Abstract
Globalization accelerates the mobilization of microorganisms via international trade and transport. Growth in population, increasing connectivity, and rapid urbanization all exacerbate the consequent risk of pandemics of zoonotic diseases. Global problems require global solutions, particularly the co-ordination of international research in biomedical sciences, global ecology, and sustainability.
Globalization accelerates the mobilization of microorganisms via international trade and transport. Growth in population, increasing connectivity, and rapid urbanization all exacerbate the consequent risk of pandemics of zoonotic diseases. Global problems require global solutions, particularly the co-ordination of international research in biomedical sciences, global ecology, and sustainability.
Main Text
Globalization Promotes Pandemic Disease
The coronavirus disease 2019 (COVID-19) pandemic is a clear example of how globalization speeds the planetary-scale mobilization of emerging infectious diseases (EIDs). The devastating impacts on human health and global economies arose as a consequence of a single novel viral transmission event, which was first reported on December 31, 2019, in Wuhan City, China.1 Assisted by human transport, the virus spread across the entire globe in as little as 3 months, leading to the announcement of a global pandemic by the World Health Organization (WHO) on March 11, 2020.
The COVID-19 pandemic is not an isolated event; viruses and microorganisms constantly move between humans, animals, plants, and the environment (Figure 1 ), and now they do so on a planetary scale at an increasing and unprecedented pace driven by international transport of goods and produce, ballast water, and exponentially growing human travel via air, sea, and land.2 The scale of the global movement of people and potential spread of infectious diseases during outbreaks is exemplified by the number of global airline passengers—a key factor in the rapid dissemination of viruses and microorganisms around the planet—which has increased by an order of magnitude in recent decades (Figure 2 ).
Figure 1.
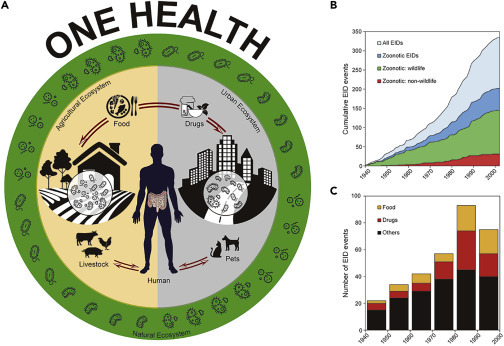
Human and Emerging Infectious Diseases
(A) Humans, animals, and the environment share a complex microbial world.
(B) The cumulative EIDs and zoonotic EIDs of humans since 1940, in which non-wildlife and wildlife represent the zoonotic EID event caused by a pathogen with no known and known wildlife origin, respectively.
(C) Effects of food and drug drivers on the number of EID events per decade.
For (B) and (C), data were collected from the Jones et al. database.3
Figure 2.
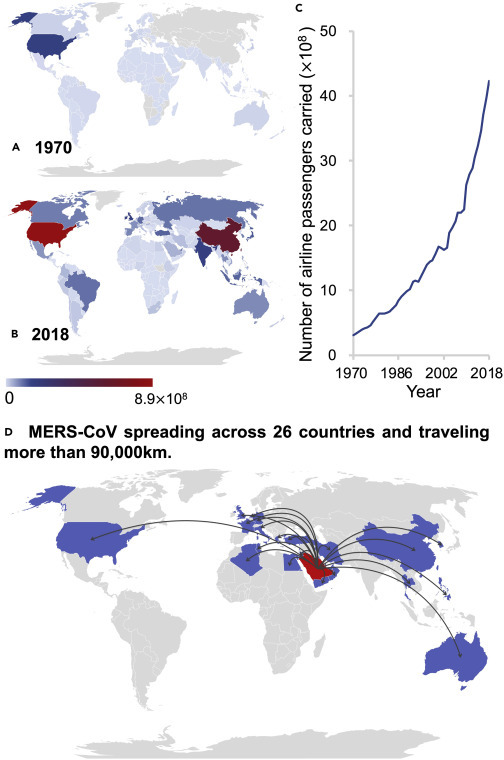
Airline Passengers Carried Include Both Domestic and International Passengers of Air Carriers Registered in the Country
(A–C) Number of airline passengers carried in 1970 (A), 2018 (B), and 1970–2018 (C). Data sources: https://www.indexmundi.com/facts/indicators/IS.AIR.PSGR and https://data.worldbank.org/indicator/IS.AIR.PSGR.
