Abstract
Rickettsia rickettsii, a bacterial tickborne pathogen that causes Rocky Mountain spotted fever (RMSF), stains poorly or not at all with conventional tissue Gram techniques, and contemporary visualization of the pathogen in formalin-fixed, paraffin-embedded tissues has relied almost entirely on immunohistochemical staining methods that are generally limited to specialized research laboratories or national reference centers. To our knowledge, previously described argyrophil-based histochemical techniques have not successfully detected rickettsiae in formalin-fixed, paraffin-embedded tissues. To investigate the ability of standard silver impregnation techniques to demonstrate the occurrence and distribution of R. rickettsii in tissues of patients with RMSF confirmed by molecular and immunohistochemical methods, three widely recognized and commercially available silver impregnation methods (Warthin–Starry, Steiner, and Dieterle’s) were applied to various tissues obtained at autopsy from 10 patients with fatal RMSF. R. rickettsii bacteria were demonstrated in one or more tissues of all patients, using each of the argyrophil-based methods, and appeared as small, dark brown-to-black lanceolate rods, often in pairs and occasionally surrounded by a faint halo. Rickettsiae were identified most consistently in small arteries and arterioles of liver, kidney, and leptomeninges, and were localized predominantly to the cytoplasm of endothelial cells and less often within the internal elastic lamella and smooth muscle of the media. This validation of argyrophilic techniques to detect R. rickettsii demonstrates the utility of inexpensive core histochemical methods in the diagnosis of infectious agents in pathology specimens and may have utility in certain resource-limited settings where RMSF is endemic.
Keywords: Argyrophil, Dieterle’s, Rickettsia rickettsii, Steiner, Rocky Mountain spotted fever, Warthin–Starry
Introduction
Rocky Mountain spotted fever (RMSF), a nationally notifiable disease caused by the tickborne bacterium Rickettsia rickettsii, is one of the most lethal infectious diseases in the western hemisphere. During 1983–2012, 300 fatal cases of RMSF were reported to the Centers for Disease Control and Prevention, which likely represents an underestimate of the actual number of deaths in the United States attributable to this disease.1–3 In several countries of Latin America where RMSF is endemic, including Argentina, Brazil, Colombia, Costa Rica, Mexico, and Panama, the case fatality rates for RMSF are considerably higher than in the United States.4–12 Efforts to assess the magnitude of fatal RMSF in the Americas are curtailed by limited access to costly diagnostic techniques in many of these regions.
Most bacterial species have the ability to adsorb silver ions from a solution. When the adsorbed silver is chemically reduced, the metallic form becomes visible and the morphology of the bacterium is revealed. Several histochemical techniques, including those developed during the early twentieth century by Warthin and Starry,13 Dieterle,14 and Steiner,15 use this principle to detect bacteria that otherwise stain weakly or not at all by conventional tissue Gram stains. During the last 100 years, argyrophil-based methods have been used widely by pathologists to demonstrate various pathogenic bacteria, including Treponema pallidum, Legionella pneumophila, Bartonella henselae, Borrelia burgdorferi, Helicobacter pylori, and Klebsiella (Calymmobacterium) granulomatis in formalin-fixed, paraffin-embedded patient tissues.16–20
Shortly following the discovery of RMSF during the early 1900s, the pathologist S.B. Wolbach used a modified Giemsa stain to identify R. rickettsii in endothelial and smooth muscle cells in small blood vessels of patients with fatal RMSF (Fig. 1(A)).21,22 Other pathologists effectively used this technique during the first half of the twentieth century to further characterize the pathology of fatal RMSF.23–25 Nonetheless, Giemsa stain has not been consistently or successfully used in contemporary histopathological studies of RMSF, and some investigators have noted limitations in its interpretation, particularly the non-specific staining of cellular debris.26,27 R. rickettsii and other species of pathogenic spotted fever group Rickettsia (SFGR) bacteria stain poorly with tissue Gram methods and, during the last 40 years, pathologists relied almost exclusively on antibody-based staining techniques, including immunofluorescence, immunoperoxidase, and immunoalkaline phosphatase methods, to demonstrate R. rickettsii in tissues of patients with RMSF.28–30 More recently, pathologists have developed molecular methods to detect SFGR in formalin-fixed tissues.31 Although antibody-based and molecular assays are highly sensitive and specific for SFGR, these methods characteristically require specialized reagents, instrumentation, and expertise that are often limited to research institutions and national or international reference centers.
