Abstract
Background
In light of the coronavirus disease 2019 (COVID-19) pandemic, cancer centres in the United Kingdom and Europe re-organised their services at an unprecedented pace, and many patients with cancer have had their treatments severely disrupted. Patients with cancer were considered at high risk on sparse evidence, and despite a small number of emerging observational studies, the true incidence and impact of COVID-19 in the ‘at-risk’ population of patients with cancer is yet to be defined.
Methods
Epidemiological and clinical data were collected prospectively for patients attending the Royal Marsden Hospital and three network hospitals between March 1st and April 30th 2020 that were confirmed to have Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection. Significance of clinical and pathological characteristics was assessed using the Fisher's exact test and Wilcoxon rank sum test, whilst univariate and multivariate logistic regression models were used to further assess risk. The number of patients attending in March/April 2020 for face-to-face attendances was also extracted.
Findings
During the 2-month study period, 867 of 13,489 (6.4%) patients met the criteria leading to swab testing. Of the total at-risk population, only 113 of 13,489 (0.84%) were swab positive, 101 of 13,489 (0.75%) required hospital admission and 29 of 13,489 (0.21%) died of COVID-19. Of the patients that attended the hospital to receive cytotoxic chemotherapy alone or in combination with other therapy, 59 of 2001 (2.9%) were admitted to the hospital for COVID-19–related issues and 20 of 2001 (1%) died. Of the patients that collected targeted treatments, 16 of 1126 (1.4%) were admitted and 1 of 1126 (0.1%) died. Of the 11 patients that had received radiotherapy, 6 of 1042 (0.6%) required inpatient admission and 2 of 1042 (0.2%) died.
Interpretations
Administration of systemic anticancer therapy appears to be associated with a modest risk of severe COVID-19 infection. Based on this snapshot taken as the first wave of COVID-19 hit our practice, we conclude that continuation of active cancer treatment, even in the palliative setting, is appropriate.
Keywords: COVID-19, Cancer, Systemic anticancer therapy, Chemotherapy, Radiotherapy
Highlights
-
•
Of 13,489 cancer patients, 867 were tested and 113 were coronavirus disease 2019 (COVID-19) swab-positive.
-
•
Of these only 101 patients required hospital admission and 29 died of COVID-19.
-
•
Systemic anticancer therapy (SACT) and radiotherapy confer only a modest risk of significant COVID-19 infection.
-
•
Incidence of clinically significant COVID-19 was as in the general population.
-
•
We encourage oncology centres to continue to deliver SACT and radiotherapy.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has compelled oncologists worldwide to restructure cancer care to contain the spread of the virus and mitigate infection risk to patients by reducing hospital attendances, inpatient admissions and therapy-induced complications, where possible, without compromising cancer-specific outcomes. Cancer centres in the United Kingdom and in Europe re-organised their services at an unprecedented pace and scale to deal with the impact of COVID-19 [1]. The measures adopted were largely based on expert opinions, influenced or supported by information extrapolated from other infectious diseases, but with the central assumption that anticancer treatments might increase the severity of COVID-19 [2]. The National Institute for Health and Care Excellence produced guidelines for categorising and prioritising patients for systemic anticancer therapies, radiotherapy and surgery [3]. Similarly, the European Society for Medical Oncology produced specific guidance to mitigate the negative effects of the COVID-19 pandemic on the diagnosis and treatment of patients with cancer [4]. Consequently, many patients with cancer have had their planned or current cancer treatments severely disrupted, even when this is likely to result in worse cancer-specific outcomes.
There are limited but increasing data regarding the epidemiologic characteristics and clinical features of infected patients with a cancer diagnosis. According to an initial study of Chinese patients with COVID-19, those with cancer had a higher risk of severe events, defined as requiring admission to an intensive care unit (ICU) or death, compared with patients without cancer (odds ratio [OR] 5.4, 95% confidence interval [CI] 1.8–16.2) [5]. This study was limited by inclusion of only eighteen patients with cancer. Half of the cancer diagnoses were over four years before COVID-19 infection, suggesting that these patients may not have been on active treatment, and it was not clear if the multivariate analysis adequately accounted for the confounding effects of comorbidities. A subsequent Chinese study on 105 patients with cancer and 536 age-matched patients without cancer reported an OR of 2.84 for ICU admission and 2.34 for death [6]. An early study from Italy focussing on COVID-19–related deaths also reported that 20% of patients had a diagnosis of cancer in the previous 5 years [7]. In contrast, a UK study comparing outcomes of patients with COVID-19 and cancer with those with no history of cancer reported no increase in severe outcomes or mortality, although this analysis was limited by a small sample size [8]. The results of larger studies are now becoming available, such as a UK-based study of 800 patients conducted by the UK Coronavirus Cancer Monitoring Project, as well a large cohort study in the US, both of which focused on the impact of recent anticancer therapy and COVID-19–related morbidity and mortality but did not identify a significantly higher risk linked with recent immunotherapy, hormonal therapy, targeted therapy, or radiotherapy [[9], [10], [11]].