(D) Global spread of MERS-CoV across 26 countries.4
Unit: ×1012 passengers.
The pace and scale of the current pandemic are not simply the result of this exponentially increased mobilization. They also result from other human-related risk factors, including population density, anthropogenic disturbance of ecosystems, and zoonotic transmission. Anthropogenic impacts can cascade via the disturbance of ecosystems into the microbial world, changing pathogen hosts and transmission routes and increasing the likelihood of disease outbreaks in humans. Understanding the complexities of these interactions is central to predicting and managing the continuously increasing number of EID events (Figure 1).
Infectious Diseases and Sustainability
Historical data and modeling confirm the increasing incidence of EIDs being driven by deforestation, food production, and climate change.3 , 5 These risk factors are likely to be exacerbated given that the world’s population is projected to increase by two billion, nearly 70% of whom will live in cities, by 2050. Rapid urbanization and associated increases in population density provide fertile ground for the rapid spread of novel pathogens in pathogen-naive populations. Meanwhile, population growth and rising food demand drive agricultural expansion and intensification and thus deforestation and habitat loss, all of which are associated with an increased incidence of EIDs.6 The majority of recently emerged infectious diseases are zoonoses, and of these, 70% originate from wildlife3 (Figure 1). Hunting and widespread trading in wildlife are therefore also major drivers for the emergence and spread of infectious diseases.3 , 7
As discussed above, all of these sustainability issues (such as rapid population growth, urbanization, increasing consumption of nature-derived commodities, and land conversion) are interconnected and interact with each other to increase risks of pandemic disease at the global scale. Novel pathogens, such as new coronaviruses, are often zoonotic3 and consequently are likely to emerge and spread as human land use expands into nature, as food distribution and human traveling are globalized, and as population density increases. Although sustainability issues have been included in large-scale modeling of EID incidences, they have yet to be fully integrated into systems for managing and preventing infectious diseases. We therefore call for urgent consideration of EIDs in the global sustainability agenda. We need to track the way that microorganisms cycle between the environment, animals, and humans at various temporal and spatial scales. We can no longer afford to ignore the complex dynamics and ecology of the microbial world. Incorporating ecological understanding, environmental management, and disentanglement of human-animal interactions will help inform decision-making and preventative actions around infectious diseases.
Systems Approaches to Managing Infectious Diseases
Monitoring and assessing microbial ecology should enlighten the complex multifactorial relationship among sustainable development, human health, and microorganisms. Such an understanding will make it possible to develop the required complex and integrated solutions to manage future emerging infectious diseases. One Health8 is one such systems approach to global health security. One Health is dedicated to achieving optimal human and environmental health and aims to link research disciplines, sectors, and public health organizations to advance a holistic view of the interconnections among humans, animals, plants, microbes, and ecosystems as a single integrated system. It particularly examines the role of microbial dynamics in socio-ecological systems from local to global scales.
Realizing these aims and effectively mitigating emerging infectious diseases, however, still require a paradigm shift in scientific research. We need improved collaboration among the biomedical sciences, global ecology, and sustainability. In general (although not universally), modern medicine is often practiced as though humans are separate from the natural world, and evolutionary processes are often ignored.9 Efficient data sharing, and an appreciation of research that crosses traditional discipline boundaries, would help in disease prevention and control by allowing faster and multi-pronged responses to outbreaks. An increased appreciation in medical circles of the importance of microbial ecology and evolution in disease dynamics will be critical to managing future pandemics. The evolutionary processes of mutation and selection and the evolutionary dynamics between virulence and transmission are important for understanding how diseases spread and adapt to their hosts. The One Health approach8 offers a platform for collaboration, but fundamental integration among biomedical science, global ecology, and sustainability is still needed. Ecological and evolutionary disciplines have often been seen as irrelevant to human health, but they are central to understanding disease emergence and risk.
