Abstract
The growing field of immune metabolism has revealed promising indications for metabolic targets to modulate anti-cancer immunity. Combination therapies involving metabolic inhibitors with immune checkpoint blockade (ICB), chemotherapy, radiation, and/or diet now offer new approaches for cancer therapy. However, it remains uncertain how to best utilize these strategies in the context of the complex tumor microenvironment (TME). Oncogene-driven changes in tumor cell metabolism can impact the TME to limit immune responses and present barriers to cancer therapy. These changes also reveal opportunities to reshape the TME by targeting metabolic pathways to favor immunity. Here we explore current strategies that shift immune cell metabolism to pro-inflammatory states in the TME and highlight a need to better replicate physiological conditions to select targets, clarify mechanisms, and optimize metabolic inhibitors. Unifying our understanding of these pathways and interactions within the heterogenous TME will be instrumental to advance this promising field and enhance immunotherapy.
Introduction
Cancer metabolism has been studied extensively over the past two decades and it is widely accepted that oncogenic transformation can cause cancer cells to adapt a well-characterized metabolic phenotype that can profoundly influence the tumor microenvironment (TME) (Vander Heiden & DeBerardinis, 2017). The TME is composed of diverse cell populations in a complex matrix, which often has limited or poorly differentiated vasculature, creating inefficiencies of nutrient and/or oxygen delivery as well as waste removal. This poor vascular exchange can result in nutrient limitation in the TME, while the bioenergetic demands of rapidly proliferating cancer and immune cells compete for nutrients necessary to carry out an anti-tumor defense (De Berardinis & Chandel, 2016; Pavlova & Thompson, 2016). In these settings, the metabolic microenvironment of tumors themselves can present an immune suppressive environment to be overcome. Notably, this harsh environment forces infiltrating immune cells to undergo metabolic adaptations associated with tolerant phenotypes. Ultimately these metabolic changes in immune cells can undermine the effectiveness of the anti-tumor immune response.
Strategies to alter cell metabolism now offer promising opportunities for cancer therapies. Specifically, identifying targets that suppress or alter cancer metabolism to improve the TME nutrient availability or that modulate immune metabolism to bolster inflammation will help maximize the efficacy of cancer therapies. A key barrier is that many metabolic pathways used by cancer cells are also important for inflammatory immune function and blocking those pathways may be counterproductive. Further, many metabolic pathways can have context specific effects, such that different microenvironments may lead to different outcomes. Despite these apparent challenges, in vitro models that enforce assay uniformity are often used to identify metabolic targets which then often fail to translate to the heterogenous TME in vivo or in diverse cancer types or patients. The inability to replicate physiologic conditions in vitro and lack of relevant ex vivo model systems thus ultimately delays the development of effective treatments. This review explores current strategies and molecular targets under investigation to modulate immune metabolism in the TME and describe the limitations of in vitro culture conditions in metabolic studies. Improving our understanding of these therapeutic targets through both mechanistic and global systems-level views of the heterogenous TME will help advance the translation of these approaches.
Metabolic profiles and nutrient requirements of activated immune cells
Immune cells develop specific metabolic profiles during times of activation, adaptation to different tissue environments, and during periods of inflammation or disease (Andrejeva & Rathmell, 2017). Importantly, metabolic changes also occur within immune cells following migration into a TME. Different types and subsets of immune cells have distinct nutrient requirements for their metabolic programming and therefore are faced with unique challenges upon migration to the TME (Figure 1).
Figure 1. Tumor microenvironment and associated immune cell metabolism.
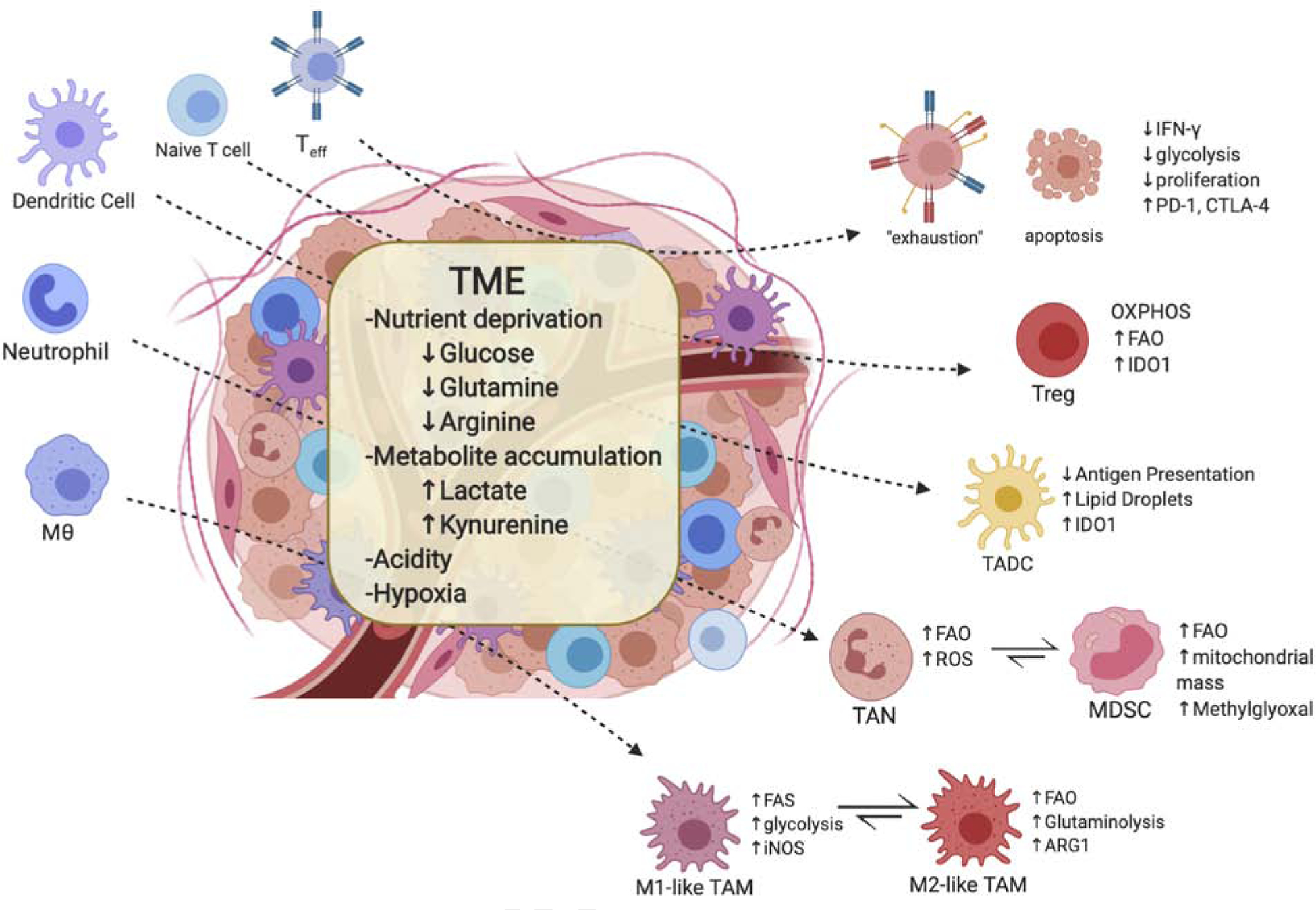
The tumor microenvironment (TME) is often characteristic of nutrient competition, low pH, limited oxygen, and accumulation of metabolites. Such conditions, in general, results in immunosuppressive or tolerogenic phenotypes of immune cells and encourages metabolism that rely more on oxidative phosphorylation and fatty acid oxidation to fulfill energy needs. Additionally, the TME accelerates T effector cell exhaustion followed by increased immune checkpoint expression on these cells. These conditions also promote differentiation and accumulation of Treg, M2-like macrophages, and MDSCs. The TME also produces unique subsets of myeloid cells known as tumor-associated dendritic cells (TADC) and tumor associated neutrophils (TAN) that have yet to fully characterized but are suggested to have suppressive or tolerant phenotypes. (MDSCs, myeloid-derived dendritic cells; Teff, effector T cell)
T cell subsets serve as well-characterized examples of these adaptations within a TME due to their distinct metabolic programs. Briefly, activated T cells ramp up both glycolytic and glutaminolytic metabolism, preferentially using aerobic glycolysis over TCA-coupled OXPHOS for ATP production and biosynthesis for clonal expansion (E. L. Pearce, Poffenberger, Chang, & Jones, 2013). Activation with CD28 co-stimulation, however, can increase spare mitochondrial capacity and enhance respiration under low glucose conditions (Frauwirth et al., 2002; Klein Geltink et al., 2017). By contrast, regulatory T cells (Tregs) rely on OXPHOS and fatty acid oxidation (FAO) to support their survival and differentiation (Beier et al., 2015; P. C. Ho & Liu, 2016; Michalek et al., 2011) and may uniquely activate AMPK signaling that promotes mTOR Complex I activity to drive catabolic processes (Delgoffe et al., 2009; Kishton et al., 2016).
Myeloid cells also exhibit characteristic metabolic phenotypes upon activation. Tumor antigens activate DCs through Toll-like receptor (TLR) signals which lead to rapid increases in glycolysis and fatty acid synthesis (FAS). DCs remain glycolytic, which is essential for continued survival and is controlled by mTOR and hypoxia-inducible factor 1α (HIF1α) (E. J. Pearce & Everts, 2015). Similarly, macrophages reprogram their metabolism in response to TME through activated glycolysis, FAS and altered nitrogen cycle metabolism while maintaining a tumor promoting function of cytokine production and angiogenetic factor secretion. Tumor associated macrophages (TAMs) are categorized into M1-like and M2-like phenotypes, although this dichotomy does not fully appreciate the heterogeneity of TAM subsets. M1-like TAMs reflect a more inflammatory phenotype and use glycolysis, FAS and amino acid metabolism to support their function. Conversely, M2-like TAMs exhibit a more suppressive phenotype, utilizing the TCA cycle and FAO (Netea-Maier, Smit, & Netea, 2018). Of note, however, limited information is available on human macrophage phenotypes and macrophage metabolism data have been largely derived from murine studies. Despite this, there appears to be a clear distinction between the metabolic programs of M1-like and M2-like TAMs which could pose unique targeting strategies requiring further investigation in primary human macrophages (Al-Khami, Rodriguez, & Ochoa, 2017).
Neutrophils and myeloid derived suppressor cells (MDSC) have complex roles in the TME that can promote cancer progression. Neutrophils are often described as purely glycolytic to support ATP generation and microbial killing. However, under glucose-restricted conditions as may occur in TMEs, neutrophils engage in oxidative mitochondrial metabolism and FAO to support NADPH oxidase-dependent ROS production (Rice et al., 2018). Although neutrophils are generally inflammatory they have been implicated in cancer progression, possibly through association or plasticity to MDSCs (Rapoport, Steel, Theron, Smit, & Anderson, 2020), which can potently suppress innate and adaptive immunity. The MDSC subset of myeloid cells and their metabolic profile has yet to be fully characterized but are considered a therapeutic target particularly within tumors. In vitro generated MDSCs display an increase in glycolysis, glutaminolysis, and TCA cycle activity with an increased AMPK activation (Hammami et al., 2012). Interestingly, MDSCs in vivo display unique phenotypes depending on tissue origin and microenvironment. For example, tumor-infiltrating MDSCs have increased mitochondrial mass and preferentially use FAO over glycolysis as a primary source of energy compared to peripheral MDSCs (Al-Khami, Rodriguez, & Ochoa, 2016; Hossain et al., 2015). Recently, the glycolytic by-product methylglyoxal has been identified as a more specific marker for MDSCs and may play a key role in the suppression of T effector function (Baumann et al., 2020).
Targeting hypoglycemia within the TME
The enhanced glycolytic activity of cancer cells combined with poor vascular exchange may result in a limited availability of glucose within the TME, although this depends on tumor type. While glucose can be available in some tumors (Siska et al., 2017), it may be partially depleted in the interstitial fluid of others compared to plasma or healthy tissue (M. R. Sullivan et al., 2019). Co-culture of lymphoma cells and T cells revealed that lymphoma cells impose a nutrient deprivation or a metabolic competition on T cells that limits their ability to produce effector cytokines (C.-H. Chang et al., 2013; C. Chang et al., 2015). Reduced glucose availability can limit T cell expansion and effector function through competition, altered apoptosis sensitivity (Voss, Larsen, & Snow, 2017), or lead to eventual “exhaustion”. Low glucose environments also induce FOXP3 expression, increasing T cell differentiation from effector T cells to Tregs (Macintyre et al., 2014). Additionally, accelerated glycolytic metabolism in cancer cells increases tumor-derived granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF to promote infiltration of MDSCs, which further suppress effector T cell function (W. Li et al., 2018).
Targeting low glucose availability in the TME has had mixed results. Reducing glycolytic metabolism in cancer cells by either inhibiting glycolytic regulatory enzymes or using the competitive glucose analog 2-DG may be effective at reducing cancer cell proliferation and has been shown to support the formation of long term memory CD8+ T cells, but can also suppress the proliferation and function of tumor infiltrating effector immune cells (Bonuccelli et al., 2010; Kouidhi, Ayed, & Elgaaied, 2018; Sukumar et al., 2013; D. Zhang et al., 2014). For example, Ho et. al discovered that overexpression of a gluconeogenic enzyme PCK1 can improve T cell antitumor responses upon infiltration into a glucose deprived TME (P.-C. Ho et al., 2015) to support an important role for glycolytic intermediates in the proliferation and function of effector T cells. Most recently, neutralization of the dicarbonyl activity of methylglyoxal produced by MDSC could improve efficacy of cancer immune therapy (Baumann et al., 2020).