Given the severe disruption to normal medical care triggered by concerns about adverse outcomes of COVID-19 in patients with cancer, there is an urgent need to collect and share patient data to assess the safety and relative risk of continuing anticancer therapies during the COVID-19 pandemic. Such information will help to design a risk-based framework for cancer healthcare that minimises the chances of patients contracting the infection while simultaneously avoiding inferior cancer-specific outcomes. In this study, we share our experience of the incidence of COVID-19 in a large population of patients receiving on-going care in our oncology service and report the specific outcomes for 113 patients with cancer and reverse transcription polymerase chain reaction (RT-PCR) confirmed infections.
2. Methods
Institutional approval was obtained following local information governance processes. The first approval by the Committee for Clinical Research was granted on the 9th of April 2020 (SE937), with two subsequent minor amendments approved on the 11th and 22nd of May.
3. Data collection
The number of patients attending the Royal Marsden Hospital (RMH) in March/April 2020 for face-to-face attendances, including intravenous systemic anticancer therapy (SACT), collection of oral SACT, radiotherapy, surgical procedures, venepuncture or imaging, and visits for non-elective assessment at the clinical assessment unit (CAU) and COVID-19 hub was extracted from the hospital electronic patient records in an anonymised fashion by a dedicated information development manager. Equivalent data were also collected for March/April 2019 as an indication of variance in such interactions during the pandemic.
From within this patient population, we conducted a prospective study in eligible patients aged 18 years or older with a diagnosis of solid or haematological malignancy who were diagnosed with COVID-19 between March 1st and April 30th 2020 at the RMH and three network hospitals. The presence of novel SARS-CoV2 infection was established by real-time RT-PCR from a nose and/or throat swab. In contrast to the present situation of widespread community-based testing in the UK, during the 2-month study period, swab tests were only available in hospitals and applied to patients who met pre-specified criteria. We applied the following selection criteria for testing: (i) fever of 37.8 °C and/or upper respiratory tract symptoms in a patient who had received chemotherapy or radiotherapy within the last 6 weeks or immunotherapy within the last three months, (ii) pre-assessment for surgery or stem cell transplantation and (iii) incidental radiological findings compatible with COVID-19 on imaging performed as part of cancer management. Prospective epidemiological, demographic, clinical, laboratory, treatment and outcome data were extracted from electronic medical records.
4. Statistical analysis
The incidence of swab-positive SARS-CoV2 infection and COVID-19 in the total patient population and in the sub-groups undergoing specific interventions was expressed as percentages. Significance of clinical and pathological characteristics and treatment differences between patients were assessed using the Fisher's exact test for categorical variables and the Wilcoxon rank sum test for continuous variables where appropriate. To explore the OR and 95% CIs for the risk factors associated with death, univariate and multivariate logistic regression models were used in R (version 3.4.4 for Windows).
5. Results
To estimate the true risk of continued management of patients' underlying malignant conditions, our analyses focused on defining the total ‘at-risk’ population of patients with cancer in our practice. To do this as accurately as possible, we counted physical attendances at the hospital. In the months of March and April 2020, a total of 13,489 individual patients, aged 18 years and older, had face-to-face attendances at the RMH. A breakdown of the underlying diagnoses for patients who attended is presented in Table 1 . Of those, 2205 patients received intravenous SACT, including 1713 patients receiving cytotoxic chemotherapy, alone or in combination with other agents (including immunotherapy, anti-HER2, anti–vascular endothelial growth factor, anti–epidermal growth factor receptor, anti-CD20 and anti-CD38 agents). Of those who received immunotherapy (n = 325), 137 did so in combination with cytotoxic chemotherapy and 188 had monotherapy or combination immunotherapy. Oral SACT was collected by 1790 patients, with 664 collecting oral chemotherapy and 1126 a targeted treatment, with tyrosine kinase inhibitors alone or in combination (n = 559), Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors (n = 257) and Poly(ADP-ribose) polymerase (PARP) inhibitors (n = 79) being the commonest groups. Oral SACT was part of a regimen that included intravenous SACT in 376 cases. In total, 2001 patients received cytotoxic chemotherapy, oral or intravenous, alone or in combination with other agents. One thousand forty-two patients received radiotherapy; of these, 735 (70.5%) were treated with a radical and 307 (29.5%) with a curative intent. The majority had multiple attendances for fractionated regimens with a combined total of 10,132 visits in March and April 2020. The treatment-related characteristics and primary tumour sites of patients receiving radiotherapy are summarised in Table 2 . One thousand eighty-one had a surgical intervention; 8153 had at least one blood test; and 9033 attended for an imaging investigation. There were also 397 patients that attended the CAU and 226 the COVID-19 hub for a non-elective assessment. Many patients had multiple attendances for intravenous or oral SACT, radiotherapy, venepuncture and/or imaging investigations.