Preventing disease outbreaks at their sources will also require fundamental innovations. Agile surveillance and warning systems are now within practical reach given that the cost of DNA sequencing analysis is rapidly dropping, and we can effectively screen wild animal populations to understand viral composition, evolution, and dynamics10 before animal viruses become a danger to human populations. Indeed, pandemic risk should be explicitly considered within the overall framework of global ecology and sustainable development,11 and research teams should be assembled to monitor and understand viral dynamics in natural ecosystems.
In the longer term, understanding the impact of consumption-driven changes in ecosystems could also help to inform fundamental changes in policy. For example, it is estimated that about 20% of malaria risk is driven by the international trade of commodities implicated in defeorestation, such as timber, wood products, tobacco, cocoa, coffee, and cotton.12 This linking of EID risk to the final consumers of commodities strongly justifies demand-side policy measures with important co-benefits for reducing deforestation and forest disturbance.12 Demand-side measures can be established through the engagement of consumers, legislation of certification and other producer standards, and/or establishment of fiscal instruments to reduce deforestation and EID risk at the same time.
With our increasing awareness of pandemics, preparedness should become a priority for the global health agenda. Preparedness for disease outbreaks will require expanded reserves in diagnostic infrastructure, in the population of medical personnel, and in healthcare consumables. But preparedness will also require full consideration of the nexus between social and ecological systems. Thus, greater public literacy about infectious diseases and their dynamics—such as awareness of the interconnections among pandemics, population growth, urbanization, and consumption—is also warranted. We therefore strongly propose that the dynamics of the planet’s microorganisms be incorporated as a key issue into the United Nations Sustainable Development Goals (SDGs). Currently, the issue of infectious disease is included in SDG 3 (Good Health and Well-Being). However, because the emergence and spread of infectious diseases are the integral outcome of intricate interactions between human and natural ecosystems, SDG 3 must promote a strong coordination of international research in biomedical sciences, global ecology, and sustainability. Furthermore, strong interactions between SDG 3 and the other SDGs—such as SDG 1 (No Poverty), SDG 2 (Zero Hunger), SDG 11 (Sustainable Cities and Communities), SDG 12 (Responsible Consumption and Production), SDG 13 (Climate Action), and SDG 15 (Life on Land) (see https://sustainabledevelopment.un.org/sdgactions)—will further contribute to the mitigation of EID risks.
Long-term preparedness will ultimately rely on advances in our fundamental understanding of the composition, dynamics, and functions of the microbial world, in which pathogens co-exist and interplay with beneficial microorganisms. Such advances will assist rapid and rational monitoring of microbial cycling between humans and the environment by using molecular and big-data approaches. Better understanding of Earth’s microbial inhabitants will help to predict likely sources of pandemics and novel disease agents and facilitate vaccine development in a timely and targeted manner. For example, spatially explicit models can be developed to diagnose and predict the regions where humans are more exposed to wildlife and their associated microbiomes.13 We urgently need to assess what species (e.g., those with the highest viral loads), locations (e.g., regions projected to experience rapid urbanization by 2050), and practices (e.g., wildlife traffic) pose the greatest risk for zoonotics and plan or act accordingly.14 , 15
Concluding Remarks
Despite our attempts to insulate ourselves from disease agents, we are living in an ever more tightly connected microbial world, regardless of wealth or location. Human activity has caused dramatic changes in the world, potentially to the point of irreversibility. These changes extend into the microbial world, much of which has yet to be explored or understood. This understanding can be achieved only through a strong coordination of international research in biomedical sciences, global ecology, and sustainability.
