Abstract
Background
Rab7 belongs to the Ras oncogene family. Many studies have shown that its dysfunction is associated with many types of malignant tumors, but its effect on the pathogenesis of gastric cancer (GC) is still unknown. Therefore, we investigated the effect and mechanism of Rab7 in GC.
Material/Methods
The expression of Rab7 in GC and adjacent tissues was detected by immunohistochemistry, Western blot analysis, and qRT-PCR. The relationship of Rab7 with clinicopathological parameters and prognosis was analyzed. The expressions of Rab7, PI3K, and AKT in GC cells were assessed by Western blot. Overexpressed and silenced GC cell lines were constructed and AGS cells were treated with LY294002. The proliferation capacity of GC cells was detected by CCK8 assay, cell cycle changes were detected by flow cytometry, and the invasion and migration abilities of GC cells were assessed by transwell assay.
Results
The expression of Rab7 was upregulated in the samples and cells, and was positively correlated with lymph node metastasis but negatively correlated with histological differentiation and clinical prognosis. In cell function experiments, overexpression of Rab7 induced the transition from S phase to G2 phase and promoted the proliferation, invasion, and migration of GC cells. Our assessment of the molecular mechanism showed that Rab7 promoted the phosphorylation of PI3K and AKT in GC cells. Incubation with the PI3K inhibitor Ly294002 impaired the enhanced effect of Rab7 overexpression on proliferation, migration, and invasion abilities of GC cells. These results show that the Rab7 affects GC cell progression by modulating the PI3K/AKT pathway.
Conclusions
Rab7 could be a prognostic biomarker and therapeutic target of the PI3K/AKT pathway in GC.
MeSH Keywords: Arabinofuranosyluracil; Promoter Regions, Genetic; Proto-Oncogene Proteins c-akt; Stomach Neoplasms
Background
The incidence and mortality rates of gastric cancer (GC) rank fifth and second, respectively, among all cancers. There were more than 1 000 000 new cases and an estimated 783 000 deaths (accounting for 1 in every 12 deaths globally) in 2018 [1]. Although diagnosis and treatment have made great progress in recent decades, the burden of GC remains very high in Asia, Latin America, and Central and Eastern Europe [2]. Because most patients with GC are in the middle and late stage of disease at diagnosis, the prognosis remains poor [3,4]. Determining the molecular mechanism underlying the occurrence and development of GC will help find new therapeutic targets and improve the prognosis of GC.
The Ras-related protein Rab7 is a small GTPase and is the basis of lysosomal biogenesis, positioning, and functions, as well as the transport and degradation of several signal receptors, thus also affecting transduction of the signal pathway [5,6]. The literature suggests that Rab7 participates in a variety of physiological processes, such as cell apoptosis [7–9], neurotrophin transportation, signal pathway, and neurite outgrowth [10,11], phagocytosis [12], and autophagy and mitophagy [13,14]. The dysfunction of Rab7 is associated with development of many diseases. For instance, the occurrence of Charcot-Marie-Tooth type 2B peripheral neuropathy is closely associated with Rab7 gene mutation [15,16]. Recently, the role of Rab7 in cancer has been reported. Many studies have found that Rab7 is a tumor promotor. The weakening of Rab7 suppresses the invasion of breast cancer and cholangiocarcinoma [17,18]. However, the anti-tumor effect of Rab7 has been reported in prostate cancer and glioblastoma [19,20]. Interestingly, Rab7 has a bi-directional regulatory effect on some tumors. For example, Alonso-Curbelo et al. found that Rab7 was selectively upregulated in melanoma and partially downregulated in highly invasive melanoma cells [21]. However, the role of Rab7 in the pathogenesis of GC is unknown. Therefore, it is of great significance to reveal the role of Rab7 in the carcinogenesis and development of GC.
In the present study, we collected tissue samples from 115 patients with GC and analyzed the relationship of Rab7 with clinicopathological characteristics and survival prognosis. Through a series of cell function experiments, we also explored the effects of Rab7 on the progression of GC.
Material and Methods
Clinical samples
A total of 115 GC carcinoma and adjacent tissues were collected at the Second Affiliated Hospital of Fujian Medical University (Quanzhou, China) from 2013 to 2015, and the clinicopathological characteristics and survival time were recorded. The tissues were stored in a freezer at −80°C for further research. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University, and all patients signed an informed consent form.
Cell lines
Six human GC cell lines (MKN74, N87, SNU216, MGC803, HGC-27, and AGS) were purchased from the Chinese Academy of Sciences (Shanghai, China). All cell lines were cultured in RPMI-1640 (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), except for AGS cells cultured in Ham’s F12 medium (Cellgro, Manassas, VA, USA), and incubated them in an atmosphere containing 5% CO2 at 37°C.
Immunohistochemistry (IHC)
Paraffin-embedded sample tissues were sliced at a thickness of 5 um for HE staining and immunohistochemical analysis. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 15 min. After washing 3 times with PBS, anti-Rab7 primary antibody was added, and samples were incubated at 4°C for 30 mins, then the secondary antibody was added, followed by incubation at 37°C. AEC was used for immunohistochemical staining, and the resin was observed under a microscope. The positive staining of Rab7 protein was evaluated as follows: 0 points for ≤5% positive cells, 1 point for 5–25% positive cells, 2 points for 26–50% positive cells, 3 points for 51–75% positive cells, and 4 points for ≥75% positive cells. The total score was calculated according to the staining intensity score: 0 points for no staining, 1 point for weak staining (light yellow color), 2 points for moderate staining (yellow-brown color), and 3 points for strong staining (brown color). All samples were divided into a low-expression group (score ≤3) or a high-expression group (4≤ score ≤12). Yellow-to-brownish staining of Rab7 in more than 10% of cells was considered as a positive reaction.
