Abstract
Purpose
To investigate the effects of topical application of ophthalmic 5% povidone-iodine eye drops, which has been reported to cause apnea in spontaneously breathing children during general anesthesia.
Methods
The authors conducted a randomized, controlled, single-blinded study comparing the effect of balanced salt solution eye drops and povidone-iodine eye drops on respiration in spontaneously breathing children during general anesthesia with sevoflurane via a laryngeal mask airway. Fifty patients received balanced salt solution eye drops and 50 patients received 5% povidone-iodine eye drops.
Results
None of the control patients had a significant change in respiration. Thirty of the 50 (60%) povidone-iodine patients had a slowing of respiration within the first 6 breaths after eye drop instillation (P < .001). The median time of respiratory pause in those 30 patients was 18.5 seconds (range: 4.36 to 96.2 seconds). Among the povidone-iodine patients, children with a history of a prior tonsillectomy and adenoidectomy and/or bilateral myringotomy had a 7.2 times greater chance of experiencing a change in respiration after instillation of the povidone-iodine eye drops.
Conclusions
Topical application of 5% povidone-iodine eye drops causes a slowing and pause in spontaneous ventilation in a majority of children prior to strabismus surgery. This may represent activation of the diving reflex.
INTRODUCTION
An observational study published by Emhardt et al.1 demonstrated that 54% of spontaneously breathing children under general anesthesia developed apnea after the topical application of 5% povidone-iodine ophthalmic antibacterial solution to the eye. Due to the observational nature of their study, no mechanism for the apnea could be delineated. We were concerned that the mechanical stimulation of the povidone-iodine eye drops on the eye may trigger a reflex response manifested as apnea. To more clearly assess changes in respiration caused by povidone-iodine eye drops, we designed a randomized, single-blinded, controlled study with more robust power.
PATIENTS AND METHODS
After approval by the institutional review board of the Medical University of South Carolina, informed parental consent, and patient assent (when appropriate), 100 patients (2 to 17 years of age) scheduled for strabismus surgery were enrolled in the study. Patients were randomized in 1:1 allocation using simple randomization to receive either ophthalmic balanced salt solution (control group) or 5% buffered ophthalmic povidone-iodine solution (povidone-iodine group). Patients were blinded to treatment assignment. Although the study was single-blinded, the study statistician was blinded to treatment assignment for the analyses and was only unblinded after results were reported. All decisions concerning clinical care of each patient were rendered by the anesthesiologist of record. Exclusion criteria included a history of adverse reaction to iodine, history of thyroid disease, patients requiring tracheal intubation, a contraindication to sevoflurane, or an inability or unwillingness by the patient and/or legal guardian to give informed consent.
Anesthesia was induced with sevoflurane in oxygen by face mask. After an appropriate depth of anesthesia was attained, an intravenous catheter was inserted into a peripheral vein and an appropriately sized laryngeal mask airway was inserted. The study intervention commenced once the laryngeal mask airway was secured and the patient reached a steady state of anesthesia. Patients were randomized as described above to one of two groups. The povidone-iodine group received three drops of 5% buffered ophthalmic solution (Alcon Laboratories, Inc., Fort Worth, TX) instilled into each eye (right eye first) and the control group received three drops of ophthalmic balanced salt solution (BSS; Alcon Laboratories, Inc.) instilled into each eye (right eye first). Both solutions were at room temperature.
Because the definition of apnea is arbitrary and varies from study to study, we measured changes in the respiratory rate by timing the peak-to-peak intervals on the end-tidal carbon dioxide waveform on the capnograph.2 This technique is similar to calculating the heart rate by measuring the R to R interval on the electrocardiograph. The time measurements were obtained by using the lap feature on the stopwatch of an Apple iPhone (Apple, Inc., Cupertino, CA) and observing the capnograph. Physiologic and pharmacologic data collected included heart rate, blood pressure, respiratory rate, arterial oxygen saturation, end-tidal carbon dioxide, and inspired and expired sevoflurane. Medical history information collected during the preoperative interview from each patient included age, weight, gender, coexisting diseases, prior surgery, and any history of prematurity.
Statistical Analysis
An a priori size calculation was conducted prior to the study. Assuming no change in the peak-to-peak end-tidal carbon dioxide interval time after eye drop instillation in the control group, and a peak-to-peak interval time variability of ±4 seconds, a sample size of 50 patients per group (100 patients total) provided greater than 80% power to detect an increase in change in peak-to-peak interval of 2.5 seconds in the povidone-iodine group compared to the control group assuming equal variance between the two groups using a two-sided test with a significance level at alpha = 0.05.