Table 1.
Tumour sub-sites in those diagnosed with COVID-19 (n = 113) and the at-risk population of adults presenting for face-to-face consultations (n = 13,489) at the RMH in March/April 2020.
| Tumour subtype | COVID-19–positive patients n = 113 (%) | Patients attending RMH n = 13,489 (%) |
|---|---|---|
| Gastrointestinal | 32 (28.31%) | 1773 (13.9%) |
| Breast | 18 (15.92%) | 3141 (23.28%) |
| Haematological | 18 (15.92%) | 1085 (8%) |
| Thoracic | 15 (13.24%) | 778 (5.76%) |
| Urological | 12 (12.63%) | 2188 (16.2%) |
| Gynaecological | 6 (5.39%) | 973 (7.2%) |
| Central Nervous System (CNS) | 4 (3.54%) | 342 (2.5%) |
| Head & Neck | 3 (2.65%) | 449 (3.3%) |
| Skin | 3 (2.65%) | 450 (3.32%) |
| Sarcoma | 1 (0.88%) | 1064 (7.88%) |
| Thyroid | 1 (0.88%) | 247 (1.83%) |
| Other | 0 (0%) | 999 (7.4%) |
| Total | 113 | 14,007 |
| Over 18 years | 113 | 13,489 |
COVID-19 = coronavirus disease 2019; RMH = Royal Marsden Hospital.
Table 2.
Treatment-related characteristics and sub-sites of patients receiving radiotherapy at the RMH in March/April 2020.
| Treatment-related characteristics of patients (n = 1042 treatments) on radiotherapy | |
|---|---|
| Total number of visits | 10,132 |
| Treatment intent | |
| Curative | 735 (70.5%) |
| Palliative | 307 (29.5%) |
| Primary tumour | |
| Gastrointestinal | 102 (9.8%) |
| Breast | 289 (27.7%) |
| Haematological | 44 (4.2%) |
| Thoracic | 117 (11.2%) |
| Urological | 186 (17.8%) |
| Gynaecological | 74 (7%) |
| Central Nervous System (CNS) | 81 (7.8%) |
| Head & Neck | 68 (6.5%) |
| Skin | 37 (3.5%) |
| Sarcoma | 35 (3.6%) |
| Thyroid | 4 (0.5%) |
| Other | 5 (0.5%) |
RMH = Royal Marsden Hospital.
To put these numbers into context, we collected the same data for patients who had face-to-face attendances at the RMH in March and April 2019. A total of 18,087 patients aged 18 years and older attended in that period, which was higher by 25% compared with March and April 2020. The reasons for hospital attendance in the two time periods are summarised in Table 3 .
Table 3.
Face-to-face interventions at the RMH in March/April 2019 and 2020.
| Intervention | March/April 2019 | March/April 2020 | Change (%) |
|---|---|---|---|
| IV SACT | 2047 | 2205 | +8 |
| Immunotherapy | 386 | 325 | −16 |
| PO SACT | 1683 | 1790 | +6 |
| Radiotherapy (RT) | 1156 | 1042 | −10 |
| Surgery | 1393 | 1081 | −22 |
| Blood test | 11,054 | 8153 | −26 |
| Imaging | 12,457 | 9033 | −27 |
| ALL | 18,666 | 14,007 | −25 |
| Adults | 18,087 | 13,489 | −25 |
SACT = systemic anticancer therapy; RMH = Royal Marsden Hospital; IV = intravenous; PO = oral.
