Graphical abstract

Abbreviations: ASP, total polysaccharides from Angelica sinensis; AUF1, an adaptor protein AU-binding factor 1; BALF, bronchoalveolar lavage fluid; BLM, bleomycin; BMSCs, bone marrow mesenchymal stem cells; COVID-2019, Coronavirus disease-2019; DANCR, differentiation antagonizing non-protein coding RNA; DOP, a neutral heteropolysaccharides from Dendrobium officinale; EMT, epithelial-mesenchymal transition; FMP-1, a polysaccharide from Morchella esculenta; FN, Fibronectin; HEPF cell, human embryo pulmonary fibrosis cell; IFN-γ, Interferon gamma; IL, Interleukin; IL-1RA, interleukin-1 receptor antagonist; LMWF, Low-molecular weight fucoidan; MCP-1, Monocyte chemotactic protein 1; MDA, malondialdehyde; MS80, a novel sulfated oligosaccharide extracted from seaweed; Mw, molecular weight; Nrf2, nuclear factor-erythroid 2-related factor 2; POL, Total polysaccharide from O. lanpingensis; OSM, Oncostatin M; FYGL-1, a neutral heteropolysaccharide from G. lucidum; PF, Pulmonary fibrosis; RLE-6TN cell, alveolar type Ⅱ epithelial cell; ROS, reactive oxygen species; α-SMA, α-smooth muscle actin; SOD, superoxide dismutase; TGF-β1, transforming growth factor β1; TNF-α, Tumor necrosis factor-α
Keywords: Polysaccharides, Pulmonary fibrosis, Mechanisms, Corona virus disease 2019
Abstract
Pulmonary fibrosis (PF) is a lung disease with highly heterogeneous and mortality rate, but its therapeutic options are now still limited. Corona virus disease 2019 (COVID-19) has been characterized by WHO as a pandemic, and the global number of confirmed COVID-19 cases has been more than 8.0 million. It is strongly supported for that PF should be one of the major complications in COVID-19 patients by the evidences of epidemiology, viral immunology and current clinical researches. The anti-PF properties of naturally occurring polysaccharides have attracted increasing attention in last two decades, but is still lack of a comprehensively understanding. In present review, the resources, structural features, anti-PF activities, and underlying mechanisms of these polysaccharides are summarized and analyzed, which was expected to provide a scientific evidence supporting the application of polysaccharides for preventing or treating PF in COVID-19 patients.
1. Introduction
Pulmonary fibrosis (PF) is a worldwide disease, along with progressive and permanent fibrotic “scar” in alveolus and bronchioles of the lung parenchyma (Jacob et al., 2018), which could be induced by genetic susceptibility and various environmental risk factors including virus, bacteria, cigarette smoke, wood dust, stone dust etc. (Martinez et al., 2017; Richeldi, Collard, & Jones, 2017). Interana and pirfenidone were approved by the USA Food and Drug Administration for suppressing the progression of pulmonary fibrosis (Raghu et al., 2015). In clinical, glucocorticoid (Ozaki et al., 1982), prednisone (Bickerman, Beck, & Barach, 1955), cyclophosphamide (Miniati & Cerinic, 2007), and other immunosuppressive modulators have also been used for delaying the degree of pulmonary fibrosis and prolonging the survival of patients. However, all of them cannot improve the risks of incidence and mortality of pulmonary fibrosis (Canestaro, Forrester, Raghu, Ho, & Devine, 2016), but cause a large economic burden for patient (Raimundo et al., 2016). Therefore, it is still urgent developing new therapies and drugs for treating pulmonary fibrosis.
The transformation of epithelial into myofibroblasts and the amount of extracellular matrix (ECM) generated by fibroblasts are considered as crucial developmental milestones in pulmonary, wherein a pivotal fibrogenic cytokine TGF-β is aberrantly expressed, which in turn triggers epithelial-mesenchymal transition (EMT) process thereby enhancing ECM deposition mediated by both Smad dependent and independent pathways (Bale, Venkatesh, Sunkoju, & Godugu, 2018; Liu, Lu, Kang, Wang, & Wang, 2017). Furthermore, some other mechanisms have been also found to be imperative in pulmonary fibrosis progression including inflammation, oxidative stress, deregulated ECM and EMT signaling (Liu et al., 2017).
Human coronaviruses were first described in the 1960s for patients with the common cold, which in the past two decades have caused severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), as well as COVID-19 now. A typical clinical feature associated with SARS is pulmonary fibrosis, which resulted in a high mortality and a low quality of life for recovered patients (Venkataraman & Frieman, 2017). Several long-term follow-up studies have demonstrated that more than 20 % of SARS survivors exhibited radiographic evidence of lung fibrotic changes (Hui et al., 2005; Xie et al., 2005). Recently, a retrospective analysis on the pulmonary computed tomographic imaging of fifty patients with COVID-19 pneumonia also observed the formation of fibrotic stripes during their rehabilitation period (Xu et al., 2020). It will be a high probability that pulmonary fibrosis is one of the major complications in COVID-19 patients. Therefore, there will be a huge need for effective and safety agents and therapeutic strategies for treating pulmonary fibrosis induced by COVID-19 (Wang et al., 2020).
With the broad spectrum of bioactivities and highly safety, polysaccharides have attracted more and more attention (Muhama, Zulkifli, Selvakumaran, & Lazim, 2019; Shi, 2016). In addition, several natural polysaccharides, such as polysaccharides-K (PSK) and polysaccharides-peptide (PSP) from Coriolus versicolor (Kidd, 2000), and fucoidan (Wang, Geng, Yue, & Zhang, 2019), have been applied in clinical for treating diseases and improving health. In fact, many studies have been conducted to reveal the anti-pulmonary fibrosis activities and underlying mechanisms of naturally occurring polysaccharides from medicinal plants, seaweed, and edible fungi. However, to the best of our knowledge, a comprehensively understanding of these anti-pulmonary fibrosis natural polysaccharides is still limited.
Thus, in present review, the available information about these natural polysaccharides with anti-pulmonary fibrosis activities were collected by searching in the related topics of “pulmonary fibrosis” and “polysaccharides” in the databases of Scopus (http://www.scopus.com) and China National Knowledge Infrastructure (https://www.cnki.net). In addition, the resources, structural features, physiological activities, as well as underlying mechanisms of these polysaccharides were systematically summarized and analyzed, which was expected to provide a scientific evidence for the application of polysaccharides for preventing or treating pulmonary fibrosis in COVID-19 patients.
2. Mechanisms underlying the anti-pulmonary fibrosis activities of natural occurring polysaccharides
2.1. Transforming growth factor β1 (TGF-β1)-Smad2/3 axis
TGF-β1 overproduction has been recognized as the most relevant element related to the progress of pulmonary fibrosis, which plays a crucial role in the induction of EMT, and stimulates differentiation, proliferation and migration of immature fibroblasts, as well as induces phenotypic conversion of fibroblasts into myofibroblasts (Walton, Johnson, & Harrison, 2017). Smad2 and Smad3 are the main transcription factors for TGF-β1 signals, which are phosphorylated and translocated into the nucleus upon ligand binding (Fig. 1 ).
Fig. 1.
Targeting TGF-β mediated Smad2/3 signaling for anti-pulmonary fibrosis effects of DOP, ginsan, LMWF and MS80.
DOP is a neutral heteropolysaccharides isolated from Dendrobium officinale, which consisted of Man and Glc (in a mole ration of 5.9:1) with an average molecular weight (Mw) at about 1.78 × 105 Da, and had a partial structure of O-acetylated glucomannan with β-d-configuration in pyranose sugar forms (He et al., 2016). It has been demonstrated that DOP could significantly attenuate bleomycin (BLM)-induced up-regulation of TGF-β1 expression and Smad2/3 phosphorylation in the rat lung tissues, and further suppress the transformation of rat type II alveolar epithelial cells into myofibroblasts (Chen et al., 2018) (Fig. 1).
