Abstract
Infection by SARS-CoV-2 commonly begins in the nasopharynx, and the cytologic and molecular correlates are not characterized. Fifty-eight cytologic preps (20 oral and 38 from the nasopharynx) were obtained from ten patients and analyzed in a blinded fashion for SARS-CoV-2 spike and envelope protein by immunohistochemistry and viral RNA by in situ hybridization. qRTPCR identified three positive cases and seven controls; the three cases reported mild symptoms that resolved in 2–3 days. Blinded analyses confirmed the presence of the SARS-CoV-2 spike and envelope proteins and viral RNA in the three cases and viral absence in the seven controls. A signal for the positive cases was evident in each nasopharyngeal and none of the oral samples. Viral RNA/proteins localized exclusively to glandular cells and was present in high copy number. Blinded analysis of the cytology documented that the glandular cells infected by SARS-CoV-2 showed marked degeneration with ciliocytophthoria; viral inclusions were not evident. Co-expression analysis showed viral infected cells had increased apoptosis, marked by strong expression of activated caspase 3. Weekly serial testing of two of the cases showed persistence of productive viral infection for up to 2 weeks after symptom onset. It is concluded that the target cell of SARS-CoV-2 in the head and neck region is the glandular cell of the nasal passages, that viral infection is lytic and associated with high copy number that facilitates viral spread. The method outlines a simple, rapid test for productive SARS-CoV-2 based on immunohistochemistry or in situ hybridization of the glandular cells from the nasopharynx.
Keywords: COVID-19, Nasopharynx, SARS-CoV-2, Glandular Cells
Highlights
-
•
SARS-CoV-2 infects the glandular cells of the nasopharynx.
-
•
SARS-CoV-2 RNA can be detected in situ in cytology smears.
-
•
SARS-CoV-2 capsid can be detected in situ in cytology smears.
-
•
These in situ tests can be used for rapid diagnosis of COVID-19.
1. Introduction
SARS-CoV-2, a coronavirus first identified at the end of 2019, is responsible for the most serious pandemic of the last 100 years that, as of this writing, has caused more than 2 million documented cases and, most likely, over 20 million infections when subclinical disease is included. It is well documented that the infection most commonly originates in the head and neck region with both nasopharyngeal and oral swabs being used for diagnosis of viral RNA either by qRTPCR or next generation sequencing (NGS). Although there is some disparity in the literature, it is generally agreed that nasopharyngeal swabs are superior to oral swabs for diagnostic purposes [[1], [2], [3]]. It has also been documented that people with mild symptoms can be positive for viral RNA that can persist for weeks [3]. One pitfall of the tests for the SARS-CoV-2 RNA is that they cannot determine if the virus is infectious; this would require demonstration of viral RNA and the protein coat which includes the spike protein, which attaches to the cell receptor, and the envelope protein, which serves as support for the spike protein [4].
The histopathology of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), both also due to coronaviruses, includes infection of the upper and lower respiratory tracts. However, in SARS and MERS, the primary cell target of the virus in the lung is the alveolar pneumocyte (type I and type II) which induces a strong cytokine response that includes IL6, TNF alpha, IL-1B and CCL2 [4]. The end result for 10% and 35% of infected people, respectively, is death associated with acute respiratory distress syndrome with its characteristic hyaline membrane disease [4]. Early data on the histopathology of COVID-19 suggests that the respiratory tract is indeed the primary entry/site of serious disease but that the target cell in the lung is the alveolar macrophage and endothelial cell; infection of the latter induces a fatal microangiopathy marked by activation of the complement cascade [5].
A major issue regarding the diagnosis of SARS-CoV-2 is the acute shortage of many essential ingredients for performing the standard tests from nasopharyngeal swabs (qRTPCR and NGS) [1]. These currently used tests for viral RNA cannot show the cell type(s) that are viral targets or if the infection is productive. We explored whether formalin fixed cytology smears prepared from the oral cavity or nasopharynx could be used to detect active coronavirus infection using standard immunohistochemistry and in situ based methods, much like human papillomavirus can be detected in Pap smears.
