The coronavirus disease 2019 (COVID-19) pandemic provided a unique opportunity to activate and mobilize a new approach to aligning and accelerating research activities across the Mayo Clinic enterprise. Just days after the state-wide lockdown in Minnesota, the executive deans of research and practice activated a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 Research Task Force led by medical, scientific, and administrative leaders from across Mayo Clinic. This task force was created even before molecular testing was offered to the communities in which Mayo Clinic operates.
The SARS-CoV-2/COVID-19 Research Task Force responded to vigorous efforts by the Mayo Clinic research community across the following domains:
-
•
Conducting basic laboratory research on SARS-CoV-2/COVID-19 as well as host responses
-
•
Developing a vaccine
-
•
Launching studies on the immune response and on how and whether certain immune responses protect against reinfection
-
•
Investigating the body’s innate response to COVID-19 and ways to block the inflammatory response that contributes to severity of the disease
-
•
Designing epidemiologic and artificial intelligence research to predict disease spread and aid in allocation of community resources, such as testing capabilities
-
•
Evaluating factors to help predict the risk of complications and disease severity
-
•
Engaging communities to understand the pandemic’s impact and community priorities for research
-
•
Adopting novel strategies to monitor patients with COVID-19 before and after they leave the hospital by using remote monitoring and wearable devices
-
•
Developing processes for reducing environmental risk, such as novel approaches to decontaminating personal protective equipment
-
•
Working with regulatory bodies to create new and nimble processes for study review, approval, and implementation
Besides the above, the SARS-CoV-2/COVID-19 Research Task Force was responsible for other challenges occurring in an accelerating research environment in the new SARS-CoV-2/COVID-19 field, including concerns that pockets of COVID-19 research could exist across Mayo Clinic that were not part of a unified plan or that duplicative clinical trials could be competing for the same patients. The task force leadership quickly established a structure with a design and culture that enabled a unique and very effective operational model within the rapidly evolving landscape of the COVID-19 pandemic, that is
-
•
An overall task force structure with a physician-scientist chair and an administrative co-chair
-
•
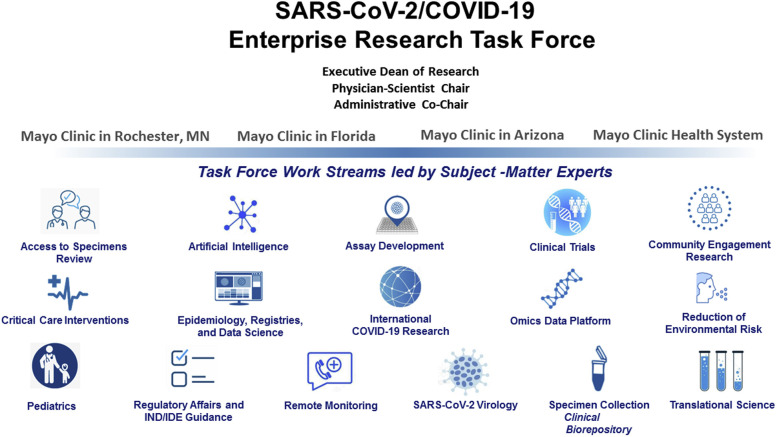
Establishment of 16 function-specific work streams, aligned to strategies of the SARS-CoV-2/COVID-19 Research Task Force and led by experts in those areas (eg, clinical trials, data science, artificial intelligence, pediatrics, and community-engaged research) (Figure )
-
•
Steering groups at each major site (Arizona, Florida, Rochester, Mayo Clinic Health System) to ensure that site-specific needs were met and that opportunities for research can be quickly shared from one campus to another
-
•
An overall culture of trust, autonomy, rapid communication, and nearly immediate access to thought leaders and scientific experts
Figure.
SARSCoV-2/COVID-19 Research Task Force Operational Structure. (Used with permission from the Mayo Foundation for Medical Education and Research.) COVID-19 = coronavirus disease 2019; IDE = investigational device exemption; IND = investigational new drug; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Study Selection Process
As the task force was launching and establishing its structure, the urgency was underscored by the sheer magnitude of patient lives at stake. At the time of task force development, no current COVID-19 treatments were shown to improve health and save lives; therefore, activating interventional trials became a top priority. The SARS-CoV-2/COVID-19 Research Task Force examined the safety and efficacy of very strategically selected experimental therapies that were not yet approved by the US Food and Drug Administration (FDA).
Hundreds of clinical trials related to COVID-19 are registered on ClinicalTrials.gov from a broad range of subject areas, for example, basic science investigating the immunology and virology of SARS-CoV-2, early vaccine development, registries, health disparities research, epidemiologic studies, chemoprophylaxis, and numerous treatment trials. The investigational treatments span a spectrum of existing antiviral agents, antimicrobial agents, convalescent plasma, and immune-modulatory and cellular therapies. Within this landscape, Mayo Clinic took a specific, values-driven, scientific approach to therapies included in their investigational portfolio. The therapies needed to show an acceptable human safety profile; biologic plausibility; in vitro anti–SARS-CoV-2 activity, where appropriate; a sufficient, secure drug supply that was compliant with FDA Good Manufacturing Practice; a funding source that covered costs; and an investigational new drug approval from the FDA.
