Age at baseline | 0.057 | 0.973 (0.945–1.001) |
| Sex | 0.315 | 1.981 (0.522–7.523) |
| Caucasian ethnicity | 0.966 | 1.014 (0.521–1.974) |
| Daily glucocorticoid dose at baseline | 0.829 | 0.998 (0.978–1.018) |
| SLICC/ACR damage index at 5 years | 0.490 | 1.111 (0.823–1.500) |
Discussion
In SLE, OPN has been proposed as a useful biomarker of disease activity (4, 10), as well as of organ damage (4, 11, 12). Most previous studies had cross-sectional design, but in the present study we aimed to dissect whether or not baseline OPN could be a predictor of future organ damage in a longitudinal cohort. Our results confirm some previous reports indicating that OPN constitute a marker of disease activity and lupus nephritis, rather than a marker of future damage accrual.
In line with previous findings by our group and others (4, 5), OPN levels were elevated in SLE patients compared with population-based healthy controls. Increased circulating OPN levels have been reported to precede increased cumulative disease activity and organ damage in SLE patients, especially in pediatric SLE (12). In a cross-sectional pilot study, we evaluated OPN in a cohort of Swedish SLE cases and found that circulating OPN reflects global organ damage (4). In the present study, OPN levels at inclusion were weakly associated with damage accrual after 5 years, and were able to predict damage using SDI≥1 as cut-off. However, when pushing the threshold to SDI≥2, the predictive value was lost. Notably, only 33 cases had an SDI score of 2 or more, why we acknowledge possible statistical power issues for this group. To study this further, a larger group of patients with more extensive damage accrual observed during a longer time period would be applicable.
Since OPN showed an inverse correlation with age, and because differences were observed between men and women, as well as between Caucasians and non-Caucasians, these factors were adjusted for in the statistical analyses. Rullo et al. (12) reported that high circulating OPN levels preceded increased cumulative disease activity and organ damage over 12 months. In contrast, the SLICC inception cohort consists mainly of adult SLE cases, and there may also be differences between the studies regarding ethnicities which potentially could have affected the divergent conclusions of OPN as a potential biomarker of future organ damage.
In line with earlier reports, we observed an association between OPN and disease activity, using the SLEDAI-2K (4, 5). In our pilot study, we noted a robust correlation between SLEDAI-2K and OPN when we restricted the analysis to recent-onset disease (4). Herein, patients with active disease (i.e. SLEDAI-2K≥5) had higher OPN levels compared to those with inactive disease (i.e. SLEDAI-2K<5). Moreover, higher OPN levels were also seen in patients with persistently raised disease activity.
We further investigated associations of baseline OPN with different clinical manifestations. Patients meeting the lupus nephritis criterion (ACR-7) displayed higher levels of OPN. An association between OPN and renal impairment has been reported previously (4, 10, 11), and lupus-prone mice with nephritis have been shown to express OPN associated with macrophage infiltration (19). Furthermore, anti-OPN therapy in nephritic rats reduces albuminuria and invasion of macrophages (20), and OPN knock-out mice have less recruitment of macrophages as well as reduced fibrosis (21). Regarding organ damage, the only separate organ domain which showed a significant positive impact on OPN levels was the musculoskeletal domain, including muscle atrophy/weakness, deforming or erosive arthritis, osteoporosis, avascular necrosis, and osteomyelitis (17). This organ domain comprises manifestations closely related to the disease itself (e.g. erosive arthritis) as well as manifestations more related to side-effects from treatments, for instance glucocorticoids (e.g. muscle atrophy and osteoporosis).
The reason for elevated OPN in SLE remains uncertain. However, the intracellular expression of OPN in plasmacytoid dendritic cells (pDCs) has been reported to be required for TLR-9-dependent production of IFN-α (8) and could be of relevance to the pathogenesis of SLE. In addition, mutations in tartrate-resistant acid phosphatase (TRAP) cause spondyloenchondrodysplasia, an unusual recessive disease associated with short stature, brain calcifications and lupus-like autoimmunity (22). OPN is a substrate for TRAP, and TRAP has been shown to co-localize and physically interact with OPN in pDCs and macrophages (23). Lack of TRAP leads to hyperphosphorylation of OPN and enhanced TLR-9 signaling in pDCs with subsequent IFN-α production, which can cause the lupus-like autoimmunity seen in spondyloenchondrodysplasia patients. Thus, future studies focusing on potential associations between IFN-α and OPN in SLE are highly warranted.
The present study has several strengths, e.g. the extremely well characterized study population. In addition, the study had a prospective design using a large international inception cohort with 5 years of follow-up data. Some limitations should also be mentioned. Although the control subjects were matched according to sex and age, the great majority were Caucasians which did not reflect the ethnicities of the SLE cases (58% Caucasians). The relatively small number of damage events over 5 years probably reflects well-controlled SLE patients, but generates uncertainties in predicting damage accrual. Finally, OPN was analyzed at baseline only and we acknowledge that the predictive value of OPN for different outcome measures (such as damage accrual) may vary over time in established disease.