Figure 1.
(A) Pen and ink drawing by E.R. Piotti of an arteriole from the skin of a man who died from Rocky Mountain spotted fever (RMSF) during the early twentieth Century. Reproduced from Wolbach, SB. Studies on Rocky Mountain spotted fever. J Med Res. 1919;41:1–197 © Public Domain. (B) Minute, rod-shaped Rickettsia rickettsii bacteria (arrowheads), demonstrated by the Warthin–Starry method, in the cytoplasm of endothelium and smooth muscle cells of the media of an interlobular artery in the kidney of an infant who died from RMSF (original magnification ×100). Image produced by author and adapted from Plate 19, Figure 66 in reference [22]
A silver impregnation technique, described as the method of Morozov, was used by early Russian investigators to examine Rickettsia species in smear preparations made from infected arthropods and animal tissues,32 but there are no descriptions of SFGR in formalin-fixed, paraffin-embedded human tissues using standard argyrophil-based techniques. Herein we report the validation of these methods to demonstrate the distribution of R. rickettsii in tissues of patients with fatal RMSF.
Methods and Materials
Patients
Tissues were obtained at autopsy from patients from 10 US states who died from RMSF during 2005–2013. The 10 patients ranged in age from 1–59 years (median, 5 years), comprised four females and six males, and died 6–12 days (median, 9 days) following illness onset. The tissues evaluated included lung, liver, kidney, spleen, skin, adrenal gland, testis, and central nervous system. Infection with R. rickettsii was confirmed in all of the decedents using immunohistochemical and molecular techniques.
Molecular methods
To confirm the presence of DNA of R. rickettsii in patient tissues, four 16-μm sections were cut from each of one or more paraffin blocks from each patient and placed in microcentrifuge tubes. The sections were de-paraffinized with xylene and washed twice with absolute ethanol. Tissues were then incubated overnight at 56 °C in 180 μL buffer ATL and 20 μL proteinase K (QIAGEN, Valencia, CA). Extraction of the supernatant was completed using a Qiagen QIAamp deoxyribonucleic acid (DNA) Micro Kit with a final elution volume of 50 μL. A real-time polymerase chain reaction (PCR) assay, adapted to detect rickettsial nucleic acids obtained from formalin-fixed tissues,31 was used to amplify a segment of a hypothetical protein (A1G_04230) gene of R. rickettsii.33 Reactions included 12.5 μl of Qiagen QuantiTect Multiplex PCR Master Mix, 0.2 μM of each of primers RRi6_F and RRi6_F and probe RRi6_P, and 2.5 μl of DNA extract in a 25 μl volume.31 Cycling conditions consisted of one cycle of 95°C for 15 min, and 45 cycles of 94°C for 1 min, and 60°C for 90 s using an Agilent Mx3005P real-time thermal cycler. All PCR reactions included appropriate positive and negative controls, and samples were considered positive if the Ct value was ≤40.
Histochemical methods
Serial sections were cut at 4 -μm thickness from formalin-fixed, paraffin-embedded tissue blocks and evaluated by Warthin–Starry, Dieterle’s, and Steiner techniques using commercially available reagents and according to the manufacturer’s recommendations. The Warthin–Starry Stain Kit was optimized for use on the Artisan™ Staining System (Dako, Carpenteria, CA) and is an automated technique comprising three reagents: gelatin in deionized water containing 0.5% ProClin™ 300, a 1% silver nitrate solution, and a 0.13% hydroquinone reducing solution. The Dieterle’s Stain Kit (American MasterTech, Lodi, CA) supports a manual procedure comprising six reagents: Wiley’s Solution, Solution A, Solution B, hydroquinone capsules, 10% zinc formalin, and alcoholic gum mastic. The Steiner Stain Kit (American Mastertech, Lodi, CA) supports a manual procedure comprising six reagents: an oxidizer solution, 0.1% silver nitrate, hydroquinone capsules, 10% zinc formalin, 2.5% gum mastic, and 1% silver nitrate. Vero E6 cells infected with R. rickettsii were pelleted by centrifugation, fixed in 10% neutral buffered formalin, and embedded in paraffin for use as a positive control sample.
