1. Introduction
The recent pandemic of COVID 19, caused by SARS-CoV-2, has been confirmed on May 8th in more than 3.5 million cases worldwide, carrying a mortality of approximately 2–7% [1]. The elderly and patients with comorbidities may develop a severe progression of COVID-19, even whether the rate risk in immunosuppressed is still unknown [2].
Multiple sclerosis (MS) is a chronic disease characterized by inflammation, demyelination, and neurodegeneration and a common cause of disability in young adults. The therapeutic landscape of MS includes immunomodulating and immunosuppressant drugs, which are associated with an increased infectious risk and require a specific surveillance [3].
We describe the course of COVID-19 in two Relapsing Remitting MS (RRMS) patients, treated with fingolimod and teriflunomide. Fingolimod is a sphingosine-1-phosphate receptor modulator which sequesters lymphocytes in lymph nodes [4]. Teriflunomide is an immunomodulatory drug inhibiting pyrimidine de novo synthesis by blocking the enzyme dihydroorotate dehydrogenase [5].
Plasma and serum aliquots were obtained by centrifugation from blood samples of patients and collected at day 7, 21, 28, and 35 after the SARS-CoV-2 infection diagnosis. Two commercially available ELISA kits (SARS-COV-2 NP IgG and SARS-CoV-2 S1RBD IgG ELISA KIT, immunodiagnostic systems, 10 Didcot Way, United Kingdom) were used to detect the presence of IgG antibodies directed against nucleocapsid protein (NP) and spike protein S1 receptor binding domain (S1RBD) of SARS-COV-2. Results were expressed as optical density measurements using a microplate reader with a 450 nm filter (Od450). The Od450 cut-off was established by performing the test on a panel of negative controls, defining a minimum cut-off of 0.199 Od450 for anti-NP and 1.000 Od450 for anti-S1RBD [6].
2. MS course
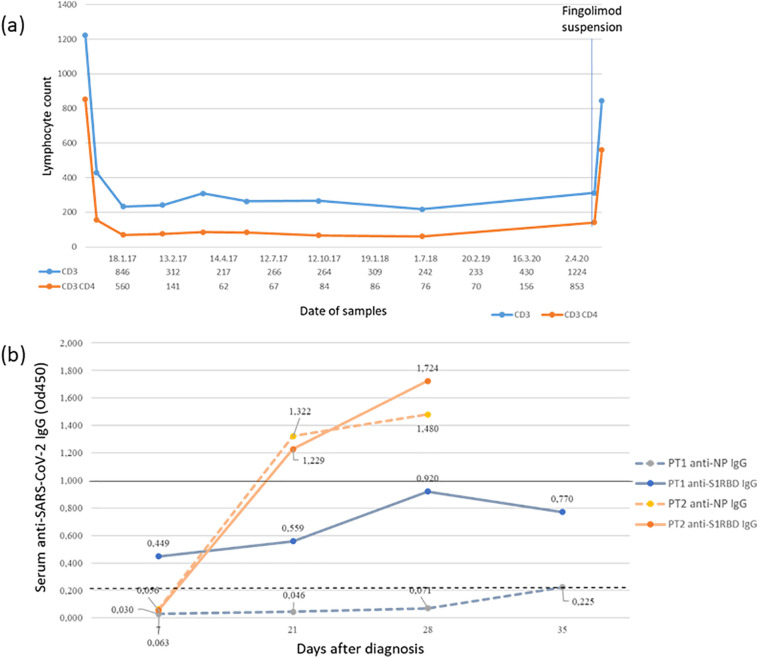
Case 1 is a 34-years old woman, who was diagnosed with RRMS in May 2016 after a myelitis. In June 2016 she started dimethyl fumarate, which was discontinued one year later due to relapse activity, and thereafter she started Fingolimod. In the following two years her lymphocyte CD3+ count waves between 220 and 430 cells/μl, of which 60–160 cells/μl CD3 + CD4+ (Fig. 1a). In January 2020, her neurological disability evaluated at the Expanded Disability Status Scale (EDSS) was 2.5 and no evidence of disease activity was reported in the previous year.
Fig. 1.
(a) CD3+ and CD3 + CD4+ lymphocyte count after start of therapy with fingolimod until the hospitalization for Covid-19 in case 1 (b) anti spike protein S1 receptor binding domain (S1RBD) and anti-nucleocapsid (NP) IgG response to SARS-CoV-2 in patient 1 and 2 detected at 7,21,28,35 days after diagnosis. Dotted line: Cut off of anti-NP IgG positivity (=0.199). Continuous line: Cut off of anti-S1RBD IgG positivity (=1.000). Od450: optical density measurements using a microplate reader with a 450 nm filter.