Western blot analysis
Following the instructions of the BCA protein analysis kit (Thermo Fisher Scientific, Waltham, MA, USA), GC cells were lysed in WESTERN & IP cell lysis buffer (Beyotime, Shanghai, China) and PMSF (AMRESCO, Solon, Ohio, USA). The supernatant was collected after centrifugation at 4°C for 10 min. The same amount of sample proteins was added to the wells for electrophoresis, and then transferred to the membrane by using 0.45 μm PVDF membrane (Amersham Hybond, GE Healthcare, Munich, Germany). Wells were incubated with rabbit antibody at 4°C overnight using anti-Rab7 (1-2000, Abcam, UK), PI3K (1-1000, Cell Signaling Technology, USA), pPI3K (1-1000, cell signal), AKT (1-1000, cell signal), pAKT (Ser308) (1-1000, Cell Signaling Technology, USA), and pAKT (Ser473) (1-1000, Cell Signaling Technology, USA). After washing for 10 min in TBST, the primary antibody was incubated with enhanced chemiluminescence substrate (Abcam, UK) for 2 h at room temperature, and then protein signal detection was performed using enhanced chemiluminescence (Lulong Biotech, Xiamen, China).
RNA extraction and quantitative real-time PCR (qRT-PCR)
The total RNA of GC tissues was extracted according to the TRIzol reagent instruction manual (Treasure Creature, Dalian, China). The concentration and purity of RNA were assessed using a ND-1000 spectrophotometer (Thermo Fisher Scientific, USA) to ensure that D260 nm/D280 nm >2.0, D230 nm/D260 nm >1.8. Adopt RevertAid Reverse transcriptase kit (Thermo Fisher Scientific, USA) reverse-transcribed RNA into cDNA. The qPCR reaction system was prepared with 25 uL 2*SGExcel SYBR Mixture+1 μL upstream primers+1 μL downstream primers+1 μL cDNA, added to ultra-pure water to 50 μL according to the instructions of the 2*SGExcel FastSYBR Mixture (Shanghai, China). Detection of relative mRNA expression of Rab7 was performed using a StepOnePlus™ PCR (ABI, USA) testing instrument. Primers used were Rab7 forward primer: 5′-CCGCTCGAGCTATGACCTCTAGGAAG- 3′, Rab7 reverse primer: 5′-GCCCCATGGTCAGCAACTGCAGCTTT-3′, internal reference primer b-actin, forward: 5′-ACGGGAAGCTTGTCATCAATGG-3′, reverse: 5′-ATGGTGGTGAAGACGCCAGTGG-3′. All primers were purchased from Shanghai Shenggong. The reaction procedures were: pre-denaturation at 95°C for 20 s, denaturation at 95°C for 3 s, and annealing/extension at 60°C for 30 s, for 35 cycles.
Plasmid construction and cell transfection
To overexpress Rab7 in AGS cells, the cDNAs encoding human Rab7 were amplified (Rab7 Forward: 5′-TCCGCTCGAGATGGCAACTGCACCATAC-3′; Rab7 Reverse: 5′-ATGGGGTACCGAGCAGCCACAGCCTTCTCTC-3′) and cloned into the pCDH (Invitrogen, Carlsbad, CA, USA) expression vector (AGS-pRab7). Empty vector pCDH was used as the control in the gain-of-function experiments (AGS-pCDH). To silence Rab7 in MCG803 and HGC-27 cells, a short hairpin RNA was designed based on the sequence 5′-TGCAAGGAATCTCACCAAT-3′ and 5′-AACAAGATTGACCTCGAAA-3′, and was cloned into pSuper-retro-puro (Oligoengine, Seattle, USA) expression vector (MCG803-psh1Rab7, HGC-27-psh1Rab7, MCG803-psh2Rab7, HGC-27-psh2Rab7). Empty vector pSuper-retro-puro was used as the control in the loss-of-function experiments (MCG803-psuperRab7 and HGC-27-psuperRab7).
Cell proliferation assay
Follow the instructions of the Cell Counting Kit-8 (CCK-8, Dojindo, Kuma-moto, Japan). Cells were incubated into a 96-well plate (103 cells/well) at 37°C for 24, 48, 72, 96, and 120 hours respectively. Then went on incubating for another 4 hours after adding CCK-8. The OD value was measured using a UV spectrometer reader (Bio-Tek, Winooski, VT, USA) at 450 nm.
Flow cytometry assay detected cell cycle
The treated cells in logarithmic growth phase were inoculated into 24-well plate. After fixed, the cells were stained with 400uL’s propidium iodide solution (PI, Thermo Fisher Scientific, USA). The PI absorbance of the cells was detected by flow cytometry, and the results were analyzed by software ModFit (Verity Software House, USA).
Cell migration and invasion assay
Cells in a serum-free medium (105 cells/L) were incubated into the upper chamber of transwell (BD Bioscience, USA). Cell culture medium containing fetal calf serum was added to the lower chamber and incubated for 48 hours. The cell culture medium was then removed, washed twice with PBS, fixed with 4% paraformaldehyde for 15 mins, and stained with 0.1% crystal violet for 15 mins. Before the cell invasion experiment, Matrigel was added to the upper chamber and incubated at 37°C for 30 mins. The other steps were the same as the migration experiment. The cell counts in 5 random fields per were evaluated by using the Qimaging MicroPublisher 5.0RTV microscope camera (OLYMPUS).
