Highlights
-
•
COVID-19 is a global pandemic that has overwhelmed health care systems around the world.
-
•
SARS-CoV-2 can affect both the central and peripheral nervous systems.
-
•
Arterial and venous ischemic strokes can occur in COVID-19 patients.
-
•
Strokes in COVID-19 patients can occur while on therapeutic dose of anticoagulation.
-
•
Elevated D-dimer and inflammatory markers are a possible cause of strokes in COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, Stroke, Cerebral venous thrombosis
Abstract
Background and purpose
Coronavirus disease 2019 (COVID-19) is a global pandemic that causes flu-like symptoms. There is a growing body of evidence suggesting that both the central and peripheral nervous systems can be affected by SARS-CoV-2, including stroke. We present three cases of arterial ischemic strokes and one venous infarction from a cerebral venous sinus thrombosis in the setting of COVID-19 infection who otherwise had low risk factors for stroke.
Methods
We retrospectively reviewed patients presenting to a large tertiary care academic US hospital with stroke and who tested positive for COVID-19. Medical records were reviewed for demographics, imaging results and lab findings.
Results
There were 3 cases of arterial ischemic strokes and 1 case of venous stroke: 3 males and 1 female. The mean age was 55 (48–70) years. All arterial strokes presented with large vessel occlusions and had mechanical thrombectomy performed. Two cases presented with stroke despite being on full anticoagulation.
Conclusions
It is important to recognize the neurological manifestations of COVID-19, especially ischemic stroke, either arterial or venous in nature. Hypercoagulability and the cytokine surge are perhaps the cause of ischemic stroke in these patients. Further studies are needed to understand the role of anticoagulation in these patients.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a global pandemic that causes flu-like symptoms. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) primarily affects the respiratory system leading to acute respiratory distress syndrome (ARDS), intubation and mechanical ventilation. Multi-organ failure and hypercoagulable states have also been observed in COVID-19 patients [1], [2], [3], [4]. There is a growing body of evidence suggesting that both the central and peripheral nervous systems can be affected by SARS-CoV-2 [5], [6]. We present three cases of arterial ischemic strokes and one venous infarction from a cerebral venous sinus thrombosis in the setting of COVID-19 infection who otherwise had low risk factors for stroke.
2. Methods
We retrospectively reviewed patients presenting to a large tertiary care academic US hospital with stroke and who tested positive for COVID-19. SARS-CoV-2 infection was confirmed in all patients by detection of viral nucleic acid in a nasopharyngeal swab, using the reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay. Medical records were reviewed for demographics, imaging results and lab findings.
2.1. Cases
2.1.1. Case 1
A 51-year-old male with history of hypertension (HTN), coronary artery disease (CAD), and hyperlipidemia (HLD) was admitted to an outside hospital (OSH) with progressive shortness of breath and cough for four days. He was confirmed COVID-19 positive and required 6 L nasal cannula oxygen. In accordance to the OSH COVID-19 treatment policy, the patient was started on therapeutic dose enoxaparin (1 mg/kg) upon admission. On hospital day 2, he was found to be hemiplegic on the left side with an NIHSS of 20. The patient did not receive IV tPA given he was on therapeutic enoxaparin. CTA head and neck demonstrated a tandem occlusion: acute thrombus in the right internal carotid artery (ICA) from its origin and an M1 occlusion. He was transferred to our hospital for endovascular intervention. Shortly after transfer, the patient developed worsening hypoxia and required mechanical intubation while in the angiography suite. He underwent mechanical thrombectomy (TICI 0 to 2B) with five stent placements to the right ICA. He was loaded with aspirin and clopidogrel and therapeutic enoxaparin was discontinued.
Post stroke day 1, a repeat CT head in the neurocritical care unit (NICU) showed a large right middle cerebral artery (MCA) territory stroke (Fig. 1 ). Table 1 details pertinent laboratory studies. Laboratory testing was significant for the presence of anticardiolipin IgA antibodies, anti-B2-glycoprotein IgA and IgG antibodies. Unfortunately, the patient had progressive hypotension requiring multiple vasopressors and worsening hypoxia. The patient’s family ultimately decided to withdraw life sustaining treatment and the patient died on hospital day four.