Case 2 is a 58-years old woman, who was diagnosed with RRMS in April 2015 after an episode of right limbs numbness. In October 2015, she started intramuscular interferon beta-1a, which was suspended in March 2016 due to lack of tolerance, and she was switched to teriflunomide. In March 2020, her EDSS was 2.5 and no evidence of disease activity was reported in the previous year.
3. Clinical report
On 12th March, case 1 reported hyperpyrexia (maximum temperature 38.5 degrees) and pharyngeal pain that were not responding adequately to paracetamol therapy. The patient resulted positive at the nasopharyngeal swab for Sars-CoV2 and she was admitted to the Infectious Diseases ward. The blood tests performed showed lymphopenia (3050 cells/μl, normal range (nr):800–5000 cells/μl), mild thrombocytopenia, high levels of C-reactive protein (CRP) (40.5 mg/L; nr:<2.9 mg/L), erythrocyte sedimentation rate (38 mm/h, nr <5 mm/h) and human IL-6 (2.3 pg/mL, nr < 1.8 pg/mL). Thoracic X-ray examination performed was normal. The patient has been treated with Lopinavir/Ritonavir (LPV/r) 200/50 mg two tablets twice a day and Hydroxychloroquine Sulphate (HCQ) 200 mg one tablet twice a day. Treatment with fingolimod was immediately discontinued after the infection was detected. During the hospitalization, case 1 never developed respiratory difficulties, the highest body temperature recorded was 37.4 degrees and blood oxygen saturation remained within normal limits. Lymphocyte count was tested at the admission time showing a reduction of lymphocytes related to immunosuppressive therapy (Fig. 1a).
On 20th March, case 2 developed fever (up to 39 °C) and diarrhea. She went to the Emergency Department and she was admitted to the COVID-19 Unit after the nasopharyngeal swab resulted positive for SARS-CoV-2. The patient was found to have diffuse interstitial pneumonia at lung ultrasonography. Blood tests showed lymphopenia (1311 cells/μL, nr: 800–5000 cells/μl) and elevated inflammation markers, CRP: 36.4 mg/L (nr <2.9 mg/L) and D-Dimers: 1045 μg/L (nr <500 μg/L), arterial gas analysis showed a mild type I respiratory failure (pH: 7.51, pO2: 58 mmHg, pCO2: 28 mmHg). Furthermore, lymphocyte CD3 + CD4+ were 1487 cells/μL (nr: 467–1563 cells/μl). An antiviral therapy was started with LPV/r 200/50 mg two tablets twice a day, HCQ 200 mg one tablet twice a day, Azithromycin 500 mg 1 tablet a day, Enoxaparin treatment was started (80 mg/die), due to the high D-Dimers levels, and an oxygen support with Venturi Mask 6 lt/min was supplied. During hospitalization, no further worsening of the respiratory function was detected. Antiviral therapy was discontinued after ten days, while the oxygen support was slowly reduced. The patient was discharged in good clinical conditions, after two consecutive negative nasopharyngeal swab tests were obtained.
We studied the IgG serum response to SARS-CoV-2 (Fig. 1b). A limited immunoglobulin response, with a slightly positive value of serum anti-NP IgG detected only at day 35 was reported in case 1 (day 7 0.03, day 21 0.046, day 28 0.071 and day 35 0.225), while a negative value was detected for anti-S1RBD IgG. Case 2 showed an adequate antibody production by the third week from the infection for both anti- NP (day 7 0.063, day 21 1.322, day 28 1.48) and anti-S1RBD (day 7 0.056, day 21 1.229, day 28 1.724). No symptoms related to COVID-19 infection were reported in family members of both patients.
4. Discussion
With this case series, we describe the clinical outcome and serum IgG antibody response to SARS-CoV2 infection in two RRMS patients treated with immunomodulatory/immunosuppressive therapies. The immunosuppression treatment is not currently recommended to be used for SARS-CoV-2 infection, despite some authors hypothesize that a timely and appropriate immunosuppressive therapy should be considered to prevent pneumonia development or worsening [2].