6. Screening
In the months of March and April 2020, a total of 867 patients who fulfilled our criteria for COVID-19 testing had nose and/or throat swabs taken for RT-PCR. Of those, 113 (13%) returned positive results, 685 (79%) were negative and 69 (8%) tests were pending at the time of data collection cut-off. Of the 113 patients, 101 required hospital admission with 10.6% (n = 12) requiring direct admission or escalation to the ICU. The median age of the 113 patients was 66 years (interquartile range (IQR) = 54 to 69, range 21–91). Most patients were men (n = 63, 55.7%) and 23.8% were of black, Asian or minority ethnic background. Comorbidities included hypertension (n = 39, 34.5%), type II diabetes (n = 18, 15.9%), cardiovascular disease (n = 13, 11.5%) and chronic obstructive pulmonary disease (n = 6, 5.3%). Twenty-seven patients (23.9%) had no previous comorbidities, and the majority of patients were of good performance status (81.4% with Eastern Cooperative Oncology Group: 0–1). Eleven (9.7%) patients were on medium- to long-term immunosuppressive corticosteroid therapy; this was part of their anticancer treatment (n = 4), for treatment of immunotherapy-induced toxicities (n = 2), for prevention of tumour lysis syndrome (n = 1), for palliation of symptoms (n = 3) and for reasons not linked with their cancer diagnosis (n = 1). Only 11 patients (9.7%) were current smokers. The demographic, clinical, laboratory and radiographic findings of patients on admission are shown in Table 4 .
Table 4.
Demographic and clinical characteristics findings of patients on admission.
|
Demographics and clinical characteristics | |
| Total n = 113 | |
| Age, years | 66 (IQR: 54–69) |
| Sex | |
| Female | 50 (44.2%) |
| Male | 63 (55.7%) |
| Current smokers | 11 (9.7%) |
| Comorbidity | 86 (76.1%) |
| Hypertension | 39 (34.5%) |
| Diabetes | 18 (15.9%) |
| Ischaemic heart disease | 13 (11.5%) |
| COPD | 6 (5.3%) |
| Other |
11 (9.7%) |
|
ECOG performance status |
n (%) |
| 0 | 16 (14.18%) |
| 1 | 76 (67.25%) |
| 2 | 11 (9.73%) |
| 3 | 9 (7.96%) |
| 4 |
1 (0.88%) |
|
Symptoms on admission |
n (%) |
| Fever | 55 (54.5%) |
| Cough | 36 (35.6%) |
| Dyspnoea | 28 (27.7%) |
| Diarrhoea | 4 (3.9%) |
| Headache | 4 (3.9%) |
COVID-19 = coronavirus disease 2019; SACT = systemic anticancer therapy; COPD = chronic obstructive pulmonary disease; ECOG = Eastern Cooperative Oncology Group; IQR = interquartile range.
The most common presenting features of COVID-19 were fever (n = 55, 48.7%), cough (n = 36, 31.9%) and shortness of breath (n = 28, 24.8%), with diarrhoea (n = 4, 3.5) and headache (n = 4, 3.5), presenting less frequently. Anosmia and/or ageusia were not routinely documented.
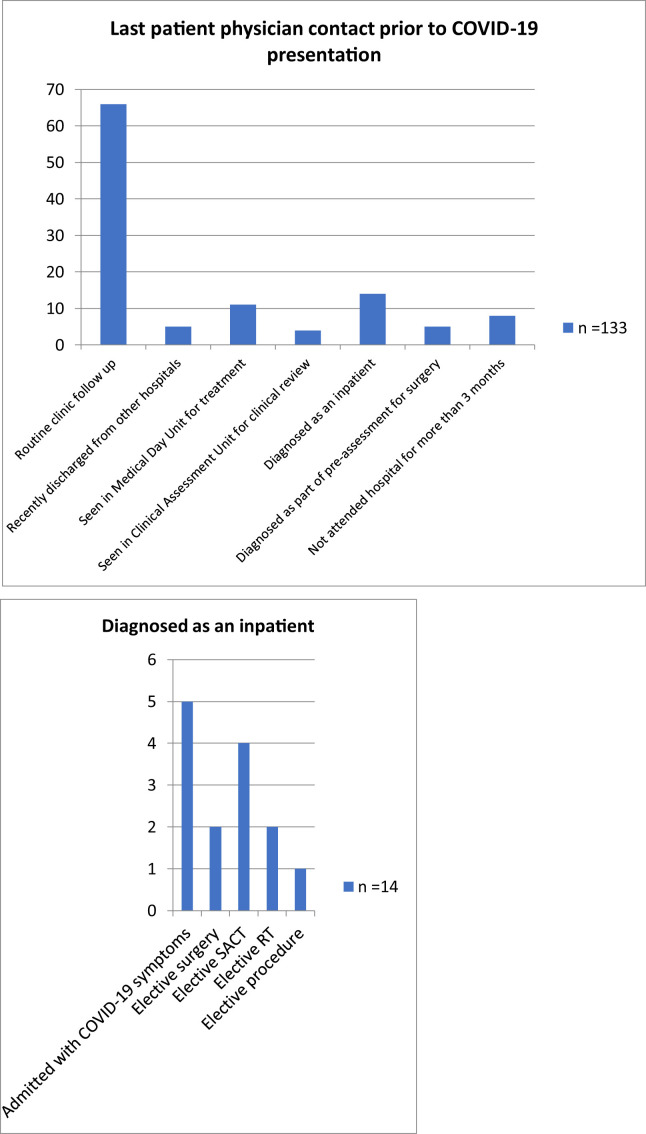
Of the 113 patients, 99 were found to be COVID-19 positive after a symptom-driven emergent or elective hospital attendance, as per the aforementioned criteria. The majority of these (n = 66, 58.4%) had previously visited the hospital for a scheduled appointment, a median of 12 (range 1–114) days before the swab was performed. Of the 66 patients, only four had upper respiratory tract symptoms and none had fever at the time of their previous scheduled appointment. Fourteen patients were swabbed and diagnosed during an inpatient hospital stay: 5 were non-elective admissions with symptoms other than those of COVID-19 and 9 were tested after developing fever during elective inpatient treatment. Details are summarised in Table 5 and Fig. 1 .