Statistical analysis
The data represent the mean (±standard deviation, SD) of three independent experiments. SPSS22.0 software was used for statistical analysis. Two-tailed t test was used to compare the measurement data of the 2 samples, Pearson χ2 test was used to compare the counting data of the 2 samples, and one-way ANOVA was used to compare the counting data of multiple samples. Kaplan-Meier method was used to draw Survival Curve. The difference was statistically significant (P<0.05).
Results
Expression of Rab7 in GC tissues and cells, and its relationship with clinical characteristics and prognosis
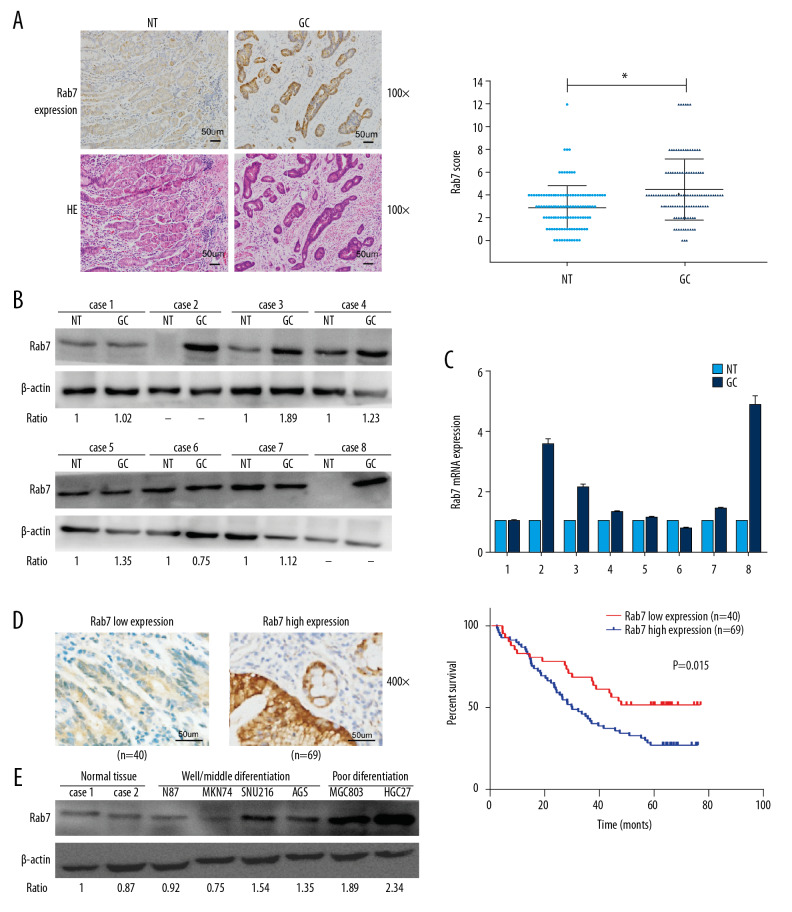
In order to determine the expression of Rab7 in GC tissues. First, we used IHC to detect the expression of Rab7 in 115 carcinoma tissues and the corresponding adjacent normal tissues (NT). It was identified that the expression of Rab7 was significantly higher in carcinoma tissues than that in the adjacent tissues (Figure 1A). The results of Western blot and qRT-PCR also showed that Rab7 was highly expressed in most cancer tissues. (Figure 1B, 1C). According to the staining score of tumor cells, the immunohistochemical results of high and low Rab7 expression of GC tissues were shown in Figure 1D. Correlation analysis between expression of Rab7 and patient’s clinicopathological features (Table 1) showed that expression of Rab7 was negatively correlated with histological differentiation (P=0.001) and positively correlated with the presence of lymph node metastasis (P=0.001). Other pathologic parameters, including age, gender, depth of tumor invasion, tumor size and clinical stage, were not significantly correlated with expression of Rab7. In addition, We Used Kaplan-Meier method to determine the relationship between survival time and expression level of Rab7 in GC patients. It was identified that the survival of patients with high Rab7 expression was significantly shorter than patients with low Rab7 expression (Figure 1D). To further confirm the upregulation of Rab7 in GC, we used Western blot analysis to detect expression of Rab7 in normal gastric tissues and 6 GC cell lines (Figure 1E). We found that the expression of Rab7 was significantly upregulated in moderately and poorly differentiated GC cells and Rab7 expression level was negatively associated with the GC cells differentiation status. Because Rab7 is highly expressed in MCG803 and HGC-27 cells, but low in AGS cells, for further explore the function of Rab7 in GC, we selected MCG803 and HGC-27 cells to construct Rab7 silent expression cell lines, and AGS cells to construct Rab7 overexpression cell lines.
Figure 1.
Upregulation of Rab7 in carcinoma tissues was associated with the prognosis. (A) Expression of Rab7 in carcinoma tissues and adjacent tissues was detected by IHC. Scale bar, 50 μm. The chart shows that the score of Rab7 in GC is significantly higher than that of NT (* P<0.05, by 2-tailed t test, n=115). (B, C) The results of Western blot and qRT-PCR showed the expression level of Rab7 protein and mRNA in cancer tissues and adjacent tissues. β-actin was used as load control (n=8). (D) According to the staining score, the high-expression group and low-expression group of Rab7 were identified. Kaplan-Meier analysis showed that the overall survival in patients(n=69) with high Rab7 expression was significantly shorter than that(n=40) with low Rab7 expression (P=0.015, by log-rank test). (E) The Rab7 expression of normal tissues and 6 GC cell lines were analyzed by Western blot analysis. β-actin was used as a loading control.