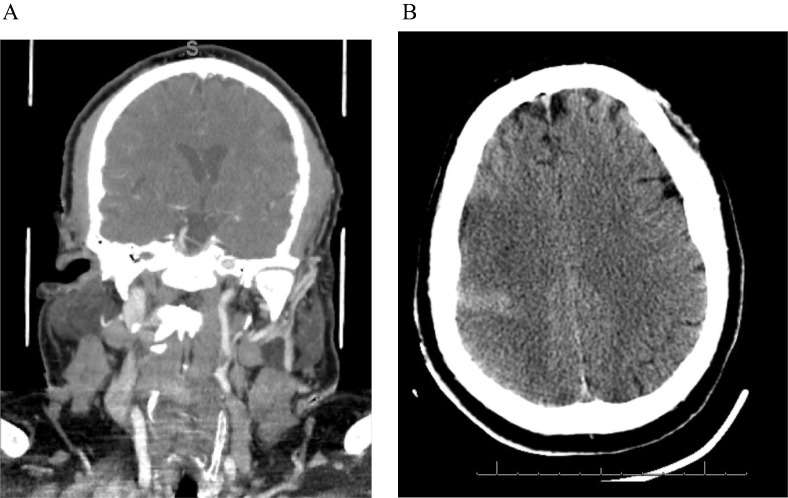
Fig. 1.
51 year old male with R MCA stroke A. CT Angiogram demonstrating R ICA occlusion. B. Non-contrast CT Head demonstrating developing R MCA stroke.
Table 1.
Baseline Characteristics.
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Demographics characteristics | ||||
| Age (years) | 51 | 70 | 54 | 48 |
| Gender | M | F | M | M |
| Initial Findings | ||||
| Medical History | HTN, HLD, CAD | No PMH | HTN | HLD |
| Respiratory Symptoms | Fever, cough, myalgias, dyspnea | Fever, cough, hypoxia | Shortness of breath, cough, hypoxia | None |
| Neurological Symptoms | L hemiplegia | L hemiplegia | Coma | Aphasia, R hemiplegia |
| Admission Chest X-ray Findings | Diffuse bilateral airspace opacities | B/L consolidations and ground glass opacities | B/L patchy airspace opacities and left lower lobe consolidation | Normal lung fields bilaterally |
| Days from disease onset to thrombotic event | 5 | 3 | 11 | 1 |
| Findings on ICU Admission | ||||
| Disease Severity | Critical | Critical | Critical | Moderate |
| Laboratory findings | ||||
| White Cell count (per mm3) | 5.8 | 17.7 | 14.6 | 10.3 |
| Platelet count (per mm3) | 273 | 483 | 372 | 237 |
| Hemoglobin (g/L) | 11.51 | 12 | 14.4 | 13.3 |
| Prothrombin time (s) | 15.7 | 14.6 | 11.8 | 12.2 |
| Activated partial thromboplastin (s) | 36 | 43 | 25 | 29 |
| Fibrinogen (g/L) | 719 | 970 | 429 | 243 |
| Fibrin degradation products (mg/L) | >20 | >20 | Not obtained | Not obtained |
| D-dimer (mg/L) | 2,476 | 11,559 | 7,873 | 6383 |
| Serum ferritin (μg/L) | 1,085 | 3500 | 508 | 270 |
| Procalcitonin (ng/ml) | 6.23 | 0.26 | 0.09 | 0.05 |
| High-sensitivity C-reactive protein (mg/L) | 21.60 | 39.90 | 3.90 | 0.30 |
| Lupus Anticoagulant (s) | ||||
| dRVVT Screen | 60.8 | 54.5 | 61.4 | 31 |
| dRVVT Mix | 42.5 | 46.2 | 45.4 | NA |
| dRVVT Confirm | 37.1 | 33.2 | 37.1 | 25.6 |
| dRVVT Normalized Ratio | 1.3 | 1.4 | 1.4 | 1.2 |
| Interleukin 6 | 185.33 | 458.41 | 24.53 | 9.6 |
| Glycated Hemoglobin (%) | 7% | 5.5 | 6.8 | 5.4 |
| Low-density lipoprotein (mg/dL) | <40 | 63 | 69 | 166 |
| Stroke Characteristics | ||||
| NIHSS | 20 | 28 | NA | 31 |
| CT Head on hospital day 2 | Large right MCA infarct in temporal, posterior frontal and parietal lobes | Large right MCA and ACA infarct | Bilateral thalamic and basal ganglia infarcts with hydrocephalus and cerebral edema | Mild attenuation of L insular ribbon |
| Vessel Imaging (CTA, CTV) | R ICA occlusion | R M2 occlusion | filling defects in the vein of Galen, straight sinus, bilateral internal cerebral veins and right basal vein of Rosenthal | L M1 occlusion |
| Treatment | Mechanical Thrombectomy | Mechanical Thrombectomy, hemicraniectomy | EVD, hypertonic therapy | TPA, Mechanical Thrombectomy |
| TICI Score | 0 to 2B | 0 to 2A | NA | 0 to 3 |
M = Male, F = Female, MCA = Middle cerebral artery, ACA = Anterior cerebral artery.