Many studies have indicated that immune checkpoints including as PD-1, PD-L1 and CTLA-4 act in part by suppressing metabolic reprogramming of immune cells and inhibit glycolysis while increasing lipolysis and FAO (Parry et al., 2005; Patsoukis et al., 2015; Qorraj et al., 2017). Blockade of these pathways with checkpoint inhibitors thus rescue the effector function of tumor infiltrating lymphocytes (TILs) in part by restoring glycolysis and favorable anabolic metabolic pathways (Figure 2). Consistent with this model for checkpoint action, antibodies against CTLA-4, PD-1, and PD-L1 corrected tumor-induced glucose restrictions on T cell metabolism, restored T cell glycolysis, and IFN-γ production (C. Chang et al., 2015). However, the effects of checkpoint blockade therapy on the metabolism of other immune cells warrants further attention. For example, myeloid cell-specific ablation of PD-1 more effectively decreased tumor growth than T cell-specific ablation, yet the metabolic impact of PD-1 blockade on myeloid cells remains uncertain (Strauss et al., 2020).
Figure 2. Potential therapeutic targets within glucose metabolism pathways.
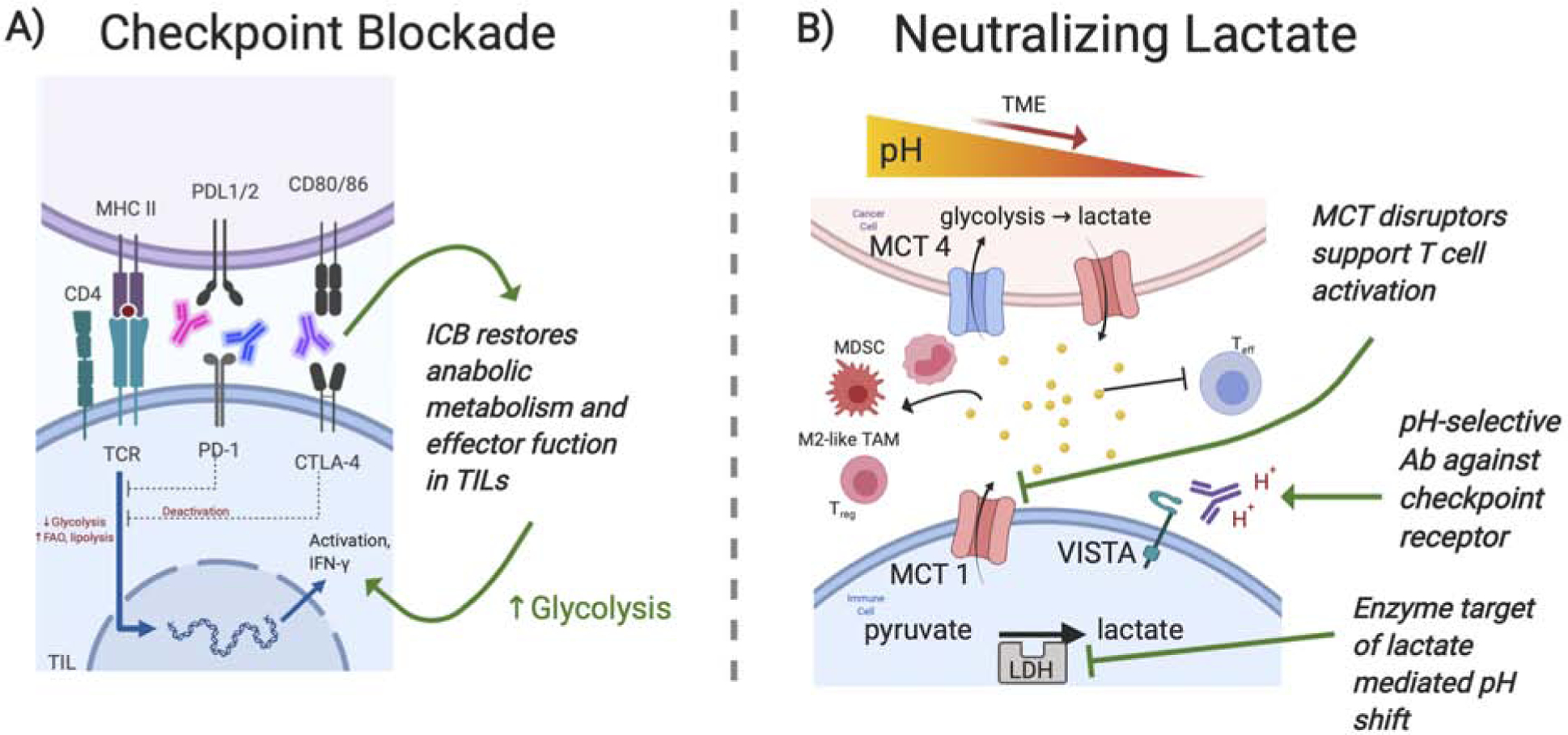
A) Immune checkpoint blockades have emerged as a promising immune targeted strategy that has been approved clinically for a variety cancer types. Increased expression of checkpoint receptors is often the result of low glucose, acidity or lactate within the tumor microenvironment and engagement of these receptors causes an immunosuppressive phenotype characteristic of decreased glycolysis and increased FAO. Antibodies targeted against these checkpoint receptors have been successful at restoring glycolysis which in turn supports anti-tumor effector functions within immune cells. However due to the variability of immune checkpoint expression on immune cells, the most promising utilization will involve combination therapies coupling ICB with one or multiple other metabolic targets. B) Accumulation of lactate in the tumor microenvironment has been found to promote immunosuppressive immune cells through M2 macrophage polarization, MDSC infiltration, Treg survival and by inhibiting T effector functions. Strategies that decrease lactate accumulation through inhibiting lactate producing enzyme LDH, inhibiting lactate transporters, MCT1/4, or neutralizing lactic acid induced acidity have proved effective at improving antitumor immune cells. Recently utilization of acidic pH selective Abs against checkpoint receptor VISTA provides an intriguing strategy that exploits the TME to improve treatment specificity. However, targeting these pathways can be TME context and immune cell specific therefore a clear mechanism for these anticancer effects is still unclear. (ICB, Immune Checkpoint Blockades; LDH, lactate dehydrogenase; MCT, monocarboxylate transporter)
Although the use of checkpoint blockades has greatly improved cancer therapy outcomes, it is important to note that the current strategies have been most effective against highly glycolytic tumors (Darvin, Toor, Sasidharan Nair, & Elkord, 2018; Najjar et al., 2019) which are characterized by a high neoantigen load, high levels of TILs, low MDSCs and increased secretion of IFN-γ. In contrast, poor responsiveness to PD-1 blockade may correlate less well with the extent of tumor glycolysis than high rates of tumor oxidative phosphorylation (Najjar et al., 2019). These data show clear links between cell metabolism and checkpoint blockades such that combinations with metabolic interventions to disrupt cancer metabolism may offer opportunities to increase the efficacy of checkpoint inhibitors across diverse tumor types. It is important to consider that each of these targets can have differential effects and are cell subset and context dependent.
Targeting Lactate in the TME
Enhanced glycolytic activity of cancer cells under hypoxic conditions increases the buildup of lactate which acidifies the TME (Brand et al., 2016; Choi, Collins, Gout, & Wang, 2013; Colegio et al., 2014). Lactate production can be increased 40-fold in tumor cells, and the lactate producing enzyme lactate dehydrogenase (LDH) is positively correlated with tumor size and clinical severity (Girgis et al., 2014; X. Sun et al., 2014). Lactic acid can inhibit effector T cell function through decreased proliferation and IFN-y production (Fischer et al., 2007; Mendler et al., 2012) and decrease the pH within the TME to further contribute to immune suppression. Low pH was also found to dramatically promote the differentiation of monocyte derived dendritic cells which exhibit reduced glucose consumption, upregulated mitochondrial respiration genes and inhibited mTORc1 activity (Erra Díaz et al., 2020). Hence, neutralization of low pH in the TME may have a meaningful impact on improving the efficacy and outcomes of anticancer immunotherapy (Figure 2). Notably, buffering the TME with oral bicarbonate can inhibit tumor growth when combined with anti-PD-1 immunotherapy in a melanoma model, and improve survival when combined with adoptive T-cell transfer (Koltai, 2016; Kouidhi et al., 2018). More recently, VISTA was found to selectively suppress T cells under acidic pH and that the development of acid pH selective antibodies against VISTA or its receptor, PSGL-1, reversed immune suppression in vivo (Johnston et al., 2019). In addition, co-blockade of VISTA and PD-1 resulted in tumor rejection within the MC38 tumor model. This work supports a promising approach that exploits the acidic TME to improve the selectivity of treatments and enhance ICB therapy.
In addition to maintaining physiologic pH in the TME, lactate accumulation also has direct immunosuppressive effects on immune cells. First, lactate inhibits monocyte activation and dendritic cell differentiation (Gottfried et al., 2006; Nasi et al., 2013) and promotes M2-polarization through increased arginase and HIF-1α stabilization (Colegio et al., 2014). Lactate also inhibits NK cell function and increases MDSCs further contributing to the suppressive microenvironment (Husain, Huang, Seth, & Sukhatme, 2013). Additionally, cancer cell-generated lactate activates the G protein coupled receptor (GPR81) on immune cells and endothelial cells, which promotes angiogenesis and immune evasion (Brown & Ganapathy, 2019). Knockdown of this signaling pathway in mice reduced the production of IL-10 and suppressed the generation of Tregs (Feichtinger & Lang, 2019; Ranganathan et al., 2018). Finally, lactic acid can also boost Treg survival in the TME given their robust ability to oxidize exogenous lactate (Angelin et al., 2017). These data suggest that targeting lactate-responsive pathways or receptors such as GPR81 may provide a promising approach to cancer therapeutics (Faubert et al., 2017).
One target of particular interest is the lactate producing enzyme lactate dehydrogenase (LDH). Targeting LDH has shown promising anticancer effects (Yeung et al., 2019), but can have differential effects to immune cell function. While inhibition of LDH using small-molecules or siRNA may ameliorate intratumor lactate levels and tumor regression (de la Cruz-López, Castro-Muñoz, Reyes-Hernández, García-Carrancá, & Manzo-Merino, 2019), these preclinical results require additional study. Additionally, LDH inhibition can lead to a decrease in T cells and IFN-γ production (Kouidhi et al., 2018). While inhibition of lactate may provide a novel therapeutic in cancer, clinical evaluation of these drugs and contradictory effects on immune function must be considered.
A different approach to targeting lactate is to inhibit lactate transporters. To this end, monocarboxylate transporter (MCT) 1 and/or 4 inhibitors have been effective at increasing intracellular lactate and resulted in decreased glycolytic rate with enhanced cell death of cancer cell lines (de la Cruz-López et al., 2019). These treatments have been reported to enhance IL-2 and IFN-γ secretion in T cells (Chirasani et al., 2012), suggesting that MCT disruptors could suppress tumor cell proliferation while supporting T cell activation (Kouidhi et al., 2018). Redundancy between these two transporters, however, makes a dual strategy likely necessary to achieve significant broad impact. For example, dual inhibition of MCT 1 and 4 with syrosingopine showed promise in combination with metformin by causing a lethal energy crisis in cancer cells (Benjamin et al., 2018).
Targeting amino acid metabolism
Amino acids provide components and substrates for a variety of critical processes in cell metabolism and physiology. While each individual amino acid plays multiple specific roles, glutamine, arginine, and tryptophan have been best described to modulate both tumor progression and immunity (Figure 3).
Figure 3. Promising strategies that alter amino acid metabolism within the TME.
