Abstract
The organic compounds found in carbonaceous chondrite meteorites provide insight into primordial solar system chemistry. Evaluating the formation and decomposition mechanisms of meteoritic amino acids may aid our understanding of the origins of life and homochirality on Earth. The amino acid glycine is widespread in meteorites and other extraterrestrial environments; other amino acids, such as isovaline, are found with enantiomeric excesses in some meteorites. The relationship between meteoritic amino acids and other compounds with similar molecular structures, such as aliphatic monoamines and monocarboxylic acids is unclear; experimental results evaluating the decomposition of amino acids have produced inconclusive results about the preferred pathways, reaction intermediates, and if the conditions applied may be compatible with those occurring inside meteoritic parent bodies. In this work, we performed extensive tandem metadynamics, umbrella sampling, and committor analysis to simulate the neutral mild hydrothermal decomposition mechanisms of glycine and isovaline and put them into context for the origins of meteoritic organic compounds. Our ab initio simulations aimed to determine free energy profiles and decomposition pathways for glycine and isovaline. We found that under our modeled conditions, methylammonium, glycolic acid, and sec-butylamine are the most likely decomposition products. These results suggest that meteoritic aliphatic monocarboxylic acids are not produced from decomposition of meteoritic amino acids. Our results also indicate that the decomposition of L-isovaline prefers an enantioselective pathway resulting in the production of (S)-sec-butylamine.
Keywords: ab initio molecular dynamics, enhanced sampling, meteoritic organics, parent body processes, glycine, isovaline
Graphical abstract

INTRODUCTION
Carbonaceous chondrites are natural reaction vessels carrying within their mineral matrix the chemical fingerprint of the early solar system. Their study provides insights into the prebiotic organic synthesis that occurred in the presolar nebula, the protoplanetary disk, and during asteroidal aqueous and thermal processing.1,2 Meteoritic aliphatic amino acids have been found in representatives of all carbonaceous chondrite groups, have become the most heavily studied organic compounds in meteorites3–5 because of their contribution to the prebiotic inventory available for the origins of life on Earth. The organic matter accreted and/or synthesized inside asteroids and comets could have been an important source of substrates for the origin and early evolution of life on Earth (and possibly elsewhere). Amino acids can be chiral (exhibit handedness) and only left-handed amino acids (L-enantiomers) are translated in biology (homochirality). In the absence of a chiral driving force, the abiotic synthesis of amino acids results in racemic mixtures (1:1 ratio of L-and D-enantiomers). The transition from racemic abiotic chemistry to homochiral life remains an unanswered key question in origins-of-life research.6,7 Some meteoritic amino acids possess L-enantiomeric excess, with the best studied example of these being isovaline (e.g., present at up to 18% L-excessǂ in the Murchison,8 and other meteorites)9. The presence of non-racemic meteoritic amino acids suggests that homochirality on Earth could have been seeded and developed from the delivery of L-enantioenriched amino acids by carbonaceous chondrites.
Low-temperature aqueous alteration of meteoritic parent bodies may have occurred in melting water ices that resulted from heating caused by radioactive decay of short-lived radionuclides and/or by impacts throughout the history of the asteroid parent body.10–12 These icy inclusions may have contained the starting materials needed for the formation of meteoritic amino acids through the Strecker synthesis, Michael addition, and/or other synthetic routes.13–15 Aqueous processes in the parent body of the Murchison and other carbonaceous chondrites belonging to varying petrologic types have been proposed to occur at pH ranging from 6–12,16 however, this value may greatly depend on the temperature of the system, adsorption of ions, flows of gases and fluids, and the specific mineral composition of the sample, among other parameters.17 Additionally, continuous heating of these aqueous inclusions may have resulted in the decomposition of synthesized amino acids through interactions with liquid water and mineral.18–20 It has been suggested that the decomposition of amino acids through deamination and decarboxylation driven by aqueous and thermal processes inside the asteroid parent body may result in the formation of carboxylic acids and amines.21–23 Both aliphatic carboxylic acids and amines have been isolated from carbonaceous chondrites.21–26 To date, however, the role of amino acids in the production of carboxylic acids and amines through their decomposition under asteroid-like conditions remains poorly understood.27
In this work, we modeled the effects of asteroidal aqueous processing on the decomposition of amino acids leading to the formation of monocarboxylic acids and monoamines (hereafter called “carboxylic acids” and “amines”). We selected the amino acids glycine and isovaline as proxies for studies of amino acid decomposition under meteoritic conditions using full atomistic molecular dynamics (MD) simulations based on density functional theory (DFT). Glycine is a common achiral amino acid which has been identified in all carbonaceous chondrites and in other extraterrestrial environments, including lunar regolith and comets.28–31 The chiral amino acid isovaline is rare in the terrestrial biosphere but common in many carbonaceous chondrites and is of interest because excesses of its L-enantiomer have been measured in several meteorites,8,9,32–34 creating a potential link between its exogenous delivery to Earth and the origins of terrestrial biological homochirality. Our computational study aimed to unveil prospective synthetic relationships between meteoritic amino acids, carboxylic acids, and amines and the maturation of meteoritic organics prior to, during, and after their formation in the asteroid parent body.