2. Materials and methods
2.1. Patient selection and sample preparation
Standard nasopharyngeal and oral swabs were prepared from ten people being tested for SARS-CoV-2RNA using a standard qRTPCR test. Three of the people had reported mild symptoms (low-grade fever up to 100F, mild cough, mild fatigue) that lasted from 1 to 3 days. The other people had possible exposure to SARS-CoV-2 infected people, though in no case was contact with a verified infected person documented. Four swabs of each region were done per site, then spread on four unstained PLUS slides, and air-dried for 30 min. Then the slides were placed in 10% buffered formalin overnight, washed in distilled water, and air-dried. One swab/site was stained with hematoxylin and eosin for cytologic analysis. The other three slides were used for molecular/viral testing. One additional swab was taken/site, and placed in the viral collection media for standard qRTPCR testing.
2.2. Cytology review
Cytology review of the hematoxylin and eosin stains was done blinded to the viral results. A nasopharyngeal swab was considered satisfactory if at least 25 glandular cells were evident per slide. Each slide was scored for the following variables: squamous cell atypia, inflammation (degree, and cell types), amount of mucous, glandular cell degeneration which was defined either as cellular swelling, nuclear degeneration, or loss of integrity of the cellular membrane.
2.3. Immunohistochemistry
All testing was done blinded to the qRTPCR data. Four slides per patient (two oral, two from the nasopharynx) were tested for the envelope and spike proteins of SARS-CoV-2, respectively for a total of 40 slides. The protocol was based on prior published studies [[6], [7], [8]]. In brief, the primary antibodies versus the spike and envelope proteins were provided by ProSci (Poway, CA). The optimal pretreatment conditions included antigen retrieval for 30 min with an EDTA buffer (pH 9.0), incubation of the primary antibody for 30 min, and use of the horseradish peroxidase – anti-rabbit conjugate from Enzo Life Sciences as this reduced background [7]. Optimal dilutions of the spike and envelope antibodies were 1:5000 and 1:500, respectively,
2.4. In situ hybridization
In situ hybridization was done using our published protocol for RNA viruses based on the RNAscope assay of ACD, which provided the SARS-CoV-2 RNA probe [6,8]. In brief, pretreatment included peroxide block, target retrieval for 15 min, protease digestion for 10 min, hybridization overnight, and detection following the manufacturer's protocol. Lung tissue from two different people who died of respiratory failure from COVID-19 and normal lung obtained from cases prior to 2018 were used as the positive and negative controls, respectively, for both the immunohistochemistry and in situ hybridization.
2.5. Co-expression analysis
Co-expression analyses were done using the Nuance system (CRI) as previously published [6,8]. In brief, a given tissue was tested for two different antigens using fast red as the chromogen for one target followed by immunohistochemistry using DAB (brown) as the second chromogen with hematoxylin as the counterstain. The results were then analyzed by the Nuance system where each chromogenic signal is separated, converted to a fluorescence based signal, then mixed to determine what percentage of cells were expressing the two proteins of interest.