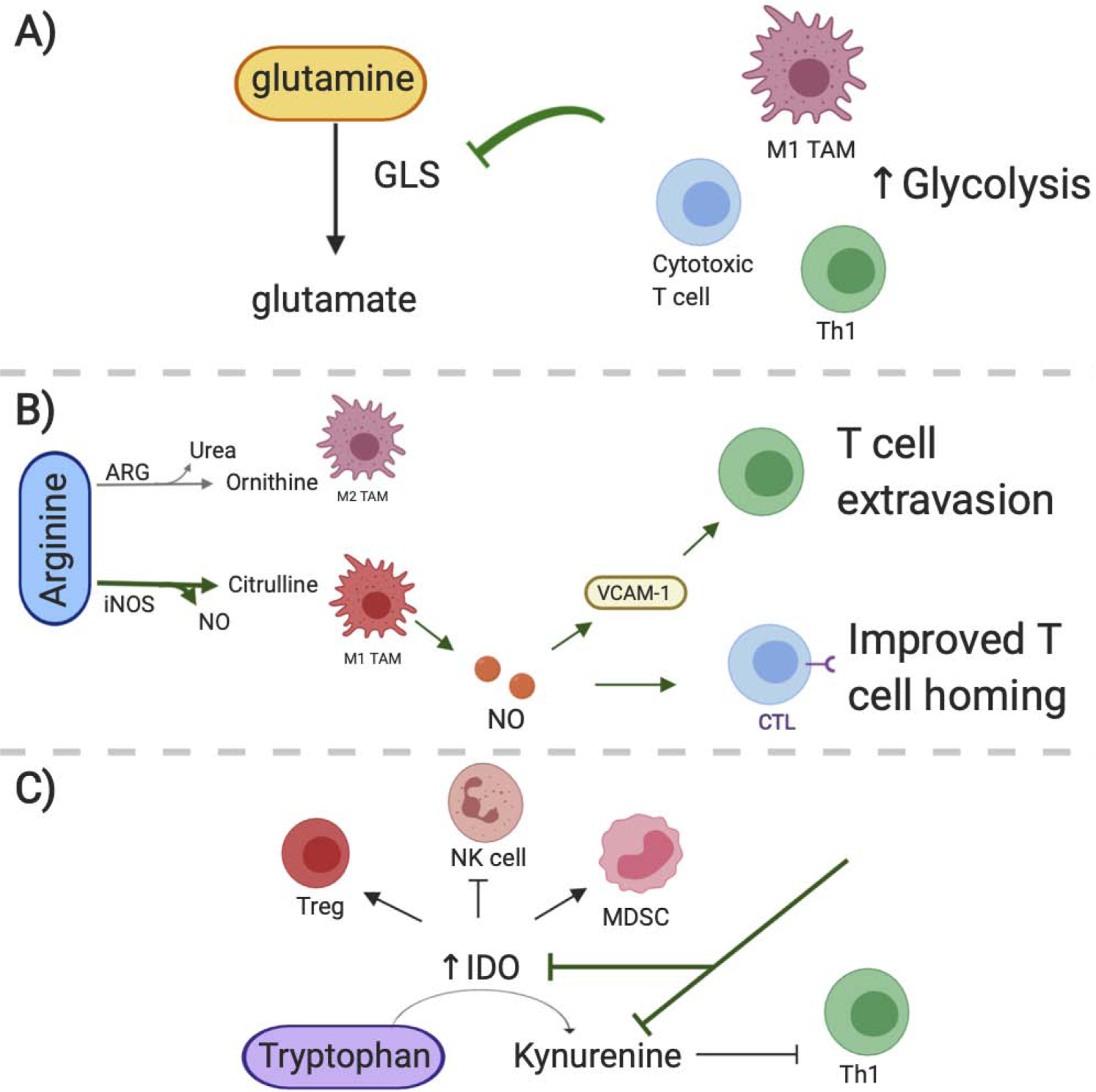
A) Glutamine metabolism is altered as a result of the tumor microenvironment. Inhibiting glutaminolysis through blocking glutaminase (GLS) has proved to promote a proinflammatory macrophage phenotype. However specific T cell subsets can be GLS dependent or independent and thus GLS targets can have differential effects in T cell populations. B) Therapies that facilitate the metabolism of arginine through iNOS have beneficial effects in cancer therapies. Macrophages utilizing iNOS as opposed to ARG exhibit an M1 phenotype and their NO secretion promotes T cell extravasion and homing against tumors. C) Adaptive immune cell subsets, including Tregs, tolerogenic DCs and MDSCs have increased IDO expression which is the enzyme responsible for metabolizing tryptophan to kynurenine. Targeting IDO activity may suppress these adaptive immune subsets within the tumor. Further reducing kynurenine accumulation in the microenvironment can ameliorate its immune suppressive effects against T cells. (iNOS, inducible nitric oxide synthase; ARG, arginase; IDO, indoleamine 2,3 dioxygenase)
Glutamine and Glutamate
After glucose, glutamine is the most abundant amino acid in circulation and the most rapidly consumed nutrient by many cultured cancer cells and (Lukey, Katt, & Cerione, 2017). Glutamine is used as an anaplerotic fuel source to maintain TCA flux in cancer cells undergoing aerobic glycolysis or can be used in reductive carboxylation as a source of citrate for lipid synthesis. Additionally glutaminolysis can suppress oxidative stress, and maintain mitochondrial membrane integrity contributing to the survival of proliferating cells (Ananieva, 2015). Glutaminase (GLS) converts glutamine to glutamate and is now being targeted to suppress the metabolism of some cancers (Bott, Maimouni, & Zong, 2019; Ren et al., 2020). Blocking GLS activity can reduce glutamate which leads to redox and mitochondrial stress, to which cells respond with an increased dependence on aspartate as a means to regenerate glutamate and maintain cellular redox (Alkan et al., 2018). Targeting this pathway may also decrease cancer cell glucose consumption. However, resistance to GLS inhibition in some cancers is mediated by adapting mechanisms which increase glucose metabolism to anaplerotically maintain mitochondrial flux.
Unlike glucose which is required by both cancer and immune cells for anabolic growth, glutamine may be differentially utilized by cancer and immune cells. Interestingly, inflammatory anti-tumor immune cells appear to be less dependent or even restrained by this pathway. Macrophage subsets demonstrate inherent differences in their dependencies for glutamine. For example, M2 macrophages consume more glutamine than naive macrophages while, pro-inflammatory M1 macrophages can be suppressed by glutaminolysis (P. S. Liu et al., 2017). This differential effect appears in part due to a product of glutaminolysis, α-KG, which can change gene expression programs to favor more anti-inflammatory M2 like states. In a breast cancer model, inhibiting glutamine metabolism, decreased tumor growth through reduced MDSC recruitment and promoted their conversion to inflammatory M1 like macrophages (Oh et al., 2020). Additionally, reducing intracellular glutamine in macrophages through inhibition or genetic ablation of glutamine synthetase (GLUL) promoted an M1-like phenotype consistent with enhanced glycolysis and subsequent activation of HIF-1α (Bott et al., 2019; Palmieri et al., 2017). These data suggest that targets aimed at inhibiting glutaminolysis or reducing intracellular glutamine can inhibit suppressive myeloid subsets and provide a potential target that repolarizes TAMs from M2-like into M1-like states.
Glutamine metabolism is also increased in effector T cell activation to provide intermediates necessary for growth (O’Neill, Kishton, & Rathmell, 2016). Deprivation of glutamine has been found to alter Th1 differentiation and shift naïve CD4+ T cells towards a Treg phenotype (Klysz et al., 2015). Additionally, genetic loss of the glutamine transporter protein ASCT2 in T cells resulted in impaired generation and function of Th1 and Th17 cells, whereas Tregs were not altered (Nakaya et al., 2014). Blockade of glutamine metabolism via GLS targeting to prevent glutamine-dependent production of glutamate, however, lead to adaptive subset-specific metabolic reprogramming in T cells to differentially regulate survival, proliferation, and effector function. While Th17 cells remain dependent on GLS and were suppressed by GLS inhibition or genetic deletion, Th1 and cytotoxic T cells adapted to become GLS-independent through upregulation of glycolysis (Araujo, Khim, Mkhikian, Mortales, & Demetriou, 2017; Johnson et al., 2018; Kono et al., 2019). Indeed, broad blockade of glutamine metabolism combined with anti-PD-1 dramatically improved antitumor effects by suppressing tumor metabolism while promoting T cell glucose metabolism, epigenetic reprogramming, and cytotoxic function (Leone et al., 2019). When tested in a Chimeric Antigen Receptor (CAR)-T cell model, however, genetic deletion of GLS enhanced effector differentiation but eventually led to loss of T cell effector function (Johnson et al., 2018). This effect appeared in part due to terminal T cell effector differentiation or potential suppression of T cells through induction and activation of inhibitory receptors including PD-1. Together, studies of glutamine metabolism do highlight a potential method to separate the distinct dependencies of cancer cells and inflammatory immune cells that may allow specific immune cell targeting in immunotherapy.
Arginine and Nitric Oxide
Arginine plays important roles in a variety of biological functions including proliferation, survival and protein synthesis in both cancer and immune cells. CD8+ T cells especially benefit from L-arginine uptake by enhanced survival, memory formation and anti-tumor efficacy (Geiger et al., 2016). Arginine metabolism predominately relies on the activity of nitric oxide synthetase (NOS) and arginase (ARG) enzyme families. Due to its dual roles in both cancer and immune cells, the modulatory effect of arginine within the TME is complicated. Depletion of arginine is generally well tolerated and has been shown to have anti-tumor effects in arginine-dependent cancer types. However, many cancer cells can activate the arginine-succinate synthetase (ASS1) pathway to synthesizes arginine from citrulline to compensate for arginine starvation (Mondanelli, Iacono, Allegrucci, Puccetti, & Grohmann, 2019; Zou, Wang, Liu, Ke, & Xu, 2019). Despite being effective in some cancer types, arginine depletion can also negatively impact the immune response through increasing accumulation of MDSCs and inhibiting T cell function (Raber, Ochoa, & Rodríguez, 2012). More specifically, MDSCs expressing ARG contribute to arginine depletion in a TME to suppress antitumor T cell responses (Fletcher et al., 2015). Furthermore, increased lactate in the TME favors the catabolism of arginine by ARG over NOS, resulting in increased TAM secretion of tumor-supporting factors (Carmona-Fontaine et al., 2017; Colegio et al., 2014). Indeed, inhibiting ARG can restore arginine levels resulting in tumor regression and improved T cell function (Miret et al., 2019).
Alternatively, metabolism of arginine through NOS results in the release of NO and generally promotes an inflammatory phenotype in myeloid cells. Macrophage-derived NO induces the expression of the adhesion molecule VCAM-1 in melanoma xenografts, which is important for T-cell extravasation. In addition, NO produced by tumor-infiltrating myeloid cells was found to be important for activation of adoptively transferred cytotoxic T cells (Marigo et al., 2016). Co-transfer of CD8+ T cells with wild-type macrophages, but not with NOS-depleted macrophages, increased T-cell homing to the tumor and consequently enhanced to tumor rejection (Keshet & Erez, 2018; Sektioglu et al., 2016). Taken together these studies suggest that targeting ARG and NOS activity in myeloid cells could provide a potential for immune therapies in cancer, although the mechanisms of ARG and NOS in immune cell regulation need further elucidation.
Tryptophan and kynurenine
Unlike glutamine and arginine, tryptophan in an essential amino acid that must be taken from the diet to support physiological processes including cell growth and maintenance. Tryptophan also acts as the substrate for the kynurenine pathway dictated by the rate limiting enzymes indoleamine-2,3-dioxygenase (IDO1), IDO2 and tryptophan-2,3-dioxygenase (TDO). In cancer, dysregulated IDO1 and TDO activation may suppress antitumor immunity. In addition to potential tryptophan depletion, kynurenine can accumulate in IDO positive cancers and is, in turn, associated with poor prognosis in cancer patients (Heng et al., 2016; Ino et al., 2008; Platten, Nollen, Röhrig, Fallarino, & Opitz, 2019). Specifically, increased kynurenine correlates with defective interleukin-2 (IL-2) signaling in memory CD4+ T cells. Increased IDO1 activity prevents the activation of effector T cells, inhibits NK cell function, supports Treg activation, and promotes the expansion and activation of DCs and MDSCs (Fallarino et al., 2003, 2006; Frumento et al., 2002; Munn et al., 2005). Despite this promising pre-clinical data suggesting inhibiting IDO or reducing kynurenine may improve immune function within the TME, IDO inhibitors showed disappointing efficacy in monotherapy clinical trials (Labadie, Bao, & Luke, 2019).
IDO inhibitors may, however, still provide benefit in some combinations with chemotherapy, radiotherapy, or other immunotherapy. Indeed, IDO1 inhibitors have been investigated as a metabolic adjuvant for immune cells to boost anti-tumor immunity (M. Liu et al., 2018) and are currently under being evaluated in combination with anti-PD-1 or anti-CTLA-4 in a number of cancer settings. Previously, IDO1 inhibition combined with anti-PD-1 improved objective response rates in unresectable stage 3 and 4 melanoma patients (Lanitis, Dangaj, Irving, & Coukos, 2017). In a murine tumor model, the combination of an IDO inhibitor with an anti-tumor dendritic cell vaccine resulted in conversion of Tregs into Th17 cells that enhanced CD8+ T cell anti-tumor function (Riera-Domingo et al., 2020; Sharma et al., 2009). This area remains controversial but highlights the need for effective and physiologically relevant pre-clinical studies to best identify the conditions in which IDO1 inhibitors and modulation of tryptophan metabolism in the TME may add therapeutic value.
Targeting Fatty Acid Metabolism
Highly proliferative cancer cells show a strong lipid and cholesterol avidity, which they satisfy by either increasing uptake of exogenous lipids or hyper-activating synthesis pathways. Indeed, many cancer cells have been found to upregulate enzymes involved in lipid and cholesterol biosynthesis (Beloribi-Djefaflia, Vasseur, & Guillaumond, 2016; Wegiel, Vuerich, Daneshmandi, & Seth, 2018). Excessive lipids and cholesterol are stored in lipid droplets, which are quantitatively correlated with cancer aggressiveness (Beloribi-Djefaflia et al., 2016). For these reasons, inhibitors of fatty acid synthesis (FAS) have been an area of focus for cancer therapy, especially targeting of fatty acid synthase (FASN) (Lu et al., 2018; Mullen & Yet, 2015). However, inhibition of fatty acid metabolism in cancer cells will also have indirect consequences on immune cell function (Figure 4).
Figure 4. Manipluating fatty acid metabolism within the TME.
