ABSTRACT
Objective:
The objective of this article was to understand and decode the mystery of the formation of para-chloroaniline (PCA). The ingredient of the brown precipitate after mixing sodium hypochlorite (NaOCl) and chlorhexidine gluconate (CHX) is still in debate.
Materials and Methods:
Various studies adopt a different methodology to substantiate that it may contain PCA, which is a carcinogenic agent. The purpose of this systematic review is to evaluate the relationship between PCA and brown precipitate. Two reviewers independently conducted a comprehensive literature search. The MEDLINE, Embase, Cochrane, and PubMed databases were searched. In addition, the bibliographies were manually searched. There was no disagreement between the two reviewers. This review was reported and conducted in step with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Results:
Of 233 articles, only 13 articles met the inclusion criteria. Available scientific evidence was more supportive that the brown precipitate form after mixing NaOCl and CHX may form para-chloroamide moiety rather than free PCA, and PCA may be the by-product of CHX degradation.
Conclusion:
On the basis of the current evidence and data extracted from the various databases, it can be concluded that the mixture of sodium hypochlorite and chlorhexidine does not form PCA, and PCA may be the by-product of high concentrated chlorhexidine. Further studies are required to substantiate the evidence.
KEYWORDS: Chlorhexidine, para-chloroaniline, sodium hypochlorite, spectroscopy
INTRODUCTION
Persistent infection due to remnant infected dentinal debris bacteria is the cause of root canal failure.[1] Therefore, to obtain a sterilized canal, irrigation plays a crucial role.[2] The most common irrigant with antimicrobial property is sodium hypochlorite (NaOCl), but the increased concentration of NaOCl can be potentially toxic to the periapical tissue.[3,4]
Chlorhexidine gluconate (CHX) was introduced as an alternative against NaOCl with the good antimicrobial property but a lack of tissue dissolution capacity.[5,6,7] The introduction of chlorhexidine in the presence of NaOCl into the canal leads to the formation of brown precipitate due to acid–base reaction that extends up to 139–684 µm, which deteriorate the sealing of the root canal.[8] Some of the previously published studies claim the presence of the para-chloroaniline (PCA) in the brown precipitate,[9,10,11,12] but another study denies the concept of the formation of PCA.[13,14]
Previous study was unable to substantiate the formation of PCA. It is important to understand that mixing NaOCl and CHX forms PCA or not because PCA known to be carcinogenic and may cause methemoglobinemia.[15,16,17,18,19]
To date, the formation of PCA due to NaOCl and CHX remains vague. Therefore, the purpose of this systematic review was to evaluate whether PCA is formed through the reaction of mixing NaOCl and CHX.
MATERIALS AND METHODS
The protocol for this systematic review was developed following established guidelines.[20] The protocol was prepared and matched the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews. The focused question was adjusted according to the PICO (Population, Intervention, Comparator, Outcome) criteria for comparing laboratory studies as follows: Population (specimens or extracted teeth); Intervention and Comparison (interaction between NaOCl and CHX); Outcome (PCA formation).[21]
SEARCH STRATEGY
A literature search was performed using the U.S. National Library of Medicine (MEDLINE), Embase, Cochrane, and PubMed databases. Articles were included up to and including August 29, 2019.
In addition, bibliography of articles and textbooks were manually searched. Two reviewers (M.S. and B.A.) independently searched the article and abstract based on inclusion and exclusion criteria. All potential studies were analyzed and assessed completely. Only studies published in English were included.
INCLUSION CRITERIA
In vitro studies were considered and studies evaluating PCA formation were included.
EXCLUSION CRITERIA
Case series, cell culture laboratory studies, or animal studies were excluded.
