Abstract
Background and Purpose
Determinants for molecular and structural instability, that is, impending growth or rupture, of intracranial aneurysms (IAs) remain uncertain. To elucidate this, we endeavored to estimate the actual turnover rates of the main molecular constituent in human IA (collagen) on the basis of radiocarbon (14C) birth dating in relation to IA hemodynamics.
Methods
Collagen turnover rates in excised human IA samples were calculated using mathematical modeling of 14C birth dating data of collagen in relation to risk factors and histological markers for collagen maturity/turnover in selected IA. Hemodynamics were simulated using image-based computational fluid dynamics. Correlation, logistic regression, and receiver operating characteristic analyses were performed.
Results
Collagen turnover rates were estimated in 46 IA (43 patients); computational fluid dynamics could be performed in 20 IA (20 patients). The mean collagen turnover rate (γ) constituted 126% (±1% error) per year. For patients with arterial hypertension, γ was greater than 2600% annually, whereas γ was distinctly lower with 32% (±1% error) per year for patients without risk factors, such as smoking and hypertension. There was a distinct association between histological presence of rather immature collagen in human IA and the presence of modifiable risk factors. Spatial-temporal averaged wall shear stress predicted rapid collagen turnover (odds ratio, 1.6 [95% CI, 1.0–2.7]). Receiver operating characteristic analysis demonstrated a good test accuracy (area under the curve, 0.798 [95% CI, 0.598–0.998]) for average wall shear stress with a threshold ≥4.9 Pa for rapid collagen turnover.
Conclusion
Our data indicate that turnover rates and stability of collagen in human IA are strongly associated with the presence of modifiable risk factors and aneurysmal hemodynamics. These findings underline the importance of strict risk factor modification in patients with unruptured IA. Future should include more detailed risk factor data to establish a more causal understanding of hemodynamics and the rupture risk of individual IA.
Keywords: intracranial aneurysms, hemodynamics, birth dating, collagen turnover, wall shear stress
Introduction
Unruptured, saccular intracranial aneurysms (IAs) can remain stable for long periods, undergo structural change such as growth or remodeling within the aneurysm wall, or even rupture, resulting in subarachnoid hemorrhage [1]. The assessment of the structural stability/risk of rupture of IA remains a major clinical challenge. Aberrant hemodynamics play an important role in IA pathogenesis and are increasingly studied using computational fluid dynamics (CFD) [2]. However, CFD simulations are rarely associated with actual biological data derived from human IA.
We previously conducted radiocarbon (14C) birth dating, an ex vivo method, of the main molecular constituent in human IA (collagen type I) to estimate its age, as an indicator for the developmental chronology [3]. 14C birth dating uses atmospheric 14C concentrations with its sharp increase between 1950 and 1963 (nuclear bomb tests) and nearly exponential decrease (Nuclear Ban Treaty 1963) as a molecular time stamp [4]. We found that collagen age in human IA is generally young (<5 years), which might reflect ongoing collagen turnover [3]. In this study, we endeavored to quantify actual collagen turnover rates in IA using mathematical modeling on the basis of 14C birth dating and investigate its relation to collagen histology, risk factors for IA rupture, and aneurysmal hemodynamics.
Methods
The authors declare that all supporting data are available within the article. The Ethics Committees of the Medical Faculty of the University Düsseldorf, Germany (ID 3365), Medical Faculty Mannheim, Germany (ID 2016–592 N-MA), St. Michael’s Hospital, Toronto, Canada (ID 09–309), and Lawrence Livermore National Laboratory, Livermore, CA (ID 10–108), approved the underlying study.
Collagen turnover rates were calculated by a mathematical model based on F14C values from 14C birth dating of collagen in IA samples derived from patients undergoing surgical repair for IA. Aneurysmal hemodynamics were calculated by CFD using segmented 3-dimensional computed tomography angiographic data from patients before surgery. Risk factors for IA instability, such as arterial hypertension, current smoking, IA irregularity, size, and site, were assessed. For visualization of collagen maturity, linear-polarized microscopy was performed in cryosections of IA samples stained with Picrosirius Red. Correlation, logistic regression, and receiver operating characteristic analyses were performed to identify associations between collagen turnover, risk factors, and hemodynamics.