Ginsan, a polysaccharide isolated from the roots of Panax ginseng with an average Mw of 1.5 × 106 Da, composed mainly of Glc and Gal (over 90 %, w/w), and 5–8 % Man and Ara (Ahn et al., 2011; Lee et al., 1997). In mouse lung fibroblasts (NIH/3T3 cells) or human lung fibroblasts (IMR-90 and WI-38 cells), ginsan could significantly reduce the phosphorylation of Smad2 and Smad3 induced by TGF-β via inhibiting induction of R-Smads but not inhibitory Smads (Smad6 or Smad7). In addition, ginsan significantly reduced the phosphorylation of ERK and Akt induced by TGF-β, but did not affect the phosphorylation of either JNK or p38, which indicated that ginsan downregulated both Smad-dependent and independent signaling pathways induced by TGF-β. Furthermore, ginsan could obviously reduce the increases in protein expression of TGF-β receptors (TβRI and TβRII), and block the decrease in protein expression of TβRIII, a known coreceptor of TβRII (Ahn et al., 2011) (Fig. 1).
ERK signaling is another important mechanism implicated in the process of TGF-β1induced EMT during the pulmonary fibrosis. Wang, Zhang et al. (2019) had demonstrated that a sulfated low-molecular weight fucoidan isolated from brown seaweed (LMWF) can ameliorate TGF-β1 induced EMT both in vivo and in vitro models of pulmonary fibrosis by downregulating ERK signaling, which significantly inhibited the over expression of TGF-β1 and p-ERK1/2 in mice induced by BLM, as well as suppressed the over expression of p-ERK1/2 in TGF-β1induced A549 cells (Fig. 1)
The high binding affinity for TGF-β1 have revealed to be closely related to the anti-pulmonary fibrosis activities of MS80, a marine-derived sulfated oligosaccharide (1→4 α-d-glucose) isolated from seaweed with the average Mw of 8 × 103 Da, which via competitively inhibiting the heparin/ HS-TGF-β1 interaction, can arrest TGF-β1-induced human embryonic pulmonary fibroblast (HEPF) cell proliferation, collagen deposition and matrix metalloproteinase activity, as well as deactivate both the ERK and p38 signaling pathways (Jiang & Guan, 2009).
2.2. DANCR/AUF-1/FOXO3 regulatory axis
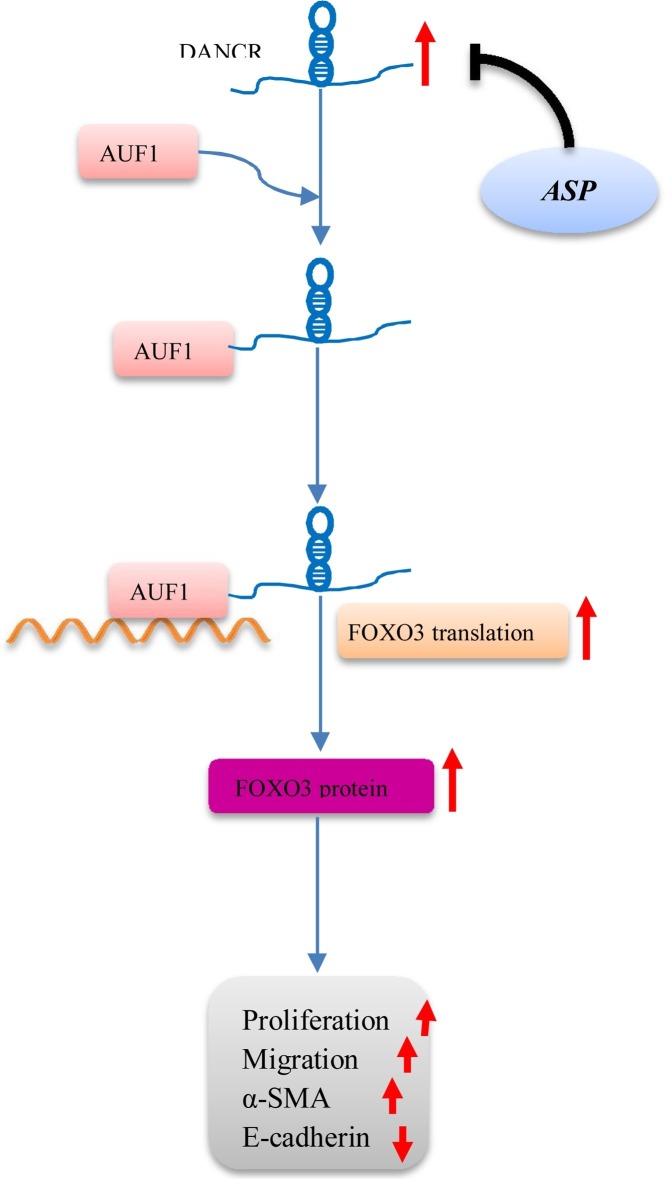
The differentiation antagonizing non-protein coding RNA (DANCR) is a newly identified long noncoding RNA (lncRNA) with pivotal roles in cell proliferation, migration, invasion, and stem cell differentiation, which was shown to promote EMT progression and invasion capability of malignant cells (Yang, Sun, Gao, Meng, & Yang, 2018). DANCR cooperated with an adaptor protein AU-binding factor 1 (AUF1) has been demonstrated to regulate EMT and fibrogenesis by upregulating FOXO3 protein levels without influencing its mRNA expression. The root of Angelica sinensis is a well-known traditional Chinese medicine, which has been used for thousands of years to prevent and treat various diseases. The polysaccharides have been proved as one of the major effective ingredients in A. sinensis, and more than 30 polysaccharides have been identified from A. sinensis, most of which were heteropolysaccharides (Jin, Zhao, Huang, Xu, & Shang, 2012). Treatment with total polysaccharide of Angelica sinensis (ASP) exhibited significant downregulatory effects on the expression of DANCR, which in turn represses AUF1-mediated FOXO3 translation to suppress the EMT and pulmonary fibrosis both in in vitro and in vivo (Qian, Cai, Qian, Wang, & Zhang, 2020) (Fig. 2 ).
Fig. 2.
Total polysaccharides from A. sinensis (ASP) suppress the pulmonary fibrosis via DANCR/AUF-1/FOXO3 regulatory axis.
2.3. Antioxidant ability
The pathological progresses of pulmonary fibrosis are complex, but oxidative stress injury plays an important role (Cameli et al., 2020). FYGL-1 is a neutral heteropolysaccharide isolated from Ganoderma lucidum, consisted of Gal, Rha and Glc (in a mole ration of 1.00:1.15:3.22) with an average Mw of 7.8 × 106 Da, and the backbone structure consisted mainly of 1,2-linked-β-LRhap, 1,3,6-linked-α-d-Galp, 1,2,6-linked-α-d-Glcp and 1-linked-α-d-Glcp (Pan et al., 2012). Treatment with FYGL-1 (at 100 and 300 mg/kg for 28 days) led to a markedly reducing in the pulmonary index, inflammatory cell infiltration and collagen deposition in rats induced by BLM, which was associated with increased levels of glutathione, glutathione peroxidase, catalase and superoxide dismutase and decreased contents of malondialdehyde and hydroxyproline in the lung tissues (Chen et al., 2016).