Descriptive statistics by treatment group were estimated for patient and procedural characteristics. Differences in peak-to-peak interval time between the control and povidone-iodine groups were examined using a linear mixed model approach. Based on the a priori analysis plan, the model included fixed effects for treatment, breath number, a treatment by breath number interaction, and a random subject effect to account for correlation between measures collected on the same patient. The model also controlled for factors thought to be associated with respiratory rate, including age, preterm birth, history of apnea, and breath duration prior to eye drop installation. Comparisons of breath duration between treatment groups at each of 15 breaths after eye drop instillation were estimated from the model using Bonferroni corrected significance.
A secondary outcome of interest was occurrence of a significant change in respiration. Although apnea has been defined in the literature as a breath duration of 10 to 20 seconds, this definition is arbitrary and fails to account for within- and between-subject variability of breath duration. To account for within- and between-subject variability, we defined changes in respiration as having a breath duration greater than twice the interquartile range above the 75th percentile for breaths within-subject for the 10 breaths prior to and 15 breaths after instillation of eye drops. Associations between occurrence of change in respiration with patient and procedural characteristics were evaluated using univariate logistic regression models. Notably, no participants in the control group experienced a change in respiration; therefore, all additional univariate tests were conducted on the subset of patients who received povidone-iodine eye drops. All analyses were conducted in SAS software (version 9.4; SAS Institute, Cary, NC).
RESULTS
Participant and procedural characteristics of the two groups are reported in Table 1.
TABLE 1.
Participant and Procedural Characteristics by Treatment Group
| Variable | Povidone-Iodine (n = 50) | BSS (n = 50) | P |
|---|---|---|---|
| Demographics | |||
| Sex | .221 | ||
| Male, n (%) | 17 (34%) | 23 (46%) | |
| Female, n (%) | 33 (66%) | 27 (54%) | |
| Age, years, mean ± SD | 6.62 ± 3.88 | 6.40 ± 3.36 | .805 |
| Weight, kg, mean ± SD | 23.5 ± 10.4 | 26.9 ± 13.4 | .153 |
| Medical history | |||
| Preexisting condition, yes, n (%) | 15 (30%) | 16 (32%) | .829 |
| Asthma, yes, n (%) | 4 (8%) | 6 (12%) | .505 |
| History of apnea, yes, n (%) | 4 (8%) | 4 (8%) | 1.000 |
| Preterm birth, yes, n (%) | 8 (16%) | 8 (16%) | 1.000 |
| Prior surgery, yes, n (%) | 27 (54%) | 32 (64%) | .309 |
| Before strabismus surgery, yes, n (%) | 8 (16%) | 19 (38%) | .013 |
| Before T&A surgery, yes, n (%) | 7 (14%) | 5 (10%) | .538 |
| Before BMT surgery, yes, n (%) | 7 (14%) | 3 (6%) | .182 |
| Before other surgeries, yes, n (%) | 15 (30%) | 10 (20%) | .248 |
| Preoperative measures | |||
| Any preoperative medications, yes, n (%) | 35 (70%) | 35 (70%) | 1.000 |
| Preoperative midazolam, yes, n (%) | 34 (68%) | 34 (68%) | 1.000 |
| Dose midazolam (all), median (IQR) | 7 (12) | 9 (10) | .709 |
| Dose midazolam if yes, median (IQR) | 10 (5) | 10 (3) | |
| Other preoperative medication, yes, n (%) | 1 (2%) | 2 (4%) | 1.000 |
| Preinstillation average breath duration, sec, mean ± SD | 1.75 ± 0.45 | 1.83 ± 0.49 | .357 |
| Preinstillation % expired sevoflurane, mean ± SD | 4.64 ± 1.46 | 4.40 ± 1.29 | .372 |
| Change in respiration after drop installation, yes, n (%) | 30 (60%) | 0 (0%) | < .001 |
BSS = balanced salt solution; SD = standard deviation; T&A = tonsillectomy and adenoidectomy; BMT = bilateral myringotomy; IQR = interquartile range
The only participant difference in the two groups was that the control group had a higher incidence of prior strabismus surgery (P = .013). Depth of anesthesia based on expired sevoflurane concentration at the time of eye drop instillation was not different between the two groups. The only significant difference in vital signs before and after eye drop instillation was a decrease in diastolic blood pressure, but this decrease was not different between the two groups.