Table 1.
Clinicopathologic characteristics of 115 GC patients according to the Rab7 expression.
| Variant | Total | Rab7 p value | |
|---|---|---|---|
| Low (44) | High (71) | ||
| Age (years) | 0.876 | ||
| <60 | 69 | 26 | 43 |
| ≥60 | 46 | 18 | 28 |
| Sex | 0.622 | ||
| Male | 84 | 31 | 53 |
| Female | 31 | 13 | 18 |
| Tumor size (cm) | 0.125 | ||
| <5 | 70 | 24 | 46 |
| ≥5 | 45 | 20 | 25 |
| Depth of tumor invasion | 0.255 | ||
| T2 | 9 | 4 | 5 |
| T3 | 14 | 8 | 6 |
| T4 | 92 | 32 | 60 |
| Lymph node metastasis | 0.001* | ||
| N0 | 22 | 9 | 13 |
| N1 | 23 | 5 | 18 |
| N2 | 20 | 2 | 18 |
| N3 | 50 | 28 | 22 |
| Clinical stage | 0.757 | ||
| I/II | 28 | 10 | 18 |
| III/IV | 87 | 34 | 53 |
| Differentiation | 0.001* | ||
| High | 16 | 10 | 6 |
| Middle | 8 | 7 | 1 |
| Low/undifferentiation | 91 | 27 | 64 |
Significant difference.
Effect of Rab7 on GC cell proliferation capacity and cell cycle
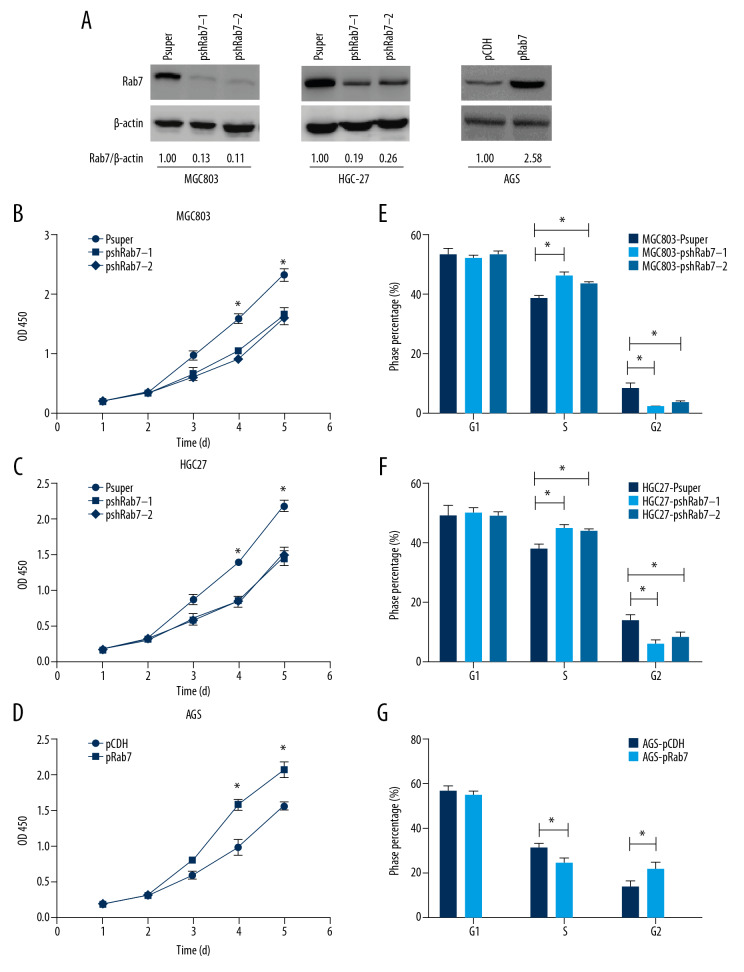
On account of the evidence had shown that Rab7 expression was associated with the prognosis of GC, Therefore, we tried to ascertain whether Rab7 can affect the malignant behavior of GC in vitro. We first studied the effect that Rab7 gene on GC cell proliferation capacity. Gene overexpression and silencing methods were used to establish Rab7 overexpressing or silencing GC cell lines. Western blot analysis confirmed that Rab7 was stably knockdown in MCG803 and HGC-27 cells and overexpressed in AGS cells (Figure 2A). As shown by CCK-8 assay, Rab7 knockdown results in suppressed MCG803 and HGC-27 cells proliferation (Figure 2B, 2C). Inversely, overexpression of Rab7 significantly increased the proliferation rate of AGS cells (Figure 2D). The result of flow cytometry (Figure 2E–2G) showed that Rab7 knockdown resulted in increasing S phase distribution and decreasing G2 phase distribution in MCG803 and HGC-27 cells. On the contrary, overexpression of Rab7 led to the opposite result in AGS cells. However, the overexpression and silencing of Rab7 did not significantly change the G1 phase distribution. These results suggest that Rab7 can induce the transition from S phase to G2 phase.
Figure 2.