Table 5.
Last patient-physician contact before COVID-19 presentation.
| Point of contact prior COVID-19 presentation | n = 113 | Median time period and range between last contact and presentation (days) |
|---|---|---|
| Routine clinic follow-up | 66 | 12 (1–114) |
| Recently discharged from other hospitals | 5 | |
| Seen in medical day unit for treatment | 11 | 9 (1–64) |
| Seen in clinical assessment unit for clinical review | 4 | 6 (1–11) |
| Diagnosed as an inpatient | 14 | |
| Admitted unwell with non-COVID-19 symptoms | 5 | |
| Elective surgery | 2 | |
| Elective SACT | 4 | |
| Elective Radiotherapy (RT) | 2 | |
| Elective procedure (drain insertion) | 1 | |
| Diagnosed as part of pre-assessment for surgery | 5 | |
| Not attended hospitals for more than 3 months | 8 |
COVID-19 = coronavirus disease 2019; SACT = systemic anticancer therapy.
Fig. 1.
Last patient-physician contact before COVID-19 presentation. COVID-19 = coronavirus disease 2019
7. Cancer characteristics
Of the 113 patients, eighteen had haematological malignancies and 95 had solid tumours. Of the latter group, the commonest primary sites were the gastrointestinal system (n = 32), breast (n = 18) and thorax (n = 15) and most had metastatic disease (n = 59/113, 52.2%) (Table 1). Most had received SACT within six weeks of admission (n = 85/113, 75.22%), mostly in the form of cytotoxic chemotherapy (n = 58/113, 52.2%): forty-eight patients received intravenous chemotherapy only, 23 oral only, eleven a combination of intravenous and oral and one patient received intrathecal chemotherapy. Only four patients (3.5%) had received immune checkpoint inhibitors within three months of presentation, and this was in all cases combined with cytotoxic therapy. The majority of patients were receiving treatment with a palliative intent (n = 73/113, 64.6%), and of these, most were on first-line treatment (n = 26/73, 35.6%). Only eleven patients (9.7%) had received radiotherapy within six weeks of presentation (Table 6 ).
Table 6.
Treatment-related characteristics of COVID-19–positive patients.
| Treatment-related characteristics (n = 113) | |
|---|---|
| Treatment intent | |
| Curative | 40 (35.4%) |
| Palliative | 73 (64.6%) |
| SACT within 6 weeks of admission | 85 |
| Cytotoxic chemotherapy | 58 |
| Tyrosine kinase inhibitors | 11 |
| Other small molecule inhibitors | 1 |
| CDK4-6 inhibitors | 4 |
| PARP inhibitors | 2 |
| Trial | 1 |
| Cytotoxic and CPI | 4 |
| CPI only | 0 |
| Non-CPI monoclonal antibody | 2 |
| Hormone therapy only | 2 |
| Radiotherapy within 6 weeks | 11 (9.7%) |
COVID-19 = coronavirus disease 2019; SACT = systemic anticancer therapy; CPI = checkpoint inhibitor; CDK4/6 = Cyclin-dependent kinase 4 and 6; PARP = Poly(ADP-ribose) polymerase.