Immunohistochemistry
Three-μm sections cut from formalin-fixed, paraffin-embedded tissue specimens were stained using an immunoalkaline phosphatase technique with naphthol fast-red and Mayer’s hematoxylin counterstain. The primary antibody for this assay is a rabbit polyclonal anti-R. rickettsii antiserum diluted at 1/500.30 This technique is specific for SFGR species and does not react with other bacterial pathogens of vascular endothelium, including typhus group Rickettsia species or Orientia tsutsugamushi (the agent of scrub typhus).
Results
DNA of R. rickettsii was detected using a real-time PCR assay and rickettsiae were identified by immunohistochemical (IHC) stain in tissue specimens of each of the ten patients evaluated in this study. Rickettsiae were also demonstrated in one or more tissues of all patients, as well as the R. rickettsii-infected Vero E6 cell control, using argyrophil-based methods. With these techniques, R. rickettsii appeared as small, dark brown-to-black lanceolate rods, often in pairs and occasionally surrounded by a faint halo. The appearance and distribution of rickettsiae using argyophil techniques resembled closely those seen by the IHC method (Fig. 2), except for additional IHC staining of fragmented antigens that co-localized frequently with intact bacteria.
Figure 2.
Demonstration of Rickettsia rickettsii bacteria (arrowheads) in the liver of a man who died from Rocky Mountain spotted fever using the Warthin–Starry silver impregnation method (A and C) and an immunohistochemical stain specific for spotted fever group rickettsiae (B and D). The distribution and appearance of the minute, rod-shaped rickettsiae, localized to the cytoplasm of endothelial cells in a portal area capillary (A and B) and portal arteriole (C and D), are identical in sequentially stained sections (original magnifications ×158)
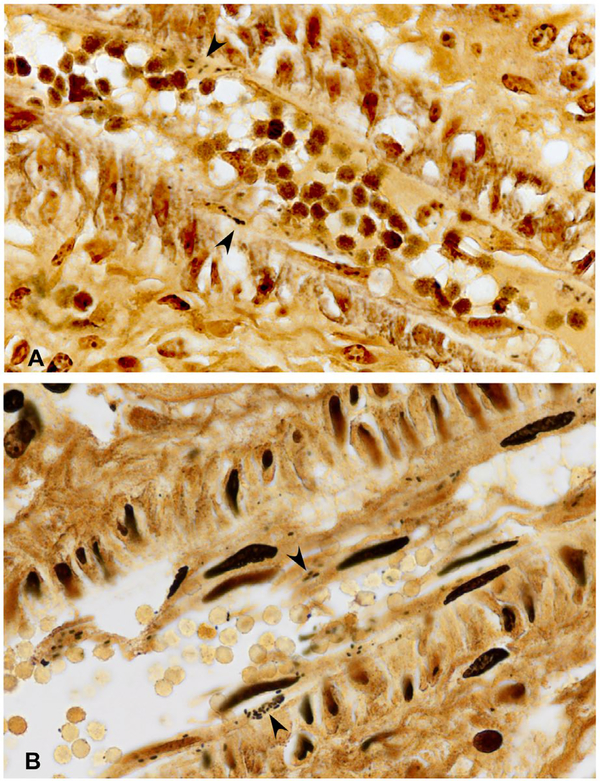
By use of Warthin–Starry, Dieterle’s and Steiner methods, R. rickettsii bacteria were found most consistently in small arteries and arterioles of infected tissues. In these vessels, rickettsiae localized to the cytoplasm of endothelial cells and less often within the internal elastic lamella and smooth muscle of the media (Figs. 1(B), 2(A and C), 3(A and B). Rickettsiae were also clearly identified in endothelium lining alveolar capillaries (Fig. 4(A)) and hepatic sinusoids (Fig. 4(B)). Abundant intracellular rickettsiae were frequently identified in sloughed, spindle-shaped cells within the lumens of small arteries and arterioles and occasionally in sinusoids of the liver (Fig. 5). Of the multiple tissues evaluated, rickettsiae were most easily detected in arterioles and small arteries in the portal areas of the liver, interlobular arteries of the kidneys, and arterioles and venules of the leptomeninges. No distinct rickettsiae were identified in the single segments of skin evaluated in this study. Of the methods assessed, the Warthin–Starry technique generally resulted in the least background staining, particularly in the spleen and liver, making it easier to interpret the slides.