Rab7 promoted the GC cells proliferation ability and changed the cell cycle. (A) Western blot analysis confirmed that Rab7 was overexpressed in AGS cells and was knocked down in MCG803 and HGC-27 cells. μ-actin acted as loading control. (B–D) Proliferation rate of GC cells after depleting or strengthening Rab7 expression, as determined by the CCK8 assay. The results revealed that knockdown of Rab7 suppressed MCG803 and HGC-27 cells proliferation and overexpression of Rab7-promoted AGS cells proliferation. The data represent the mean±SD of 3 independent experiments, * P<0.05, by 2-tailed t test). (E, F) the cycle changes of GC cells were detected by flow cytometry (data represent the mean±SD of 3 independent experiments, * P<0.05, by 2-tailed t test).
Effect of Rab7 on GC cell migration and invasion capacity
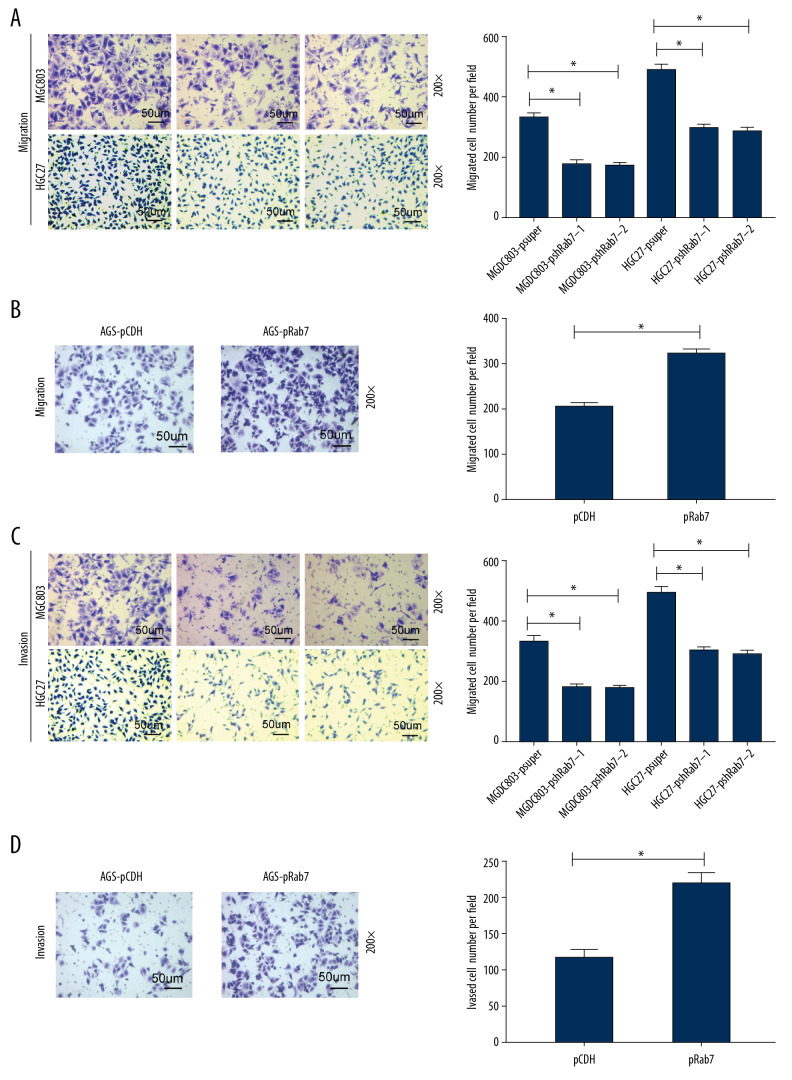
We used the transwell chamber assay to verify the effect of Rab7 on the GC cells invasion and migration. The results of transwell chamber migration assay (Figure 3A, 3B) showed that knockdown of Rab7 suppressed the migration ability of MCG803and HGC-27 cells compared with empty vector transfected cells, while the overexpression of Rab7 promoted the migration ability of AGS cells. The result of transwell chamber invasion assay (Figure 3C, 3D) revealed that knockdown of Rab7 significantly weakened the invasive ability of MCG803and HGC-27 cells compared with the cells transfected with empty vector. In contrast, the overexpression of Rab7 in AGS cells obviously enhanced their invasive ability.
Figure 3.
Rab7 regulated GC cells migration and invasion potential. (A, B) Migration ability of GC cells was measured by transwell chamber migration assay. Scale bar, 50 μm. The results showed that Rab7 knockdown results in suppressed MCG803 and HGC-27 cells migration ability, while the overexpression of Rab7 promoted the AGS cells migration ability. Data represent the mean±SD of 3 independent experiments, * P<0.05, by 2-tailed t test). (C, D) Invasion ability of gastric cancer (GC) cells was measured by transwell chamber invasion assay. Scale bar, 50 μm. The results showed that Rab7 knockdown results in suppressed MCG803 andHGC-27cells invasion ability, while the overexpression of Rab7 promoted the invasion ability of AGS cells. Statistical analysis revealed that, compared with p-super groups, the psh-Rab7 groups had significantly fewer invading and migrating cells, and, compared with p-CDH groups, the p-Rab7 groups had significantly more invading and migrating cells. Data represent the mean±SD of 3 independent experiments. * P<0.05, n=5 random fields, by 2-tailed t test).