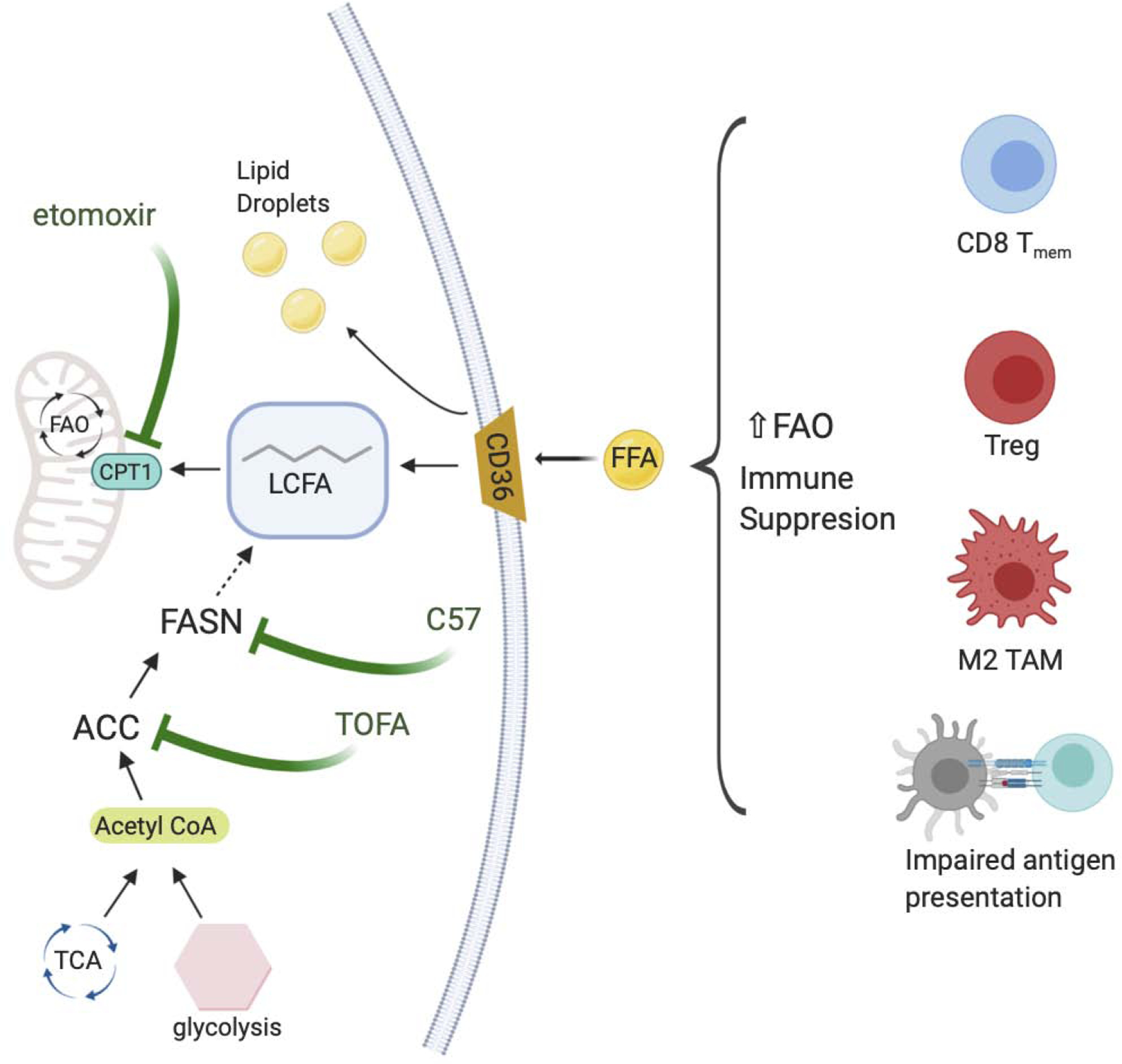
Increased fatty acids within a tumor microenvironment can result in accumulation of lipid droplets within immune cells or promote FAO. Immune suppressive phenotypes typically rely on FAO as a means to produce energy. Targets that inhibit CD36, the accumulation of lipid droplets, or the synthesis of FA via FASN have been found to ameliorate the FAO reliant immune suppressive metabolism. (FAO, fatty acid oxidation; FA, fatty acid; FASN, fatty acid synthase)
Fatty acids and lipid accumulation have individual consequences for different immune cell subsets, but lipid accumulation generally leads to immunosuppressive effects. In myeloid cells, intracellular accumulation of lipids enhances oxidative metabolism and promotes immunosuppressive function (Le Bourgeois et al., 2018). Presence of the unsaturated fatty acid oleate polarizes bone marrow-derived myeloid cells into an immunosuppressive M2 like TAM (Wu et al., 2019). Abnormal lipid accumulation of tumor infiltrating DCs (TIDCs) impairs their antigen presentation (Herber et al., 2011). In a murine model of ovarian cancer, TIDCs accumulated excess lipids exogenously from the TME resulting in poor T cell priming and activation (Jiang, Fang, Wang, Li, & Wang, 2018). Accumulation of fatty acids therefore has direct consequences within the immune cell populations in the TME, and strategies aimed to ameliorate lipid abundance either through targeting FAS or FAO have been beneficial in murine tumor models. For example, inhibition of FAS by an acetyl-CoA carboxylase (ACC) inhibitor, TOFA, normalized lipid levels in DCs to restore their activity and substantially enhanced the effects of a cancer vaccine (Herber et al., 2011). Inhibition of FASN also showed partial rescue of TIDC function in an ovarian cancer model (Jiang et al., 2018). Alternatively, inhibition of FAO may limit the immunosuppressive function of M2 macrophages (Q. Zhang et al., 2018).
Targeting fatty acid metabolism in T cells may allow for tuning of the T cell response by eliciting different effects in distinct T cell populations. On the one hand, FAO is needed for the development of CD8+ memory cells (D. O. Sullivan et al., 2014), however it is also important for the differentiation of Tregs. Thus, FAO blockade could prevent the accumulation of this immunosuppressive T cell population (Beier et al., 2015; Michalek et al., 2011) In mouse melanoma models, CD8+ TILs enhanced PPAR-α signaling and catabolism of fatty acids to preserve their effector functions (Y. Zhang et al., 2017), suggesting fatty acid uptake could be beneficial in certain TMEs. However, it has been recently found that the lipid transporter CD36 promotes Treg survival and function in tumors (Wang et al., 2020). Further inhibition of the fatty acid binding protein exclusively expressed in T cells, FABP5, enhances Treg suppression (Field et al., 2020). In a study of fatty acid metabolism and apoptosis sensitivity in primary human T cells, inhibition of FASN with C75 or siRNA knockdown significantly protected T cells from restimulation-induced cell death (RICD) by reducing the induction of FAS Ligand (Voss, Luthers, Pohida, & Snow, 2019). Therefore, FASN inhibition could be used to protect T cells from apoptosis in the TME caused by repeated TCR activation and boost T cell immunity in addition to its anti-tumor efficacy on cancer cells directly. Additionally, disruption of FAO was dispensable for RICD, highlighting the specificity of different fatty acid metabolism pathways in programming apoptosis sensitivity of immune cells. Although in a separate study, FAO inhibition exhibited delayed tumor growth and enhanced anti-tumor efficacy of T cells (Q. Zhang et al., 2018). Lipid and cholesterol metabolism are clearly complex but do offer intriguing opportunities to modulate the TME and specific immune cell populations.
Targeting Nutrient and Oxygen Sensing Pathways in the TME
AMPK
One approach to modify glucose metabolism in the TME is to focus on signaling pathways that respond to altered nutrient availability and regulate cell metabolism and fate (Figure 2). In low glucose environments, the cellular AMP:ATP ratio shifts as ATP levels decrease to activate the 5’-AMP-activated Protein Kinase (AMPK), which then induces a metabolic switch toward catabolism and OXPHOS (Eichner et al., 2019). High glucose consumption in the TME that exceeds the vascular exchange capacity may promote AMPK activation, which may aid the persistence of suppressive immune cells including TAMs, MDSCs, and Tregs (Blagih et al., 2015; Eichner et al., 2019; Michalek et al., 2011; Siska & Rathmell, 2015). Tregs specifically depend on an OXPHOS-dominant metabolism in order to function in glucose-limited, lactate-rich environments (Angelin et al., 2017). Effector T cells can also be limited by AMPK activation, through inhibition of the mTORC1 pathway and catabolic metabolism (Ma, Poffenberger, Wong, & Jones, 2017). Furthermore, AMPK activation in DCs can hinder their ability to elicit CD8+ T cell activation (Challier, Bruniquel, Sewell, & Laugel, 2013; Giovanelli, Sandoval, & Cubillos-Ruiz, 2019).
AMPK activity must be balanced, however, as while excessive AMPK may inhibit effector T cell responses, disabling AMPK activation may enhance glycolysis and inflammation while sensitizing cells to metabolic stress. In support of role, AMPKα-deficient macrophages and DCs exhibit heightened inflammatory function through increased production of inflammatory cytokines and an enhanced capacity for antigen presentation favoring the promotion of Th1 and Th17 responses (Carroll, Viollet, & Suttles, 2013). Similarly, AMPKα-deficient T cells exhibit increased glycolysis and elevated interferon-γ (IFN-γ) production (MacIver et al., 2011). However, AMPK-deficient T cells also became more sensitive to stress and apoptosis, as shown by their decreased efficacy in vivo in settings such as lung inflammation (Blagih et al., 2015). These studies suggest that AMPK disruption has shown promise to promote inflammation but may limit survival of inflammatory cells and should be carefully balanced.
One of the most well-known drugs to target AMPK is metformin, which activates AMPK signaling and is widely used for diabetes. In mice, metformin treatment can reduce the risk of certain cancers and decrease tumor burden in breast cancer models (Anisimov et al., 2005; Bodmer, Meier, Krähenbühl, Jick, & Meier, 2010). In human cohorts, metformin has also shown particular promise in colorectal cancer patients, although these analyses are complicated by comorbidities such as diabetes (Cheng et al., 2020). AMPK activation is thought to reduce tumor burden in part by slowing tumor growth, while concurrently supporting the expansion and survival of TIL in the TME (Blagih et al., 2015; Eikawa et al., 2015; Wegiel et al., 2018). Of note, metformin can also reduce glycolytic flux of cancer cells by blocking phosphofructokinase-1 (PFK1) (Hu et al., 2019) and other glycolytic enzymes induced by HIF-1α (Tyszka-Czochara, Bukowska-Strakova, Kocemba-Pilarczyk, & Majka, 2018). In multiple mouse models of AMPK-deficient leukemia, increased tumor cell death and mouse survival was observed (Chan et al., 2017; Kishton et al., 2016; Saito, Chapple, Lin, Kitano, & Nakada, 2015). Thus, while AMPK signaling may support survival of suppressive immune cell populations, it may also mitigate cell stress to support cancer cell survival. The conflicting outcomes of AMPK manipulation show that the AMPK pathway plays an important role in the TME, although it moderates a context and time dependent balance over cancer cell stress and anti-tumor immunity. Therefore, AMPK-directed therapeutics must consider both the direct effects on cancer cell death as well as the immune cell phenotype effects within the TME.
mTOR
The mTOR pathway also senses nutrients and plays a central role to promote glucose and anabolic metabolism while opposing AMPK. mTOR complex 1 (mTORC1) becomes activated in response to increased levels of glucose and amino acids, such as the essential amino acids leucine and isoleucine, to promote protein synthesis, cell proliferation and growth pathways that are important for immune cells and many cancer cell types (Saxton & Sabatini, 2017). In T cells, mTORC1 sustains glycolysis and cytokine-driven differentiation and T cell subset effector functions. Similar to the AMPK pathway, mTOR signaling occurs in a cell specific and context dependent manner which may present many challenges in the use of targeted immunotherapy in cancer. For example, the mTORC1 inhibitor rapamycin enhances CD8+ memory T cell formation (Araki et al., 2009), but is immune suppressive and abolished CD8+ antitumor responses in combination with vaccine therapy (Chaoul et al., 2015). Conversely, Treg suppressive activity can be impaired by over-activation of mTORC1 activity yet is enhanced by low levels of mTORC1 (Chapman et al., 2018; Gerriets et al., 2016). Although inhibiting mTORC1 may boost cancer therapy short-term, it raises the possibility of developing autoimmunity long-term, because the generation of effector Tregs (eTregs) is dependent on mTORC1 signaling (I.-H. Sun et al., 2018). It is important to find the right mTORC1 activity level conducive for memory formation without compromising effector T cells activity or promoting Treg. Whereas mTOR targeting in macrophages also appears to be complex and context-dependent, NK cells may offer the most promising target for mTORC1 mediated antitumor effects. Mao et al found that a high dose of IL-15 activates mTORC1 to sustain NK cell development and proliferation, and suggest that adoptive transfer of IL-15 primed NK cells could be applied clinically (Mao et al., 2016; Marçais et al., 2014). Rapamycin analogues (rapalogues) are approved for cancer therapy and can reduce cancer aerobic glycolysis and growth. These treatments, however, may not only suppress anti-tumor immunity, but also have the potential to actively promote immune suppressive environments. Similar to AMPK pathway, targeting mTOR pathway will require a nuanced approach to elicit beneficial immune responses without leading to a tumor protecting immune environment.
Hypoxia and HIF1-α in TME
Hypoxia itself has been shown to inhibit the differentiation, proliferation and IFN-γ production of T effector cells (Cho et al., 2016; Westendorf et al., 2017). Importantly, TCR activation upregulates hypoxia inducible factor (HIF) expression in CD4+ T cells, and is required for glycolytic flux and inflammation during hypoxic conditions (Chen et al., 2020; Cho et al., 2019). In reduced oxygen conditions, such as an inadequately vascularized TME, HIFs are stabilized to promote the transcription of glycolytic genes and cytokines that promote anaerobic metabolism and angiogenesis. HIF-1 and HIF-2 are attractive targets to suppress tumor metabolism by blocking this physiological response to inadequate oxygenation yet can also strongly influence potential anti-tumor responses (Figure 5). Furthermore, inadequate vascularization of some TMEs contribute to dysregulated immune cell trafficking. Hypoxia itself has conflicting effects on the function of CTLs. For example, HIF-1α-deficient CD8+ T cells had reduced tumor infiltration and killing, whereas activation in reduced oxygen conditions could enhance subsequent anti-tumor function (Gropper et al., 2017; Palazon et al., 2017). Treg are also sensitive to hypoxia and may be enhanced under low oxygen condition, as HIF-1α can bind to the promoter region of the Treg transcription factor, FOXP3, in CD4+ T cells to increase transcription and was required for Treg suppression in vivo (Clambey et al., 2012). Conversely, loss of HIF-1α in Tregs under hypoxic conditions also resulted in increased suppressive capacity, suggesting that targeting and complete loss of HIF-1α in Tregs could enhance suppressive function and impair anti-tumor immunity (Miska et al., 2019). This dual regulation of Treg function may be due in part to changes in mitochondrial pyruvate uptake and oxidative metabolism, which is consistent with increased Treg function in elevated lactate (Angelin et al., 2017).