8. Investigations and treatment during hospital admission
Of the 101 admitted patients, radiological investigations included plain chest radiograph (86/101 patients) and high-resolution computed tomography/computed tomographic pulmonary angiography (20/101). Of these, 47 (46.5%) patients had typical pulmonary infiltrates consistent with COVID-19 infection. Empiric antibiotics were commenced in 103 of 113 (91.2%) swab-positive patients. These were commenced at presentation to cover for possible bacterial infection and were stopped when clinically deemed appropriate. Superimposed bacterial infection was confirmed in only three patients on blood cultures. Two patients had an additional viral infection on polymerase chain reaction (PCR) (one metapneumovirus and one adenovirus). Intravenous corticosteroids were administered to only two patients. We captured complete data for clinical, laboratory and radiological variables. Major laboratory markers were collected at presentation, the day the COVID-19 swab was obtained (key data are presented in Table 7 ).
Table 7.
Clinical characteristics and laboratory findings of COVID-19–positive patients linked with mortality.
|
Characteristics |
All patients n = 113 |
Alive n = 84 |
Dead n = 29 |
OR (95% CI) |
p value |
| Median age (in years) | 66 (21–91) | 62 | 72 | 1.06 (1.02–1.09) | 0.00434∗ |
| Male | 63 | 43 | 20 | ||
| Female | 50 | 41 | 9 | ||
| Infiltrates on chest imaging |
47 |
28 |
19 |
6.78 (2.43–22.28) |
0.0005∗ |
|
Key laboratory findings |
Median (IQR) |
Median |
Median |
||
| Lymphocyte count (x 109 per L) | 0.74 (0–3.15) | 0.81 | 0.54 | 0.20 (0.05–0.66) | 0.0176∗ |
| Platelets (x 109 per L) | 193 (12–752) | 203 | 123 | 0.99 (0.98–0.99) | 0.0459∗ |
| C-reactive protein (CRP) | 102 (2–483) | 68 | 152 | 1.01 (1.00–1.01) | 0.00249∗ |
ORR = odds ratio; CI = confidence interval; COVID-19 = coronavirus disease 2019; IGR = interquartile range.
Twelve patients (median age 64 years) required admission to ICU for a median stay of eight days. Eight patients required invasive ventilation, but none required renal support. Most had a haematological malignancy (n = 8, 66.6%). Of the four patients admitted to ICU with a solid malignancy, the intent of SACT was curative in three patients and palliative in one. Mortality among those admitted to ICU was high, with six patients (50%) dying.
Of the 101 patients (median age: 72 years) admitted to the hospital, 29 (28.7%) died due to COVID-19 with site-specific diagnoses of haematological (11/29 patients, 37.9%), gastrointestinal (6/29, 20.6%), thoracic (4/29, 13.8%), urological (4/29, 13.8%), breast (2/29, 6.89%), gynaecological (1/29, 3.4%) and Central Nervous System (CNS) (1/29, 3.4%) malignancies.
On univariate analysis (Table 7), patients who died were significantly older (median: 72 versus 62 years, OR = 1.06, CI [1.02–1.08], p = 0.00434), more likely to have characteristic infiltrates on chest imaging (OR = 6.78, CI [2.43–22.28], p = 0.0005), had a lower lymphocyte count (median: 0.54 versus 0.81 × 109/L, OR = 0.20, CI [0.05–0.66], p = 0.0176), a lower platelet count (median: 123 versus 203, OR = 0.99, CI [0.98–0.99], p = 0.0459) and a higher C-reactive protein (CRP) (median: 152 versus 68, OR = 1.01, CI [1.00–1.01], p = 0.00249). There was no significant difference in terms of comorbidities or presenting symptoms between patients who died and those who survived. On multivariate analysis, the odds of death were only significantly increased with a lower platelet count (OR = 0.99, CI [0.98–0.99], p = 0.0395) and the presence of inflammatory infiltrates on chest imaging (OR = 13.1, CI [1.53–341.4], p = 0.0447), following adjustments for all the factors that were significant in univariate analysis.
Of 685 patients with negative swabs for SARS-CoV2, 49 had PCR-based evidence of a different viral infection. Thirty-seven had a positive bacterial blood culture, nineteen of whom had a positive culture from a central venous catheter, and the presumed diagnosis was line sepsis. For the remainder, there was no confirmed source of infection.
9. Outcomes in total at-risk population
Putting all the data into context, during the 2-month study period, only 867 of 13,489 (6.4%) patients met the criteria leading to swab testing. Of the total at-risk population, only 113 of 13,489 (0.84%) were swab positive, 101 of 13,489 (0.75%) required hospital admission and 29 of 13,489 (0.21%) died of COVID-19. Of the patients that attended the hospital to receive cytotoxic chemotherapy alone or in combination with other therapy, 59 of 2001 (2.9%) were admitted to the hospital for COVID-19–related issues and 20 of 2001 (1%) died. Of the patients that collected targeted treatments, 16 of 1126 (1.4%) were admitted and 1 of 1126 (0.1%) died. Of the patients on immunotherapy, only 4 of 325 were admitted (1.2%) and 1 of 325 died (0.3%). Of the eleven patients that received radiotherapy within six weeks of presentation, 6 of 1042 patients (0.6%) required inpatient admission and only 2 of 1042 (0.2%) died.