2.4. Reducing the recruitment of macrophagesand neutrophil in the lung tissues
Pulmonary macrophages express several fibrotic mediators and play important roles in lung injury healing and fibrosis, which are currently classified into two phenotypes, classically activated macrophages (M1) with secretion of Th1-related cytokines (including TNF-α, IL-1β, and IL-6) and alternatively activated macrophages (M2) with release Th2-related cytokines such as IL-10 and IL-13. However, the migration and invasion ability of both M1 and M2 macrophages are promoted by monocyte chemotactic protein 1 (MCP-1), a chemokine mainly expresses in alveolar macrophages and participates in the chemotaxis of cells. Zhou et al. (2020) found that the total polysaccharides extracted from Ophiocordyceps lanpingensis (POL) could suppress the increasing level of MCP-1 expression during the process of BLM-induced pulmonary fibrosis, and consequently reduce the increased amount of M1 and M2 macrophages, as well as the expression levels of relative cytokines. Finally, the generation of myofibroblasts was alleviated based on the reduced expression of α-smooth muscle actin (α-SMA) protein (Fig. 3 ). According to Yu et al. (2018) studies, the increased expression of TIMP-1, CXCL1, MCP-1, MIP-2, and interleukin-1 receptor antagonist (IL-1RA) were strongly associated with radiotherapy-induced lung fibrosis through the induction of M2 macrophages and neutrophils. However, the administration of fucoidan isolated from Sargassum hemiphyllum (FSH) in irradiated mice significantly attenuated these cytokines expression in the collected pleural fluid and reduced pleural fluid-induced collagen expression in fibroblasts, which were correlated with the reduction of neutrophil and macrophage infiltration in lung tissues (Fig. 3).
Fig. 3.
Natural polysaccharides suppressed pulmonary fibrosis via reducing the recruitment of macrophages and neutrophils in the lung tissues.
3. The alleviating effects of naturally occurring polysaccharides on pulmonary fibrosis
3.1. Polysaccharides from plants
In present review, nine kinds of polysaccharides from plants were collected. The resources, structural features, and anti-pulmonary fibrosis activities of these polysaccharides were displayed in Table 1 .
Table 1.
The sources, structural characterization and anti-pulmonary fibrosis activities of natural polysaccharides from plants.
| Name/source | Structural characterization | Experimental model | Effects | Reference |
|---|---|---|---|---|
| ASP/A. sinensis | Mw: 5.1−740 kDa; Containing Glc, Gal, GalA, Ara, Rha, Man, Fuc, Xyl; Linkage: backbone →4)-α- d-Glcp-(1→ with α-1,6-d-Glcp, β-l-Araf residues at a branching position of O-6; backbone →3/6)-α-d-Glcp-(1→;backbone→4)-α-d-GalpA-(1→2)-α-l-Rhap-(1→ with β-1,6- and β-1,4-Galp and α-1,5-Araf at a branching position of O-4; backbone→4)-α-d-Glcp-(1→4)-α-d-Glcp-(1→6)-α-d-Glcp-(1→4)-α-d-Glcp-(1→4)-α-d-Glcp-(1→. | In vivo: BLM-induced Sprague-Dawley rats; In vitro: TGF-β1 induced alveolar type Ⅱ epithelial (RLE-6TN) cells. | In vivo, ASP administration suppressed the increasing of collagen deposition accompanying with restoring collagen-1 protein levels by inhibiting the DANCR/AUF1/FOXO3 pathway in BLM-induced PF model rats. | Jin et al. (2012), Luo et al. (2017), Qian et al. (2020) |
| In vitro, ASP restored intercellular junction and spindle-like structure with downregulation of growth rate, migratory ability and α-SMA expression and upregulation of E-cadherin in RLE-6TN cells with TGF-β1 treatment. | ||||
| RAP/Astragali radix | Mw: 5.6∼7600 kDa; Containing Glc, Rha, Gala, Ara, Xyl, Man, GlcA, Gala; Linkage: backbone 1,2,4-Rhap, α-1,4-Glcp, α-1,4-GalAp6Me, β-1,3,6-Galp with 1,2,4-Rhap, β-1,3,6-Galp, α-T-Araf, α-1,5-Araf at branching positions of O-4/O-2/O-3. | In vivo: BLM-induced Wistar rats; | In vivo Astragalus polysaccharides administration inhibited inflammation of pulmonary alveoli and upregulated the serum level of IFN-γ with downregulated the serum levels of IL-4 and TNF-α in BLM-induced PF model rats. | Li et al. (2011), Yin et al. (2012) |
| PBS/B. striata | Mw: 401.3 kDa;Containing Man and Glc. | In vivo: BLM-induced adult male Sprague Dawley rats; | In vivo, administration of PBS improved pulmonary histomorphology and histopathology with inhibiting collagen deposition, decreased pulmonary index and hydroxyproline contents in BLM-induced PF model rats. | Guo et al. (2016) |
| DOP/D. officinale | Mw: 178 kDa; Containing Man and Glc (5.9:1); Linkage: acetylated glucomannan with β-d configuration in pyranose. | In vivo: BLM-induced adult male Sprague Dawley rats; In vitro: TGF-β1 induced rat type II alveolar epithelial cells. | After DOP administration in BLM-induced PF model rats, the pulmonary index, hydroxyproline expression and serum TGFβ1 concentrations were lower, with the improvement of histopathology, morphology and the inhibition of neutrophil-dominant inflammation, expression on mRNA and protein of TGFβ1, Smad2, Smad3 and α-SMA protein expression. | Chen et al. (2018), He et al. (2016) |
| In vitro, DOP decreased the expression of α-SMA, Smad 2/3 protein, pSmad 2/3 protein and the synthesis of collagen 1 and fibronectin with increasing the expression of E-cadherin. | ||||
| BHP-1/L. davidii var. unicolor | Mw: 1.93 × 105 Da; consisted of Glc and Man in a relative molar ration of 5.9: 2.0; Backbone: mainly contained α-1,4-linked d-Glcp and β-1,4-linked d-Manp: the branches were probably linked at the O-2 and or O-3 of the Man and Glc residues, with T-α-d-Glcp as a terminal structure | In vivo: BLM-induced SPF Kunming mice. | Combined with bone marrow mesenchymal stem cells transplantation, BHP-1 decreased the pulmonary index and improved the pulmonary histopathology and collagen deposition with downregulating the protein expression of TNF-α and NF-κB in BLM-induced PF model mice. | Luo et al. (2013), Hui et al. (2019), Hu et al. (2019) |
| TPOB/O. basilicum | Containing Glc, Xyl, Gal; Linkage: backbone β-d-Glcp with α-d-Xylp; β- d-Galp-(1→2)-α-d-Xylp at a branching position of O-6. | In vitro: TGF-β1 induced human A549 cells. | In vitro, TPOB changed less in morphology and reduced hydroxyproline contents with upregulating the expression levels of E-cadherin and downregulating the expression levels of Vimenth, α-SMA and COL1 in humanA549 cells with TGF-β1 treatment. | Hoffman et al. (2005), Yan et al. (2017) |
| Ginsan/P. ginseng C.A. Meyer | Mw: 3.5∼160 kDa; Containing Glc, Gal, Man, Ara; Linkage: backbone →5)-α-l-Arap-(1, →3)-α-d-Galp-(1→ with α-3,5-L-Ara; β-1,4-d-Gal at branching positions ofO-3/O-4 /O-6. | In vivo: BLM-induced male C57BL/6 mice; In vitro: TGF-β1 induced NIH/3T3 cells and IMR-90 and WI-38 cells | In vivo, Ginsan administration changed nothing in morphology and attenuated TGF-β expression in lung tissue with downregulating the levels of collagen and α-SMA in BLM-induced PF model mice. | Ahn et al. (2011), Sun (2011) |
| In vitro, Ginsan suppressed the expression of α-SMA, FN, procollagen type 1 and downregulated both Smad-dependent and -independent signaling pathways with modulates TGF-β receptor levels (downregulating the TβRI and TβRⅡ protein expression levels and upregulated the TβRⅢ protein expression level) in mouse and human lung fibroblasts with TGF-β1 treatment. | ||||