Figure 5. Immune cell response to hypoxia within the tumor microenvironment.
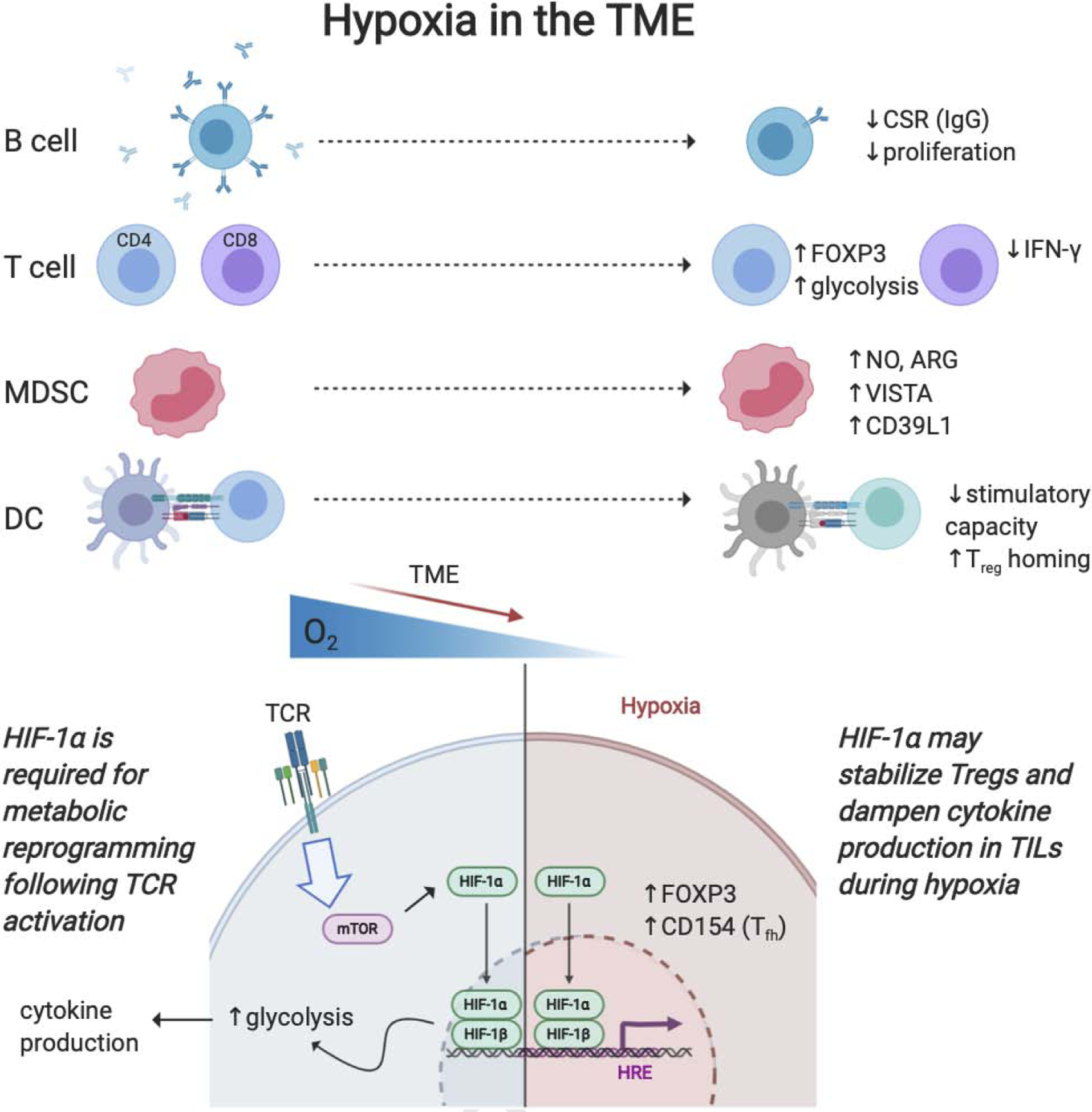
Due to limited vasculature of a rapidly growing tumor, areas within a tumor become hypoxic which alters immune metabolism to suppressive phenotypes. Hypoxic conditions promote T regulatory stimulation and reduce effector cytokine production. Hypoxia shifts macrophages towards an M2 like phenotype, promotes MDSC suppressive function, and reduces dendritic cell stimulatory effects. However, targeting hypoxia sensing pathway, HIF, proves to be immune cell specific and results in differential effects anti-tumor therapies. (HIF, hypoxia inducible factor)
Intratumoral hypoxia alters other tumor-associated immune cells to favor immunosuppressive phenotypes. HIF-1α induced arginase activity and production of nitric oxide in MDSCs leads to induction of immune suppressive molecules such as VISTA or CD39L1 (Chiu et al., 2017; Deng et al., 2019). Similarly, hypoxia inhibits the stimulatory capacity of DCs for activating T cells and instead promotes Treg homing (Winning & Fandrey, 2016). It has also been indicated that hypoxia can alter B cell function through reduced proliferation and impaired antibody class-switching (Cho et al., 2016). Targeting downstream hypoxia signaling has uncovered a promising approach for some cancer therapies and provided opportunities as a prognostic marker in tumor imaging (Huizing et al., 2019; J. Li, Zhang, Wang, & Li, 2015). Carbonic anhydrase IX (CAIX) maintains extracellular acidity under hypoxic conditions, and CAIX-targeting antibodies have been found to improve TME pH and enhance immune-mediated killing of CAIX+ tumor cells (D. K. Chang et al., 2015). Recently, anti-CAIX CAR T cells have been successful against glioblastoma in vitro and in vivo (Cui et al., 2019). Alternatively, targets aimed toward stimulating angiogenesis to better deliver oxygen to tissue may improve immunotherapy as well as drug delivery and/or immune cell infiltration. However, while considered an attractive target, modifying both the expression of immune checkpoints (Noman et al., 2019) and cytotoxic T cell function, in the context of modulating hypoxia pathways may be critical.
It is important to note that hypoxia is typically replicated in vitro as less than or equal to 5% however this determination is controversial as it based on the standard tissue culture conditions which adopt atmospheric oxygen levels ~20% rather than physiological relevant oxygen levels. In fact, oxygenation in tissues vary but generally average around 5% while most tumors exhibit <2% oxygen (Campillo et al., 2019; McKeown, 2014). Given the central role of hypoxia in the regulation of tumor progression and immune suppression, this uncertainty surrounding the definition of hypoxia needs to be better characterized in the field. Also, because hypoxia can lead to physiological changes independent of HIF it will be important to investigate how alternative pathways may be affected and to identify the level of hypoxia in their target organ and replicate these tissue specific conditions in their experimental models.
Physiologic media as a strategy to improve the relevance of in vitro studies
While immune metabolism offers a plethora of opportunities to enhance cancer therapy, a barrier to translation of pre-clinical in vitro studies of the TME has been that tissue culture conditions under which many key findings depend have been optimized for maximal cell growth rather than to mimic in vivo physiology. This is particularly evident given the shared roles for some pathways in both cancer and immune cells and the context-specific effects of many metabolic pathways discussed above. Classic in vitro cell culture conditions rely on base media such as RPMI or DMEM, which contain excessive concentrations of nutrients that can alter the metabolism of cultured cells and lead to discrepancies in assessing metabolic mechanism for translational studies. Recently, human plasma-like media (HPLM) and similar physiological media have been developed to more closely resemble nutrient and metabolite levels found in human plasma. Such developments have begun to uncover striking differences in metabolic phenotype and drug efficacy compared to classic media. For example, the low abundance of uric acid found in RPMI resulted in an increased sensitivity of cancer cells to 5FU which overinterpreted the therapeutic effects of chemotherapies (Cantor et al., 2017). Further, the proliferation of cancer cells has been shown to depend less on mitochondrial respiration when cultured with high concentrations of pyruvate than HPLM, as indicated by their decreased sensitivity to metformin (Gui et al., 2017). Additionally, increased cystine found in classic media enhances glutamine consumption and dependency of cancer cells in culture (Muir et al., 2017). In cells cultured in traditional base media, abnormally high concentrations of arginine reverses the direction of the reaction catalyzed by the urea cycle enzyme arginosuccinate lyase, which is a metabolic feature not observed in cancer cells grown in physiological media, nor in mammary orthotopic xenografts (Ackermann & Tardito, 2019). These collective observations have been remarkable and underscore the importance of improving cell culture conditions to better reflect the TME.
The striking observations made in traditional media compared to physiologically relevant media have not been limited to cancer cells. Recently it was demonstrated that HPLM improves human T cell activation compared RPMI due to physiologic levels of calcium, which otherwise was present at low levels in traditional RPMI. It was also reported that T cell activation in HPLM increases the expression of genes involved in several amino acid metabolism pathways that can favor inflammatory function (Leney-Greene, Boddapati, Su, Cantor, & Lenardo, 2020). This exciting discovery involving physiologic media in immune cell phenotypes offers a new appreciation for considering base media for in vitro conditions, particularly when assessing metabolic function. Interestingly, we have noted that while HPLM supports primary T cell activation from healthy human donors, it does not appear as effective at supporting the activation of naïve CD4+ T cells from mice (our observations). This additionally raises the concern of interpretations made from metabolic immune cell studies conducted in murine systems alone. As discussed throughout this review, it is becoming more apparent that immune cell metabolism is highly dependent on microenvironment dictated nutrient availability, further adding to the need to assess immune cell metabolism in more physiologic culture conditions in order to uncover clinically translational cancer therapies.
Refinement of media formulations and the introduction of appropriate oxygen levels and three-dimensional tissue culture systems, such as organoids or spheroid cultures will most likely reveal additional insight and improve the predictive power of in vitro systems. Recently, cancer-on-a-chip models have emerged as a tool to better study the heterogenous tumor microenvironment. Using microfluidic chips that contain small chambers for cell culture enable tissue mechanics that better reflect the local environment. Culturing cancer cells, macrophages and epithelial cells in a compartmentalized chips have uncovered TAMs significantly increase the ability of cancer cells to impair the endothelial barrier and that different TAM subtypes can disperse cancer cell aggregates via different mechanisms (Bai et al., 2015; Zervantonakis et al., 2012). Ultimately, in vitro systems aimed at better mimicking heterogeneity and nutrient availability relevant to the TME will drive further understanding of the metabolism of tumor associated immune cells and more nuanced translational approaches to cancer therapies.
Summary and Future Directions
Immunometabolism provides a wide opportunity to identify new targets to improve cancer therapies through modulation of the TME. It is clear that while studies focusing on a single metabolic pathway in cancer or immune cells are helpful to provide a better understanding of metabolism within the TME, it is unlikely that a single enzyme or transporter of a specific pathway will provide a catch-all solution. Instead, efforts that focus on combination approaches using metabolic targets coupled with chemotherapeutic or targeted interventions including immune checkpoint blockade offers the greatest potential to improve clinical efficacy. For example, metformin reduces the hypoxic state of xenograft tumors, rendering them responsive to ICB and enhancing immune cell function (Scharping, Menk, Whetstone, Zeng, & Delgoffe, 2018). In addition, phosphorylation of a serine residue on programmed cell death 1 ligand 1 (PD- L1) is AMPK-dependent, which enhances its degradation. A significant correlation was observed between the clinical response, AMPK activation and lower levels of PD- L1 expression in biopsy samples from patients with breast cancer (Cha et al., 2018). Another important consideration for TME-specific therapies is the impact of the TME on immune cell trafficking (Nicolas-Boluda & Donnadieu, 2019). Finally, effects of host microbiome composition has been strongly implicated in the success of cancer immune therapies- specifically immune checkpoint blockades (Routy et al., 2018), creating yet another layer of complexity.
Given the effects of cell metabolism in cancer therapy efficacy, appreciation has grown for nutritional and dietary interventions. Interestingly, very low carb or ketogenic diets have boosted some anti-cancer immunotherapies (Soldati et al., 2018) whereas high-fat diets may exacerbate malignancies (Pascual et al., 2017). Similarly, caloric restriction (CR) can ameliorate the incidence and severity of certain cancers (O’Flanagan, Smith, McDonell, & Hursting, 2017). Specifically, improved outcomes from ketogenic diets, CR or intermittent fasting were associated with increased percentages of TILs and decreased Tregs (Turbitt, Demark-Wahnefried, Peterson, & Norian, 2019). The ketogenic diet depletes glucose levels, thereby lowering lactate production by glycolytic tumors. This subsequently resulted in smaller tumors, decreased MDSC frequency, and an overall improved anti-tumor immune response (Husain et al., 2013). However, manipulation of diets ultimately modulates the gut microbiome which in turn affects tumor and immune metabolism and can even play a significant role in modulating tumor responses to immunotherapy across multiple cancer types (Gopalakrishnan et al., 2018; Matson et al., 2018; Routy et al., 2018; Vetizou et al., 2015; Zheng et al., 2019). Although microbiome perturbations either indirectly by altering diet or directly by probiotics and/or fecal transplant could offer a promising strategy for a more personalized approach to immunotherapy, it still requires much caution and refinement due to the diverse functions of the gut microbiome as a whole in human health.