The key component of this work was assessing the decomposition mechanisms of amino acids in hydrothermal aqueous solution and identifying their decomposition products to establish potential links for the origins of some meteoritic soluble organic compounds. Experimental results on the decomposition of amino acids often produce controversial and, in some cases, contradictory results,35 leaving unclear whether amino acids would preferentially decompose through decarboxylation or deamination and what the identity of their reaction intermediates are under neutral hydrothermal conditions that may be compatible with those experienced inside the asteroid parent body. Our recently developed approach, which defines a class of general topological reaction coordinates, is particularly well suited for complex multistep mechanisms in aqueous media.36 We performed ab initio molecular dynamics simulations (Born-Oppenheimer) combined with advanced techniques for enhanced sampling of rare events and the reconstruction of free energy landscapes and kinetics (metadynamics, umbrella sampling, weighted histogram analysis, committor analysis)37–38. Our simulation clarifies the decomposition products and synthetic origins of prebiotic molecules in meteorites related to glycine and isovaline, shedding new light on fundamental atomic-level mechanisms behind meteoritic prebiotic chemistry.
METHODS
Ab initio molecular dynamics and free energy calculations
We explored the decomposition products of glycine and isovaline using ab initio MD, which has well demonstrated its predictive power in describing amino acid synthesis in computer simulations of the Miller experiments.39 The asteroidal conditions were constrained based on previous meteoritic petrologic analyses, which suggest that aqueous alteration occurred between 0 and 340 °C).16,40–43 Amino acids may be present as neutral zwitterions inside the parent body based on large abundances of ionic species (cation and anions from mineral complexes and water-soluble salts). To model asteroid-like hydrothermal processing, we selected a temperature of 100 °C and an aqueous environment in which amino acids would be present as zwitterions. We acknowledge that the conditions for hydrothermal processing situates the reactions in bulk water, without explicit consideration of minerals and the presence of species such as a CO2, CO, and ammonia commonly found in meteorites;24,44 however, the chosen conditions provide fundamental information on amino acid reaction pathways that may serve as a reference for future experimental and computational works focusing on, for example, the specific effects of particular mineral surfaces on amino acid decomposition.
Born-Oppenheimer MD simulations (at T = 373 K) were performed using interatomic forces computed at DFT level (Perdew-Burke-Ernzerhof functional with Grimme’s van-der-Waals corrections).45,46 Kohn-Sham valence wave functions were expanded on a plane wave basis, as implemented in the CPMD code47 optimally designed for massively parallel computers. Free energy landscapes were reconstructed employing in tandem metadynamics48 and umbrella sampling;49 the former for a fast discovery of reaction pathways and preliminary estimation of barriers, and the latter for a precise sampling of selected pathways. A key component of our approach are path collective variables based on coordination patterns,38 that allow us to discover unforeseen reaction mechanisms and to obtain reversible pathways without human prejudice about transition states or intermediates. The model consisted of a periodically repeated box including one amino acid molecule (glycine or isovaline) solvated with 79 water molecules. Our simulations followed protocols similar to those previously published,40 and exploited the plugin Plumed;50 further details are provided in Supplementary Information. We cannot exclude the existence of amino acid decomposition pathways different from those described in the following sections. However, the reaction mechanisms characterized in this work are the result of extensive ab initio simulations, totaling more than 3 ns (80 ps of equilibration trajectories, 300 ps of exploratory metadynamics, 2000 ps of umbrella sampling, and 1000 ps of committor analysis), equivalent to 133 to 1.3 million Intel® Xeon® E5–2690v3 hours. For comparison, the majority of published ab initio molecular 134 dynamics studies of chemical reactivity are limited to a total simulation length of tens of ps.