| TPRH/Radix Hedysari | Mw: 1.2−668 kDa; Containing Glc, Gal, Ara, Rha, Xyl, Gala, Man and some ester sulfate; Linkage: backbone →4)-α-d-Glcp-(1→ and→1)-α-d-Glcp-(6→1)-α-d-Glcp-(6→1)-α-l-Rhap-(2→ with α-l-Araf, α-d-Glc, α-d-Gal at branching positions of O-4/ O-6; backbone →4)-β-d-Galp-(1→ and →4)-α-d-Glcp-(1→ with α-L-Araf, α-L-Rhap, α-d-Glc at a branching position of O-6; backbone →6)-α-d-Glcp-(1→ and →5)-α-l-Araf-(1→ with α-d-Glc, α-l-Araf-(1→2)-α-l-Rhap, α-l-Araf at branching positions of O-2/O-3. | In vivo: BLM-induced Wistar rats. | In vivo, TPRH administration improved pulmonary histopathological morphology, inflammation of pulmonary alveoli and proliferation and deposition of collagen fibrils with reducing the contents of hyaluronic acid and laminin in lung tissue of BLM-induced PF model rats. | Lei et al. (2008), Qiang, Wang, Li, Wang & Li (2018), Su et al. (2016) |
| In vivo combined small dose prednisone, TPRH administration alleviated alveolar inflammation, fibrosis degree and histopathology changes with downregulating the expression levels of collagen and TGF-β1 in BLM-induced PF model rats. | ||||
| RSA/R. sachalinensis | Ara, Rha, Xyl, Glc, Gal, and GalA in the mole ration of 1.00: 3.23: 0.26: 0.34: 0.84: 10.24 | In vitro: TGF-β1 induced human lung carcinoma type Ⅱ epithelial (A549) cells. | In vitro, RSA improved morphological change, inhibited cell mortality, increased cell livability and downregulated the expression of fibronectin-EDA (Fn-EDA) with the inhibition of transformation to ectomesenchymal cells in A549 cells with TGF-β1 treatment. | Han et al. (2002), Li et al. (2016) |
The root of A. sinensis is a well-known traditional Chinese medicine, which has been used for thousands of years to prevent and treat various diseases. The polysaccharides have been proved as one of the major effective ingredients in A. sinensis, and more than 30 kinds of polysaccharides have been identified from A. sinensis, most of which were heteropolysaccharides (Jin et al., 2012). Recently, Qian et al. (2020) have demonstrated that treatment with total polysaccharides from A. sinensis (ASP) significantly reversed BLM-induced collagen deposition and restored collagen-1 levels in lung tissue of SD rat. In addition, ASP treatment dramatically suppressed the increase growth rate and migratory ability of alveolar type Ⅱ epithelial (RLE-6TN) cell stimulated by TGF-β1, and further abrogated TGF-β1-induced upregulation of α-SMA and downregulation of E-cadherin expression in RLE-6TN cells. The similar inhibitory effect of ASP was also observed in TGF-β1 induced HEPF cell assay, which showed that ASP (at 25 μg/mL) significantly reduced the elevated content of hydroxyproline and the upregulated protein expressions of α-SMA and CTGF (Luo et al., 2017).
Chen et al. (2018) have demonstrated that DOP (a neutral heteropolysaccharides isolated from D. officinale) treatment significantly ameliorated indices for both pulmonary inflammation and fibrosis in a BLM-induced pulmonary fibrosis model in rats, which can significantly lower BLM-induced elevations of pulmonary index, total cell numbers and differential neutrophil counts in bronchoalveolar lavage fluid (BALF), as well as attenuate the average scores of BLM-induced histopathological changes, and decrease the BLM-induced increase in hydroxyproline content in lung tissue. A polysaccharide isolated from rhizome of Bletilla striata (PBS), with an average Mw of 4.01 × 105 Da and mainly consisted of Man and Glc, also exhibited the similar anti-pulmonary fibrosis activities in BLM-induced SD rat model, which obviously improved the BLM-induced histopathological changes of lung tissues and significantly lowered the pulmonary index and hydroxyproline content (Guo et al., 2016). In addition, the PBS did not cause any side-effect in vivo with oral administration of 4000 mg/kg/day (He et al., 2017).
RAP was a water-soluble polysaccharide purified from Radix astragali and composed of Rha, Ara, Glc, Gal, and GalA in a mole ratio of 0.03: 1.00: 0.27: 0.36:0.30 with an average Mw at 1.3 × 105 Da. The backbone of RAP consisted of 1,2,4-linked Rhap, α-1,4-linked Glcp, α-1,4-linked GalAp, β-1,3,6-linked Galp, with branched at O-4 of the 1,2,4-linked Rhap, and O-3 or O-4 of β-1,3,6-linked Galp, while the side chains mainly consisted of α-T-Araf and α-1,5-linked Araf with O-3 as branching points and trace Glc and Gal (Yin et al., 2012). The potential inhibitory effects of RAP against BLM-induced pulmonary fibrosis had been demonstrated in a Wistar rats model, which significantly inhibited the alveolar inflammation, downregulated the serum levels of interleukin-4 (IL-4) and tumor necrosis factor-α (TNF-α), but upregulated the serum level of interferon gamma(IFN-γ) (Li et al., 2011).
A total polysaccharides (TPRH) was isolated and purified from Radix hedysari, mainly composed of Ara, Man, Gal and Glc in a mole ratio of 0.42: 0.53: 0.34: 1.00, with an average Mw of 1.9 × 105 Da, and the purity of which was 94.3 % (Lei, Zhao, Wang, Yao, & Ding, 2008). Treatment with TPRH (200 mg/kg), combined with prednisone (3 mg/kg), exhibited a significant inhibitory effect against alveolar inflammation in BLM-induced Wistar rat pulmonary fibrosis, and further alleviated fibrosis degree, histopathology changes, as well as the expression levels of collagen and TGF-β1 with reducing the contents of hyaluronic acid and laminin in lung tissues of rats exposed to BLM (Lei et al., 2008). Meanwhile, TPRH demonstrated to possess the similar ameliorate effects on pulmonary histopathological morphology, inflammation of pulmonary alveoli and proliferation and deposition of collagen fibrils with reducing the contents of hyaluronic acid and laminin in lung tissue of BLM-induced pulmonary fibrosis model rats (Su et al., 2016).
In both BLM-induced C57BL/6 mice and TGF-β1-induced murine and human normal lung fibroblasts models, ginsan (a polysaccharide isolated from the roots of P. ginseng) exhibited the significant inhibitory effects against pulmonary fibrosis, which changed nothing in morphology, attenuated TGF-β expression, and reduced the levels of collagen and α-SMA in lung tissues, as well as suppressed the expression of α-SMA, fibronectin (FN) and collagen type 1 (Ahn et al., 2011). In addition, administration of ginsan (6 g/day) showed no significant adverse effect in a 14-week randomized, placebo-controlled, double-blind clinical trial (Cho, Son, & Kim, 2014).
BHP-1, a polysaccharide isolated from the bulbs of Lilium davidii var. unicolor, was mainly consisted of Glc and Man in a mole ration of 5.9: 2.0, with the average Mw of 1.93 × 106 Da. The backbone of BHP-1 mainly contained α-1,4-linked d-Glcp and β-1,4-linked d-Manp, and the branches were probably linked at the O-2 and or O-3 of the Man and Glc residues, with T-α-d-Glcp as a terminal structure (Hu et al., 2019; Hui et al., 2019). Administration of BHP-1 with or without bone marrow mesenchymal stem cells (BMSCs) transplantation could significantly reduce the pulmonary index, improve the pulmonary histopathology and collagen deposition, and downregulate the protein expression of TNF-α and NF-κB in lung tissues of Kunming mice exposed to BLM. In addition, the effects of combined intervention are better than that of BHP-1 or BMSCs transplantation alone (Luo et al., 2013).