During the hospitalization, the fingolimod exposed patient did not develop clinical and radiological signs of pneumonia and the treatment has been resumed after two negative nasopharyngeal swabs. Despite the fingolimod discontinuation and the increase of lymphocytes count, the patient did not develop a rebound of MS activity and no steroid therapy was needed. The patient exposed to teriflunomide just develop a slight dyspnoea, and the therapy was reinitiated at hospital discharge.
Computed Tomography (CT) scan of chest was not performed in our patients since the high-resolution imaging was reserved, due to the elevated number of COVID-19 patients, to those with more severe disease.
The IgG serum response to SARS-CoV-2 was quite different: the patient treated with fingolimod demonstrated a limited immunoglobulin response and, conversely, the teriflunomide treated patient showed an adequate antibody production which was consistent with seroconversion time demonstrated in immunocompetent patients [7]. Notably, the anti-nucleocapsid response is particularly useful to demonstrate an immune response to a past infection; conversely, the production of “spike protein” (S1RBD) of Sars-Cov-2 is considered pivotal in terms of SARS-Cov-2 immune neutralizing potential, although controversies on possible risk of re-infection in patients with high anti-S1RBD IgG titre still persist. Interestingly previous studies regarding the preservation of protective immune responses after seasonal influenza vaccination shown similar differences in patients treated with fingolimod and teriflunomide [8,9].
Assuming that COVID-19 will be circulating until vaccination and/or herd immunity is achieved active measures should be implemented in the management of MS patients in order to prevent future COVID-19 outbreak (i.e. increasing the use of telemedicine) [9].
In conclusion, even considering the protective factors as the young age of our patients [10] and within the limits of a case report, the role of immunomodulation and immunosuppression therapies may have been protective at the expense of an adequate seroconversion (Case 1). However, possible benefits of reducing inflammation should be carefully weighed up against the risk of inhibiting antiviral immune response, with a consequent perpetuation and worsening of the illness.
Declaration of Competing Interest
Pietro Iaffaldano has served on scientific advisory boards for Biogen Idec and has received funding for travel and/or speaker honoraria from Sanofi-Aventis, Biogen Idec, Teva and Novartis. Maria Trojano has received honoraria for consultancy or speaking from Biogen, Sanofi-Aventis, Merck Serono and Bayer-Schering and research grants from Merck Serono, Biogen and Novartis. Damiano Paolicelli received honoraria for consultancy and/or speaking from Biogen Idec, Merck-Serono, SanofiAventis, TEVA, Novartis and Genzyme. No conflicts of interests need to be declared for Luca Bollo, Tommaso Guerra, Davide Fiore Bavaro, Laura Monno, Annalisa Saracino and Gioacchino Angarano.
References
- 1.COVID-19 Situation Reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (Accessed May 17, 2020)
- 2.Conforti C., Giuffrida R., Dianzani C., Di Meo N., Zalaudek I. COVID-19 and psoriasis: is it time to limit treatment with immunosuppressants? A call for action. Dermatol. Ther. 2020:e13298. doi: 10.1111/dth.13298. Published online March 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bavaro D.F., Fiordelisi D., Angarano G., Monno L., Saracino A. Targeted therapies for autoimmune/idiopathic nonmalignant diseases: risk and management of opportunistic infections. Expert Opin. Drug Saf. 2020:1–25. doi: 10.1080/14740338.2020.1767585. Published online May 31. [DOI] [PubMed] [Google Scholar]
- 4.Blanc C.A., Grist J.J., Rosen H., Sears-Kraxberger I., Steward O., Lane T.E. Sphingosine-1-phosphate receptor antagonism enhances proliferation and migration of engrafted neural progenitor cells in a model of viral-induced demyelination. Am. J. Pathol. 2015;185(10):2819–2832. doi: 10.1016/j.ajpath.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott L.J. Teriflunomide: a review in relapsing-remitting multiple sclerosis. Drugs. 2019;79(8):875–886. doi: 10.1007/s40265-019-01135-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhao R., Li M., Song H. Early detection of SARS-CoV-2 antibodies in COVID-19 patients as a serologic marker of infection. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa523. Published online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J., Yuan Q., Wang H. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa344. Published online March 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-Or A., Freedman M.S., Kremenchutzky M. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology. 2013;81(6):552–558. doi: 10.1212/WNL.0b013e31829e6fbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kappos L., Mehling M., Arroyo R. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015;84(9):872–879. doi: 10.1212/WNL.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 10.Di Gennaro F., Pizzol D., Marotta C. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int. J. Environ. Res. Public Health. 2020;17:8. doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]