Figure 3.
Abundant intracellular Rickettsia rickettsii bacteria (arrowheads) in endothelial cells of an interlobular artery from the kidney of a child who died from Rocky Mountain spotted fever, demonstrated using Warthin–Starry (A) and Dieterle’s (B) silver impregnation methods (original magnifications ×158)
Figure 4.
A. Rickettsia rickettsii bacteria (arrowhead) in alveolar capillary endothelial cells from the lung of a child who died from RMSF, as demonstrated using the Dieterle’s method. B. R. rickettsii bacteria (arrowhead) in sinusoidal endothelial cells in the liver of a man with fatal RMSF, Warthin–Starry method (original magnifications ×158)
Figure 5.
Minute rod-shaped Rickettsia rickettsii bacteria (arrowheads) in sloughed endothelial cells in a portal arteriole (upper right) and in an adjacent hepatic sinusoid of a man with fatal RMSF (Steiner method, original magnification ×158)
Discussion
Silver impregnation methods provide detailed resolution of R. rickettsii in intact vascular endothelium and smooth muscle, as well as in detached intravascular endothelial and mononuclear cells in multiple tissues of patients with fatal RMSF. The morphology and distribution of R. rickettsii in the microvasculature of the patients described in this study are identical to the features documented by Wolbach nearly a century ago.21,22 Wolbach’s meticulously detailed descriptions provided the first definitive identification of the pathogen in human tissues and established the microbiological and histological foundation for the pathogenesis of this infection. Wolbach accomplished these observations using a slightly alkalinized preparation of Giemsa’s stain applied to tissues fixed in Zenker’s fluid. Wolbach emphasized that it was almost impossible to demonstrate the bacteria using this stain if the tissues were processed in Schaudin’s fixative, as originally recommended by Giemsa.22 In this context, it is likely that subtleties of an effective and relatively simple histochemical technique were lost over time, prompting rickettsiologist and pathologist D.H. Walker to comment that the ‘… diagnosis of rickettsial disease by staining of the organisms in tissues … is virtually a lost art’.34 Indeed, more recently, argyrophil techniques have been applied, albeit unsuccessfully, to cases of fatal RMSF.35,36 To our knowledge, this is the first application of these methods to consistently identify SFGR bacteria in formalin-fixed human tissues.
Wolbach did not address specifically the process by which R. rickettsii are disseminated to various tissues and organs of the body, and even today, the sequence of events leading to systemic infection remains incompletely characterized.37,38 Infection of endothelium by SFGR causes varied disruptions in the form and function of these cells, including discontinuity of the intercellular adherens junctions >36 h following infection.39 This study and others identified R. rickettsii within detached, intraluminal and intrasinusoidal endothelial cells. Detached endothelial cells are frequently packed with rickettsiae, creating infectious ‘rafts’ that might be distributed to other tissues and organs to lodge in capillary beds, or adhere to uninvolved endothelium of distant small-caliber vessels. Indeed, investigators have detected circulating endothelial cells infected with Rickettsia conorii in the peripheral blood of patients with Mediterranean spotted fever.40
One or more of these techniques is available to most diagnostic pathology laboratories; these methods may be useful in establishing a presumptive diagnosis of RMSF, particularly when autopsy tissues are available for evaluation in cases of fatal disease, and in resource-challenged settings where rapid access to immunohistochemical staining or molecular techniques to detect spotted fever group Rickettsia species is limited or non-existent.4,5,9–12,41,42 The automated Warthin–Starry technique was the most simple to perform and provided the least background; nonetheless, many laboratories may not have access to this type of instrumentation. In the absence of automated techniques, the Steiner method was the simplest of these methods to perform.