RESULTS
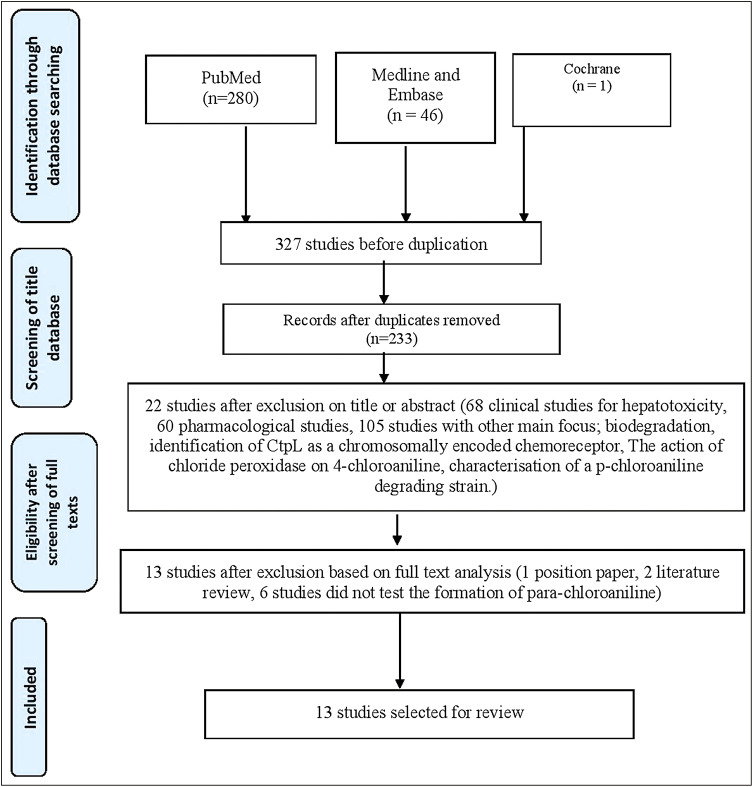
Flowchart of this systematic review based on PRISMA guidelines is presented in Figure 1. Of 233 studies, 13 met the inclusion criteria.
Figure 1.
Flow diagram based on PRISMA guideline
STUDY SELECTION
After the database screening and the removal of duplicates, 233 studies were identified. After screening the titles, 22 were left. It was further reduced to 13 after the examination of abstracts and full texts.
CHARACTERISTICS OF INCLUDED ARTICLES
This review includes studies published in English. The characteristics of 13 included studies are listed in Tables 1 and 2. Ten articles focused on the combination of NaOCl and CHX to analyze the formation of PCA, and the remaining three articles analyzed degradation and stability of CHX at various concentrations.
Table 1.
Studies focused on NaOCl and CHX mixture to analyze PCA formation
| Study ID | Study parameters | Analysis method | Outcome | ||
|---|---|---|---|---|---|
| Study design | Identification of the precipitate | Removal/ prevention of the precipitate | |||
| Basrani et al.[9] | In vitro | Yes | No | XPS and TOF-SIMS | PCA or isomers found in the precipitate |
| Basrani et al.[10] | In vitro | Yes | No | GC-MS | PCA was found in the precipitate |
| Thomas and Sem[25] | In vitro | Yes | No | 1H NMR spectroscopy | No PCA was found in the precipitate |
| Krishnamurthy and Sudhakaran[28] | In vitro | Yes | Yes | Stereomicroscopy, NMR, the Beilstein and HCl solubility tests | Presence of chlorine in the para position of the benzene ring. Intermediate flushing with saline distilled water or using absolute alcohol can prevent precipitate formation |
| Mortenson et al.[13] | In vitro | Yes | Yes | GC/MS | PCA was found. Citric acid is recommended as an intermittent irrigant |
| Kolosowski et al.[11] | In vitro | Yes | No | TOF-SIMS | Presence of PCA in dentinal tubule |
| Arslan et al.[26] | In vitro | Yes | No | 1H NMR spectroscopy | Presence of PCA in NaOCl and CHX mixture. PCA was not found in NaOCl and Qmix mixture |
| Orhan et al.[29] | In vitro | Yes | No | HPLC, proton NMR, gas chromatography, TLC, IR spectroscopy, and GC/MS | No PCA was found in the precipitate |
| Irmak et al.[27] | In vitro | Yes | No | 1H proton NMR and IR spectroscopy | No PCA was found in the precipitate |
| Siddique et al.[12] | In vitro | Yes | No | TLC, HPLC, CC, ESIMS, UV, and NMR (1H NMR and C-13 NMR) | PCA was found in the precipitate |
CC = column chromatography, ESI-MS = electron spray ionization mass spectrometry, GC-MS = gas chromatography–mass spectrometry, HPLC = high-performance liquid chromatography, IR = infrared, TLC = thin-layer chromatography, TOF-SIMS = time-of-flight secondary ion mass spectrometry, UV = ultraviolet, XPS = X-ray photon spectroscopy
Table 2.