Total polysaccharides from Ocimum basilicum (TPOB) mainly consisted of Man, Rha, Glc, Fru and Ara (the mole ratio of these monosaccharides was not reported) with an average Mw of 8−10 × 104 Da (Zhan, An, Wang, Sun, & Zhou, 2020). TPOB showed the potential suppression of pulmonary fibrosis in TGF-β1-induced human A549 cells, with improving morphological change and reduction in hydroxyproline contents, upregulating the expression levels of E-cadherin and downregulating the expression levels of Vimentin, α-SMA, type I collagen and fibronectin-EDA (Fn-EDA) (Yan et al., 2017). RSA, an acidic heteropolysaccharide isolated from Rhodiola sachalinensis, was mainly consisted of Ara, Rha, Xyl, Glc, Gal, and GalA in a mole ration of 1.00: 3.23: 0.26: 0.34: 0.84: 10.24, which showed a potential suppression of pulmonary fibrosis in TGF-β1-induced human A549 cells, with improving morphological change and reduction in hydroxyproline contents, upregulating the expression levels of E-cadherin and downregulating the expression levels of Vimenth, α-SMA, COL-1 and fibronectin-EDA (Fn-EDA) (Han et al., 2002; Li, Gao, Zhao, & Hong, 2016).
In addition, the crude polysaccharides from three different complex prescriptions of Traditional Chinese Medicine (Fei Kang Ling, Gua Lou Xie Bai decoction, and Yu Ping Feng) also exhibited potential anti-pulmonary fibrosis activities in different in vitro and in vivo assays (Gao, 2016; Jiang, 2008; Xu et al., 2014).
3.2. Polysaccharides from algae
MS80 is a marine-derived sulfated oligosaccharide (1→4 α-d-glucose) isolated from seaweed with the average Mw at 8 × 103 Da. MS80 showed a significant inhibitory effect against pulmonary fibrosis induced by BLM in Wistar rats without toxicity, with improving pathological settings and decreasing lung collagen contents through competitively inhibition of heparin/heparan sulfate-TGF-β1 interaction (Jiang & Guan, 2009). In addition, MS80 could arrest TGF-β1-induced HEPF cell proliferation, collagen deposition and matrix metalloproteinase activity (Jiang & Guan, 2009) (Table 2 ).
Table 2.
The sources, structural characterization and anti-pulmonary fibrosis activities of natural polysaccharides from seaweeds.
| Source/name | Structural characterization | Experimental model | Effects | Reference |
|---|---|---|---|---|
| L. japonica/ LMWF | Mw: 8−10 kDa; Containing Rha, Fuc, Xly, Man, Glc, Gal, GlcA, Gala; Linkage: backbone →3)-Galp-(1→, →6)-Glcp-(1→, →6)-Galp-(1→, →3,6)-manp-(1→ with →3)-Fucp-(1→, →4)-Glcp-(1→ and sulfated end units. | In vivo: BLM-induced male C57BL/6 mice; In vitro: TGF-β1 induced A549 cells. | After LMWF administration in BLM-induced PF model mice, lung fibrotic histopathology and lung hydroxyproline content was significantly improved, levels of TGF-β1 expression (in BALF and lung tissue) and the lung EMT phenotype (the expression trends of E-cadherin, α-SMA and fibronectin) was attenuated, as well as ERK signaling was downregulated. | Cui et al. (2016), Wang et al. (2019) |
| In vitro TGF-β1-induced A549 cells, LMWF significantly inhibited the cell morphologic alterations and proliferation, attenuated EMT phenotype (less expression of E-cadherin and over expression of vimentin, α-SMA and fibronectin mRNA), and downregulated the over expression of p-ERK1/2 induced by TGF-β1. | ||||
| S. hemiphyllum/ FSH | Mw: 0.8 kDa; Containing rich l-fucose and sulfated ester groups with some d-xylose, d-galactose, d-mannose, glucuronic acid and a mixture of fatty acid methyl esters. | In vivo: radiotherapy (10 Gy/shot)-induced pneumonitis and lung fibrosis in C57BL/6 mice. | Fucoidan administration attenuated the increasing of pro-collagen 1α deposition, neutrophil (over expression levels of Ly6G mRNA) and macrophages (over expression levels of F4/80 mRNA) infiltration in lung tissues, reduced cytokine expression (TIMP-1, CXCL1, MCP-1, MIP-2, IL-1Ra, TREM-1, SDF-1/CXCL12 and IL-16) in the pleural fluid induced by radiation in mice. | Yu et al. (2018), Zheng, Li, Liu, Yuan & Lu (2001) |
| A kind of seaweed/ MS80 | Mw: 8 kDa; Backbone →4)-α-d-Glcp-(1→ with sulfated residues and hydroxymethylated group. | In vivo: BLM-induced pathogen-free adult Wistar rats; In vitro: TGF-β1 induced HEPF cells. | After MS80 administration in BLM-induced PF model rats, there were some improvements in morphology and increasing hydroxyproline content of lung tissue. | Jiang & Guan (2009) |
| In vitro, MS80 inhibited the combining capacity of TGF-β1 with heparin examined by surface plasm on resonance and the proliferation, collagen deposition and matrix metalloproteinase activity of HEPF cells with TGF-β1 or BALF. |
LMWF, another sulfated polysaccharide extracted from brown seaweed, has also demonstrated to possess a significant inhibition of BLM-induced pulmonary fibrosis in C57BL/6 mice, as evidenced by improved lung histopathology and hydroxyproline content, attenuated the expression levels of TGF-β1 in BALF and lung tissue, and the lung EMT phenotype including the expression trends of E-cadherin, α-SMA and fibronectin (Wang et al., 2019) (Table 2). Furthermore, LMWF displayed no mutagenicity by either the bacterial reverse mutation or chromosomal aberration assays in vitro (at 5000 μg/mL), as well as no toxicological indications in vivo by repeated oral administration of LMWF (2000 mg/kg/day) for 28 days (Hwang, Yan, Lin, Li, & Lin, 2016). In addition, LMWF could significantly inhibited the morphologic alterations and proliferation of A549 cells induced by TGF-β1, and attenuated TGF-β1-induced EMT phenotype such as less expression of E-cadherin and over expression of vimentin, α-SMA and fibronectin (Wang et al., 2019).
The inhibitory effects of FSH has been reported against radiation pneumonitis and relative lung fibrosis in C57BL/6 mice treated by irradiated (10 Gy/shot) (Yu et al., 2018), which indicated that administration of FSH significantly attenuated the increasing in pro-collagen 1α deposition, neutrophil (over expression levels of Ly6G mRNA) and macrophages (over expression levels of F4/80 mRNA) infiltration in lung tissues, and reduced cytokine expression (TIMP-1, CXCL1, MCP-1, MIP-2, IL-1RA, TREM-1, SDF-1/CXCL12 and IL-16) in the pleural fluid induced by radiation in mice (Table 2).
3.3. polysaccharides from fungi
Four kinds of natural polysaccharides from fungi were collected in the present review. The resources, structural features, and anti-pulmonary fibrosis activities of these polysaccharides were displayed in Table 3 .
Table 3.