10. Discussion
Across the world, the COVID-19 pandemic has led to significant re-configuration of cancer care services with major disruption of standard-of-care treatments involving surgery, radiotherapy and SACT. In the UK, National Health Service (NHS) England released guidelines for the management of patients with cancer during the COVID-19 pandemic, warning about the possibility of cancer services being compromised due to a combination of factors including staff sickness and supply chain shortages [12]. To comply with the government's lockdown policy and with the goal of reducing exposure to and spread of COVID-19, many patients have seen their follow-up visits replaced by telephone consultations, scheduled investigations have been deferred or delayed and new or worsening symptoms are frequently being managed conservatively. For many, there have also been treatment delays, breaks or interruptions and, even when treatment has proceeded, it has frequently been modified from intravenous to subcutaneous or oral delivery with selection of regimens whose administration is of shorter duration and compatible with outpatient therapy. To implement urgent measures for the protection of subjects, recruitment to clinical trials has been halted [13]. Further prolongation of precautionary measures, while we await the development of effective anti-coronavirus therapies or vaccines, is likely to have profound adverse effects on patients with cancer.
Thus far, the evidence on the impact of COVID-19 on patients with cancer has been limited to reports from small case series drawn from areas worst hit in the early stages of the pandemic. Initial studies suggested that patients with pre-existing malignancies were more susceptible to infection with COVID-19 but also confirmed greater rates of severe events, including hospitalisation, mechanical ventilation and death [5,14,15]. Such studies have influenced the current situation in which both patients and their doctors have been fearful of proceeding with standard-of-care cancer therapies because of an uncertain risk of patients contracting COVID-19, especially of nosocomial origin, and suffering severe, adverse outcomes from such infections. More recent reports of larger cohorts of patients have aimed to characterise the outcomes of patients with cancer and COVID-19 and identify potential prognostic factors for mortality and severe illness but have focussed less so to address the issue of the incidence of COVID-19 in this population [9,11]. To make informed decisions, clinicians and patients need to understand the risks faced by the total population of patients with cancer, not just those unfortunate enough to contract COVID-19.
In this study, for the first time, we describe the incidence and outcomes of COVID-19 in a large population of patients with cancer receiving treatment at a single large centre (across three sites). To derive the ‘at-risk population', we chose the total population of patients with cancer who attended for face-to-face encounters at the hospital in the months of March and April 2020. By including only those patients who attended the hospital during this timeframe, rather than including those who had telemedicine-based follow-ups, we have avoided falsely underestimating the risk by including patients who had completely and adequately self-isolated or shielded during this time. For a large part of the analysis, we focused specifically on the on-treatment patients, looking at the type of treatment and its mode of administration, to assess the impact of different types of treatment on COVID-19–related outcomes.
As anticipated, our data show overall numbers of patients attending in March/April 2020 were markedly reduced (by 25%) when compared with the same period last year. The number of patients attending for surgical procedures, blood tests and imaging attendances was all greatly reduced (by 22–27%) and those receiving radiotherapy fell by 8%. These changes explain, in the main, the overall reduction in the number of patients attending. Interestingly, the year-to-year comparison revealed a modest increase in the number of people attending to receive or collect SACT. The increases of 8% and 6% in intravenous and oral SACT, respectively, probably reflect an overall increase in clinical activity within the hospital over the last year and most likely would have been even greater in the absence of the COVID-19 outbreak. In contrast to chemotherapy and/or targeted therapy, there was a 16% reduction in patients who received immunotherapy, most likely due to the fact that this treatment is usually given in the palliative setting and reflecting early concerns that immunomodulatory therapy would render patients more susceptible to COVID-19.
Despite concerns about the risks of continuing standard cancer therapies, our study suggests that the overall incidence of clinically significant COVID-19 amongst patients with cancer attending our hospital in March/April 2020 was no higher than that in the general population [13]. Crucially, during the height of the outbreak in London, administration of chemotherapy was associated with only a relatively modest risk of significant COVID-19 infection. Of the patients on cytotoxic chemotherapy alone, or in combination, and of the patients on targeted treatments, only 20 of 2001 (1%) and 1 of 1126 (0.1%), respectively, died of COVID-19. Of the patients on immunotherapy, only 1 of 325 died (0.3%). Of the patients on radiotherapy, only 2 of 1042 died (0.2%). The low admission rates to ICU can be explained by careful selection of patients that should be escalated to critical care. Patients on palliative chemotherapy with a life expectancy of shorter than 6 months were deemed as not appropriate candidates for ICU, and therefore, despite hospital admission and treatment, upon deterioration, the ceiling of care was ward based or in certain cases patients were discharged to a hospice.