Studies focused on CHX concentration to analyze PCA formation
| Study ID | Study design | Identification of the precipitate | Removal/ prevention of the precipitate | Analysis method | Outcome |
|---|---|---|---|---|---|
| Barbin et al.[22] | In vitro | No | No | Mass spectrometry, HPLC | ROS liberation was seen with the combination of CHX and Ca(OH)2 |
| Barbin et al.[23] | In vitro | No | No | GC-MS | PCA and ROS were the by-products of the 2% CHX aqueous solution |
| Câmara De Bem et al.[24] | In vitro | No | No | GC-MS | 2% CHX gel and solution may degrade into PCA, oCA, mCA, ROS, and organochlorines (ortho-chlorophenyl isocyanate and 2-amino-5-chlorobenzonitrile) regardless of storage conditions or time |
GC-MS = gas chromatography–mass spectrometry, HPLC = high-performance liquid chromatography, mCA = meta-chloroaniline, oCA = ortho-chloroaniline, ROS = reactive oxygen species
The various analytical methodologies used to detect PCA formation are listed in Table 3.
Table 3.
Various analytical methodology used to detect PCA formation
| Analytical method | Articles identified |
|---|---|
| GC-MS | Barbin et al.[22] |
| Basrani et al.[10] | |
| Mortenson et al.[13] | |
| Barbin et al.[23] | |
| Câmara De Bem et al.[24] | |
| Orhan et al.[14] | |
| 1H NMR spectroscopy | Thomas and Sem[25] |
| Arslan et al.[26] | |
| Orhan et al.[14] | |
| Irmak et al.[27] | |
| Siddique et al.[12] | |
| HPLC and TLC | Orhan et al.[14] |
| Siddique et al.[12] | |
| TOF-SIMS | Basrani et al.[9] |
| Kolosowski et al.[11] | |
| Infrared spectroscopy | Orhan et al.[14] |
| Irmak et al.[27] | |
| CC | Siddique et al.[12] |
| ESI-MS | |
| Ultraviolet | |
| Stereomicroscopy | Krishnamurthy and Sudhakaran[28] |
| XPS | Basrani et al.[9] |
CC = column chromatography, ESI-MS = electron spray ionization mass spectrometry, GC-MS = gas chromatography–mass spectrometry, HPLC = high-performance liquid chromatography, TLC = thin-layer chromatography, TOF-SIMS = time-of-flight secondary ion mass spectrometry, XPS = X-ray photon spectroscopy
DISCUSSION
This systematic review focused on any association between the identification of PCA and the analytical methods used for identification in various literature.
GAS CHROMATOGRAPHY/MASS SPECTROMETRY
Three articles were identified to address a relationship between PCA formation and gas chromatography–mass spectrometry.[10,13,14,22,23,24] Two articles reported that the combination of NaOCl and CHX leads to the formation of PCA.[10,13] Previous studies analyzed that CHX degradation at various concentrations liberates PCA,[22,23,24] whereas Orhan et al.14 reported that for the detection of PCA, mass spectrometry is not an appropriate method.
1H NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
Five articles were identified, which assessed the PCA formation.[12,14,25,26,27] Even though 1H nuclear magnetic resonance (NMR) spectroscopy is a nondestructive method for detecting PCA, results of various studies vary. Three studies clearly mentioned the absence of free PCA in the NaOCl and CHX reaction.[14,25,27] On the contrary, two studies supported the formation of PCA.[12,26] The spectrum interpretation performed between the range of 6.5–8.5 ppm followed by concluded that 7.0–8.0 ppm represent PCA but spectra result of the amino signal was included in the discussion. We contemplate that the inaccuracy of the results could be due to misinterpretation of para-chloro amide moiety of CHX or any derivative of CHX in the brown precipitate.[14] Orhan et al. single out the methodological error of the study by Arslan et al.[26] and bring down the curtain with the statement that the 1H NMR analysis was misunderstood and the result was misinterpreted.[29]
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY AND THIN LAYER CHROMATOGRAPHY
Two articles assessed the PCA formation in NaOCl and CHX reaction.[12,14] Even tough high-performance liquid chromatography is a nondestructive method to analyze the PCA formation, the results of both studies are completely different. Siddique et al.[12] supported the formation of free PCA but Orhan et al.[14] opposed it. However, Siddique et al.[12] were unable to mention para-chloro amide moiety of CHX. Therefore, their verification to justify free PCA formation may be inaccurate.