Average Breath Duration After Eye Drop Instillation
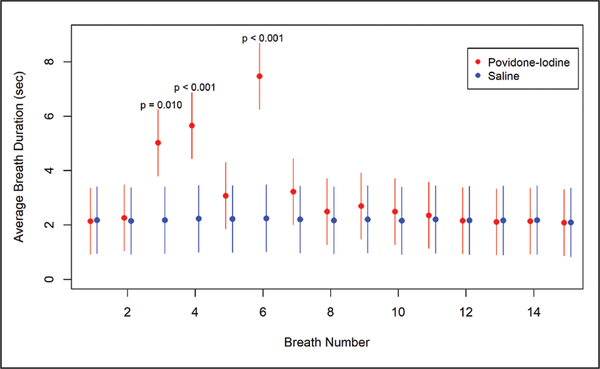
Treatment group, breath number, treatment group by breath number interaction, and average breath duration prior to eye drop instillation were significant (P < .001; type III F test). Figure 1 shows the mean difference in breath duration in seconds after eye drop instillation by treatment group controlling for covariates in the model.
Figure 1.
Least square mean (95% confidence intervals) for breath duration at breaths 1 to 15 by treatment group (control or povidone-iodine) after eye drop instillation estimated from a linear mixed model including fixed effects for treatment, breath number, a treatment by breath number interaction, average breath duration prior to instillation, patient age, history of apnea, and preoperative midazolam use. The model also included a random subject effect to account for the correlation between measures collected on the same patient. Significant differences in breath duration between treatment groups were noted at breaths 3, 4, and 6. The P values shown on the figure are the Bonferroni adjusted P values for these three pairwise comparisons.
All significant differences between the treatment groups in breath duration occurred between the third and sixth breaths. Specifically, patients in the povidone-iodine group had significantly longer breath duration at breaths 3, 4, and 6 following eye drop instillation (P = .012, < .001, and < .001, respectively) (Figure 1). Breath duration between the treatment groups was not different before the third breath or after the seventh breath.
Occurrence of a Significant Change in Respiration
Thirty of the 50 povidone-iodine patients showed at least one episode of a change in respiration in the 15 breaths after eye drop instillation compared to none of the 50 control patients (P < .001). The median time to a change in respiration among those patients who experienced a change was 6.9 seconds (interquartile range: 5.31, range: 2.60 to 25.7 seconds). The median duration of respiratory pause was 18.5 seconds (interquartile range: 22.5, range: 4.36 to 96.2 seconds).
We also examined the associations between a significant change in respiration with secondary characteristics in the povidone-iodine patients. Of note, depth of anesthesia based on expired sevoflurane concentration at the time of eye drop instillation in the povidone-iodine patients was not different between those who experienced a significant change in respiration compared to those who did not (P = .378, mean ± standard deviation % expired sevoflurane for no change was 4.9 ± 1.5 and for those who had a change was 4.5 ± 1.5. Patients in the povidone-iodine group with a history of tonsillectomy and adenoidectomy and/or bilateral myringotomies had a 7.2 times the likelihood of respiratory change compared to those without a history of tonsillectomy and adenoidectomy and/or bilateral myringotomies. Sex was marginally associated with a respiratory change, with females having 2.4 times the likelihood of respiratory change compared to males (P = .091, odds ratio = 2.43, 95% confidence interval: 0.87, 6.79).