2.1.2. Case 2
A 70-year-old female with no significant past medial history was admitted to an OSH for shortness of breath (SOB), fever, hypoxia and new onset atrial fibrillation (A-Fib). She was confirmed to be COVID-19 positive and started on hydroxychloroquine and azithromycin. Her hypoxia was treated with non-rebreather mask. Per the OSH policy, she was initiated on therapeutic enoxaparin (1 mg/kg) for the new onset A-Fib. On hospital day 3, she was found to be altered with acute left hemiparesis and facial droop with an NIHSS of 28. The patient did not receive IV tPA due to therapeutic enoxaparin. CTA head and neck demonstrated an acute right M2 occlusion. She was transferred to our hospital and underwent a successful thrombectomy (TICI 0 to 2A). While in the interventional suite, she became increasingly hypoxic and required intubation and mechanical ventilation. Table 1 details pertinent laboratory studies. Patient was treated with prone positioning for ARDS.
CT head was obtained post stroke day 1 demonstrating a hemispheric right MCA stroke with mass effect and right to left herniation (Fig. 2 ). Despite a decompressive hemicraniectomy and hypertonic therapy, the patient’s neurological exam continued to decline and the family ultimately decided to withdraw life sustaining treatment.
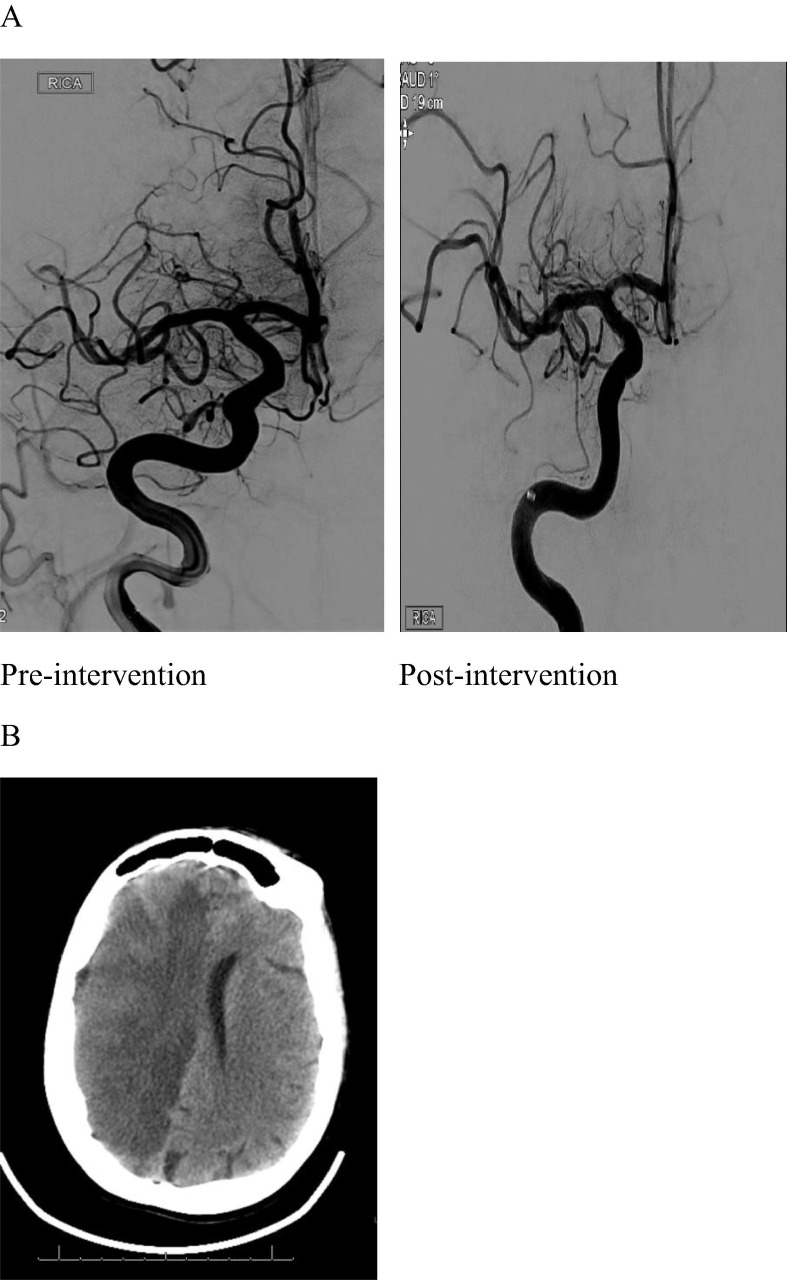
Fig. 2.
70 year old female with R MCA stroke. A. Angiogram pre-intervention demonstrating lack of flow consistent with a right M2 occlusion and post-intervention showing recanalization. B. Non-contrast CT Head demonstrating large hemispheric R MCA stroke with development of midline shift and cerebral edema.