A new emphasis on establishing model systems that better replicate physiological conditions will help expedite our efforts to uncover clear mechanisms behind metabolic pathways and overcome the context dependent aspects of cell metabolism that are not well reflected in standard culture conditions. The majority of the immunometabolism field has drawn broad conclusions based on studies conducted in media optimized for rapid proliferation under in vitro culture conditions or has been limited to in vivo observations of murine models. Whenever possible, findings need to be challenged in multiple models, taking into account the physiological conditions of the treatment area or tumor type. This also requires documenting phenotypic changes in multiple immune cell types simultaneously in addition to cancer cells, to consider the specificity of the treatment regimen given the complexity of the TME. The tumor metabolism, immunometabolism, and immunotherapy fields have come closer in recent years, enabling a deeper understanding of these pathways and advancing combinational approaches to improve cancer therapy.
Acknowledgements
We thank members of the Rathmell lab for support. This work was supported by K00 CA234920 (J.E.B.), T32 DK101003 (K.V.), and R01 CA217987 and R01 DK105550 (J.C.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no direct conflict of interest with the contents of this manuscript. JCR has hold stock equity in Sitryx and within the past two years has received unrelated research support, travel, and honorarium from Calithera, Caribou, Incyte, Kadmon, Merck, Mitobridge, Pfizer, Sitryx, and Tempest.
Bibliography
- Ackermann T, & Tardito S (2019). Cell Culture Medium Formulation and Its Implications in Cancer Metabolism. Trends in Cancer, 5(6), 329–332. 10.1016/j.trecan.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khami AA, Rodriguez PC, & Ochoa AC (2016). Metabolic reprogramming of myeloid-derived suppressor cells (MDSC) in cancer. OncoImmunology, 5(8), 1–2. 10.1080/2162402X.2016.1200771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khami AA, Rodriguez PC, & Ochoa AC (2017). Energy metabolic pathways control the fate and function of myeloid immune cells. Journal of Leukocyte Biology, 102(2), 369–380. 10.1189/jlb.1VMR1216-535R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan HF, Walter KE, Luengo A, Madreiter-Sokolowski CT, Stryeck S, Lau AN, … Bogner-Strauss JG (2018). Cytosolic Aspartate Availability Determines Cell Survival When Glutamine Is Limiting. Cell Metabolism, 28(5), 706–720.e6. 10.1016/j.cmet.2018.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananieva E (2015). Targeting amino acid metabolism in cancer growth and anti-tumor immune response. World Journal of Biological Chemistry, 6(4), 281 10.4331/wjbc.v6.i4.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrejeva G, & Rathmell JC (2017). Similarities and Distinctions of Cancer and Immune Metabolism in Inflammation and Tumors. Cell Metabolism, 26(1), 49–70. 10.1016/j.cmet.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, … Beier UH (2017). Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metabolism, 25(6), 1282–1293.e7. 10.1016/j.cmet.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, … Franceschi C (2005). Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Experimental Gerontology, 40(8–9), 685–693. 10.1016/j.exger.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, … Ahmed R (2009). mTOR regulates memory CD8 T-cell differentiation. Nature, 460(7251), 108–112. 10.1038/nature08155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo L, Khim P, Mkhikian H, Mortales CL, & Demetriou M (2017). Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. ELife, 6, 1–16. 10.7554/eLife.21330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Adriani G, Dang TM, Tu TY, Penny HXL, Wong SC, … Thiery JP (2015). Contact-dependent carcinoma aggregate dispersion by M2a macrophages via ICAM-1 and ß2 integrin interactions. Oncotarget, 6(28), 25295–25307. 10.18632/oncotarget.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann T, Dunkel A, Schmid C, Schmitt S, Hiltensperger M, Lohr K, … Höchst B (2020). Regulatory myeloid cells paralyze T cells through cell–cell transfer of the metabolite methylglyoxal. Nature Immunology, 21(5), 555–566. 10.1038/s41590-020-0666-9 [DOI] [PubMed] [Google Scholar]
- Beier UH, Angelin A, Akimova T, Wang L, Liu Y, Xiao H, … Hancock WW (2015). Essential role of mitochondrial energy metabolism in Foxp3+ T-regulatory cell function and allograft survival. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 29(6), 2315–2326. 10.1096/fj.14-268409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloribi-Djefaflia S, Vasseur S, & Guillaumond F (2016). Lipid metabolic reprogramming in cancer cells. Oncogenesis, 5(1), e189–e189. 10.1038/oncsis.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D, Robay D, Hindupur SK, Pohlmann J, Colombi M, El-Shemerly MY, … Hall MN (2018). Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin due to NAD+ Depletion in Cancer Cells. Cell Reports, 25(11), 3047–3058.e4. 10.1016/j.celrep.2018.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vázquez G, Yurchenko E, … Jones RG (2015). The Energy Sensor AMPK Regulates T Cell Metabolic Adaptation and Effector Responses InVivo. Immunity, 42(1), 41–54. 10.1016/j.immuni.2014.12.030 [DOI] [PubMed] [Google Scholar]
- Bodmer M, Meier C, Krähenbühl S, Jick SS, & Meier CR (2010). Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care, 33(6), 1304–1308. 10.2337/dc09-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, … Lisanti MP (2010). The reverse Warburg effect: Glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle, 9(10), 1960–1971. 10.4161/cc.9.10.11601 [DOI] [PubMed] [Google Scholar]
- Bott AJ, Maimouni S, & Zong WX (2019). The pleiotropic effects of glutamine metabolism in cancer. Cancers, 11(6), 1–16. 10.3390/cancers11060770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, … Kreutz M (2016). LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metabolism, 24(5), 657–671. 10.1016/j.cmet.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Brown TP, & Ganapathy V (2019). Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacology and Therapeutics, 107451 10.1016/j.pharmthera.2019.107451 [DOI] [PubMed] [Google Scholar]
- Campillo N, Falcones B, Otero J, Colina R, Gozal D, Navajas D, … Almendros I (2019). Differential oxygenation in tumor microenvironment modulates macrophage and cancer cell crosstalk: Novel experimental settingand proof of concept. Frontiers in Oncology, 9(February), 1–12. 10.3389/fonc.2019.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor JR, Abu-Remaileh M, Kanarek N, Freinkman E, Gao X, Louissaint A, … Sabatini DM (2017). Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell, 169(2), 258–272.e17. 10.1016/j.cell.2017.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Deforet M, Akkari L, Thompson CB, Joyce JA, & Xavier JB (2017). Metabolic origins of spatial organization in the tumor microenvironment. Proceedings of the National Academy of Sciences of the United States of America, 114(11), 2934–2939. 10.1073/pnas.1700600114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KC, Viollet B, & Suttles J (2013). AMPKα1 deficiency amplifies proinflammatory myeloid APC activity and CD40 signaling. Journal of Leukocyte Biology, 94(6), 1113–1121. 10.1189/jlb.0313157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J-H, Yang W-H, Xia W, Wei Y, Chan L-C, Lim S-O, … Hung M-C (2018). Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Molecular Cell, 71(4), 606–620.e7. 10.1016/j.molcel.2018.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challier J, Bruniquel D, Sewell AK, & Laugel B (2013). Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8+ T-cell priming capacity. Immunology, 138(4), 402–410. 10.1111/imm.12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LN, Chen Z, Braas D, Lee JW, Xiao G, Geng H, … Müschen M (2017). Metabolic gatekeeper function of B-lymphoid transcription factors. Nature, 542(7642), 479–483. 10.1038/nature21076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-H, Curtis JD, Maggi LB, Faubert B, Villarino AV, O’Sullivan D, … Pearce EL (2013). Posttranscriptional Control of T Cell Effector Function by Aerobic Glycolysis. Cell, 153(6), 1239–1251. 10.1016/j.cell.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Qui J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, … Pearce EL (2015). Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell, 162(6), 1229–1241. 10.1016/S0960-9822(00)80071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DK, Moniz RJ, Xu Z, Sun J, Signoretti S, Zhu Q, & Marasco WA (2015). Human anti-CAIX antibodies mediate immune cell inhibition of renal cell carcinoma in vitro and in a humanized mouse model in vivo. Molecular Cancer, 14(1), 1–13. 10.1186/s12943-015-0384-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaoul N, Fayolle C, Desrues B, Oberkampf M, Tang A, Ladant D, & Leclerc C (2015). Rapamycin impairs antitumor CD8p T-cell responses and vaccine-induced tumor eradication. Cancer Research, 75(16), 3279–3291. 10.1158/0008-5472.CAN-15-0454 [DOI] [PubMed] [Google Scholar]
- Chapman NM, Zeng H, Nguyen TLM, Wang Y, Vogel P, Dhungana Y, … Chi H (2018). MTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis. Nature Communications, 9(1). 10.1038/s41467-018-04392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Wilson PC, Shyer JA, Veselits M, Steach HR, Cui C, … Craft J (2020). Kidney tissue hypoxia dictates T cell – mediated injury in murine lupus nephritis. 1620(April). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Chen Y, Zhou C, Shen L, Tu F, Xu J, & Liu C (2020). For colorectal cancer patients with type II diabetes, could metformin improve the survival rate? A meta-analysis. Clinics and Research in Hepatology and Gastroenterology, 44(1), 73–81. 10.1016/j.clinre.2019.06.009 [DOI] [PubMed] [Google Scholar]
- Chirasani SR, Leukel P, Gottfried E, Hochrein J, Stadler K, Neumann B, … Grauer OM (2012). Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. International Journal of Cancer, 132(4), 843–853. 10.1002/ijc.27712 [DOI] [PubMed] [Google Scholar]
- Chiu DKC, Tse APW, Xu IMJ, Di Cui J, Lai RKH, Li LL, … Wong CCL (2017). Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nature Communications, 8(1), 1–12. 10.1038/s41467-017-00530-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Raybuck AL, Blagih J, Kemboi E, Haase VH, Jones RG, & Boothby MR (2019). Hypoxia-inducible factors in CD4+ T cells promote metabolism, switch cytokine secretion, and T cell help in humoral immunity. Proceedings of the National Academy of Sciences of the United States of America, 116(18), 8975–8984. 10.1073/pnas.1811702116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Raybuck AL, Stengel K, Wei M, Beck TC, Volanakis E, … Boothby MR (2016). Germinal Center hypoxia and regulation of antibody qualities by a hypoxia response system. Nature, 537(7619), 234–238. 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SYC, Collins CC, Gout PW, & Wang Y (2013). Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? Journal of Pathology, 230(4), 350–355. 10.1002/path.4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clambey ET, McNamee EN, Westrich JA, Glover LE, Campbell EL, Jedlicka P, … Eltzschig HK (2012). Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proceedings of the National Academy of Sciences of the United States of America, 109(41). 10.1073/pnas.1202366109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegio OR, Chu N-QQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, … Medzhitov R (2014). Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature, 513(7519), 559–563. 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Zhang Q, Song Q, Wang H, Dmitriev P, Sun MY, … Zhuang Z (2019). Targeting hypoxia downstream signaling protein, CAIX, for CAR T-cell therapy against glioblastoma. Neuro-Oncology, 21(11), 1436–1446. 10.1093/neuonc/noz117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvin P, Toor SM, Sasidharan Nair V, & Elkord E (2018). Immune checkpoint inhibitors: recent progress and potential biomarkers. Experimental & Molecular Medicine, 50(12), 165 10.1038/s12276-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Berardinis RJ, & Chandel NS (2016). Fundamentals of cancer metabolism. Science Advances, 2(5). 10.1126/sciadv.1600200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, & Manzo-Merino J (2019). Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Frontiers in Oncology, 9(November). 10.3389/fonc.2019.01143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, … Powell JD (2009). The mTOR Kinase Differentially Regulates Effector and Regulatory T Cell Lineage Commitment. Immunity, 30(6), 832–844. 10.1016/j.immuni.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Li J, Sarde A, Lines JL, Lee YC, Qian DC, … Mabaera R (2019). Hypoxia-induced VISTA promotes the suppressive function of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Immunology Research, 7(7), 1079–1090. 10.1158/2326-6066.CIR-18-0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner LJ, Brun SN, Herzig S, Young NP, Curtis SD, Shackelford DB, … Svensson RU (2019). Genetic Analysis Reveals AMPK Is Required to Support Tumor Growth in Murine Kras-Dependent Lung Cancer Models. Cell Metabolism, 29(2), 285–302.e7. 10.1016/j.cmet.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, & Udono H (2015). Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proceedings of the National Academy of Sciences of the United States of America, 112(6), 1809–1814. 10.1073/pnas.1417636112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erra Díaz F, Ochoa V, Merlotti A, Dantas E, Mazzitelli I, Gonzalez Polo V, … Geffner J (2020). Extracellular Acidosis and mTOR Inhibition Drive the Differentiation of Human Monocyte-Derived Dendritic Cells. Cell Reports, 31(5). 10.1016/j.celrep.2020.107613 [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, … Puccetti P (2003). Modulation of tryptophan catabolism by regulatory T cells. Nature Immunology, 4(12), 1206–1212. 10.1038/ni1003 [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, … Puccetti P (2006). The Combined Effects of Tryptophan Starvation and Tryptophan Catabolites Down-Regulate T Cell Receptor ζ-Chain and Induce a Regulatory Phenotype in Naive T Cells. The Journal of Immunology, 176(11), 6752–6761. 10.4049/jimmunol.176.11.6752 [DOI] [PubMed] [Google Scholar]
- Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, … DeBerardinis RJ (2017). Lactate Metabolism in Human Lung Tumors. Cell, 171(2), 358–371.e9. 10.1016/j.cell.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feichtinger RG, & Lang R (2019). Targeting L-Lactate Metabolism to Overcome Resistance to Immune Therapy of Melanoma and Other Tumor Entities. Journal of Oncology, 2019(Figure 1). 10.1155/2019/2084195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CS, Baixauli F, Kyle RL, Puleston DJ, Cameron AM, Sanin DE, … Pearce EL (2020). Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function. Cell Metabolism, 31(2), 422–437.e5. 10.1016/j.cmet.2019.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, … Kreutz M (2007). Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood, 109(9), 3812–3819. 10.1182/blood-2006-07-035972 [DOI] [PubMed] [Google Scholar]
- Fletcher M, Ramirez ME, Sierra RA, Raber P, Thevenot P, Al-Khami AA, … Rodriguez PC (2015). L-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Research, 75(2), 275–283. 10.1158/0008-5472.CAN-14-1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, … Thompson CB (2002). The CD28 Signaling Pathway Regulates Glucose Metabolism ability of resting cells to take up and utilize nutrients at levels sufficient to maintain viability (Rathmell et al in fat and muscle cells insulin induces glucose uptake in excess of that required. Immunity, 16, 769–777. [DOI] [PubMed] [Google Scholar]
- Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, & Ferrara GB (2002). Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. Journal of Experimental Medicine, 196(4), 459–468. 10.1084/jem.20020121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, … Lanzavecchia A (2016). L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell, 167(3), 829–842.e13. 10.1016/j.cell.2016.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, … Rathmell JC (2016). Foxp3 and Toll-like receptor signaling balance T reg cell anabolic metabolism for suppression. Nature Immunology, 17(12), 1459–1466. 10.1038/ni.3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli P, Sandoval TA, & Cubillos-Ruiz JR (2019). Dendritic Cell Metabolism and Function in Tumors. Trends in Immunology, 40(8), 699–718. 10.1016/j.it.2019.06.004 [DOI] [PubMed] [Google Scholar]
- Girgis H, Masui O, White NMA, Scorilas A, Rotondo F, Seivwright A, … Yousef GM (2014). Lactate Dehydrogenase A is a potential prognostic marker in clear cell renal cell carcinoma. Molecular Cancer, 13(1), 1–10. 10.1186/1476-4598-13-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, … Wargo JA (2018). Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science, 359(6371), 97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, … Kreutz M (2006). Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood, 107(5), 2013–2021. 10.1182/blood-2005-05-1795 [DOI] [PubMed] [Google Scholar]
- Gropper Y, Feferman T, Shalit T, Salame TM, Porat Z, & Shakhar G (2017). Culturing CTLs under Hypoxic Conditions Enhances Their Cytolysis and Improves Their Anti-tumor Function. Cell Reports, 20(11), 2547–2555. 10.1016/j.celrep.2017.08.071 [DOI] [PubMed] [Google Scholar]
- Gui DY, Sullivan LB, Luengo A, Hosios AM, Bush LN, Gitego N, … Heiden MG Vander. (2017). Sensitivity To Metformin. 24(5), 716–727. 10.1016/j.cmet.2016.09.006.Environment [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami I, Chen J, Murschel F, Bronte V, De Crescenzo G, & Jolicoeur M (2012). Immunosuppressive activity enhances central carbon metabolism and bioenergetics in myeloid-derived suppressor cells in vitro models. BMC Cell Biology, 13(1), 1 10.1186/1471-2121-13-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng B, Lim CK, Lovejoy DB, Bessede A, Gluch L, & Guillemin GJ (2016). Understanding the role of the kynurenine pathway in human breast cancer immunobiology. Oncotarget, 7(6), 6506–6520. 10.18632/oncotarget.6467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, … Gabrilovich DI (2011). Lipid accumulation and dendritic cell dysfunction in cancer. Nature Medicine, 16(8), 880–886. 10.1038/nm.2172.Lipid [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P-C, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, … Kaech SM (2015). Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell, 162(6), 1217–1228. 10.1016/j.cell.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PC, & Liu PS (2016). Metabolic communication in tumors: A new layer of immunoregulation for immune evasion. Journal for ImmunoTherapy of Cancer, 4(1), 1–9. 10.1186/s40425-016-0109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, … Ochoa AC (2015). Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunology Research, 3(11), 1236–1247. 10.1158/2326-6066.CIR-15-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Zeng Z, Xia Q, Liu Z, Feng X, Chen J, … Liu J (2019). Metformin attenuates hepatoma cell proliferation by decreasing glycolytic flux through the HIF-1α/PFKFB3/PFK1 pathway. Life Sciences, 239(October). 10.1016/j.lfs.2019.116966 [DOI] [PubMed] [Google Scholar]
- Huizing FJ, Garousi J, Lok J, Franssen G, Hoeben BAW, Frejd FY, … Heskamp S (2019). CAIX-targeting radiotracers for hypoxia imaging in head and neck cancer models. Scientific Reports, 9(1), 1–10. 10.1038/s41598-019-54824-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain Z, Huang Y, Seth P, & Sukhatme VP (2013). Tumor-Derived Lactate Modifies Antitumor Immune Response: Effect on Myeloid-Derived Suppressor Cells and NK Cells. The Journal of Immunology, 191(3), 1486–1495. 10.4049/jimmunol.1202702 [DOI] [PubMed] [Google Scholar]
- Ino K, Yamamoto E, Shibata K, Kajiyama H, Yoshida N, Terauchi M, … Kikkawa F (2008). Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: Its association with disease progression and survival. Clinical Cancer Research, 14(8), 2310–2317. 10.1158/1078-0432.CCR-07-4144 [DOI] [PubMed] [Google Scholar]
- Jiang L, Fang X, Wang H, Li D, & Wang X (2018). Ovarian Cancer-Intrinsic Fatty Acid Synthase Prevents Anti-tumor Immunity by Disrupting Tumor-Infiltrating Dendritic Cells. Frontiers in Immunology, 9(December), 2927 10.3389/fimmu.2018.02927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC, … Rathmell JC (2018). Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell, 175(7), 1780–1795.e19. 10.1016/j.cell.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Su LJ, Pinckney J, Critton D, Boyer E, Krishnakumar A, … Korman AJ (2019). VISTA is an acidic pH-selective ligand for PSGL-1. Nature, 574(7779), 565–570. 10.1038/s41586-019-1674-5 [DOI] [PubMed] [Google Scholar]
- Keshet R, & Erez A (2018). Arginine and the metabolic regulation of nitric oxide synthesis in cancer. DMM Disease Models and Mechanisms, 11(8). 10.1242/dmm.033332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishton RJ, Barnes CE, Nichols AG, Cohen S, Gerriets A, Siska PJ, … Hill C (2016). AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metabolism, 23(4), 649–662. 10.1016/j.cmet.2016.03.008.AMPK [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein Geltink RI, O’Sullivan D, Corrado M, Bremser A, Buck MD, Buescher JM, … Pearce EL (2017). Mitochondrial Priming by CD28. Cell, 171(2), 385–397.e11. 10.1016/j.cell.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klysz D, Tai X, Robert PA, Craveiro M, Cretenet G, Oburoglu L, … Taylor N (2015). Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Science Signaling, 8(396). 10.1126/scisignal.aab2610 [DOI] [PubMed] [Google Scholar]
- Koltai T (2016). Cancer: Fundamentals behind pH targeting and the double-edged approach. OncoTargets and Therapy, 9, 6343–6360. 10.2147/OTT.S115438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Yoshida N, Maeda K, Suárez-Fueyo A, Kyttaris VC, & Tsokos GC (2019). Glutaminase 1 Inhibition Reduces Glycolysis and Ameliorates Lupus-like Disease in MRL/lpr Mice and Experimental Autoimmune Encephalomyelitis. Arthritis and Rheumatology, 71(11), 1869–1878. 10.1002/art.41019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouidhi S, Ayed F Ben, & Elgaaied AB (2018). Targeting tumor metabolism: A new challenge to improve immunotherapy. Frontiers in Immunology, 9(February), 1–11. 10.3389/fimmu.2018.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labadie BW, Bao R, & Luke JJ (2019). Reimagining IDO Pathway Inhibition in Cancer Immunotherapy via Downstream Focus on the Tryptophan-Kynurenine-Aryl Hydrocarbon Axis. Clinical Cancer Research, 25(5), 1462–1471. 10.1158/1078-0432.CCR-18-2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanitis E, Dangaj D, Irving M, & Coukos G (2017). Mechanisms regulating T-cell infiltration and activity in solid tumors. Annals of Oncology, 28(September), xii18–xii32. 10.1093/annonc/mdx238 [DOI] [PubMed] [Google Scholar]
- Le Bourgeois T, Strauss L, Aksoylar HI, Daneshmandi S, Seth P, Patsoukis N, & Boussiotis VA (2018). Targeting T cell metabolism for improvement of cancer immunotherapy. Frontiers in Oncology, 8(August). 10.3389/fonc.2018.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leney-Greene MA, Boddapati AK, Su HC, Cantor JR, & Lenardo MJ (2020). Human Plasma-like Medium Improves T Lymphocyte Activation. IScience, 23(1), 100759 10.1016/j.isci.2019.100759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, … Powell JD (2019). Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science, 366(6468), 1013–1021. 10.1126/science.aav2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang G, Wang X, & Li XF (2015). Is carbonic anhydrase IX a validated target for molecular imaging of cancer and hypoxia? Future Oncology, 11(10), 1531–1541. 10.2217/fon.15.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, … Zou W (2018). Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metabolism, 28(1), 87–103.e6. 10.1016/j.cmet.2018.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S, & Li Y (2018). Targeting the IDO1 pathway in cancer: From bench to bedside. Journal of Hematology and Oncology, 11(1), 1–12. 10.1186/s13045-018-0644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, … Ho PC (2017). Α-Ketoglutarate Orchestrates Macrophage Activation Through Metabolic and Epigenetic Reprogramming. Nature Immunology, 18(9), 985–994. 10.1038/ni.3796 [DOI] [PubMed] [Google Scholar]
- Lu T, Schubert C, Cummings MD, Bignan G, Connolly PJ, Smans K, … Meerpoel L (2018). Design and synthesis of a series of bioavailable fatty acid synthase (FASN) KR domain inhibitors for cancer therapy. Bioorganic and Medicinal Chemistry Letters, 28(12), 2159–2164. 10.1016/j.bmcl.2018.05.014 [DOI] [PubMed] [Google Scholar]
- Lukey MJ, Katt WP, & Cerione RA (2017). Targeting amino acid metabolism for cancer therapy. Drug Discovery Today, 22(5), 796–804. 10.1016/j.drudis.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EH, Poffenberger MC, Wong AHT, & Jones RG (2017). The role of AMPK in T cell metabolism and function. Current Opinion in Immunology, 46, 45–52. 10.1016/j.coi.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, … Rathmell JC (2014). The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metabolism, 20(1), 61–72. 10.1016/j.cmet.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, Rathmell JC, & Jones RG (2011). The Liver Kinase B1 Is a Central Regulator of T Cell Development, Activation, and Metabolism. The Journal of Immunology, 187(8), 4187–4198. 10.4049/jimmunol.1100367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Van Hoef V, Zhang X, Wennerberg E, Lorent J, Witt K, … Lundqvist A (2016). IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood, 128(11), 1475–1489. 10.1182/blood-2016-02-698027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marçais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, … Walzer T (2014). The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nature Immunology, 15(8), 749–757. 10.1038/ni.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo I, Zilio S, Desantis G, Mlecnik B, Agnellini AHR, Ugel S, … Bronte V (2016). T Cell Cancer Therapy Requires CD40-CD40L Activation of Tumor Necrosis Factor and Inducible Nitric-Oxide-Synthase-Producing Dendritic Cells. Cancer Cell, 30(3), 377–390. 10.1016/j.ccell.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, … Gajewski TF (2018). The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science, 359(6371), 104–108. 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown SR (2014). Defining normoxia, physoxia and hypoxia in tumours - Implications for treatment response. British Journal of Radiology, 87(1035), 1–12. 10.1259/bjr.20130676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, & Noessner E (2012). Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. International Journal of Cancer, 131(3), 633–640. 10.1002/ijc.26410 [DOI] [PubMed] [Google Scholar]
- Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, … Rathmell JC (2011). Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4 + T Cell Subsets. The Journal of Immunology, 186(6), 3299–3303. 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miret JJ, Kirschmeier P, Koyama S, Zhu M, Li YY, Naito Y, … Akbay EA (2019). Suppression of Myeloid Cell Arginase Activity leads to Therapeutic Response in a NSCLC Mouse Model by Activating Anti-Tumor Immunity. Journal for ImmunoTherapy of Cancer, 7(1), 1–12. 10.1186/s40425-019-0504-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska J, Lee-Chang C, Rashidi A, Muroski ME, Chang AL, Lopez-Rosas A, … Lesniak MS (2019). HIF-1α Is a Metabolic Switch between Glycolytic-Driven Migration and Oxidative Phosphorylation-Driven Immunosuppression of Tregs in Glioblastoma. Cell Reports, 27(1), 226–237.e4. 10.1016/j.celrep.2019.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondanelli G, Iacono A, Allegrucci M, Puccetti P, & Grohmann U (2019). Immunoregulatory interplay between arginine and tryptophan metabolism in health and disease. Frontiers in Immunology, 10(July), 1–6. 10.3389/fimmu.2019.01565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A, Danai LV, Gui DY, Waingarten CY, Lewis CA, & Vander Heiden MG (2017). Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. ELife, 6, 1–27. 10.7554/eLife.27713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen GE, & Yet L (2015). Progress in the development of fatty acid synthase inhibitors as anticancer targets. Bioorganic and Medicinal Chemistry Letters, 25(20), 4363–4369. 10.1016/j.bmcl.2015.08.087 [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, & Mellor AL (2005). GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity, 22(5), 633–642. 10.1016/j.immuni.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Najjar YG, Menk AV, Sander C, Rao U, Karunamurthy A, Bhatia R, … Delgoffe GM (2019). Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight, 4(5), 1–11. 10.1172/jci.insight.124989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya M, Xiao Y, Zhou X, Chang J-H, Chang M, Cheng X, … Sun S-C (2014). Inflammatory T Cell Responses Rely on Amino Acid Transporter ASCT2 Facilitation of Glutamine Uptake and mTORC1 Kinase Activation. Immunity, 40(5), 692–705. 10.1016/j.immuni.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi A, Fekete T, Krishnamurthy A, Snowden S, Rajnavölgyi E, Catrina AI, … Rethi B (2013). Dendritic Cell Reprogramming by Endogenously Produced Lactic Acid. The Journal of Immunology, 191(6), 3090–3099. 10.4049/jimmunol.1300772 [DOI] [PubMed] [Google Scholar]