The sources, structural characterization and anti-pulmonary fibrosis activities of natural polysaccharides from fungi.
| Name/source/ | Structural characterization | Experimental model | Effects | Reference |
|---|---|---|---|---|
| Cordyceps | Mw: 2 × 105 Da | In vivo: pingyangmycin-induced ICR mice. | In vivo, cordyceps polysaccharide increased the IL-1RA levels, decreased the hydroxyproline content and shrank the fibrosis area in pingyangmycin-induced PF model mice. | Hu et al. (2019) |
| FYGL-1/G. lucidum | Mw: 7.8 × 104 Da; consisted of Gal, Rha and Glc in a mole ratio of 1.00: 1.15: 3.22; backbone structure: 1,2-linked β-l-Rhap, 1,3,6-linked α-d-Galp, 1,2,6-linked α-d-Glcp and 1-linked α- d-Glcp | In vivo: BLM-induced adult male Sprague-Dawley rats. | After FYGL-1 administration in BLM-induced PF model rats, the pulmonary index, inflammatory cell infiltration and collagen deposition reduced with upregulating the levels of glutathione, glutathione peroxidase, catalase, superoxide dismutase and downregulating the levels of malondialdehyde and hydroxyproline in the lung. | Chen et al. (2016), Pan et al. (2012) |
| FMP-1/M. esculenta | Mw: 4.7 × 103 Da; consisted of Man, Glc and Gal in a mole ratio of 1.00: 7.84: 1.24; backbone structure: →4)-α-d-Glcp-(1→,→6)-α- d-Galp-(1→ with α-1,6-d-Glcp, α-1,4-d-Glcp, β-1,6-d-Manpat a branching position of O-6. | In vitro: H2O2-induced human alveolar epithelial cells (A549). | In vitro, FMP-1 increased the cell viability with attenuating LDH release, decreased the cell apoptosis with attenuating the release of Cytochrome c and caspase-3, downregulated the expression levels of ROS and MDA, upregulated the activities of SOD and T-AOC in A549 cells, underlying antioxidative effect with PI3K/AKT/Nrf2/HO-1 signaling pathway. | Cai et al. (2018), Li et al. (2018) |
| POL/O. lanpingensisa | Mw: 3.2 × 105 Da; Containing Gal, Man and Glc in a ratio of 5.30: 13.38: 81.31 | In vivo: BLM-induced male mice (C57BL/6). | In vivo, POL improved histopathological changes, reduced collagen deposition and the accumulation of macrophages (inhibiting the expression levels of NOS2, CXCL10/IP10, MARCO and ST2), downregulated the expression levels of pro-inflammatory and pro-fibrogenic factors (TNF-α, IL-1β, IL-6, OSM,IL-10, IL-13, α-SMA, MCP-1 and TGF-β1) and inhibited MDA production with promoting SOD level in BLM-induced PF model mice. | Zhou et al. (2020) |
Polysaccharides from Cordyceps (polysaccharide content > 64 %, average Mw < 2 × 105 Da) exhibited a significant inhibition of pingyangmycin-induced pulmonary fibrosis in mice, which could increase the IL-1RA levels, and reduce the hydroxyproline content and fibrosis area (Hu, Yang, Bai, & Fu, 2019). FYGL-1, a neutral heteropolysaccharide isolated from G. lucidum, showed the similar inhibitory effect on BLM-induced pulmonary fibrosis in rats, which could suppress the pulmonary index, inflammatory cell infiltration and collagen deposition, as well as ameliorate the oxidative stress in the lung tissue, such as upregulating the levels of glutathione, glutathione peroxidase, catalase, superoxide dismutase (SOD) and downregulating the levels of malondialdehyde (MDA) (Chen et al., 2016).
POL is a homogeneous polysaccharide isolated from O. lanpingensis, primarily composed of Gal, Man and Glc in the mole ration of 5.30: 13.38: 81.31, with an average Mw of 3.2 × 105 Da (Zhou et al., 2020). POL could improve histopathological changes, collagen deposition and the accumulation of macrophages (via inhibiting the expression levels of NOS2, CXCL10/IP10, MARCO and ST2), downregulate the expression levels of pro-inflammatory and pro-fibrogenic factors including TNF-α, IL-1β, IL-6, OSM, IL-10, IL-13, α-SMA, MCP-1 and TGF-β1, as well as inhibit oxidative stress (such as MDA production and SOD level) in BLM-induced pulmonary fibrosis mice (Zhou et al., 2020).
FMP-1 is a heteropolysaccharide from the fruiting bodies of Morchella esculenta, which has an average Mw of 4.7 × 103 Da and consisted of Man, Glc and Gal (in a mole ratio of 1.00:7.84:1.24), with the backbone made up of 1,4-linked Glcp and 1,6-linked Galp (Cai et al., 2018). At the doses of 0−300 μg/mL, FMP-1 exhibited a potential anti-pulmonary fibrosis activity in H2O2-induced human alveolar epithelial A549 cells, without no inhibitory effect on cells’ proliferation, which could attenuate H2O2-induced cytochrome c and Caspase-3 release to prevent cell apoptosis via inhibition of MDA and ROS levels, and enhancement the enzymatic activities of SOD and total antioxidant capacity (Li et al., 2018). In addition, the high degree of branching, low molecular weight and favorable structures (e.g. 1,4-linked Glcp, 1,6-linked Galp and 1.6-linked Manp) are supposed to play an essential role in excellent antioxidant activities (Cai et al., 2018).
4. Conclusion
In present review, the structural features, physiological activities, and underlying mechanisms of 16 kinds of natural polysaccharides from plant, algae and fungi were systematically summarized and analyzed. The anti-pulmonary fibrosis activities of natural polysaccharides such as ASP, BHP-1, ginsan, TPRH, MS80, LMWF, and FYGL-1, have been demonstrated in different in vivo and in vitro assays, which can significantly ameliorate the pulmonary index, histopathological changes, and collagen deposition in rats or mice induced by BLM. And the main mechanisms of the anti-pulmonary fibrosis activities of these polysaccharides were targeting the TGF-β/Smad2/3 and DANCER/AUF-1/FOXO3 regulatory axis, and reducing the recruitment of macrophages and neutrophil. Furthermore, the sources of these polysaccharides are most edible materials, and the safety of ginsan, PBS and LMWF have been demonstrated in different in vivo and in vitro assays. Therefore, these polysaccharides maybe considerate as the safety and effective alternative agents for preventing or treating pulmonary fibrosis in COVID-19 patients.
Compliance with ethical standards
The authors declare that they have no conflict of interests.
Acknowledgements
This work was financed by the National Natural Science Foundation of China (No. 81602991) and GDAS' Project of Science and Technology Development (No. 2019GDASYL-0103039).