For details, see the Data Supplement, including Table I in the Data Supplement.
Results
F14C values of 46 IA samples (10 unruptured and 36 ruptured) from 43 patients were used for calculation of collagen turnover rates. Computed tomography angiography data sufficient for CFD modeling were available in 20 patients with 20 IA (6 unruptured and 14 ruptured; Table II in the Data Supplement).
Collagen Turnover in Association With Risk Factors
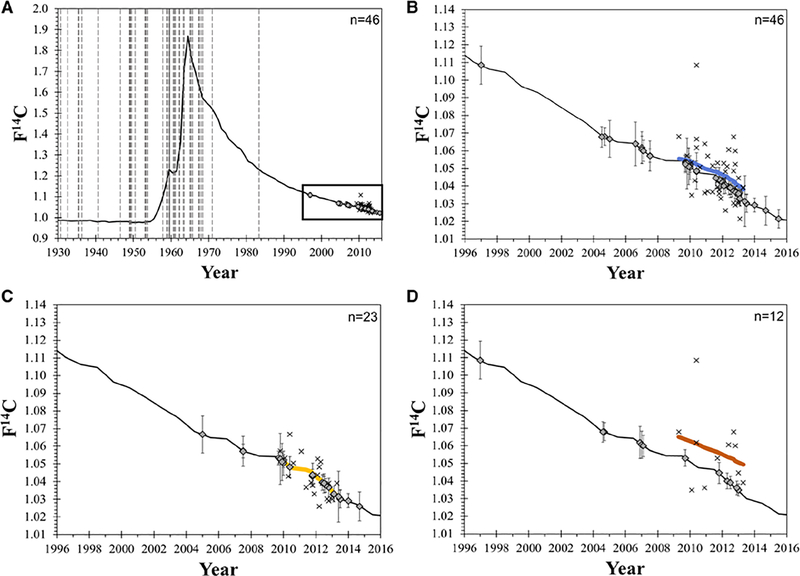
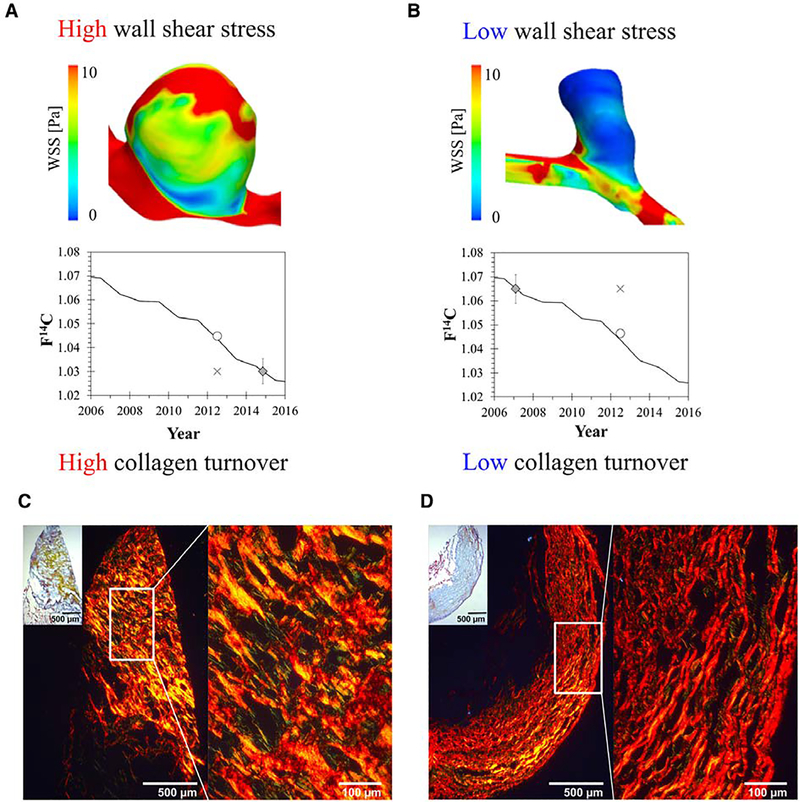
The mean collagen turnover rate (γ) constituted 126% (±1% error) per year for all 46 IA, which corresponded to a mean collagen age of 9.3 months (Table; Figure 1; Table III in the Data Supplement). The mean collagen turnover rate in IA of patients with risk factors (smoking or hypertension) constituted 1410% (±1% error) per year (corresponding mean collagen age of 3 weeks), compared with 32% (±1% error) per year (corresponding mean collagen age of 3.1 years) in patients without risk factors (smoking or hypertension). For patients with arterial hypertension only, the model predicted F14C values were equal to or lower than the actual atmospheric F14C values resulting in a mean collagen turnover rate >2600% per year and a mean collagen age <2 weeks (Figure 1). IA collagen turnover in irregular-shaped, ruptured IA was significantly higher compared with irregular-shaped, unruptured IA (940±960% versus 84±38%; P=0.002). Histological stainings of selected IA samples illustrated the presence of rather unstructured/immature collagen in unruptured IA of patients with risk factors, whereas structured/mature collagen was rather found in unruptured IA of patients without risk factors (Figure 2).