2.1.3. Case 3
A 54-year-old male with history of HTN was admitted to an OSH with SOB and cough 10 days after testing positive for COVID-19 infection. Over the next 24 h, he complained of worst headache of his life and had progressively worsening mental status into coma. MRI brain showed diffuse T2 hyperintensities in the bilateral thalami and basal ganglia, 4 mm right to left shift, cerebral edema, and hydrocephalus with transependymal flow (Fig. 3 ). MRI brain and CT venogram demonstrated filling defects in the vein of Galen, straight sinus, bilateral internal cerebral veins and right basal vein of Rosenthal consistent with thrombosis. Patient was transferred to our facility and immediately had an external ventricular drain (EVD) placed along with hypertonic therapy. After which, the patient was started immediately on a heparin drip for cerebral venous sinus thrombosis. Patient’s pertinent labs show elevated D-dimer and other inflammatory markers (Table 1). CSF analysis showed RBC 401, WBC 10, glucose 38, and protein 1104 and his CSF SARS-CoV-2 RNA PCR was negative twice. Despite aggressive EVD drainage and medical management of intracranial pressures (ICP), the patient continued to have refractory ICPs. There was no evidence for meningitis or encephalitis. The patient’s neurological exam continued to decline and the family ultimately decided to withdraw life sustaining treatment.