Conclusion
OPN is elevated in early SLE and appears to be associated with renal involvement, higher disease activity, as well as with continuously raised disease activity. In addition, we observed a weaker association with organ damage accumulation during the first 5 years. As such, raised OPN at SLE onset indicate cases with ‘severe disease’ and may as a biomarker help to guide more targeted attempts to control disease activity over time.
Acknowledgements
We would like to thank the EIRA Study personnel for providing us with information and sera from healthy controls, Nicole Anderson for biobank handling and logistics, and Lars Valter for advice on statistical analyses.
References
- 1.Clemente N, Raineri D, Cappellano G, Boggio E, Favero F, Soluri MF, et al. Osteopontin Bridging Innate and Adaptive Immunity in Autoimmune Diseases. J Immunol Res. 2016;2016:7675437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–5. [DOI] [PubMed] [Google Scholar]
- 3.Ohshima S, Yamaguchi N, Nishioka K, Mima T, Ishii T, Umeshita-Sasai M, et al. Enhanced local production of osteopontin in rheumatoid joints. J Rheumatol. 2002;29:2061–7. [PubMed] [Google Scholar]
- 4.Wirestam L, Frodlund M, Enocsson H, Skogh T, Wetterö J, Sjöwall C. Osteopontin is associated with disease severity and antiphospholipid syndrome in well-characterized Swedish cases of systemic lupus erythematosus. Lupus Sci Med. 2017;4:e000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YH, Song GG. Correlation between circulating osteopontin level in systemic lupus erythematosus and disease activity and associations between osteopontin polymorphisms and disease susceptibility: A meta-analysis. Lupus. 2017;26:132–8. [DOI] [PubMed] [Google Scholar]
- 6.Iizuka J, Katagiri Y, Tada N, Murakami M, Ikeda T, Sato M, et al. Introduction of an osteopontin gene confers the increase in B1 cell population and the production of anti-DNA autoantibodies. Lab Invest. 1998;78:1523–33. [PubMed] [Google Scholar]
- 7.Sakamoto K, Fukushima Y, Ito K, Matsuda M, Nagata S, Minato N, et al. Osteopontin in Spontaneous Germinal Centers Inhibits Apoptotic Cell Engulfment and Promotes Anti-Nuclear Antibody Production in Lupus-Prone Mice. J Immunol. 2016;197:2177–86. [DOI] [PubMed] [Google Scholar]
- 8.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronnblom L, Alm GV, Eloranta ML. The type I interferon system in the development of lupus. Semin Immunol. 2011;23:113–21. [DOI] [PubMed] [Google Scholar]
- 10.Wong CK, Lit LC, Tam LS, Li EK, Lam CW. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford). 2005;44:602–6. [DOI] [PubMed] [Google Scholar]
- 11.Quaglia M, Chiocchetti A, Cena T, Musetti C, Monti S, Clemente N, et al. Osteopontin circulating levels correlate with renal involvement in systemic lupus erythematosus and are lower in ACE inhibitor-treated patients. Clin Rheumatol. 2014;33:1263–71. [DOI] [PubMed] [Google Scholar]
- 12.Rullo OJ, Woo JM, Parsa MF, Hoftman AD, Maranian P, Elashoff DA, et al. Plasma levels of osteopontin identify patients at risk for organ damage in systemic lupus erythematosus. Arthritis Res Ther. 2013;15:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce IN, O’Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker B, Urowitz MB, Gladman DD, Lunt M, Bae SC, Sanchez-Guerrero J, et al. Clinical associations of the metabolic syndrome in systemic lupus erythematosus: data from an international inception cohort. Ann Rheum Dis. 2013;72:1308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 16.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 17.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 18.Stolt P, Bengtsson C, Nordmark B, Lindblad S, Lundberg I, Klareskog L, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis. 2003;62:835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wuthrich RP, Fan X, Ritthaler T, Sibalic V, Yu DJ, Loffing J, et al. Enhanced osteopontin expression and macrophage infiltration in MRL-Fas(lpr) mice with lupus nephritis. Autoimmunity. 1998;28:139–50. [DOI] [PubMed] [Google Scholar]
- 20.Panzer U, Thaiss F, Zahner G, Barth P, Reszka M, Reinking RR, et al. Monocyte chemoattractant protein-1 and osteopontin differentially regulate monocytes recruitment in experimental glomerulonephritis. Kidney Int. 2001;59:1762–9. [DOI] [PubMed] [Google Scholar]
- 21.Persy VP, Verhulst A, Ysebaert DK, De Greef KE, De Broe ME. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int. 2003;63:543–53. [DOI] [PubMed] [Google Scholar]
- 22.Briggs TA, Rice GI, Daly S, Urquhart J, Gornall H, Bader-Meunier B, et al. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet. 2011;43:127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An J, Briggs TA, Dumax-Vorzet A, Alarcon-Riquelme ME, Belot A, Beresford M, et al. Tartrate-Resistant Acid Phosphatase Deficiency in the Predisposition to Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017;69:131–42. [DOI] [PubMed] [Google Scholar]