Rab7 promotes the proliferation, invasion and migration of GC cells through PI3K/AKT signaling pathway
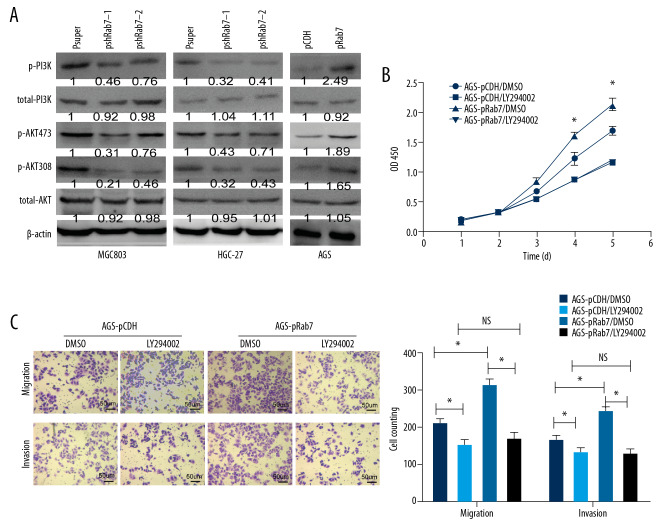
The pathogenesis of GC involves a variety of molecular mechanisms. Dysregulation in the PI3K/AKT pathway often leads to cancer, including GC [22,23]. A variety of receptors can activate this pathway, including intracellular small GTPases such as Ras [24]. Therefore, we were interested to determine whether Rab7 affects the proliferation, invasion, and migration of GC cells by mediating the PI3K/AKT signal pathway. The expression of PI3K and AKT proteins in the GC cell line was detected by Western blot analysis to evaluate the effect of Rab7 on PI3K/AKT pathway. As shown in Figure 4A, overexpression of Rab7 significantly increased the protein expression of phosphorylated PI3K and AKT (Ser308 and Ser473) in AGS cells, while the protein expression level of total PI3K and AKT remained unchanged. In contrast, knockdown of Rab7 had the opposite effects in MCG803 and HGC-27 cells. The results show that Rab7 can promote PI3K and AKT phosphorylation in GC cells. To further determine whether Rab7 affects the malignant potential of GC cells through action of the PI3K/AKT signaling pathway, we treated AGS cells with LY294002 (a PI3K inhibitor that indirectly suppresses the AKT phosphorylation level). As shown in Figure 4B and 4C, after AGS cells were treated with Ly294002, the enhanced proliferation, invasion and migration capacity stimulated by overexpression of Rab7 was significantly weakened. These results show that Rab7 promotes the progression of GC through the PI3K/AKT signaling pathway.
Figure 4.
Rab7 promotes the proliferation, invasion and migration of GC cells through PI3K/AKT signaling pathway. (A) Detection of PI3K and AKT protein expression in GC cell lines by Western blot analysis. β-actin was used as loading control. (B, C) The enhancement of Rab7-overexpressing on cell proliferation, invasion and migration was weakened by LY294002. Data represent the mean±SD of 3 independent experiments. * P<0.05; NS – no statistical significance, n=5 random fields, by 2-tailed t test).
Discussion
Gastric cancer (GC) is one of the major human cancers, and its morbidity and mortality rates are increasing steadily all over the world [25]. The survival rate is still poor in GC patients in spite of improved treatment [26,27]. The search for new tumor biomarkers is of great significance for the diagnosis and treatment of GC. Recently, an increasing number of studies have identified Rab7 as a cancer biomarker [28], but it has not been reported in GC. The objective of the present study was to assess the regulatory role of Rab7 in GC by measuring the expression of Rab7 and cell function experiments in vitro.
Previous studies found that Rab7 is upregulated in thyroid adenoma, breast cancer, lung cancer, and ovarian/primary peritoneal serous carcinoma, and is a tumor promotor [17,29–31]. The results of the present study are consistent result with those of previous studies, showing that the expression of Rab7 is upregulated in GC tissue samples and cells, and its upregulation is associated with poor clinical prognosis.
To further explore the mechanism of Rab7 underlying the progression of GC, we performed an in vitro functional experiment, showing that Rab7 can promote the proliferation of GC cells. The results of flow cytometry showed that overexpression of Rab7 induced the transition from S phase to G2 phase in GC cells, while knockdown of Rab7 led to S phase arrest, suggesting that Rab7 can accelerate the cell cycle, which may be one of the ways by which Rab7 promotes the proliferation of GC cells. These results are consistent with the results of Xie et al. in breast cancer, showing that Rab7a knockdown suppresses MDA-MB-231 cell proliferation and induces S phase arrest [17]. Zhao et al. had also found that Rab7 knockdown suppresses the proliferation of endothelial cells [32]. To further elucidate the effect of Rab7 on the growth of GC cells, it is necessary to further assess the apoptotic ability or verify it by colony formation assay in the future.
Many reports had revealed that Rab7 is involved in the invasion and migration of cancer cells. For example, in breast cancer, Xie et al. found that Knockdown of Rab7a suppressed xenografted tumorigenesis of MDA-MB-231 cells [17]. Another study on breast cancer reported that dominant inactivation of Rab7 impairs the ability of breast cancer cells to migrate and invade [33]. A report has revealed that the destruction of Rab7 protein inhibits the invasiveness of cholangiocarcinoma cells [18]. Zhao et al. found that knockdown of Rab7 reduced the migration ability of LLC cells and Ly6G+ cells across the LAL/EC monolayer [32]. In the present study, we found that overexpression of Rab7 enhanced the invasion and migration abilities of AGS cells, while knockdown of Rab7 reduced the invasion and migration abilities of MGC803 and HGC-27 cells, which also confirms the tumor-promoting effect of Rab7.