RESULTS
Decomposition of amino acids
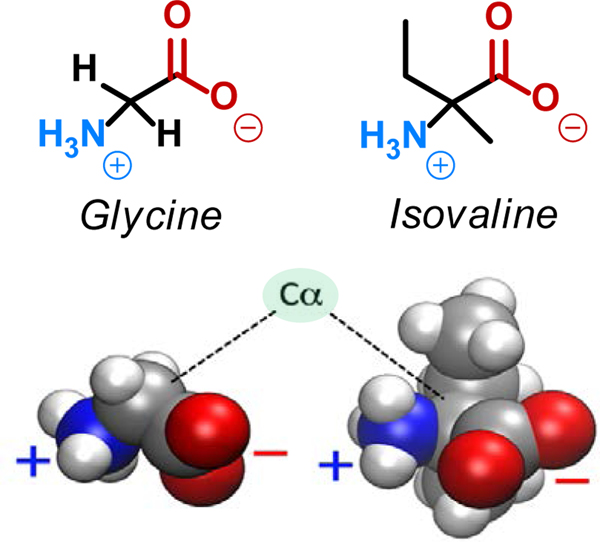
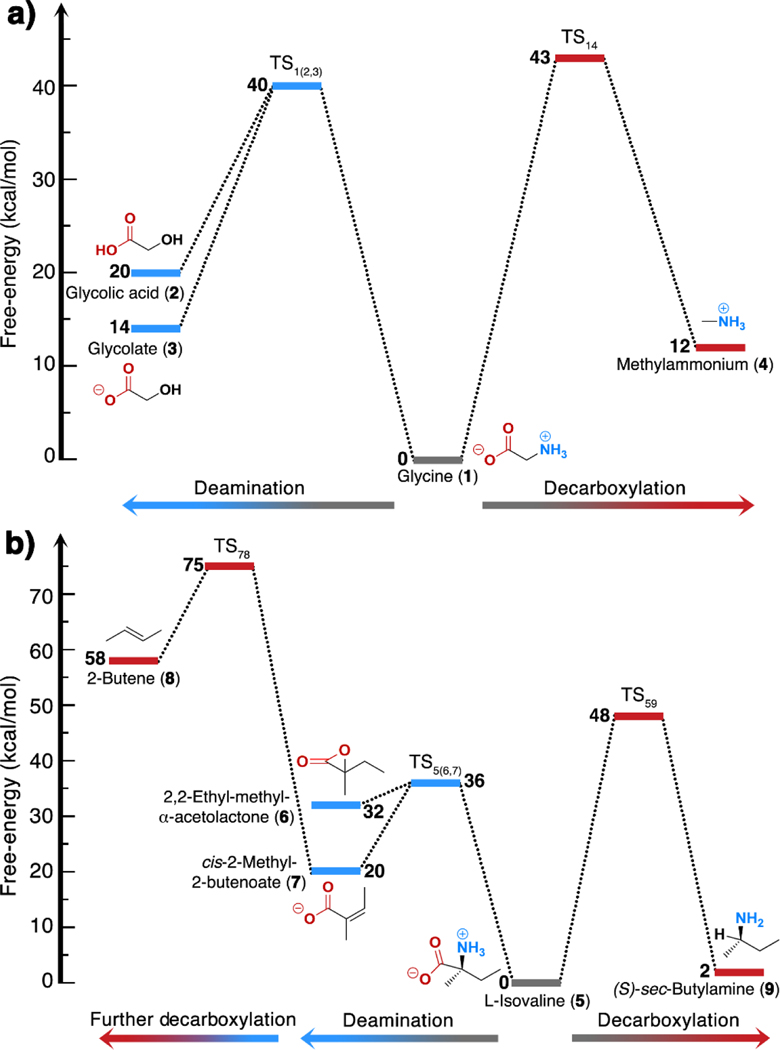
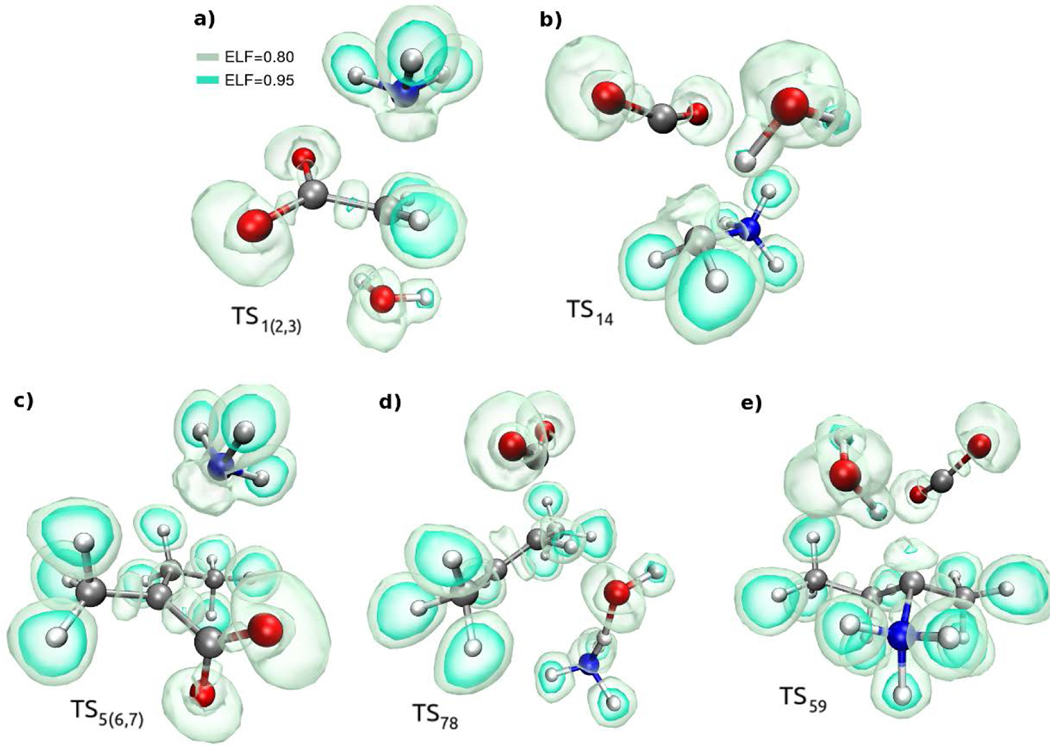
Glycine and isovaline differ at the alpha-carbon (Cα; Scheme. 1), leading to significant differences in their decomposition pathways; in glycine, the Cα is accessible to the solvent, whereas in isovaline the Cα is protected by a coordination shell of four heavy atoms. We investigated the decomposition of glycine and isovaline through deamination or decarboxylation pathways in simulated asteroidal hydrothermal processing under aqueous neutral conditions, the investigated mechanisms are shown in Figure 1 panels a and b. Additionally, we show the schematic summaries of the step-by-step reaction mechanisms for glycine and isovaline decompositions in Figures S1, S2, and Movies S1-S6 available in the Supplementary Information. Corresponding free energy profiles and landscapes are shown in Figure 2 and Figure 3 respectively, and representative transition state and electronic structures are depicted in Figures 4 and 5.
Scheme 1.
Structures of glycine and isovaline zwitterions (color highlights the amine [blue, NH2] and acid [red, CO2H] functional groups). Illustration of the different degree of steric hindrance around the alpha carbon (Cα).
Figure 1.
Decomposition reactions of glycine (A) and isovaline (B) evaluated in this work. All simulations are performed in bulk water solution at T = 373 K. Reactions marked with an X never occur in our simulations: see the related discussion in the Results section. We equate (S)-to L-enantiomers for consistency to the nomenclature used in previous meteoritic amino acid studies.
Figure 2.
Schematic free-energy profiles of a) glycine and b) isovaline hydrothermal decomposition 606 processes from ab initio simulations. Statistical uncertainties are estimated as ±1 kcal/mol.
Figure 3.