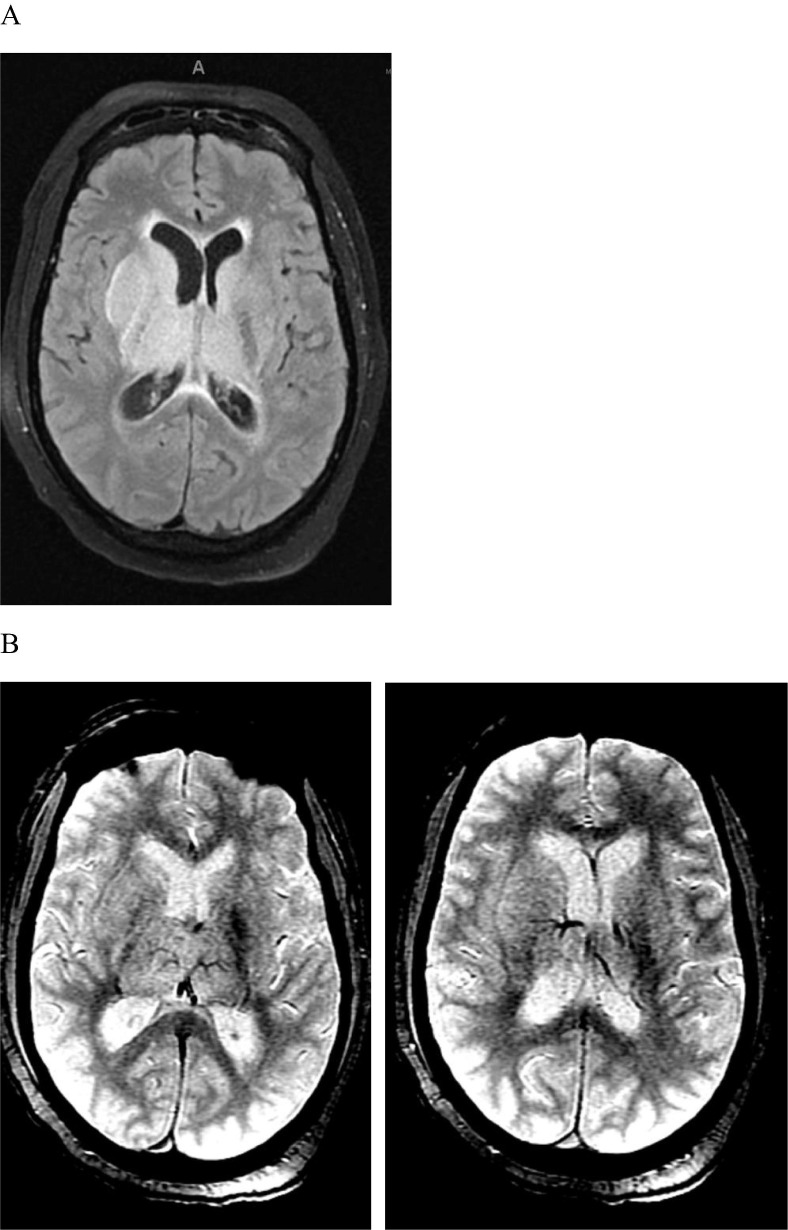
Fig. 3.
54 year old male with cerebral venous thrombosis. A. MRI brain T2 FLAIR sequence demonstrating bilateral thalamic venous infarctions and hydrocephalus. B. MRI brain GRE sequence demonstrating multiple deep internal cerebral vein thrombosis.
2.1.4. Case 4
A 48-year-old male with a history of HLD admitted to an OSH with an acute right sided hemiplegia after being found on the floor by family. On exam, the patient had global aphasia and right sided weakness with an NIHSS of 31. HCT was unremarkable but his CTA demonstrated an acute left MCA occlusion. He received IV tPA and was transferred for endovascular intervention. He underwent a successful left M1 thrombectomy (TICI 0 to 3).
In the NICU, the patient was positive for COVID-19 despite not having any recent symptoms. MRI brain showed a large acute infarct in the left MCA territory, with mass effect and mild petechial hemorrhage (Fig. 4 ). Aspirin and statin therapy have been initiated for secondary stroke prevention along with subcutaneous heparin for DVT prophylaxis. His labs are pertinent for significantly elevated D-dimer (Table 1). His NIHSS improved to 10 and currently he is in the stroke unit awaiting discharge to a rehab facility.