It is important to recognise that this study cannot provide an accurate estimate of the true prevalence of COVID-19 in patients with cancer under our care. Many patients may have had asymptomatic infections or clinically apparent disease that did not meet the strict criteria for swab testing that were in operation in our centre during March/April 2020. Having said that, all RMH patients with suspected COVID-19, fulfilling testing criteria, were encouraged to attend the COVID-19 hub for testing. It is therefore unlikely that we missed substantial numbers of clinically significant cases. Ultimately, of course, accurate determination of the real COVID-19 disease burden in patients with cancer will only emerge from carefully conducted prospective studies based on serial sample collection for virus and anti-viral antibody testing.
In the absence of such data and based on this snapshot taken as the first wave of COVID-19 hit our practice, we conclude that continuation of active cancer treatment, even in the palliative setting, is appropriate. In the coming months, we encourage oncology centres to deliver the active anticancer therapies that are vital to prevent a rise in cancer morbidity and mortality, whilst maintaining the necessary precautions to prevent transmission of COVID-19 through the use of appropriate personal protective equipment, social distancing and rationalising hospital visits.
Funding
This study received funding from the Royal Marsden Hospital NHS Trust.
Conflict of interest statement
R.J. reports receiving grants/research support from MSD and GSK and consultation fees from Adaptimmune, Athenex, Blueprint, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immunedesign, Lilly, Merck, Pharmamar and UptoDate, outside the submitted work. S.S. reports receiving grants and personal fees from Roche, outside the submitted work. All other authors declare no competing interests.
References
- 1.Spicer J., Chamberlain C., Papa S. Provision of cancer care during the COVID-19 pandemic. Nat Rev Clin Oncol. 2020;17:329–331. doi: 10.1038/s41571-020-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams M., Le Calvez K., Mi E., Chen J., Dadhania S., Pakzad-Shahabi L. Estimating the risks from COVID-19 infection in adult chemotherapy patients. medRxiv. 2020 doi: 10.1101/2020.03.18.20038067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The UK National Institute of Health and Care Excellence . 2020. COVID-19 rapid guideline: delivery of systemic anticancer treatments. [PubMed] [Google Scholar]
- 4.ESMO . 2020. Cancer patient management during the COVID-19 Pandemic.https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic Available from: [Google Scholar]
- 5.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z. Patients with cancer appear more vulnerable to SARS-CoV-2: a multi-center study during the COVID-19 outbreak. Canc Discov. 2020 doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA J Am Med Assoc. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 8.Joharatnam-Hogan N., Hochhauser D., Shiu K.-K., Rush H., Crolley V., Butcher E. Outcomes of the 2019 Novel Coronavirus in patients with or without a history of cancer - a multi-centre North London experience. medRxiv. 2020 doi: 10.1101/2020.04.16.20061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee L.Y.W., Cazier J.B., Starkey T., Turnbull C.D., Kerr R., Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020 June 20;395 doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anil I., Arnold R., Benkwitz-Beford S., Branford S., Campton N., Cazier J.B. The UK Coronavirus Cancer Monitoring Project: protecting patients with cancer in the era of COVID-19. Lancet Oncol. 2020 May;21(5):622–624. doi: 10.1016/S1470-2045(20)30230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NHS England Clinical guide for the management of non- coronavirus patients requiring acute treatment. Cancer. 2020 https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-acute-treatment-cancer-23-march-2020.pdf Available from: [Google Scholar]
- 13.Cancer services in London during the covid-19 pandemic. 2020. https://www.england.nhs.uk/london/2020/03/27/cancer-services-in-london-during-the-covid-19-pandemic/ Available from: [Google Scholar]
- 14.Dai M.-Y., Liu D., Liu M., Zhou F.-X., Li G.-L., Chen Z. Patients with cancer appear more vulnerable to SARS-CoV-2: a multi-center study during the COVID-19 outbreak. SSRN Electron J. 2020 Jun;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang X., Yang C. Full spectrum of cancer patients in SARS-CoV-2 infection still being described. Clin Oncol. 2020 Jun;32(6):407. doi: 10.1016/j.clon.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]