Table.
γ With Corresponding Mean Collagen Age and Individual γ of 46 Intracranial Aneurysm Samples
| γ (Percentage per y; ±%Error) | Mean Collagen Age | Individual γ (Percentage per y; ±SD) | P Value | ||
|---|---|---|---|---|---|
| All | 126 (±1) | 9.3 mo | |||
| Sex | Women | 114 (±1) | 10.3 mo | 970 (±970) | NS |
| Men | 169 (±1) | 6.9 mo | 850 (±930) | NS | |
| Rupture Status | Yes | 132 (±1) | 8.9 mo | 1020 (±970) | NS |
| No | 108 (±1) | 10.8 mo | 590 (±830) | NS | |
| Arterial hypertension or smoking | Yes | 1410 (±1) | 19 d | 1090 (±950) | 0.004 |
| No | 32 (±1) | 3.1 y | 470 (±810) | 0.004 | |
| Arterial hypertension | Yes | > 2600 | <2 wk | 1100 (±960) | 0.04 |
| No | 55 (±1) | 1.8 y | 750 (±920) | 0.04 | |
| Smoking | Yes | 233 (±1) | 4.9 mo | 990 (±960) | NS |
| No | 92 (±1) | 1.1 y | 890 (±950) | NS | |
| Irregularity | Yes | 135 (±1) | 8.6 mo | 850 (±950) | NS |
| No | 123 (±1) | 9.6 mo | 980 (±960) | NS |
γ indicates mean collagen turnover rate; and NS, nonsignificant.
Figure 1.
Collagen turnover in relation to risk factors in intracranial aneurysm (IA) patients. (A) The birth dates (vertical lines) of the IA patients, F14C values (diamonds), and excision dates (×) of the IA samples are projected onto the atmospheric 14C curve. (B-D) The atmospheric 14C curve is enlarged from 1996 to 2016 showing the estimated collagen turnover rate as a colored line. (B) The gray line indicates the collagen turnover rate for all IA samples. (C) The estimated collagen turnover rate in IA of patients with arterial hypertension only is nearly contemporary (on top of the curve), while (D) the turnover rate in IA of patients without risk factors such as smoking and hypertension is older (above the curve).
Figure 2.
Hemodynamics, collagen turnover, and collagen maturity in ruptured or unruptured intracranial aneurysm (IA). A and B, Association between aneurysmal time-averaged wall shear stress (WSS; top) and corresponding collagen turnover rate (bottom) is illustrated in 2 ruptured IA samples: (A) an IA of the internal carotid artery with high WSS (10.4 Pa) and high collagen turnover (≥2000% per y) is shown; (B) an IA of the anterior cerebral artery with low WSS (1.4 Pa) and low collagen turnover (20% per y) is depicted. C and D, Movat pentachrome (small inset, with x50 magnification) for overview and corresponding Picrosirius Red staining for visualization of collagen I maturity in 2 representative unruptured IA samples: in each panel, 8-μm cryosections, stained in Picrosirius Red, are visualized by polarized microscopy (left: overview with x50 magnification; right: x200 magnification). C, Rather unstructured, immature collagen is illustrated in an unruptured IA patient with arterial hypertension and smoking, whereas D highlights rather structured, mature collagen in an unruptured IA patient without risk factors. ○ indicates F14C predicted value; ◊, F14C aneurysm sample; ×, F14C aneurysm excision date.