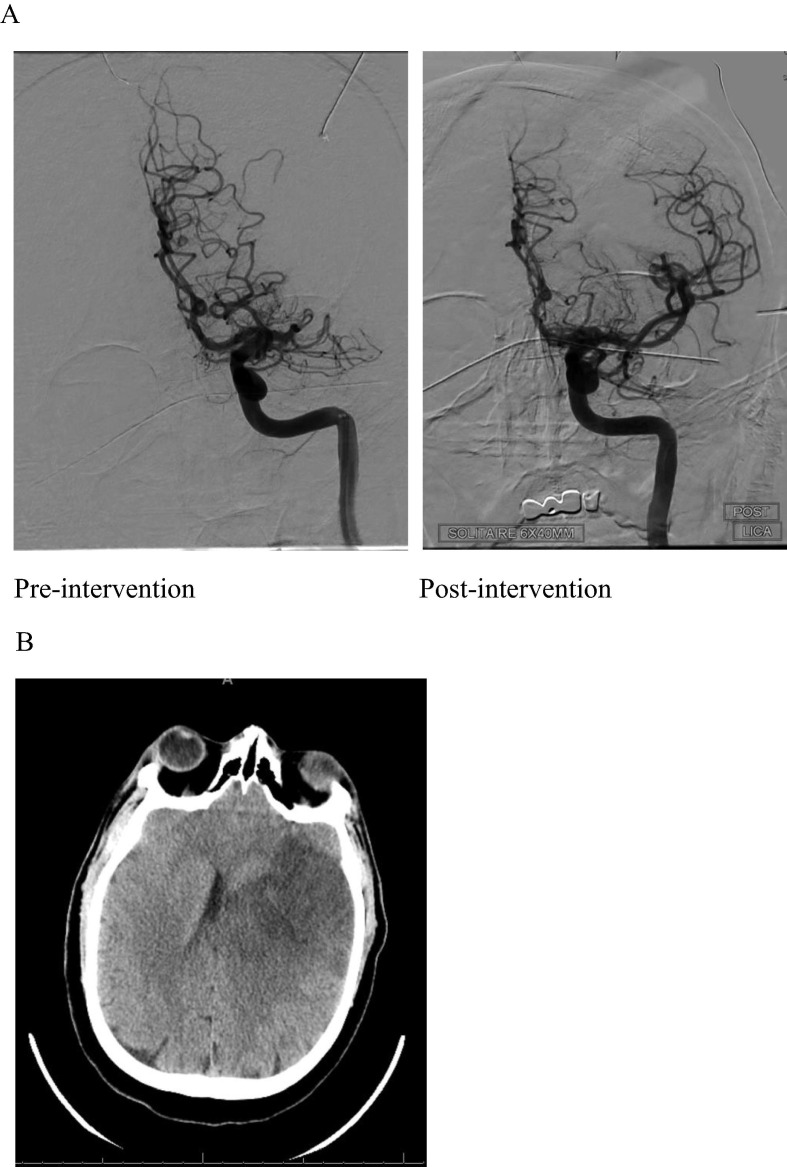
Fig. 4.
48 year old male with L MCA stroke. A. Angiogram pre-intervention showing left M1 occlusion and post-intervention showing recanalization of the vessel. B. Non-contrast CT Head demonstrating L MCA stroke with development of midline shift and cerebral edema.
3. Discussion
Coronaviridae is a family of RNA viruses that were isolated in the 1960s [5], [7]. The first cases of SARS-CoV-2 were discovered in Wuhan, China in December 2019 [8]. Since then, the infection has become a pandemic affecting over 2 million individuals with a mortality close to 7% globally. Most commonly, patients manifest an upper respiratory tract infection or flu-like symptoms. In one review, up to 71% of patients developing ARDS require intubation and mechanical ventilation [1]. These patients can also have acute kidney injury (AKI), elevated liver enzymes, and cardiac injury [2]. A majority of patients manifest coagulopathy and increased inflammation with elevated CRP (75–93%), LDH (27–92%), ESR (85%) and D-dimer (36–43%) [9]. Neurologic manifestations are more common than expected in COVID-19 patients. In a recent review in China, 36.4% of COVID-19 patients had neurologic manifestations, ranging from skeletal muscles injury to acute cerebrovascular disease. The prevalence of cerebrovascular disease was low in this review with only 1–5% of patients developing symptoms of cerebrovascular disease [6]. A multi-center prospective study in intensive care units (ICUs) in France found that close to 3% of COVID-19 patients develop ischemic strokes [3]. Patients with severe symptoms are more likely than patients with mild to moderate symptoms to have neurological manifestations [6].
In our case series, we present four cases of cerebrovascular disease who had concurrent COVID-19 infection. Our patients displayed evidence of hypercoagulability and significantly increased inflammatory markers. All four cases were positive for anticardiolipin antibodies. The first patient was positive for anti-B2-glycoprotein I IgA and IgG antibodies. A recent publication showed an association with the development of ischemic infarcts and the presence of these antibodies [10]. All patients had elevated D-Dimers and other inflammatory markers.