References
- Ahn J.Y., Kim M.H., Lim M.J., Park S., Lee S.L.O., Yun Y.S., Song J.Y. The inhibitory effect of Ginsan on TGF-beta mediated fibrotic process. Journal of Cellular Physiology. 2011;226(5):1241–1247. doi: 10.1002/jcp.22452. [DOI] [PubMed] [Google Scholar]
- Bale S., Venkatesh P., Sunkoju M., Godugu C. An adaptogen: Withaferin A ameliorates in vitro and in vivo pulmonary fibrosis by modulating the interplay of fibrotic, matricelluar proteins, and cytokines. Frontiers in Pharmacology. 2018;9 doi: 10.3389/fphar.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickerman H.A., Beck G.J., Barach A.L. The use of prednisone (Meticorten) in respiratory disease. II. Pulmonary emphysema and pulmonary fibrosis. Journal of Chronic Diseases. 1955;2(3):247–259. doi: 10.1016/0021-9681(55)90132-6. [DOI] [PubMed] [Google Scholar]
- Cai Z.N., Li W., Mehmood S., Pan W.J., Wang Y., Meng F.J.…Chen Y. Structural characterization, in vitro and in vivo antioxidant activities of a heteropolysaccharide from the fruiting bodies of Morchella esculenta. Carbohydrate Polymers. 2018;195:29–38. doi: 10.1016/j.carbpol.2018.04.069. [DOI] [PubMed] [Google Scholar]
- Cameli P., Carleo A., Bergantini L., Landi C., Prasse A., Bargagli E. Oxidant/antioxidant disequilibrium in idiopathic pulmonary fibrosis pathogenesis. Inflammation. 2020;43(1):1–7. doi: 10.1007/s10753-019-01059-1. [DOI] [PubMed] [Google Scholar]
- Canestaro W.J., Forrester S.H., Raghu G., Ho L., Devine B.E. Drug treatment of idiopathic pulmonary fibrosis systematic review and network meta-analysis. Chest. 2016;149(3):756–766. doi: 10.1016/j.chest.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Chen J.H., Lu J.H., Wang B.L., Zhang X.L., Huang Q.Y., Yuan J.…Chu H.Q. Polysaccharides from Dendrobium officinale inhibit bleomycin-induced pulmonary fibrosis via the TGF beta 1-Smad2/3 axis. International Journal of Biological Macromolecules. 2018;118:2163–2175. doi: 10.1016/j.ijbiomac.2018.07.056. [DOI] [PubMed] [Google Scholar]
- Chen J.H., Shi Y.Y., He L., Hao H.R., Wang B.L., Zheng Y.L., Hu C.P. Protective roles of polysaccharides from Ganoderma lucidum on bleomycin-induced pulmonary fibrosis in rats. International Journal of Biological Macromolecules. 2016;92:278–281. doi: 10.1016/j.ijbiomac.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Cho Y.J., Son H.J., Kim K.S. A 14-week randomized, placebo-controlled, double-blind clinic trail to evaluate the efficacy and safety of gineng polysaccharide (Y-75) Journal of Translational Medicine. 2014;12 doi: 10.1186/s12967-014-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C., Lu J.H., Sun-Waterhouse D.X., Mu L.X., Sun W.Z., Zhao M.M., Zhao H.F. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. Lwt-Food Science and Technology. 2016;73:602–608. [Google Scholar]
- Gao Y. JiLin Agricultural University; 2016. Study on Gua Lou Xie Bai decoction for inhibiting pulmonary fibrosis. (Thesis) [Google Scholar]
- Guo Q., Meng Y., Zhao Y., Cheng X.C., Lu Y.X., Zhang Q.L. Inhibitory effect of Bletilla striata extracts on bleomycin-induced pulmonary fibrosis in rats. Journal of International Pharmaceutical Research. 2016;43(3):518–523. [Google Scholar]
- Han L.P., Liang Z.Y., Zhang L.P., Han L.M., Gong R.C., Ma X.H. Purification and composition analysis of a polysaccharide RSA from Rhodiola sachalinensis A. Bor. Chinese Pharmaceutical Journal. 2002;37(6):418–421. [Google Scholar]
- He T.B., Huang Y.P., Yang L., Liu T.T., Gong W.Y., Wang X.J.……Hu J.M. Structural characterization and immunomodulating activity ofpolysaccharide from Dendrobium officinale. International Journal of Biological Macromolecules. 2016;83:34–41. doi: 10.1016/j.ijbiomac.2015.11.038. [DOI] [PubMed] [Google Scholar]
- He X.R., Wang X.X., Fang J.C., Zhao Z.F., Huang L.H., Guo H., Zheng X.H. Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. Journal of Ethnopharmacology. 2017;195:20–38. doi: 10.1016/j.jep.2016.11.026. [DOI] [PubMed] [Google Scholar]
- Hoffman M., Jia Z.H., Pena M.J., Cash M., Harper A., Blackburn A.R.…York W.S. Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydrate Research. 2005;340(11):1826–1840. doi: 10.1016/j.carres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Hu R., Yang Y., Bai H.B., Fu X.C. Effect of Cordyceps polysaccharide on pingyangmycin induced pulmonary fibrosis in mice. Chinese Journal of Modern Applied Pharmacy. 2019;36(13):1639–1642. [Google Scholar]
- Hui D.S., Wong K.T., Ko F.W., Tam L.S., Chan D.P., Woo J., Sung J.J.Y. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui H.P., Li X.Z., Jin H., Yang X.Y., Xin A.Y., Zhao R.M., Qin B. Structural characterization, antioxidant and antibacterial activities of two heteropolysaccharides purified from bulbs of Lilium davidii var. unicolor Cotton. International Journal of Biological Macromolecules. 2019;133:306–315. doi: 10.1016/j.ijbiomac.2019.04.082. [DOI] [PubMed] [Google Scholar]
- Hwang P.A., Yan M.D., Lin H.T.V., Li K.L., Lin Y.C. Toxicological evaluation of low molecular weight fucoidan in vitro and in vivo. Marine Drugs. 2016;14(7) doi: 10.3390/md14070121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Bartholmai B.J., Rajagopalan S., Karwoski R., Nair A., Walsh S.L.F.…Wells A.U. Likelihood of pulmonary hypertension in patients with idiopathic pulmonary fibrosis and emphysema. Respirology. 2018;23(6):593–599. doi: 10.1111/resp.13231. [DOI] [PubMed] [Google Scholar]
- Jiang H.D. Ocean University of China; 2008. The effects and mechanism study of TCM-based Fei KangLing (FKL) on pulmonary interstital fibrosis. (PhD thesis) [Google Scholar]
- Jiang H.D., Guan H.S. MS80, a novel sulfated oligosaccharide, inhibits pulmonary fibrosis by targeting TGF-beta 1 both in vitro and in vivo. Acta Pharmacologica Sinica. 2009;30(7):973–979. doi: 10.1038/aps.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M.L., Zhao K., Huang Q.S., Xu C.L., Shang P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydrate Polymers. 2012;89(3):713–722. doi: 10.1016/j.carbpol.2012.04.049. [DOI] [PubMed] [Google Scholar]
- Kidd P.M. The use of mushroom glucans and proteoglycans in cancer treatment. Alternative Medicine Review. 2000;5(1):4–27. [PubMed] [Google Scholar]
- Lee Y.S., Chung I.S., Lee I.R., Kim K.H., Hong W.S., Yun Y.S. Activation of multiple effector pathway of immune system by the antineoplastic immunostimulator acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Research. 1997;17(1A):323–331. [PubMed] [Google Scholar]
- Lei F.F., Zhao J.X., Wang X.X., Yao B.T., Ding K. An experimental observation of pulmonary fibrosis in rats and its transforming growth factor beta1 treated by total hedysarum polybotyssaccharide. Journal of Chinese Medicinal Materials. 2008;31(6):873–877. [PubMed] [Google Scholar]
- Li J., Zhang Y., Liu Y.Q., Li J.T., Wei S.C., Su W., Nie L. Effects of Astragalus polysaccharides on level of cytokines and pathological structure of lung tissue in rat model of pulmoanry fibrosis. Lishizhen Medicine and Materia Medica Research. 2011;22(7):1684–1685. [Google Scholar]
- Li W., Cai Z.N., Mehmood S., Wang Y., Pan W.J., Zhang W.N.…Chen Y. Polysaccharide FMP-1 from Morchella esculenta attenuates cellular oxidative damage in human alveolar epithelial A549 cells through PI3K/AKT/Nrf2/HO-1 pathway. International Journal of Biological Macromolecules. 2018;120:865–875. doi: 10.1016/j.ijbiomac.2018.08.148. [DOI] [PubMed] [Google Scholar]
- Li Z.L., Gao Y., Zhao L., Hong B. Study on rhodiola polysaccharide against pulmonary fibrosis. Journal of Molecular Science. 2016;32(1):34–39. [Google Scholar]