Leveraging Site Leaders and Mayo Clinic’s Three Shields
In addition to the scientific rigor invoked during this crisis, Mayo Clinic called upon its rich heritage of values-driven care and teamwork to support the needs of patients. This culture spans all Mayo Clinic locations and all three shields: practice, research, and education. Key experienced clinical investigators serve as leads for the practice at each Mayo Clinic campus. The clinical leaders provide support for the practice shield with guidance on off-label drug use and available trials. The education shield ensured that trainees rotated through the inpatient COVID-19 clinical services in a virtual manner, which minimized their contact with infected patients while giving them unique experiences in the operation of clinical trials and in treating COVID-19 patients as discoveries were unfolding. The physician leaders served the research shield by reviewing all research proposals, with a sharp focus on evaluating clinical interventional trials to determine appropriateness for Mayo Clinic. Clinical trials that were applicable across Mayo Clinic’s diverse geographic sites were sought, and trials with the highest scientific merit and feasibility were prioritized for review by the Mayo Clinic Institutional Review Board. Trials that were of lesser merit, duplicative, or otherwise inappropriate for Mayo Clinic were not pursued. Management of the COVID-19 research portfolio at sites outside of Rochester required some flexibility in tailoring trials to different populations and differences in regional practice and disease incidence.
Patient Selection
After the treatment trials began, Mayo Clinic initiated a new process to evaluate hospitalized patients real-time for eligibility in one or more clinical trials, which was a departure from the normal process of clinical trial enrollment. We created the approach, which is consensus-driven, because we recognized that, at the onset, no one had experience in treating COVID-19. Therefore, a consensus-driven approach would maximize the equipoise around treatment recommendations. Treatment review teams evaluated each hospitalized patient once or twice daily to determine trial eligibility, decide on appropriateness of the treatment, recommend investigative therapies, and monitor outcomes. Recommendations were shared and discussed with primary treating physicians and a decision was made regarding if and when a drug trial would be offered to each patient. This model allowed a small group to gain experience in treating and managing COVID-19, which was leveraged for the benefit of subsequent patients. As with any new process, challenges were encountered and lessons were learned.
Regulatory Management
Therapeutics showed the most promise early on, and we aligned trials to sites based on drug availability and patient need, then mobilized operational teams to create accelerated pathways to approval. Research leaders identified staff with deep expertise in clinical trials to serve as project managers along with a dedicated team to shepherd clinical trials through the regulatory and approval processes. The team spanned Code & Coverage Analysis, the Office of Sponsored Projects Administration, Legal Contract Administration, Office of Research Regulatory Support, Office of Research Finance, and the Mayo Clinic Institutional Review Board, as well as important ancillary services such as research pharmacy, biospecimen accessioning and processing, budgets and contracts, and data collection.
Bidirectional communication channels were created, reviewed, and iteratively improved to maintain information flow among principal investigators for the individual trials, the treatment review group, and the treating physicians. The treatment review teams, described above in Patient Selection, are an example of this type of bidirectional communication. Besides team evaluations and recommendations, patients and providers have opportunities to ask for clarifications or updates, and during hospitalization, the patient’s course is monitored and discussed daily with the primary care teams. This approach has enabled timely and transparent discussions to address any trial-related issues, ultimately for the betterment of patient care.
Conclusion
With a rich history and breadth of research excellence, Mayo Clinic was well positioned to respond to the COVID-19 pandemic and leveraged its deep resources across multiple disciplines to create an effective, efficient SARS-CoV-2/COVID-19 Research Task Force that spanned all research domains and all Mayo Clinic sites.
Activating the SARS-CoV-2/COVID-19 Research Task Force enabled resources and expertise to be assembled with common objectives, an understood urgency, and a collective mindset of calling upon others within the organization when needed to expedite action and outcomes. As a result, Mayo Clinic has been able to rapidly open therapeutic trials to patients in need. Other research domains within the task force have shown similar efficiencies and impact on Mayo Clinic’s response to the COVID-19 pandemic.
The Mayo Clinic SARS-CoV-2/COVID-19 Research Task Force has enabled scientific, patient care, and administrative thought leaders to accelerate discovery, translation, and application focused on the unmet needs of COVID-19 patients.
Acknowledgment
Editing and proofreading were provided by Scientific Publications, Mayo Clinic.
Footnotes
This supplement is sponsored by Mayo Clinic Foundation for Medical Education and Research and is authored by experts from multiple Departments and Divisions at Mayo Clinic.
Potential Conflicts of Interest: The authors report no potential competing interests.