Rab7 is the basis of the transport and degradation of a variety of signal receptors, which can regulate the signal transduction pathway [5,6]. The carcinogenesis of Rab7 involves a variety of molecular mechanisms. It has been reported that Rab7 regulates cell migration through Rac-1 and vimentin [34]. Vimentin is an indicator of epithelial-stromal transformation and is regulated by Rab7 in cancer cells [35,36]. Rab7 can affect the invasion and migration of cervical cancer Hela cells by affecting the internal transport and recovery of membrane-anchored membrane type 1 matrix metalloproteinase (MT1-MMP) [33]. Endothelial cells (EC) are an important type of stromal cell, which can interact with malignant cells to promote tumor angiogenesis and cancer cell extravasation [37]. Zhao et al. found that Rab7 interacts with the mTOR signal pathway to promote the tumorigenesis of EC cells [32]. Xie et al. found that Rab7a promotes the occurrence of breast cancer by regulating eukaryotic translation initiation factor 4F (eIF4F) [17]. The present study found that Rab7 plays a positive regulatory role in the development of GC, but the molecular mechanism is unclear. We plan to explore the potential molecular mechanism of Rab7 affecting the progression of GC in future research.
Recent studies reported that Rab7 can inhibit the degradation of EGFR, which is an activating molecule of the PI3K/AKT pathway [38], and maintains the level of AKT phosphorylation in epidermoid carcinoma cells, suggesting that Rab7 can maintain the AKT signaling pathway [39,40]. The PI3K/AKT signaling pathway plays an extremely important role in regulating cell survival and proliferation and thus is a key regulatory axis in tumor development and progression [41,42]. The roles of the AKT signaling pathway in GC have been revealed by several studies, and its abnormal regulation frequently results in tumorigenesis [43,44] Therefore, we speculate that Rab7 affects the progress of GC through the PI3K/AKT signaling pathway. Here, we detected the interaction between Rab7 and PI3K/AKT pathways. Our Western blot results showed that the overexpression of Rab7 significantly increased the protein expression of phosphorylated PI3K and AKT (Ser308 and Ser473), and knockdown of Rab7 significantly suppressed the expression of pPI3K and pAKT (Ser308 and Ser473), while the expression of total PI3K and AKT was not significantly changed. We also found that LY294002 eliminated the effect of overexpression of Rab7 on proliferation, migration, and invasion of AGS cells. These results further confirm that Rab7 affects the progression of GC through the PI3K/AKT signaling pathway.
Conclusions
In conclusion, the present study provides evidence for the role of Rab7 in the carcinogenesis and development of GC. We demonstrated that the expression of Rab7 was increased in GC and was associated with poor prognosis. We also found that Rab7 can promote the proliferation, invasion, and migration of GC cells, and it targets GC cells through the PI3K/AKT signaling pathway. Our findings suggest that Rab7 may be a prognostic factor and target for the treatment of GC.
Footnotes
Availability of data and materials
The datasets generated and/or analyzed during the study are available from the corresponding author on reasonable request.
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CaCancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388(10060):2654–64. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 3.Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–13. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehdev A, Catenacci DV. Gastroesophageal cancer: Focus on epidemiology, classification, and staging. Discov Med. 2013;16(87):103–11. [PubMed] [Google Scholar]
- 5.Guerra F, Bucci C. Multiple roles of the small GTPase Rab7. Cells. 2016;5(3) doi: 10.3390/cells5030034. pii: E34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucci C, Thomsen P, Nicoziani P, et al. Rab7: A key to lysosome biogenesis. Mol Biol Cell. 2000;11(2):467–80. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edinger AL. Growth factors regulate cell survival by controlling nutrient transporter expression. Biochem Soc Trans. 2005;33:225–27. doi: 10.1042/BST0330225. [DOI] [PubMed] [Google Scholar]
- 8.Edinger AL, Cinalli RM, Thompson CB. Rab7 prevents growth factor-independent survival by inhibiting cell-autonomous nutrient transporter expression. Dev Cell. 2003;5(4):571–82. doi: 10.1016/s1534-5807(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 9.Kou X, Yang Y, Jiang X, et al. Vorinostat and Simvastatin have synergistic effects on triple-negative breast cancer cells via abrogating Rab7 prenylation. Eur J Pharmacol. 2017;813:161–71. doi: 10.1016/j.ejphar.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Saxena S, Bucci C, Weis J, Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci. 2005;25(47):10930–40. doi: 10.1523/JNEUROSCI.2029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deinhardt K, Salinas S, Verastegui C, et al. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52(2):293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Harrison RE, Bucci C, Vieira OV, et al. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: Role of Rab7 and RILP. Mol Cell Biol. 2003;23(18):6494–506. doi: 10.1128/MCB.23.18.6494-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamano K, Fogel AI, Wang C, et al. Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. eLife. 2014;3:e01612. doi: 10.7554/eLife.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyttinen JM, Niittykoski M, Salminen A, Kaarniranta K. Maturation of autophagosomes and endosomes: A key role for Rab7. Biochim Biophys Acta. 2013;1833(3):503–10. doi: 10.1016/j.bbamcr.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Bucci C, De Luca M. Molecular basis of Charcot-Marie-Tooth type 2B disease. Biochem Soc Trans. 2012;40(6):1368–72. doi: 10.1042/BST20120197. [DOI] [PubMed] [Google Scholar]