TIME-OF-FLIGHT SECONDARY ION MASS SPECTROMETRY
Two articles supported the PCA formation using time-of-flight secondary ion mass spectrometry (TOF-SIMS).[9,11] The presence of PCA can be interpreted using TOF-SIMS but unable to differentiate between free PCA and amide moiety. TOF-SIMS showed the presence of PCA in the CHX group and NaOCl/CHX group. Hence TOF-SIMS cannot be a reliable method for detecting PCA.
INFRARED SPECTROSCOPY
Two published studies examined PCA formation in the NaOCl/CHX mixture, and both studies denied PCA formation in the brown precipitate.[14,27]
COLUMN CHROMATOGRAPHY, ELECTRON SPRAY IONIZATION MASS SPECTROMETRY, AND ULTRAVIOLET
Only one study used column chromatography, electron spray ionization mass spectrometry, and ultraviolet to analyze the presence of PCA in the mixture.[12] However, this study discussed the value range for PCA but not about amide moiety; thus, the result of this study cannot be a cutoff proof for finding PCA in the mixture.
STEREOMICROSCOPY
Krishnamurthy and Sudhakaran.[28] detected to confirm the presence of PCA using stereomicroscopy and NMR and concluded that intermediate flushing with saline can prevent the formation of PCA.
X-RAY PHOTON SPECTROSCOPY
Basrani et al.[9] confirmed the presence of PCA using X-ray photon spectroscopy and TOF-SIMS.
Neither Krishnamurthy and Sudhakaran[28] nor Basrani BR et al.[9] discussed para-chloro amide moiety of CHX or CHX derivative. Zong and Kirsch[30] tested the pH-dependant chlorhexidine instability and concluded that chlorhexidine degradation into PCA is more in an acidic medium as compared to the alkali medium, which could be the cutoff proof that NaOCl is not responsible for PCA formation due to its alkali nature.
NaOCl and CHX lead to an acid–base reaction that forms brown precipitate, which may contain para-chloro amide moiety indeed it disturbs the sealer penetration and may affect periapical seal,[25] or it is due to NaOCl that cause chlorination of the guanidino nitrogen of CHX.[32] But to date no conclusive methodology is available that clearly identify the by-product of NaOCl and CHX mixture. Many studies have been performed to solve the mystery of the brown precipitate; some studies supported and some opposed the PCA formation. It may be due to differences between the techniques; however, based on available data it is clear that the brown precipitate might contain aromatic ring, which might be the substitute of PCA.[25]
There is a concern that the brown precipitate, which is PCA according to some authors, is carcinogenic. Patil.[31] investigated the mutagenic potential of the brown precipitate by Ames test and concluded that the precipitate is free from carcinogenic elements.
Thus, from this systematic review, it can be concluded the PCA may be the degradation product of concentrated CHX and not the NaOCl/CHX mixture. In future, well-designed research methodologies are needed to address this question.
CONCLUSION
On the basis of the provided evidence, we can conclude that NaOCl and CHX mixture does not contain free PCA.
We should understand that leaving the brown precipitate in the canal is terrible, so intermittent flushing should be performed to avoid precipitate formation.
Well-designed studies may able to provide cutoff proof of the absence of free PCA in NaOCl and CHX mixture.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Mohd. Sibghatullah Khatib: Concepts, Design, Literature search. Bilal Ameer: Manuscript preparation. Nikita Ajit Mannur: Literature search. Amith Madi Ramalingaiahsetty: Data acquisition. Sayed Mateen Peerzade: Literature search. Amrut Bambawale: Manuscript review.
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
To the best of the author(s) knowledge, this study has been conducted in full accordance with the World Medical Association Declaration of Helsinki.