The mechanism on how the SARS-CoV-2 virus causes ischemic strokes is unclear. Several theories have been proposed. The elevated D-dimer in COVID-19 patients can be associated with the development of ischemic stroke. Elevated D-dimer increases blood coagulation, thrombin formation, and intravascular fibrin. In addition, elevated D-dimer concentrations have been reported in cerebral venous sinus thrombosis, acute pulmonary embolism, spontaneous intracerebral hemorrhage [11]. The increased hypercoagulability can directly lead to clot formation in the large intracranial vessels and increased risk for ischemic stroke. In addition, the SARS-CoV-2 virus has been associated with a cytokine surge that is linked with the development of acute cerebrobasilar disease [12]. In our patients, the LDL and HBA1C were at normal levels. Their bedside echocardiograms were unrevealing. It is possible that the hypercoagulability and inflammatory surge that accompanies COVID-19 infection can lead to ischemic infarcts through clot formation in large intracranial vessels. Moreover, the accompanying hypoxemia can lead to ischemic stroke by increasing intracellular acidosis and free radicals. This process can eventually lead to cell damage and apoptosis [13]. Damage to the central nervous system can also come from direct invasion of the virus to brain cells. Brain autopsy in deceased COVID-19 patients detected the nucleic acid of the virus in their cerebrospinal fluid and brain tissue [14]. Perhaps the invasion is a result of ACE2 receptors in cerebral blood vessels similar to the invasion that occurs in lung tissue, but further research is required to validate this [15]. Another animal model showed that the olfactory nerve could be the point of entry of the virus to the brain [15].
Tang et al. suggested that anticoagulation therapy with low molecular weighted heparin (LMWH) is associated with better prognosis in severely affected COVID-19 patients with elevated D-dimer [4]. It is important to note that the first two cases developed strokes while on full dose anticoagulation. This raises an important question on the best way to anticoagulate COVID-19 patients to prevent future strokes. Further research is warranted to help answer this question.
4. Conclusion
The SARS-CoV-2 virus has caused a pandemic that has overwhelmed healthcare providers around the world. Multiorgan failure has been described in patients with the COVID-19 infection including the central and peripheral nervous systems. It is important to recognize the neurological manifestations of COVID-19, especially ischemic stroke, either arterial or venous in nature. Hypercoagulability and the cytokine surge are perhaps the cause of ischemic stroke in these patients. Early anticoagulation may help prevent ischemic stroke in patients with elevated D-Dimer. It is unclear which agent and dose is required to help prevent the occurrence of ischemic strokes in patients who are severely ill. Further studies are needed to understand the role of anticoagulation in these patients.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
MK, TT, KS, FH, JU, MA and SOS have nothing to disclose.
PJ h is a consultant for Medtronic and MicroVention.
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. N Engl J Med. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. Epub ahead of print 4 May 2020. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed]
- 4.Tang N., Li D., Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson C, Busl K. Neurologic manifestations of severe respiratory viral contagions. Crit Care Explor. Epub ahead of print April 2020. doi: 10.1097/CCE.0000000000000107. [DOI] [PMC free article] [PubMed]
- 6.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. Epub ahead of print 10 April 2020. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed]
- 7.Tyrrell D.A., Bynoe M.L. Cultivation of a novel type of common-cold virus in organ cultures. Br Med J. 1965;1(5448):1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson R. Pandemic potential of 2019-nCoV. Lancet Infect Dis. 2020;20(3):280. doi: 10.1016/S1473-3099(20)30068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. Epub ahead of print 03 March 2020. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed]
- 10.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zi W.J., Shuai J. Plasma D-dimer levels are associated with stroke subtypes and infarction volume in patients with acute ischemic stroke. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0086465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ham P.B., 3rd, Raju R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog Neurobiol. 2017;157:92–116. doi: 10.1016/j.pneurobio.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arabi Y.M., Balkhy H.H., Hayden F.G. Middle east respiratory syndrome. N Engl J Med. 2017;376(6):584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi S, Srivastava AK, Ray U, et al. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID-19 patients?. ACS Chem Neurosci. Epub ahead of print 29 April 2020. doi: 10.1021/acschemneuro.0c00217. [DOI] [PubMed]