- 16.Cogli L, Piro F, Bucci C. Rab7 and the CMT2B disease. Biochem Soc Trans. 2009;37:1027–31. doi: 10.1042/BST0371027. [DOI] [PubMed] [Google Scholar]
- 17.Xie J, Yan Y, Liu F, et al. Knockdown of Rab7a suppresses the proliferation, migration, and xenograft tumor growth of breast cancer cells. Biosci Rep. 2019;39(2) doi: 10.1042/BSR20180480. pii: BSR20180480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suwandittakul N, Reamtong O, Molee P, et al. Disruption of endocytic trafficking protein Rab7 impairs invasiveness of cholangiocarcinoma cells. Cancer Biomark. 2017;20(3):255–66. doi: 10.3233/CBM-170030. [DOI] [PubMed] [Google Scholar]
- 19.Steffan JJ, Dykes SS, Coleman DT, et al. Supporting a role for the GTPase Rab7 in prostate cancer progression. PLoS One. 2014;9(2):e87882. doi: 10.1371/journal.pone.0087882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Zhang H, Liu S, et al. Internalized CD44s splice isoform attenuates EGFR degradation by targeting Rab7A. Proc Natl Acad Sci USA. 2017;114(31):8366–71. doi: 10.1073/pnas.1701289114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alonso-Curbelo D, Riveiro-Falkenbach E, Pérez-Guijarro E, et al. RAB7 controls melanoma progression by exploiting a lineage-specific wiring of the endolysosomal pathway. Cancer Cell. 2014;26(1):61–76. doi: 10.1016/j.ccr.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Mundi PS, Sachdev J, McCourt C, Kalinsky K. AKT in cancer: New molecular insights and advances in drug development. Br J Clin Pharmacol. 2016;82(4):943–56. doi: 10.1111/bcp.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh SS, Yap WN, Arfuso F, et al. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J Gastroenterol. 2015;21(43):12261–73. doi: 10.3748/wjg.v21.i43.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander SP, Mathie A, Peters JA. Br J Pharmacol. 5th edition. 2011. Guide to receptors and channels (GRAC) pp. S1–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 26.Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–46. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]
- 27.Strong VE, Wu AW, Selby LV, et al. Differences in gastric cancer survival between the U.S. and China. J Surg Oncol. 2015;112:31–37. doi: 10.1002/jso.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerra F, Bucci C. Role of the RAB7 protein in tumor progression and cisplatin chemoresistance. Cancers (Basel) 2019;11(8) doi: 10.3390/cancers11081096. pii: E1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croizet-Berger K, Daumerie C, Couvreur M, et al. The endocytic catalysts, Rab5a and Rab7, are tandem regulators of thyroid hormone production. Proc Natl Acad Sci USA. 2002;99(12):8277–82. doi: 10.1073/pnas.122187699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano T, Shimizu K, Kawashima O, et al. Establishment of a human lung cancer cell line with high metastatic potential to multiple organs: Gene expression associated with metastatic potential in human lung cancer. Oncol Rep. 2012;28(5):1727–35. doi: 10.3892/or.2012.1972. [DOI] [PubMed] [Google Scholar]
- 31.Davidson B, Zhang Z, Kleinberg L, et al. Gene expression signatures differentiate ovarian/peritoneal serous carcinoma from diffuse malignant peritoneal mesothelioma. Clin Cancer Res. 2006;12:5944–50. doi: 10.1158/1078-0432.CCR-06-1059. [DOI] [PubMed] [Google Scholar]
- 32.Zhao T, Ding X, Yan C, Du H. Endothelial Rab7 GTPase mediates tumor growth and metastasis in lysosomal acid lipase-deficient mice. J Biol Chem. 2017;292(47):19198–208. doi: 10.1074/jbc.M116.773093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams KC, Coppolino MG. Phosphorylation of membrane type 1-matrix metalloproteinase (MT1-MMP) and its vesicle-associated membrane protein 7 (VAMP7)-dependent trafficking facilitate cell invasion and migration. J Biol Chem. 2011;286(50):43405–16. doi: 10.1074/jbc.M111.297069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margiotta A, Progida C, Bakke O, Bucci C. Rab7a regulates cell migration through Rac1 and vimentin. Biochim Biophys Acta Mol Cell Res. 2017;1864(2):367–81. doi: 10.1016/j.bbamcr.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cogli L, Progida C, Bramato R, Bucci C. Vimentin phosphorylation and assembly are regulated by the small GTPase Rab7a. Biochim Biophys Acta. 2013;1833(6):1283–93. doi: 10.1016/j.bbamcr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weis SM, Cheresh DA. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 38.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21(2):177–84. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Lin X, Zhang J, Chen L, et al. Tyrosine phosphorylation of Rab7 by Src kinase. Cell Signal. 2017;35:84–94. doi: 10.1016/j.cellsig.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Wang T, Zhang M, Ma Z, et al. A role of Rab7 in stabilizing EGFR-Her2 and in sustaining Akt survival signal. J Cell Physiol. 2012;227(6):2788–97. doi: 10.1002/jcp.23023. [DOI] [PubMed] [Google Scholar]
- 41.Toker A. Achieving specificity in Akt signaling in cancer. Adv Biol Regul. 2012;52(1):78–87. doi: 10.1016/j.advenzreg.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Revathidevi S, Munirajan AK. Akt in cancer: Mediator and more. Semin Cancer Biol. 2019;59:80–91. doi: 10.1016/j.semcancer.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Kang MJ, Ryu BK, Lee MG, et al. NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology. 2008;135(6):2030–42. 2042.e1–3. doi: 10.1053/j.gastro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Shi R, Yang Z, Liu W, et al. Knockdown of Slit2 promotes growth and motility in gastric cancer cells via activation of AKT/β-catenin. Oncol Rep. 2014;31(2):812–18. doi: 10.3892/or.2013.2887. [DOI] [PubMed] [Google Scholar]