Many other infectious conditions, including rat bite fever, Staphylococcus aureus sepsis, meningococcemia, leptospirosis, infective endocarditis, malaria, and dengue, as well as some non-infectious diseases, such as thrombotic thrombocytopenic purpura, cause life-threatening illnesses that may be confused clinically with RMSF.43–47 Silver impregnation techniques will accentuate most pathogenic bacteria in tissues, including Streptobacillus moniliformis, Staphylococcus aureus, Neisseria meningitidis, Leptospira species, and other bacteria that cause sepsis syndromes; nonetheless, spotted fever and typhus group Rickettsia species, and O. tsutsugamushi are unique among bacterial pathogens of humans in that these organisms localize predominantly to vascular endothelium.38 In this context, identification of small, argyrophilic, rod-shaped bacilli in the endothelium of small vessels in a patient with a fatal, rash-associated illness provides strong supportive evidence of RMSF. Further studies are needed to determine if O. tsutsugamushi or other pathogenic Rickettsia species are similarly demonstrated in tissues by silver impregnation methods.
Argyrophil techniques accentuate other cellular structures and foreign material, including carbon particles, lipofuscin, and neurosecretory granules which occasionally hinders interpretation of these methods. This study was limited by a focused evaluation of tissues obtained from patients with fatal disease; the application of silver impregnation methods to skin biopsy specimens from patients with less severe disease may be far less sensitive. Nonetheless, this evaluation demonstrates the continued utility of core histochemical methods in the diagnosis of infectious agents in pathology specimens, and may be particularly applicable in many regions of the western hemisphere where RMSF is endemic but where access to immunohistochemical or molecular methods may be limited.
Footnotes
One continuing education hour can be earned by reading this article and taking a short test; for details see www.nsh.org
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 USC. 105, no copyright protection is available for such works under US Law.
References
- 1.Paddock CD, Holman RC, Krebs JW, Childs JE. Assessing the magnitude of fatal Rocky Mountain spotted fever in the United States: comparison of two national data sources. Am J Trop Med Hyg. 2002;67:349–54. [DOI] [PubMed] [Google Scholar]
- 2.Dahlgren FS, Holman RC, Paddock CD, Callinan LS, McQuiston JH. Fatal Rocky Mountain spotted fever in the United States, 1999–2007. Am J Trop Med Hyg. 2012;86:713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drexler NA, Dahlgren FS, Heitman KN, Massung RF, Paddock CD, Behravesh CB. National surveillance of spotted fever group rickettsioses in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ripoll CM, Remondegui CE, Ordonez G, Arazamendi F, Fusaro H, Hyman MJ, et al. Evidence of rickettsial spotted fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am J Trop Med Hyg. 1999;61:350–4. [DOI] [PubMed] [Google Scholar]
- 5.Paddock CD, Fernandez S, Echenique GA, Sumner JW, Reeves WK, Zaki SR, et al. Rocky Mountain spotted fever in Argentina. Am J Trop Med Hyg. 2008;78:687–92. [PubMed] [Google Scholar]
- 6.de Sá Del Fiol F, Junqueira FM, Pereira da Rocha MC, de Toledo MI, Filho SB. A febre maculosa no Brasil [Spotted fever in Brazil]. Rev Panam Salud Publ. 2010;27:461–6. [DOI] [PubMed] [Google Scholar]
- 7.Hun L, Herrero L, Fuentes L, Vargas M. Tres nuevos casos de fiebre manchada de las Montañas Rocosas en Costa Rica [Three new cases of Rocky Mountain spotted fever in Costa Rica]. Rev Costarric Cien Méd. 1991;12:51–6. [Google Scholar]