Association of Collagen Turnover With Hemodynamics
There was a distinct correlation between aneurysmal time averaged wall shear stress (WSS) and collagen turnover rate (r=0.57, P=0.009; Figure 2). The relation of hemodynamic parameters and risk factors is highlighted in Table IV in the Data Supplement). Average WSS was a significant predictor for rapid collagen turnover (likelihood ratio, 5.55; P=0.02; odds ratio, 1.6 [95% CI, 1.0–2.7]). Receiver operating characteristic analysis demonstrated a good test accuracy (area under the curve, 0.798 [95% CI, 0.598– 0.998]) for average WSS with a threshold ≥4.9 Pa for rapid collagen turnover (sensitivity, 72.7%; specificity, 77.8%; Figure I in the Data Supplement).
Discussion
This study found that actual collagen turnover rates and the histological presence of rather immature collagen in human IA are strongly associated with the presence of modifiable risk factors and aneurysm morphology and hemodynamics according to CFD.
Previous studies on human connective tissues (skin, cartilage, intervertebral disc) reported generally low collagen type I turnover rates (≈1% per year), but there are no data on collagen turnover rates in human IA [5]. One study on 14C birth dating of collagen type I in healthy tendons reported no turnover in adulthood and continuous, slow turnover due to newly synthesized collagen in tendinopathic samples (collagen age about 15 years) [6]. Further, only few studies associated hemodynamics simulated by CFD with biological data from human IA, and the clinical value of CFD remains controversial [7,8]: one study on hemodynamics in 65 human IA reported that high WSS was related to local thinning of IA walls and low WSS to hyperplastic IA regions [8]. Another study demonstrated a pathological collagen fiber appearance in IA in areas of high WSS by using 3-dimensional vascular models for mapping of local hemodynamics based on excised IA samples [9]. Thus, our study is the first to provide estimates for collagen turnover in human IA in relation to CFD.
There are different explanations for our findings: most likely, the presence of risk factors and aberrant hemodynamics results in increased collagen synthesis and rapid degradation, that is, rapid turnover, which leads to more immature collagen and less cross-linking and potentially structural instability in IA [10,11]. This is supported by previous studies and our own preliminary findings of rather immature collagen in unruptured human IA samples from patients with risk factors [9]. Importantly, our data are exclusive for human IA and cannot be applied to intracranial arteries, since these do not comprise meaningful collagen type I due to the absence of an adventitia [1,4].
Alternatively, it is also likely that there are different populations of IA, according to their molecular structure, for example, degenerative or atherosclerotic aneurysms (rather mature collagen) versus thin-walled aneurysms (rather immature and less stable collagen) or IA that occur on the basis of congenital structural defects or predisposed vulnerability of the vasculature (e.g., in bicuspid aortic valve or aortic coarctation) [2,12]. However, for IA, such associations have never been studied, and further research for these scarce subpopulations of IA patients is required.
Our study holds limitations: first, our 14C birth dating analyses comprised the entire aneurysm dome, and we could not study focal hemodynamic forces with focal collagen turnover in IA, since 14C birth dating requires IA tissue at least 3 mm in diameter. Additionally, the IA tissue required for reliable 14C birth dating prohibited concomitant histological analyses for the majority of samples. Second, the mathematical model for estimation of γ assumed collagen conservation from birth and did not include the possibility of spontaneous growth. Third, the number of preoperative computed tomography angiography data for CFD modeling in relation to F14C analyses were limited, but large-scale 14C birth dating of human IA is rather challenging. Finally, due to the cross-sectional nature of our study, we could not relate the F14C and CFD analyses, for example, with actual mean blood pressure values, number of cigarettes, serum lipids, and hemoglobin A1c levels 12 months before IA sampling to understand whether our findings of F14C and CFD are causal simple associations.