- Liu Y., Lu F., Kang L.R., Wang Z.H., Wang Y.F. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulmonary Medicine. 2017;17 doi: 10.1186/s12890-017-0405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.A., An F.Y., Li N.L., Liu Y.Q., Li C.H., Zhang Y.H., Chen J. Effects of Angelica polysaccharides and angelica small molecules on expression of α-SMA, CTGF in human embryonic lung fibroblast cells induced by TGF-β1. Pharmacology and Clinics of Chinese Materia Medica. 2017;33(3):73–78. [Google Scholar]
- Luo Y.L., Wang Y.L., Pan Z., Li N.L., Li Y., Yan X., Zhang L. Effect of lily polysaccharides combined with BMSCs transplantation on expression of TNF-alpha and NF-kappaB in bleomycin-induced pulmonary fibrosis mice. Journal of Third Military Medical University. 2013;35(5):431–434. [Google Scholar]
- Martinez F.J., Collard H.R., Pardo A., Raghu G., Richeldi L., Selman M.…Wells A.U. Idiopathic pulmonary fibrosis. Nature Reviews Disease Primers. 2017;3 doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- Miniati I., Cerinic M.M. Pulmonary fibrosis in systemic sclerosis: Is treatment with cyclophosphamide more effective than placebo? Nature Clinical Practice Rheumatology. 2007;3(7):372–373. doi: 10.1038/ncprheum0507. [DOI] [PubMed] [Google Scholar]
- Muhama I.I., Zulkifli N., Selvakumaran S.A.P., Lazim N.A.M. Bioactive algal-derived polysaccharides: Multi-functionalization, therapeutic potential and biomedical applications. Current Pharmaceutical Design. 2019;25(11):1147–1162. doi: 10.2174/1381612825666190618152133. [DOI] [PubMed] [Google Scholar]
- Ozaki T., Nakayama T., Ishimi H., Kawano T., Yasuoka S., Tsubura E. Glucocorticoid receptors in bronchoalveolar cells from patients with idiopathic pulmonary fibrosis. The American Review of Respiratory Disease. 1982;126(6):968–971. doi: 10.1164/arrd.1982.126.6.968. [DOI] [PubMed] [Google Scholar]
- Pan D., Wang L.Q., Chen C.H., Teng B.S., Wang C.D., Xu Z.X.…Zhou P. Structure characterization of a novel neutral polysaccharide isolated from Ganoderma lucidum fruiting bodies. Food Chemistry. 2012;135(3):1097–1103. doi: 10.1016/j.foodchem.2012.05.071. [DOI] [PubMed] [Google Scholar]
- Qian W.B., Cai X.R., Qian Q.H., Wang D.L., Zhang L. Angelica Sinensis polysaccharide suppresses epithelial-mesenchymal transition and pulmonary fibrosis via a DANCR/AUF-1/FOXO3 regulatory axis. Aging and Disease. 2020;11(1):17–30. doi: 10.14336/AD.2019.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang Z.Z., Wang Y., Li S., Wang M.W., Li C.Y. Structure and activity of Hedysari radix polysaccharides and its derivatives. Chinese Journal New Drugs. 2018;27(19):2271–2280. [Google Scholar]
- Raghu G., Rochwerg B., Zhang Y., Garcia C.A.C., Azuma A., Behr J.…Schunemann H.J. An official ATS/ERS/JRS/ALAT clinical practice guideline: Treatment of idiopathic pulmonary fibrosis an update of the 2011 clinical practice guideline. American Journal of Respiratory and Critical Care Medicine. 2015;192(2):E3–E19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- Raimundo K., Chang E., Broder M.S., Alexander K., Zazzali J., Swigris J.J. Clinical and economic burden of idiopathic pulmonary fibrosis: A retrospective cohort study. BMC Pulmonary Medicine. 2016:16. doi: 10.1186/s12890-015-0165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- Shi L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. International Journal of Biological Macromolecules. 2016;92:37–48. doi: 10.1016/j.ijbiomac.2016.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Zhang Y., Li J., Li X.Y., Liu Y.Q., Lin X.Y., Li J.T. Effects of active ingredients in Radix hedysari on collagen area, hyaluronic acid and laminin of lung tissues in rats with pulmonary interstitial fibrosis. Chinese Journal of Information on Traditional Chinese Medicine. 2016;23(4):72–76. [Google Scholar]
- Sun Y.X. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng CA Meyer: An overview. Carbohydrate Polymers. 2011;85(3):490–499. [Google Scholar]
- Venkataraman T., Frieman M.B. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antiviral Research. 2017;143:142–150. doi: 10.1016/j.antiviral.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K.L., Johnson K.E., Harrison C.A. Targeting TGF-beta mediated SMAD signaling for the prevention of fibrosis. Frontiers in Pharmacology. 2017;8 doi: 10.3389/fphar.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang B.J., Yang J.C., Wang M.Y., Chen C., Luo G.X., He W.F. Advances in the research of mechanism of pulmonary fibrosis induced by Corona Virus Disease 2019 and the corresponding therapeutic measures. Chinese Journal of Burns. 2020;36(0) doi: 10.3760/cma.j.cn501120-20200307-00132. [DOI] [PubMed] [Google Scholar]
- Wang J., Geng L.H., Yue Y., Zhang Q.B. In: Zhang, editor. Vol. 163. 2019. Use of fucoidan to treat renal diseases: A review of 15 years of clinic studies; pp. 95–111. (Glycans and glycosaminoglycans as clinical biomarkers and therapeutics, Pt B). [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang P., Li X.P., Zhang Y., Zhan Q.Y., Wang C. Low-molecular-weight fucoidan attenuates bleomycin-induced pulmonary fibrosis: Possible role in inhibiting TGF-beta 1-induced epithelial-mesenchymal transition through ERK pathway. American Journal of Translational Research. 2019;11(4):2590–2602. [PMC free article] [PubMed] [Google Scholar]
- Xie L.X., Liu Y.N., Fan B.X., Xiao Y.Y., Tian Q., Chen L.G.…Chen W.J. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respiratory Research. 2005;6(3–5) doi: 10.1186/1465-9921-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Li L.C., Zhao P., Qi L.W., Li P., Gao J., Fei G.H. Total polysaccharide of Yu Ping Feng protects against bleomycin-induced pulmonary fibrosis via inhibiting transforming growth factor-beta 1-mediated type I collagen abnormal deposition in rats. The Journal of Pharmacy and Pharmacology. 2014;66(12):1786–1795. doi: 10.1111/jphp.12308. [DOI] [PubMed] [Google Scholar]
- Xu Y.H., Dong J.H., An W.M., Lv X.Y., Yin X.P., Zhang J.Z.…Gao B.L. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. The Journal of Infection. 2020;80(4):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D.D., Tian J.Z., Zhang D., Hou L., Li L., Zhang C.H.…Zhu Q.J. Inhibitory effect of Basil polysaccharide on TGF-beta-induced epithelial-mesenchymal transition of A549 cells. Traditional Chinese Drug Research and Clinical Plarmacology. 2017;28(2):154–159. [Google Scholar]
- Yang J.X., Sun Y., Gao L., Meng Q., Yang B.Y. Long non-coding RNA DANCR facilitates glioma malignancy by sponging miR-33a-5p. Neoplasma. 2018;65(5):790–798. doi: 10.4149/neo_2018_170724N498. [DOI] [PubMed] [Google Scholar]
- Yin J.Y., Chan B.C.L., Yu H., Lau L.Y.K., Han X.Q., Cheng S.W.…Han Q.B. Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix astragali. Carbohydrate Polymers. 2012;87(1):667–675. doi: 10.1016/j.carbpol.2011.08.045. [DOI] [PubMed] [Google Scholar]
- Yu H.H., Ko E.C., Chang C.L., Yuan K.S.P., Wu A.T.H., Shan Y.S., Wu S.Y. Fucoidan inhibits radiation-Induced pneumonitis and lung fibrosis by reducing inflammatory cytokine expression in lung tissues. Marine Drugs. 2018;16(10) doi: 10.3390/md16100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y.F., An X.N., Wang S., Sun M.J., Zhou H.M. Basil polysaccharides: A review on extraction, bioactivities and pharmacological applications. Bioorganic & Medicinal Chemistry. 2020;28(1):115179. doi: 10.1016/j.bmc.2019.115179. [DOI] [PubMed] [Google Scholar]
- Zheng C., Li Y.S., Liu H.B., Yuan D., Lu B.R. Sulfoglycolipid from the marine brown alga Sargassum hemiphyllum. Journal of Asian Natural Products Research. 2001;3(2):117–122. doi: 10.1080/10286020108041378. [DOI] [PubMed] [Google Scholar]
- Zhou S.B., Zhou Y.C., Yu J.J., Du Y.X., Tan Y., Ke Y.M.…Ge F. Ophiocordyceps lanpingensis polysaccharides attenuate pulmonary fibrosis in mice. Biomedecine & Pharmacotherapy. 2020;126 doi: 10.1016/j.biopha.2020.110058. [DOI] [PubMed] [Google Scholar]