Acknowledgments
Y.-G.Z. is supported by the Natural Science Foundation of China (21936006) and the Ministry of Science & Technology for International Cooperation (2017YFE0107300). M.G. is supported by the Australian Research Council. J.P. is supported by European Research Council grant ERC-SyG-2013-610028 IMBALANCE-P. We thank Ms. Hui-Ling Cui and Dr. Nan Li for making the diagrams and Ms. Xuan Nie for editing the references.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y.-G., Gillings M., Simonet P., Stekel D., Banwart S., Penuelas J. Microbial mass movements. Science. 2017;357:1099–1100. doi: 10.1126/science.aao3007. [DOI] [PubMed] [Google Scholar]
- 3.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackay I.M., Arden K.E. MERS coronavirus: diagnostics, epidemiology and transmission. Virol. J. 2015;12:222. doi: 10.1186/s12985-015-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohr J.R., Barrett C.B., Civitello D.J., Craft M.E., Delius B., DeLeo G.A., Hudson P.J., Jouanard N., Nguyen K.H., Ostfeld R.S., et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019;2:445–456. doi: 10.1038/s41893-019-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faust C.L., McCallum H.I., Bloomfield L.S.P., Gottdenker N.L., Gillespie T.R., Torney C.J., Dobson A.P., Plowright R.K. Pathogen spillover during land conversion. Ecol. Lett. 2018;21:471–483. doi: 10.1111/ele.12904. [DOI] [PubMed] [Google Scholar]
- 7.Allen T., Murray K.A., Zambrana-Torrelio C., Morse S.S., Rondinini C., Di Marco M., Breit N., Olival K.J., Daszak P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017;8:1124. doi: 10.1038/s41467-017-00923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degeling C., Johnson J., Kerridge I., Wilson A., Ward M., Stewart C., Gilbert G. Implementing a One Health approach to emerging infectious disease: reflections on the socio-political, ethical and legal dimensions. BMC Public Health. 2015;15:1307. doi: 10.1186/s12889-015-2617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonovics J., Abbate J.L., Baker C.H., Daley D., Hood M.E., Jenkins C.E., Johnson L.J., Murray J.J., Panjeti V., Rudolf V.H., et al. Evolution by any other name: antibiotic resistance and avoidance of the E-word. PLoS Biol. 2007;5:e30. doi: 10.1371/journal.pbio.0050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi M., Lin X.D., Chen X., Tian J.H., Chen L.J., Li K., Wang W., Eden J.S., Shen J.J., Liu L., et al. The evolutionary history of vertebrate RNA viruses. Nature. 2018;556:197–202. doi: 10.1038/s41586-018-0012-7. [DOI] [PubMed] [Google Scholar]
- 11.Di Marco M., Baker M.L., Daszak P., De Barro P., Eskew E.A., Godde C.M., Harwood T.D., Herrero M., Hoskins A.J., Johnson E., et al. Opinion: sustainable development must account for pandemic risk. Proc. Natl. Acad. Sci. USA. 2020;117:3888–3892. doi: 10.1073/pnas.2001655117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaves L.S.M., Fry J., Malik A., Geschke A., Sallum M.A.M., Lenzen M. Global consumption and international trade in deforestation-associated commodities could influence malaria risk. Nat. Commun. 2020;11:1258. doi: 10.1038/s41467-020-14954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morse S.S., Mazet J.A., Woolhouse M., Parrish C.R., Carroll D., Karesh W.B., Zambrana-Torrelio C., Lipkin W.I., Daszak P. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han B.A., Schmidt J.P., Bowden S.E., Drake J.M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA. 2015;112:7039–7044. doi: 10.1073/pnas.1501598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daszak P., Cunningham A.A., Hyatt A.D. Emerging infectious diseases of wildlife--threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]