3. Results
3.1. qRTPCR data and cytology review
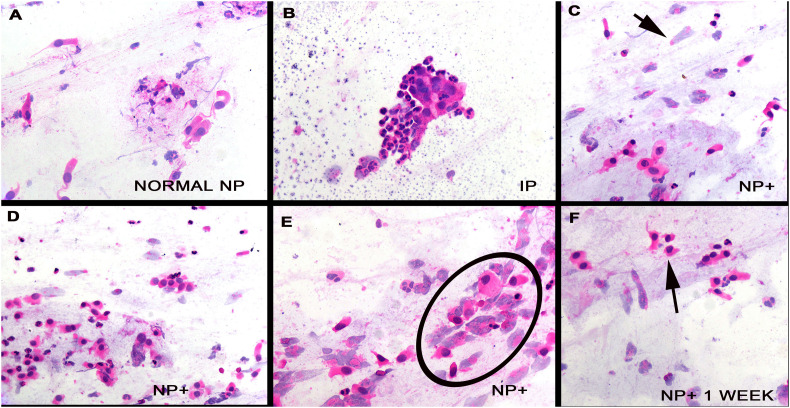
Testing by qRTPCR identified three of the people to be SARS-CoV-2 positive and the other seven to be negative for viral RNA. Thus, we had three cases and seven controls. The hematoxylin and eosin stains of the 20 slides (10 oral and 10 nasopharyngeal) were reviewed blinded to the virology data. Each of the nasopharyngeal swabs contained at least 25 glandular cells and was deemed satisfactory; many of these swabs had many more glandular cells. The glandular cells in the seven controls were well preserved, being tall cells with basal nuclei of moderate chromaticity (Fig. 1A); delicate cilia were evident. In two of the seven controls clusters of inflammatory cells, mostly neutrophils, formed aggregates that tended to trap the glandular cells that hindered but did not prevent interpretation (Fig. 1B). Each of these people had long-standing history of allergic rhinitis and nasal polyps. The oral swabs in each of the seven controls and three cases showed many squamous cells, many anucleated, with no evidence of atypia. The three nasopharyngeal swabs from the three cases showed similar findings. The squamous cells were unremarkable. The glandular cells showed marked degenerative changes that included loss of cilia, pyknotic nuclei, loss of nuclei, cytoplasmic swelling, and cell membrane rupture (Fig. 1C–F). Viral inclusions, either nuclear or cytoplasmic, were not evident.
Fig. 1.
Cytologic findings in nasopharyngeal swab cell preparations from cases and controls. Panel A shows the typical findings in the nasopharyngeal swab prep from a person negative for SARS-CoV-2. Note the tall glandular cells with basal nuclei. Panel B shows the uncommon finding that collections of inflammatory cells may obscure the glandular cells. Panels C–E and cytologic preps from the nasopharynx from the cases. Note the degenerated glandular cells (arrow, panel C; circle, panel E) where many glandular cells have lost their nuclei and others show pyknotic nuclei (panel F, arrow).
3.2. Immunohistochemistry analysis for the SARS-CoV-2 spike and envelope proteins
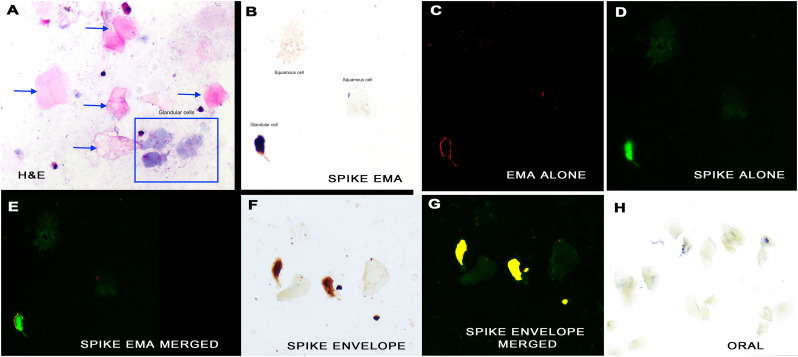
Next, two slides each from each oral and nasopharyngeal swabs were tested for the spike and envelope proteins of SARS-CoV-2, respectively, blinded to the qRTPCR data. Both the spike and envelope protein produced a signal in the nasopharyngeal swabs of the three cases and no signal was evident in the nasopharyngeal swabs of the seven controls. Fig. 2A shows representative cytology from one of the positive nasopharyngeal swabs where squamous cells (arrows) and degenerated glandular cells (box) often co-existed. Viral protein localized only to the glandular cells. Although often clear on the cytology alone, to further document the localization of SARS-CoV-2 proteins to glandular cells, co-expression was done with the protein epithelial membrane antigen (EMA). Analyses of cervical and nasopharyngeal biopsies showed that EMA localized to mature glandular cells plus metaplastic cells and not mature squamous cells (Fig. 3H). Co-expression analyze for the spike protein of SARS-CoV-2 and EMA showed strong co-localization (Fig. 2, B–E). To further demonstrate the specificity of the immunohistochemistry reaction, co-expression for SARS-CoV-2 spike and envelope proteins was performed. Strong co-localization was demonstrated (Fig. 2, panels F, G). The other indicator that the SARS-CoV-2 was infecting only glandular cells, besides the cytologic data (Fig. 1), was that each of the oral swabs (three cases and seven controls) was negative for both SARS-CoV-2 proteins (Fig. 2H). It was also demonstrated that the ACE 2 protein, the receptor for SARS-CoV-2 spike protein, localized to the glandular cell, often concentrating towards the cilia (Fig. 3G).