DISCUSSION
The results of this study are similar to those presented by Emhardt et al.1 Sixty percent of our patients in the povidone-iodine group demonstrated a change in spontaneous respiration after the instillation of povidone-iodine eye drops. In contrast to Emhardt et al.’s study, our study was a randomized, controlled study that measured multiple variables. Ophthalmic balanced salt solution eye drops were instilled into the eyes of the control group. The control group had no significant change in respiration after instillation of the eye drops. Although the precise mechanism for the effect of the povidone-iodine eye drops on respiration cannot be delineated from our study, we can exclude some influences that Emhardt et al.’s study could not. A mechanical effect of the eye drops is not likely because the technique for administration of the balanced salt solution and povidone-iodine eye drops was the same. Although studies measuring pain after the instillation of povidone-iodine eye drops have produced conflicting results, pain or discomfort from povidone-iodine eye drops was an unlikely cause of a change in respiration because all of the patients were deeply anesthetized (2.2 to 2.4 minimum alveolar concentration).3–5
Goudarzi et al.6 reported no apnea in a study of 41 children who received 10% povidone-iodine eye drops. They defined apnea as a respiratory pause of 20 seconds or more. However, their patients did experience a decrease in respiratory rate. If we applied Goudarzi et al.’s definition of apnea to our patients, only half of our 30 patients who experienced a change in respiration would have met their definition of apnea. Our study used a much more sensitive method of detecting subtle changes in respiratory rate. Another explanation of the difference in our study and Goudarzi et al.’s is the chemical composition of povidone-iodine. The bactericidal effect of povidone-iodine is secondary to the effect of free iodine. Lower concentrations of povidone-iodine release more free iodine. If free iodine is the trigger that causes a slowing of respiration, the 5% povidone-iodine solution could be expected to produce a greater effect than a 10% solution.
An unexpected, but not unsurprising, finding in our study was an increased likelihood of respiratory changes within the povidone-iodine patients in children with a history of tonsillectomy and adenoidectomy and/or bilateral myringotomies. This finding might suggest that previous tonsillectomy and adenoidectomy sensitizes pharyngeal receptors and povidone-iodine eyedrops stimulated the nasopharyngeal reflex. However, the transit time of solutions such as fluorescein from the eye to the nasopharynx via the nasolacrimal duct averages 1.4 minutes and the slowing of respiration in our patients occurred within seconds.7 It seems more likely that the underlying pathology of sleep disordered breathing in children with tonsillar hypertrophy and recurrent otitis media increases the risk of a decrease in spontaneous breathing rate during anesthesia.8 Recurrent otitis media is associated with a higher incidence of obstructive sleep apnea.9 Although tonsillectomy and adenoidectomy reduces the severity of obstructive sleep apnea, many children with sleep disordered breathing still have residual sleep apnea after tonsillectomy and adenoidectomy.10 We also found that females have a greater likelihood of respiratory slowing after povidone-iodine eye drops, but this was only a marginal association likely due to the limited power of the study for this variable. None of our patients experienced any adverse event.
We confirmed that instillation of 5% povidone-iodine ophthalmic eye drops decrease the rate of spontaneous breathing in children and causes respiratory pauses of varying duration during general anesthesia with sevoflurane. However, our study did eliminate the effect of mechanical stimulation of eye drops causing a change in respiration. Although this study was not designed to fully delineate the cause of respiratory changes, one possible mechanism for the effect of povidone-iodine eye drops on respiration is activation of a trigeminocardiac reflex. The most likely trigeminocardiac reflex is the diving reflex or the nasopharyngeal reflex. The diving reflex is characterized by a slowing of respiration after submersion of the face in water and stimulation of receptors in the forehead and eyes is a sensitive trigger of the diving reflex in humans.11 Most cases of trigeminocardiac reflex that occur during anesthesia can be effectively treated by cessation of the inciting stimulus or the administration of an anticholinergic. However, there are reports of severe cardiovascular effects from trigeminocardiac reflexes that are resistant to therapy.12–14
Carefully designed future studies that may better define the mechanism for our findings might include the effect of cold ophthalmic solutions, more dilute ophthalmic povidone-iodine solutions, application of topical anesthetics prior to instillation of povidone-iodine solutions, and the effect of the prior administration of an anticholinergic such as glycopyrrolate or atropine.
Footnotes
The authors have no financial or proprietary interest in the materials presented herein.
Registered at Clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT03349515, NCT03349515.
Contributor Information
Michelle S. Rovner, Department of Anesthesia & Perioperative Medicine, Medical University of South Carolina, Charleston, South Carolina..
Bethany Jacobs Wolf, Department of Public Health Services Sciences, Medical University of South Carolina, Charleston, South Carolina..
Melanie Rubin, Department of Anesthesia & Perioperative Medicine, Medical University of South Carolina, Charleston, South Carolina..
Alexandra Ritter, Department of Anesthesia & Perioperative Medicine, Medical University of South Carolina, Charleston, South Carolina..
Christopher L. Heine, Department of Anesthesia & Perioperative Medicine, Medical University of South Carolina, Charleston, South Carolina..
Tracy E. Wester, Department of Anesthesia & Perioperative Medicine, Medical University of South Carolina, Charleston, South Carolina..