PATIENT DECLARATION OF CONSENT
For the further development of medical treatment in, dentistry, the publishing of clinical pictures and treatment methods is indispensable. This is why I explicitly agree that all information collected during the course of the treatment, including picture, sound and video material – even if my person / child is recognizable – may be published for scientific as well as for educational purposes in the publishing group of JISPCD. The material may be linked with information about the disease pattern and the treatment methods, etc.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ACKNOWLEDGMENT
Nil.
REFERENCES
- 1.Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J. 1982;15:187–96. doi: 10.1111/j.1365-2591.1982.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 2.Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am. 2010;54:291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson RM, Kidd B, Evans GE, Moule AJ. The effect of surfactant on the dissolution of porcine pulpal tissue by sodium hypochlorite solutions. J Endod. 2012;38:1257–60. doi: 10.1016/j.joen.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Ehrich DG, Brian JD, Jr, Walker WA. Sodium hypochlorite accident: Inadvertent injection into the maxillary sinus. J Endod. 1993;19:180–2. doi: 10.1016/S0099-2399(06)80684-9. [DOI] [PubMed] [Google Scholar]
- 5.Ohara P, Torabinejad M, Kettering JD. Antibacterial effects of various endodontic irrigants on selected anaerobic bacteria. Endod Dent Traumatol. 1993;9:95–100. doi: 10.1111/j.1600-9657.1993.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 6.Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod. 2004;30:785–7. doi: 10.1097/00004770-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Okino LA, Siqueira EL, Santos M, Bombana AC, Figueiredo JA. Dissolution of pulp tissue by aqueous solution of chlorhexidine digluconate and chlorhexidine digluconate gel. Int Endod J. 2004;37:38–41. doi: 10.1111/j.1365-2591.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- 8.Bui TB, Baumgartner JC, Mitchell JC. Evaluation of the interaction between sodium hypochlorite and chlorhexidine gluconate and its effect on root dentin. J Endod. 2008;34:181–5. doi: 10.1016/j.joen.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Basrani BR, Manek S, Sodhi RN, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod. 2007;33:966–9. doi: 10.1016/j.joen.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Basrani BR, Manek S, Mathers D, Fillery E, Sodhi RN. Determination of 4-chloroaniline and its derivatives formed in the interaction of sodium hypochlorite and chlorhexidine by using gas chromatography. J Endod. 2010;36:312–4. doi: 10.1016/j.joen.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Kolosowski KP, Sodhi RN, Kishen A, Basrani BR. Qualitative analysis of precipitate formation on the surface and in the tubules of dentin irrigated with sodium hypochlorite and a final rinse of chlorhexidine or QMiX. J Endod. 2014;40:2036–40. doi: 10.1016/j.joen.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Siddique R, Sureshbabu NM, Somasundaram J, Jacob B, Selvam D. Qualitative and quantitative analysis of precipitate formation following interaction of chlorhexidine with sodium hypochlorite, neem, and tulsi. J Conserv Dent. 2019;22:40–7. doi: 10.4103/JCD.JCD_284_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mortenson D, Sadilek M, Flake NM, Paranjpe A, Heling I, Johnson JD, et al. The effect of using an alternative irrigant between sodium hypochlorite and chlorhexidine to prevent the formation of para-chloroaniline within the root canal system. Int Endod J. 2012;45:878–82. doi: 10.1111/j.1365-2591.2012.02048.x. [DOI] [PubMed] [Google Scholar]