Fig. 2.
Detection of SARS-CoV-2 proteins in nasopharyngeal swab cell preparations. A Hematoxylin and eosin stain demonstrates the large, flat pink squamous cells (arrows) and the more elongate grey glandular cells (box) (Panel A). An intense signal for covid-19 spike protein was seen in the glandular cells and not the squamous cells (panel B; signal brown due to DAB). Cilia were rarely seen in infected cells (ciliocytophthoria). The strong signal indicates high viral copy number that facilitates immunohistochemistry detection. Co-expression for epithelial membrane antigen (panel C, signal red) and spike protein (panel D, signal green) documented that signal was in glandular cells (panel E, merged signal yellow). Co-expression of spike and envelope proteins of SARS-CoV-2 (panel F, RGB image) documented localization of each protein to glandular cells with negative squamous cells two weeks after full recovery (panel G, signal yellow). No signal was seen in oral swabs of positive cases (panel H). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
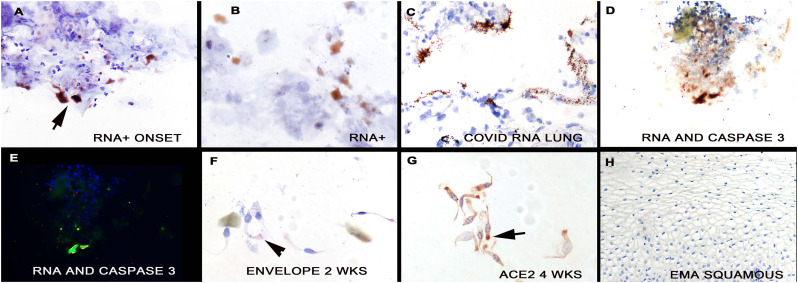
Fig. 3.
Detection of SARS-CoV-2 in nasopharyngeal swab cell preparations. SARS-CoV-2 RNA was detected in the nasopharyngeal swabs from the cases near the onset of symptoms (panel A, arrow); note the marked degenerative changes in some of the infected cells (panel B); the signal is brown (DAB) with hematoxylin counterstain. Panel C shows the positive control that was SARS-CoV-2 RNA detected in the lung of a person who died of the infection; note the high viral load. Panels D/E show co-expression of viral RNA and activated caspase-3. Note that the viral RNA (green) and caspase 3 (red) co-localize (Panel E, yellow). Panel F shows some SARS-CoV-2 infected cells, albeit with lower viral load, were evident 2 weeks after the initial swabs (panel F, signal is red with hematoxylin counterstain). Panel G shows the nasopharyngeal swab from the person in panel A tested 4 weeks after symptom onset for ACE2 protein. Note that the protein tends to localize towards the apical surface (arrow) and that the cells are now cytologically normal. Panel H shows the lack of EMA detection in mature squamous epithelia of the oral cavity. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. In situ analysis for the SARS-CoV-2 RNA and serial testing for the virus
Next, one slide from the nasopharyngeal swabs were tested for SARS-CoV-2 RNA by in situ hybridization again blinded to the qRTPCR data. The three cases showed many positive cells (more than 10 positive cells/sample that was also true for the immunohistochemistry and the seven controls were each negative. The background staining was very low with the SARS-CoV-2 in situ hybridization test and tended to be higher for the spike protein immunohistochemistry, where occasional squamous cells showed nonspecific binding. The viral RNA localized to the glandular cells (Fig. 3, panels A/B) in cells that were at times strongly degenerated. Panel C shows the positive control where high viral load was noted in the alveolar septa of a patient who died of COVID-19 pneumonia. To document that the SARS-CoV-2 infected cells were degenerated and, likely apoptotic, co-expression was done for viral RNA and activated caspase 3. The data from these experiments demonstrated that about 20% of the infected cells had activated caspase 3 (Fig. 3, panels D/E).