Cory M. Furse, Department of Anesthesia & Perioperative Medicine, Medical University of South Carolina, Charleston, South Carolina..
REFERENCES
- 1.Emhardt JD, Haider KM, Plager DA, Grundhoefer DL. Intraoperative apnea in children after buffered 5% povidone-iodine site sterilization for strabismus surgery. Paediatr Anaesth. 2015;25(2):193–195. doi: 10.1111/pan.12476 [DOI] [PubMed] [Google Scholar]
- 2.Powell MB, Ahlers-Schmidt CR, Engel M, Bloom BT. Clinically significant cardiopulmonary events and the effect of definition standardization on apnea of prematurity management. J Perinatol. 2017;37(1):88–90. doi: 10.1038/jp.2016.167 [DOI] [PubMed] [Google Scholar]
- 3.Behne M, Wilke HJ, Harder S. Clinical pharmacokinetics of sevoflurane. Clin Pharmacokinet. 1999;36(1):13–26. doi: 10.2165/00003088-199936010-00002 [DOI] [PubMed] [Google Scholar]
- 4.Ridder WH III, Oquindo C, Dhamdhere K, Burke J. Effect of povidone-iodine 5% on the cornea, vision, and subjective comfort. Optom Vis Sci. 2017;94(7):732–741. doi: 10.1097/OPX.0000000000001091 [DOI] [PubMed] [Google Scholar]
- 5.Oakley C, Allen P, Hooshmand J, Vote BJT. Pain and antisepsis after ocular administration of povidone-iodine versus chlorhexidine. Retina. 2018;38(10):2064–2066. doi: 10.1097/IAE.0000000000001800 [DOI] [PubMed] [Google Scholar]
- 6.Goudarzi M, Yaghooti AA, Marashi S. Comment to “Intraoperative apnea in children after buffered 5% povidone-iodine site sterilization for strabismus surgery.” Paediatr Anaesth. 2017;27(9):975. doi: 10.1111/pan.13192 [DOI] [PubMed] [Google Scholar]
- 7.Tucker NA, Codère F. The effect of fluorescein volume on lacrimal outflow transit time. Ophthal Plast Reconstr Surg. 1994;10(4):256–259. doi: 10.1097/00002341-199412000-00006 [DOI] [PubMed] [Google Scholar]
- 8.Havidich JE, Beach M, Dierdorf SF, Onega T, Suresh G, Cravero JP. Preterm versus term children: analysis of sedation/anesthesia adverse events and longitudinal risk. Pediatrics. 2016;137(3):e20150463. doi: 10.1542/peds.2015-0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robison JG, Wilson C, Otteson TD, Chakravorty SS, Mehta DK. Increased eustachian tube dysfunction in infants with obstructive sleep apnea. Laryngoscope. 2012;122(5):1170–1177. doi: 10.1002/lary.22473 [DOI] [PubMed] [Google Scholar]
- 10.Lee CH, Hsu WC, Chang WH, Lin MT, Kang KT. Polysomnographic findings after adenotonsillectomy in obese and non-obese children: a systematic review and meta-analysis. Clin Otolaryngol. 2015;41:498–510. doi: 10.1111/coa.12549 [DOI] [PubMed] [Google Scholar]
- 11.Schuitema K, Holm B. The role of different facial areas in eliciting human diving bradycardia. Acta Physiol Scand. 1988;132(1):119–120. doi: 10.1111/j.1748-1716.1988.tb08306.x [DOI] [PubMed] [Google Scholar]
- 12.Meuwly C, Leibundgut G, Rosemann T, Schaller B. Sinus arrest with prolonged asystole due to the trigeminocardiac reflex during application of local anaesthetic in the nasal mucosa. BMJ Case Rep. 2018;2018:bcr-2018–226427. doi: 10.1136/bcr-2018-226427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane SM, Davis J. Cardiac arrest and death attributable to the “diving response” triggered during incision and debridement of an abscess of the forehead. J Craniofac Surg. 2018;29(5):e507–e509. doi: 10.1097/SCS.0000000000004555 [DOI] [PubMed] [Google Scholar]
- 14.Nicholson D, Kossler A, Topping K, Stary CM. Exaggerated oculocardiac reflex elicited by local anesthetic injection of an empty orbit: a case report. A A Case Rep. 2017;9(12):337–338. doi: 10.1213/XAA.0000000000000609 [DOI] [PubMed] [Google Scholar]