- Netea-Maier RT, Smit JWA, & Netea MG (2018). Metabolic changes in tumor cells and tumor-associated macrophages: A mutual relationship. Cancer Letters, 413, 102–109. 10.1016/j.canlet.2017.10.037 [DOI] [PubMed] [Google Scholar]
- Nicolas-Boluda A, & Donnadieu E (2019). Obstacles to T cell migration in the tumor microenvironment. Comparative Immunology, Microbiology and Infectious Diseases, 63(December 2017), 22–30. 10.1016/j.cimid.2018.12.006 [DOI] [PubMed] [Google Scholar]
- Noman MZ, Hasmim M, Lequeux A, Xiao M, Duhem C, Chouaib S, … Janji B (2019). Improving Cancer Immunotherapy by Targeting the Hypoxic Tumor Microenvironment: New Opportunities and Challenges. Cells, 8(9), 1083 10.3390/cells8091083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Flanagan CH, Smith LA, McDonell SB, & Hursting SD (2017). When less may be more: Calorie restriction and response to cancer therapy. BMC Medicine, 15(1), 1–9. 10.1186/s12916-017-0873-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LAJ, Kishton RJ, & Rathmell J (2016). A guide to immunometabolism for immunologists. Nature Reviews Immunology, 16(9), 553–565. 10.1038/nri.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M-H, Sun I-H, Zhao L, Leone RD, Sun I-M, Xu W, … Powell JD (2020). Targeting glutamine metabolism enhances tumor specific immunity by modulating suppressive myeloid cells. Journal of Clinical Investigation. 10.1172/jci131859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazon A, Tyrakis PA, Macias D, Veliça P, Rundqvist H, Fitzpatrick S, … Johnson RS (2017). An HIF-1α/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell, 32(5), 669–683.e5. 10.1016/j.ccell.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri EM, Menga A, Martín-Pérez R, Quinto A, Riera-Domingo C, De Tullio G, … Castegna A (2017). Pharmacologic or Genetic Targeting of Glutamine Synthetase Skews Macrophages toward an M1-like Phenotype and Inhibits Tumor Metastasis. Cell Reports, 20(7), 1654–1666. 10.1016/j.celrep.2017.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, … Riley JL (2005). CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Molecular and Cellular Biology, 25(21), 9543–9553. 10.1128/MCB.25.21.9543-9553.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CSO, … Benitah SA (2017). Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature, 541(7635), 41–45. 10.1038/nature20791 [DOI] [PubMed] [Google Scholar]
- Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, … Boussiotis VA (2015). PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nature Communications, 6, 1–13. 10.1038/ncomms7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova NN, & Thompson CB (2016). The Emerging Hallmarks of Cancer Metabolism. Cell Metabolism, 23(1), 27–47. 10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EJ, & Everts B (2015). Dendritic cell metabolism. Nature Reviews Immunology, 15(1), 18–29. 10.1038/nri3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Poffenberger MC, Chang C-H, & Jones RG (2013). Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science, 342(6155), 12424–12454. 10.1097/CCM.0b013e31823da96d.Hydrogen [DOI] [Google Scholar]
- Platten M, Nollen EAA, Röhrig UF, Fallarino F, & Opitz CA (2019). Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nature Reviews Drug Discovery, 18(5), 379–401. 10.1038/s41573-019-0016-5 [DOI] [PubMed] [Google Scholar]
- Qorraj M, Bruns H, Böttcher M, Weigand L, Saul D, Mackensen A, … Mougiakakos D (2017). The PD-1/PD-L1 axis contributes to immune metabolic dysfunctions of monocytes in chronic lymphocytic leukemia. Leukemia, 31(2), 470–478. 10.1038/leu.2016.214 [DOI] [PubMed] [Google Scholar]
- Raber P, Ochoa AC, & Rodríguez PC (2012). Metabolism of L-Arginine by Myeloid-Derived Suppressor Cells in Cancer: Mechanisms of T cell suppression and Therapeutic Perspectives. Immunological Investigations, 41(6–7), 614–634. 10.3109/08820139.2012.680634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P, Shanmugam A, Swafford D, Suryawanshi A, Bhattacharjee P, Hussein MS, … Manicassamy S (2018). GPR81, a Cell-Surface Receptor for Lactate, Regulates Intestinal Homeostasis and Protects Mice from Experimental Colitis. The Journal of Immunology, ji1700604 10.4049/jimmunol.1700604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport BL, Steel HC, Theron AJ, Smit T, & Anderson R (2020). Role of the Neutrophil in the Pathogenesis of Advanced Cancer and Impaired Responsiveness to Therapy. Molecules, 25(7), 1618 10.3390/molecules25071618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L, Ruiz-Rodado V, Dowdy T, Huang S, Issaq SH, Beck J, … LeBlanc AK (2020). Glutaminase-1 (GLS1) inhibition limits metastatic progression in osteosarcoma. Cancer & Metabolism, 8(1), 1–13. 10.1186/s40170-020-0209-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CM, Davies LC, Subleski JJ, Maio N, Gonzalez-Cotto M, Andrews C, … McVicar DW (2018). Tumour-elicited neutrophils engage mitochondrial metabolism to circumvent nutrient limitations and maintain immune suppression. Nature Communications, 9(1). 10.1038/s41467-018-07505-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera-Domingo C, Audigé A, Granja S, Cheng WC, Ho PC, Baltazar F, … Mazzone M (2020). Immunity, Hypoxia, and Metabolism-the Ménage à Trois of Cancer: Implications for Immunotherapy. Physiological Reviews, 100(1), 1–102. 10.1152/physrev.00018.2019 [DOI] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, … Zitvogel L (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science, 359(6371), 91–97. 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- Saito Y, Chapple RH, Lin A, Kitano A, & Nakada D (2015). AMPK Protects Leukemia-Initiating Cells in Myeloid Leukemias from Metabolic Stress in the Bone Marrow. Cell Stem Cell, 17(5), 585–596. 10.1016/j.stem.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, & Sabatini DM (2017). mTOR Signaling in Growth, Metabolism, and Disease. Cell, 169(2), 361–371. 10.1016/j.cell.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Scharping NE, Menk AV, Whetstone RD, Zeng X, & Delgoffe GM (2018). Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunology Research, 5(1), 9–16. 10.1158/2326-6066.CIR-16-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sektioglu IM, Carretero R, Bender N, Bogdan C, Garbi N, Umansky V, … Hämmerling GJ (2016). Macrophage-derived nitric oxide initiates T-cell diapedesis and tumor rejection. OncoImmunology, 5(10), 1–13. 10.1080/2162402X.2016.1204506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, … Munn DH (2009). Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood, 113(24), 6102–6111. 10.1182/blood-2008-12-195354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siska PJ, Beckermann KE, Mason FM, Andrejeva G, Greenplate AR, Sendor AB, … Rathmell JC (2017). Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight, 2(12), 1–13. 10.1172/jci.insight.93411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siska PJ, & Rathmell JC (2015). T cell metabolic fitness in antitumor immunity. Trends in Immunology, 36(4), 257–264. 10.1016/j.it.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati L, Di Renzo L, Jirillo E, Ascierto PA, Marincola FM, & De Lorenzo A (2018). The influence of diet on anti-cancer immune responsiveness. Journal of Translational Medicine, 16(1), 1–18. 10.1186/s12967-018-1448-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, … Boussiotis VA (2020). Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Science Immunology, 5(43), 1–15. 10.1126/sciimmunol.aay1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar M, Restifo NP, Sukumar M, Liu J, Ji Y, Subramanian M, … Finkel T (2013). Inhibiting glycolytic metabolism enhances CD8 + T cell memory and antitumor function Find the latest version : Inhibiting glycolytic metabolism enhances CD8 + T cell memory and antitumor function. Journal of Clinical Investigation, 123(10), 4479–4488. 10.1172/JCI69589.tor [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan DO, Windt G. J. W. Van Der, Huang SC, Jonathan D, Chang C, Buck MD, … Erika L (2014). Memory CD8+ T Cells use cell intrinsic lipolysis. Immunity, 41(1), 75–88. 10.1016/j.immuni.2014.06.005.Memory [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MR, Danai LV, Lewis CA, Chan SH, Gui DY, Kunchok T, … Muir A (2019). Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. ELife, 8, 1–27. 10.7554/eLife.44235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun I-H, Oh M-H, Zhao L, Patel CH, Arwood ML, Xu W, … Powell JD (2018). mTOR Complex 1 Signaling Regulates the Generation and Function of Central and Effector Foxp3+ Regulatory T Cells. Journal of Immunology (Baltimore, Md. : 1950), 201(2), 481–492. 10.4049/jimmunol.1701477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Sun Z, Zhu Z, Guan H, Zhang J, Zhang Y, … Sun M (2014). Clinicopathological significance and prognostic value of lactate dehydrogenase A expression in gastric cancer patients. PLoS ONE, 9(3). 10.1371/journal.pone.0091068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbitt WJ, Demark-Wahnefried W, Peterson CM, & Norian LA (2019). Targeting glucose metabolism to enhance immunotherapy: Emerging evidence on intermittent fasting and calorie restriction mimetics. Frontiers in Immunology, 10(June), 1–8. 10.3389/fimmu.2019.01402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszka-Czochara M, Bukowska-Strakova K, Kocemba-Pilarczyk KA, & Majka M (2018). Caffeic acid targets AMPK signaling and regulates tricarboxylic acid cycle anaplerosis while metformin downregulates HIF-1α-induced glycolytic enzymes in human cervical squamous cell carcinoma lines. Nutrients, 10(7). 10.3390/nu10070841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, & DeBerardinis RJ (2017). Understanding the Intersections between Metabolism and Cancer Biology. Cell, 168(4), 657–669. 10.1016/j.cell.2016.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, … Zitvogel L (2015). Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science, 350(6264), 1079–1084. 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss K, Larsen SE, & Snow AL (2017). Metabolic reprogramming and apoptosis sensitivity: Defining the contours of a T cell response. Cancer Letters, 408, 190–196. 10.1016/j.canlet.2017.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss K, Luthers CR, Pohida K, & Snow AL (2019). Fatty Acid Synthase Contributes to Restimulation-Induced Cell Death of Human CD4 T Cells. Frontiers in Molecular Biosciences, 6(October), 1–16. 10.3389/fmolb.2019.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Franco F, Tsui Y, Xie X, Trefny MP, Zappasodi R, … Ho P (2020). CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nature Immunology, 21(3), 298–308. 10.1038/s41590-019-0589-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel B, Vuerich M, Daneshmandi S, & Seth P (2018). Metabolic switch in the tumor microenvironment determines immune responses to anti-cancer therapy. Frontiers in Oncology, 8(August), 1–9. 10.3389/fonc.2018.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf AM, Skibbe K, Adamczyk A, Buer J, Geffers R, Hansen W, … Jendrossek V (2017). Hypoxia enhances immunosuppression by inhibiting CD4+ Effector T cell function and promoting treg activity. Cellular Physiology and Biochemistry, 41(4), 1271–1284. 10.1159/000464429 [DOI] [PubMed] [Google Scholar]
- Winning S, & Fandrey J (2016). Dendritic Cells under Hypoxia: How Oxygen Shortage Affects the Linkage between Innate and Adaptive Immunity. Journal of Immunology Research, 2016 10.1155/2016/5134329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Han Y, Rodriguez Sillke Y, Deng H, Siddiqui S, Treese C, … Glauben R (2019). Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Molecular Medicine, 11(11), 1–17. 10.15252/emmm.201910698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung C, Gibson AE, Issaq SH, Oshima N, Baumgart JT, Edessa LD, … Heske CM (2019). Targeting glycolysis through inhibition of lactate dehydrogenase impairs tumor growth in preclinical models of Ewing sarcoma. Cancer Research, 79(19), 5060–5073. 10.1158/0008-5472.CAN-19-0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, & Kamm RD (2012). Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proceedings of the National Academy of Sciences of the United States of America, 109(34), 13515–13520. 10.1073/pnas.1210182109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Li J, Wang F, Hu J, Wang S, & Sun Y (2014). 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Letters, 355(2), 176–183. 10.1016/j.canlet.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang H, Mao C, Sun M, Dominah G, Chen L, & Zhuang Z (2018). Fatty acid oxidation contributes to IL-1β secretion in M2 macrophages and promotes macrophage-mediated tumor cell migration. Molecular Immunology, 94, 27–35. 10.1016/j.molimm.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kurupati R, Liu L, Zhou XY, Zhang G, Hudaihed A, … Ertl HCJ (2017). Enhancing CD8+ T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell, 32(3), 377–391.e9. 10.1016/j.ccell.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, … Fang W (2019). Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. Journal for ImmunoTherapy of Cancer, 7(1), 1–7. 10.1186/s40425-019-0650-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Wang X, Liu P, Ke C, & Xu S (2019). Arginine metabolism and deprivation in cancer therapy. Biomedicine and Pharmacotherapy, 118(June), 109210 10.1016/j.biopha.2019.109210 [DOI] [PubMed] [Google Scholar]