In summary, our data indicate rapid collagen turnover and histologically rather immature collagen or structurally deficient IA wall composition in IA of patients who have raised blood pressure or currently smoke. These findings underline the increasing body of data on the importance of strict risk factor modification in patients with IA [13,14]. Average WSS is associated with rapid collagen turnover in IA, but further studies, especially with increasing computing power/ artificial intelligence, should investigate F14C and CFD analyses in relation to numerical risk factor data and histological analyses to establish a more causal understanding on the rupture risk of individual IA.
Supplementary Material
Acknowledgments
We thank the Medical Research Center, Mannheim, Germany, for provision of the Leica Microscope.
Sources of Funding
Drs. Buchholz and Navid were supported by NIH NIGMS P41GM103483 and LLNL LDRD 18-LW-037. Dr Siddiqui, Meng, and H. Rajabzadeh-Oghaz were supported by NIH R01NS091075.
Footnotes
Disclosures
This work was performed, in part, under the auspices of the US Department of Energy by Lawrence National Laboratory under contract DE-AC52-07NA27344; reviewed and released as LLNLJRNL-787658. The authors report no conflicts of interests related to the topic of the present study.
References
- 1.Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. 2016;12:699–713. doi: 10.1038/nrneurol.2016.150 [DOI] [PubMed] [Google Scholar]
- 2.Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol 2014;35:1254–1262. doi: 10.3174/ajnr.A3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etminan N, Dreier R, Buchholz BA, Beseoglu K, Bruckner P, Matzenauer C, et al. Age of collagen in intracranial saccular aneurysms. Stroke. 2014;45:1757–1763. doi: 10.1161/STROKEAHA.114.005461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028 [DOI] [PubMed] [Google Scholar]
- 5.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200 [DOI] [PubMed] [Google Scholar]
- 6.Heinemeier KM, Schjerling P, Øhlenschl.ger TF, Eismark C,Olsen J, Kj.r M. Carbon-14 bomb pulse dating shows that tendinopathy is preceded by years of abnormally high collagen turnover. FASEB J. 2018;32:4763–4775. doi: 10.1096/fj.201701569R [DOI] [PubMed] [Google Scholar]
- 7.Cebral J, Ollikainen E, Chung BJ, Mut F, Sippola V, Jahromi BR, et al. Flow conditions in the intracranial aneurysm lumen are associated with inflammation and degenerative changes of the aneurysm wall. AJNR Am J Neuroradiol. 2017;38:119–126. doi: 10.3174/ajnr.A4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cebral JR, Detmer F, Chung BJ, Choque-Velasquez J, Rezai B, Lehto H, et al. Local hemodynamic conditions associated with focal changes in the intracranial aneurysm wall. AJNR Am J Neuroradiol. 2019;40: 510–516. doi: 10.3174/ajnr.A5970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cebral JR, Duan X, Gade PS, Chung BJ, Mut F, Aziz K, et al. Regional mapping of flow and wall characteristics of intracranial aneurysms. Ann Biomed Eng. 2016;44:3553–3567. doi: 10.1007/s10439-016-1682-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frösen J, Cebral J, Robertson AM, Aoki T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus. 2019;47:E21. doi: 10.3171/2019.5.FOCUS19234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3 pt 2):581–587. doi: 10.1161/hy09t1.096249 [DOI] [PubMed] [Google Scholar]
- 12.Wågsäter D, Paloschi V, Hanemaaijer R, Hultenby K, Bank RA, Franco-Cereceda A, et al. Impaired collagen biosynthesis and crosslinking in aorta of patients with bicuspid aortic valve. J Am Heart Assoc. 2013;2:e000034. doi: 10.1161/JAHA.112.000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korja M, Lehto H, Juvela S, Kaprio J. Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology. 2016;87:1118–1123. doi: 10.1212/WNL.0000000000003091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76:588–597. doi: 10.1001/jamaneurol.2019.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.