- 8.Arguello AP, Hun L, Rivera P, Taylor L. A fatal urban case of Rocky Mountain spotted fever presenting as an eschar in San José, Costa Rica. Am J Trop Med Hyg. 2012;87:345–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hidalgo M, Miranda J, Heredia D, Zambrano P, Vesga JF, Lizarazo D, et al. Outbreak of Rocky Mountain spotted fever in Córdoba, Colombia. Mem Inst Oswaldo Cruz. 2011;106:117–8. [DOI] [PubMed] [Google Scholar]
- 10.De Lara Huerta J, Barragán RC. Fiebre manchada de las Montañas Rocosas en pediatría. Revisión clínica de una serie de 115 casos [Rocky Mountain spotted fever in children. Clinical review of a series of 115 patients]. Rev Enferm Infec Ped. 2008;22:4–9. [Google Scholar]
- 11.A lvarez-Hernandez G, Murillo-Benitez C, Candia-Plata MC, Moro M. Clinical profile and predictors of fatal Rocky Mountain spotted fever in children from Sonora, Mexico. Pediatr Infect Dis J. 2015;34: 125–30. [DOI] [PubMed] [Google Scholar]
- 12.Tribaldos M, Zaldivar Y, Bermudez S, Samudio F, Mendoza Y, Martinez AA, et al. Rocky Mountain spotted fever in Panama: a cluster description. J Infect Dev Ctries. 2011;5:737–41. [DOI] [PubMed] [Google Scholar]
- 13.Warthin AS, Starry AC. A more rapid and improved method of demonstrating spirochetes in tissues (Warthin and Starry’s cover-glass method). Am J Syph Gon Ven Dis. 1920;4:97–103. [Google Scholar]
- 14.Steiner G Ueber eine neue Spirochätendarstellung im Gefrierschnitt [About a new spirochete representation in frozen sections]. München Med Wochenschr. 1922;69:121. [Google Scholar]
- 15.Dieterle RR. Method for demonstration of Spirochaeta pallida in single microscopic sections. Arch Neurol Psych. 1927;18:73–80. [Google Scholar]
- 16.Chandler FW, Hicklin MD, Blackmon JA. Demonstration of the agent of Legionnaires’ disease in tissue. N Engl J Med. 1977;297:218–1220. [DOI] [PubMed] [Google Scholar]
- 17.Wear DJ, Margileth AM, Hadfield TL, Fischer GW, Schlagel CJ, King FM. Cat scratch disease: a bacterial infection. Science. 1983;221:1403–5. [DOI] [PubMed] [Google Scholar]
- 18.Berger BW, Clemmensen OJ, Ackerman AB. Lyme disease is a spirochetosis. Am J Dermatopathol. 1983;5:111–24. [PubMed] [Google Scholar]
- 19.Duray PH, Kusnitz A, Ryan J. Demonstration of the Lyme disease spirochete by a modified Dieterle stain method. Lab Med. 1985;16:685–7. [Google Scholar]
- 20.Rollason TP, Stone J, Rhodes JM. Spiral organisms in endoscopic biopsies of the human stomach. J Clin Pathol. 1984;37:23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.W olbach SB. The etiology and pathology of Rocky Mountain spotted fever (third preliminary report). The occurrence of the parasite and the pathology of the disease in man. Additional notes on the parasite. J Med Res. 1918;37:499–508. [PMC free article] [PubMed] [Google Scholar]
- 22.Wolbach SB. Studies on Rocky Mountain spotted fever. J Med Res. 1919;41:1–197. [PMC free article] [PubMed] [Google Scholar]
- 23.Pinkerton H, Maxcy KF. Pathological study of a case of endemic typhus in Virginia with demonstration of Rickettsia. Am J Pathol. 1931;7:95–104. [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PN. Histological study of a case of the Eastern type of Rocky Mountain spotted fever. Am J Pathol. 1933;9:91–103. [PMC free article] [PubMed] [Google Scholar]
- 25.Lillie RD. Pathology of Rocky Mountain spotted fever. Nat Inst Hlth Bull. 1941;177:1–59. [Google Scholar]
- 26.Castleman B, Scully RE, McNeely BU. Case records of the Massachusetts General Hospital. Case 26–1973. N Engl J Med. 1973;288:1400–4. [DOI] [PubMed] [Google Scholar]
- 27.Conlon PJ, Procop GW, Fowler V, Eloubeidi MA, Smith SR, Sexton DJ. Predictors of prognosis and risk of acute renal failure in patients with Rocky Mountain spotted fever. Am J Med. 1996;101:621–6. [DOI] [PubMed] [Google Scholar]
- 28.Walker DH, Cain BG. A method for specific diagnosis of Rocky Mountain spotted fever on fixed, paraffin-embedded tissue by immunofluorescence. J Infect Dis. 1978;137:206–9. [DOI] [PubMed] [Google Scholar]