- 14.Orhan EO, Irmak Ö, Hür D, Yaman BC, Karabucak B. Does para-chloroaniline really form after mixing sodium hypochlorite and chlorhexidine? J Endod. 2016;42:455–9. doi: 10.1016/j.joen.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Boehncke A, Kielhorn J, Könnecker G, Pohlenz-Michel C, Mangelsdorf I. Concise International Chemical, Assessment, Document 48: 4-Chloroaniline. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 16.Chhabra RS, Huff JE, Haseman JK, Elwell MR, Peters AC. Carcinogenicity of p-chloroaniline in rats and mice. Food Chem Toxicol. 1991;29:119–24. doi: 10.1016/0278-6915(91)90166-5. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay DH, Harvey CC. Markingink poisoning: An outbreak of methaemoglobin cyanosis in newborn babies. Lancet. 1959;1:910–2. [PubMed] [Google Scholar]
- 18.Messmer AS, Nickel CH, Bareiss D. P-chloroaniline poisoning causing methemoglobinemia: A case report and review of the literature. Case Rep Emerg Med. 2015;2015:208732. doi: 10.1155/2015/208732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni BS, Acharya VN, Khanna RM, Nath S, Mankodi RP, Raghavan P. Methemoglobinemia due to nitro-aniline intoxication. Review of the literature with a report of 9 cases. J Postgrad Med. 1969;15:192–200. [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Forrest JL, Miller SA. Evidence-based decision making in action: Part 1—Finding the best clinical evidence. J Contemp Dent Pract. 2002;3:10–26. [PubMed] [Google Scholar]
- 22.Barbin LE, Saquy PC, Guedes DF, Sousa-Neto MD, Estrela C, Pécora JD. Determination of para-chloroaniline and reactive oxygen species in chlorhexidine and chlorhexidine associated with calcium hydroxide. J Endod. 2008;34:1508–14. doi: 10.1016/j.joen.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Barbin LE, Estrela C, Guedes DF, Spanó JC, Sousa-Neto MD, Pécora JD. Detection of para-chloroaniline, reactive oxygen species, and 1-chloro-4-nitrobenzene in high concentrations of chlorhexidine and in a mixture of chlorhexidine and calcium hydroxide. J Endod. 2013;39:664–8. doi: 10.1016/j.joen.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Câmara De Bem SH, Estrela C, Guedes DF, Sousa-Neto MD, Pécora JD. Determination of chemical components derived from 2% chlorhexidine gel degradation using gas chromatography-mass spectrometry. Acta, Odontol, Scand. 2014;72:630–8. doi: 10.3109/00016357.2014.880941. [DOI] [PubMed] [Google Scholar]
- 25.Thomas JE, Sem DS. An in vitro spectroscopic analysis to determine whether para-chloroaniline is produced from mixing sodium hypochlorite and chlorhexidine. J Endod. 2010;36:315–7. doi: 10.1016/j.joen.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arslan H, Uygun AD, Keskin A, Karatas E, Seçkin F, Yıldırım A. Evaluation of orange-brown precipitate formed in root canals after irrigation with chlorhexidine and QMix and spectroscopic analysis of precipitates produced by a mixture of chlorhexidine/NaOCl and QMix/NaOCl. Int Endod J. 2015;48:1199–203. doi: 10.1111/iej.12427. [DOI] [PubMed] [Google Scholar]
- 27.Irmak Ö, Orhan EO, Görgün K, Yaman BC. Nuclear magnetic resonance spectroscopy and infrared spectroscopy analysis of precipitate formed after mixing sodium hypochlorite and QMix 2in1. PLoS One. 2018;13:e0202081. doi: 10.1371/journal.pone.0202081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnamurthy S, Sudhakaran S. Evaluation and prevention of the precipitate formed on interaction between sodium hypochlorite and chlorhexidine. J Endod. 2010;36:1154–7. doi: 10.1016/j.joen.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Orhan EO, Irmak O. Comments on misinterpretation of the proton nuclear magnetic resonance spectroscopic data of a previous study. Int Endod J. 2019;52:1397–8. doi: 10.1111/iej.13167. [DOI] [PubMed] [Google Scholar]
- 30.Zong Z, Kirsch LE. Studies on the instability of chlorhexidine, part I: Kinetics and mechanisms. J Pharm Sci. 2012;101:2417–27. doi: 10.1002/jps.23151. [DOI] [PubMed] [Google Scholar]
- 31.Patil P, Aminoshariae A, Harding J, Montagnese TA, Mickel A. Determination of mutagenicity of the precipitate formed by sodium hypochlorite and chlorhexidine using the Ames test. Aust Endod J. 2016;42:16–21. doi: 10.1111/aej.12100. [DOI] [PubMed] [Google Scholar]
- 32.Prado M, Santos Júnior HM, Rezende CM, Pinto AC, Faria RB, Simão RA, et al. Interactions between irrigants commonly used in endodontic practice: A chemical analysis. J Endod. 2013;39:505–10. doi: 10.1016/j.joen.2012.11.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.