Finally, serial weekly nasopharyngeal swabs were available from two of the cases over a 4-week period. The nonspecific changes of glandular cell degeneration were still evident 1–2 weeks after the initial swab (Fig. 1, panel F). Viral RNA and envelope protein were still evident at 1 week and 2 weeks after the initial swab, respectively (Fig. 3, panel F) though the amount of virus was much less than at the initial swab. Note that the viral signal localized to the area of the terminal plate of the cilia.
4. Discussion
The main findings of this study were twofold: 1) the in situ based SARS-CoV-2 testing of the nasopharyngeal and oral swabs identifies the viral cell target which is the glandular cell of the nasal passages. This gives cytologic clarity to the repeated demonstration that the nasopharyngeal swab is superior to the oral swab for viral detection by qRTPCR of NGS [2,3] and also suggests that nasal secretions and sneezing are likely the most infectious though, of course, post nasal drainage would also make the oral secretions infectious. 2) SARS-CoV-2 infection of the glandular cells is high copy and lytic which clearly facilitates viral transmission. Another finding of this study is that infectious virus, defined by detection of both viral RNA and key SARS-CoV-2 proteins, is still evident up to two weeks after mild symptoms are no longer present, giving a pathologic basis to the demonstration that asymptomatic people can be infectious [3]. Our data also elucidates why disorders of smell are typical of COVID-19 disease since glandular cells in the nasal cavity play a key role in this process [3].
There are many publications where nasopharyngeal swabs are used for detection of viral and bacterial infections [[9], [10], [11], [12], [13], [14], [15]]. Clearly, this reflects the fact that many infections start in the nasal passageways. However, basically all of these studies lyse all of the cells and then do solution-based testing for viral proteins or RNA/DNA. There are very few papers that describe the cytological changes associated with the viral infections of the nasopharynx. This may reflect, in part, the realization that most viruses that infect the nasal passages do not induce viral specific inclusions that allow the cytologic diagnosis on a swab. To our knowledge, this paper is the first description of the cytology of the primary site of SARS-CoV-2 infection, the nasal passage. The cytologic changes were indeed dramatic with widespread changes in glandular cells that included pyknosis, degeneration, and loss of cilia. However, these changes are best deemed as nonspecific. A careful and thorough search failed to detect any viral inclusions, even in cells that contained abundant viral RNA or proteins. This suggests that claims of viral inclusions with covid-19 disease [16] may, rather, represent nonspecific cytopathic effect.
The immunohistochemistry and in situ hybridization assays described in this paper could provide a rapid, easy to do assay for detecting productive infection from nasopharyngeal swabs. The instrumentation and reagents for immunohistochemistry or the RNAscope for viral RNA are available in any diagnostic pathology laboratory. The test from swab to read-out for the immunohistochemistry test can be completed in less than 3 h. Cytotechnologists and cytopathologists are well trained to review large numbers of such specimens and the process has been partly automated for many years. Given the marked backlog in the current testing methods for SARS-CoV-2 from such specimens (qRTPCR and NGS) [1], it would be advantageous for other pathology laboratories to consider developing their own cytology-based assays for SARS-CoV-2. The key technical points in this regard included: 1) fixation in 10% buffered formalin that would immediately inactivate the virus; 2) for immunohistochemistry the use of the Enzo Life Sciences horseradish peroxidase conjugate as this reduced background [7], 3) our observation that the in situ hybridization assay tended to yield cleaner results with no background issues suggests trying both assays and deciding which one gives the best signal to noise ratio in your laboratory setting. With regard to the formalin fixation, our protocol initially used overnight fixation. But testing nasopharyngeal swabs after 1 h of fixation in 10% buffered formalin documented a strong signal for proteins such as pan-cytokeratin and, thus, suggests that short fixations would be adequate when developing such assays.