- 29.Dumler JS, Gage WR, Pettis GL, Azad AF, Kuhadja FP. Rapid immunoperoxidase demonstration of Rickettsia rickettsii in fixed cutaneous specimens from patients with Rocky Mountain spotted fever. Am J Clin Pathol. 1990;93:410–4. [DOI] [PubMed] [Google Scholar]
- 30.Paddock CD, Greer PW, Ferebee TL, Singleton J Jr, McKechnie DB, Treadwell TA, et al. Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically unconfirmed disease. J Infect Dis. 1999;179:1469–76. [DOI] [PubMed] [Google Scholar]
- 31.Denison AM, Amin BD, Nicholson WL, Paddock CD. Detection of Rickettsia rickettsii, Rickettsia parkeri, and Rickettsia akari in skin biopsy specimens using a multiplex real-time polymerase chain reaction assay. Clin Infect Dis. 2014;59:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Z drodovskii PF, Golinevich HM. The rickettsial diseases. New York, NY: Permagon Press, Inc.; 1960. p. 21. [Google Scholar]
- 33.Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, Massung RF. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol. 2013;51:314–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker DH. Diagnosis of rickettsial diseases. Pathol Ann. 1988;2: 69–96. [PubMed] [Google Scholar]
- 35.G reen WR, Walker DH, Cain BG. Fatal viscerotropic Rocky Mountain spotted fever. Report of a case diagnosed by immunofluorescence. Am J Med. 1978;64:523–8. [DOI] [PubMed] [Google Scholar]
- 36.Samuels MA, Newell KL. Case 32–1997. A 43-year-old woman with rapidly changing pulmonary infiltrates and markedly increasing intracranial pressure. N Engl J Med. 1997;337:1149–56. [DOI] [PubMed] [Google Scholar]
- 37.Walker DH. Pathology and pathogenesis of the vasculotropic rickettsioses In: Walker DH, editor. Biology of rickettsial diseases. Boca Raton, FL: CRC Press; 1988. p. 115–38. [Google Scholar]
- 38.V albuena G, Walker DH. Infection of the endothelium by members of the order Rickettsiales. Thromb Haemost. 2009;102:1071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valbuena G, Walker DH. Changes in the adherens junctions of human endothelial cells infected with spotted fever group rickettsiae. Virchows Archiv. 2005;446:379–82. [DOI] [PubMed] [Google Scholar]
- 40.Drancourt M, George F, Brouqui P, Sampol J, Raoult D. Diagnosis of Mediterranean spotted fever by indirect immunofluorescence of Rickettsia conorii in circulating endothelial cells isolated with monoclonal antibody-coated immunomagnetic beads. J Infect Dis. 1992;166:660–3. [DOI] [PubMed] [Google Scholar]
- 41.Zavala-Castro JE, Zavala-Velázquez JE, Walker DH, Arcila EER, Laviada-Molina H, Olano JP, et al. Fatal human infection with Rickettsia rickettsii, Yucatán, Mexico. Emerg Infect Dis. 2006;12:672–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estripeaut D, Aramburú MG, Sáez-Llorens X, Thompson HA, Dasch GA, Paddock CD, et al. Rocky Mountain spotted fever, Panama. Emerg Infect Dis. 2007;13:1763–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portnoy BL, Satterwhite TK, Dyckman JD. Rat bite fever misdiagnosed as Rocky Mountain spotted fever. South Med J. 1979; 72:607–8. [DOI] [PubMed] [Google Scholar]
- 44.Milunski MR, Gallis HA, Fulkerson WJ. Staphylococcus aureus septicemia mimicking fulminant Rocky Mountain spotted fever. Am J Med. 1987;83:801–3. [DOI] [PubMed] [Google Scholar]
- 45.Z avala-Velasquez JE, Yu XJ, Walker DH. Unrecognized spotted fever group rickettsiosis masquerading as dengue fever in Mexico. Am J Trop Med Hyg. 1996;55:157–9. [DOI] [PubMed] [Google Scholar]
- 46.B aggett MV, Turbett SE, Schwartzenberg SS, Stone JR. Case 5–2014: a 59-year-old man with fever, confusion, thrombocytopenia, rash, and renal failure. N Engl J Med. 2014;307:651–60. [DOI] [PubMed] [Google Scholar]
- 47.Turner RC, Chaplinski TJ, Adams HG. Rocky Mountain spotted fever presenting as thrombotic thrombocytopenic purpura. Am J Med. 1986;81:153–7. [DOI] [PubMed] [Google Scholar]