Acknowledgments
Acknowledgements
The authors are most appreciative of the photodocumentation provided by Dr. Margaret Nuovo.
Declaration of competing interest
The authors have no conflicts of interest to report.
References
- 1.https://www.webmd.com/lung/news/20200330/covid19-testing-hits-second-wall-lack-of-supplies
- 2.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai Y., Yao L., Wei T. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gralinski L.E., Baric R.S. Molecular pathology of emerging coronavirus infections. J Pathol. 2015;235(2):185–195. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magro CM, . Mulvey J, Berlin D, Nuovo GJ, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement activation is associated with the thrombotic microvascular injury of severe COVID-19 disease. Transl Res (in Press) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7158248/. [DOI] [PMC free article] [PubMed]
- 6.Nuovo G.J. 2nd ed. Elsevier; NY: 2020. In situ molecular pathology and co-expression analyses. [Google Scholar]
- 7.Nuovo G. False-positive results in diagnostic immunohistochemistry are related to horseradish peroxidase conjugates in commercially available assays. Ann Diagn Pathol. 2016;25:54–59. doi: 10.1016/j.anndiagpath.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Thirukkumaran C.M., Shi Z.Q., Nuovo G.J., Luider J., Kopciuk K.A., Dong Y. Oncolytic immunotherapy and bortezomib synergy improves survival of refractory multiple myeloma in a preclinical model. Blood Adv. 2019;3(5):797–812. doi: 10.1182/bloodadvances.2018025593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturgis C.D., Burkey B.B., Momin S., Hoschar A.P. High grade (large cell) neuroendocrine carcinoma of the nasopharynx: novel case report with touch preparation cytology and positive EBV encoded early RNA. Case Rep Pathol. 2015;(23):1070. doi: 10.1155/2015/231070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho H.T., Chang M.S., Wei T.Y., Hsieh W.S., Hung C.C., Yang H.M. Colonization of severe acute respiratory syndrome-associated coronavirus among health-care workers screened by nasopharyngeal swab. Chest. 2006;129(1):95–101. doi: 10.1378/chest.129.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juarez D., Guevara C., Wiley M., Torre A., Palacios G., Halsey E.S. Isolation of complete equine encephalitis virus genome from human swab specimen, Peru. Emerg Infect Dis. 2018;24(8):1578–1580. doi: 10.3201/eid2408.171274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torretta S., Marchisio P., Colombo M.R., Rosazza C., Pignataro L. Paediatric nasopharyngeal cytology: a new diagnostic opportunity? Eur J Clin Microbiol Infect Dis. 2016;35(7):1097–1099. doi: 10.1007/s10096-016-2637-5. [DOI] [PubMed] [Google Scholar]
- 13.Leber A.L., Everhart K., Daly J.A., Hopper A., Harrington A., Schreckenberger P. Multicenter evaluation of BioFire FilmArray respiratory panel 2 for detection of viruses and bacteria in nasopharyngeal swab samples. J Clin Microbiol. 2018;56(6):601–611. doi: 10.1128/JCM.01945-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elfving K., Shakely D., Andersson M., Baltzell K., Msellem M.I., Björkman A. Lindh M Pathogen clearance and new respiratory tract infections among febrile children in Zanzibar investigated with multitargeting real-time polymerase chain reaction on paired nasopharyngeal swab samples. Pediatr Infect Dis J. 2018;37(7):643–648. doi: 10.1097/INF.0000000000001876. [DOI] [PubMed] [Google Scholar]
- 15.Stensballe L.G., Trautner S., Kofoed P.E., Nante E., Hedegaard K., Jensen I.P. Comparison of nasopharyngeal aspirate and nasal swab specimens for detection of respiratory syncytial virus in different settings in a developing country. Trop Med Int Health. 2020;7(4):317–321. doi: 10.1046/j.1365-3156.2002.00867.x. [DOI] [PubMed] [Google Scholar]
- 16.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. https://marlin-prod.literatumonline.com/pb-assets/Lancet/pdfs/S0140673620309375.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]