Free-energy landscapes of chemical reactions at T=373 K as a function of path collective variables based on distances between atomic coordination patterns36. The landscapes are obtained from weighted histogram analysis applied to ab initio umbrella sampling simulations, a) glycine deamination to (2), b) glycine deamination to (3), c) glycine decarboxylation to (4), d) isovaline deamination to (7), e) (7) decarboxylation to (8), and f) isovaline decarboxylation to (9).
Figure 4.
Representative transition state structures, identified by means of committor analysis on umbrella sampling trajectories for a) glycine deamination, b) glycine decarboxylation, c) isovaline deamination, d) angelic acid decarboxylation, and e) isovaline decarboxylation.
Figure 5.
Electronic structure of the transition state configurations, represented by means of different isovalues of the electron localization function.73 Solvent water molecules are present in the calculations but not depicted here, except for those undergoing bond breaking/formation. The corresponding reactions are a) glycine deamination, b) glycine decarboxylation, c) isovaline deamination, d) angelic acid decarboxylation, and e) isovaline decarboxylation. The carbocation character of the Cα is evident in panels a) and c), whereas its carbanion character is evident in panels b) and e).
The two decomposition mechanisms of solvated glycine (1) and isovaline (5) start from their favored zwitterionic species (Figure 1). The deamination of glycine does not show the formation of acetate (2’), which is the simplest aliphatic deamination product one could envisage; indeed, the loss of neutral ammonia from the glycine zwitterion would leave a carbocation species that, formally, would need the addition of a hydride (H–) to form acetate. Note, however, that this reaction is not viable in practice: even if a hydride were present in solution, it would probably undergo a different fate (reduction of the glycine carboxylate group; reaction not shown here). Instead, the deamination of glycine results in two possible end products: neutral glycolic acid (2) and ammonia, or their conjugate base/acid glycolate (3) ammonium salt (Figure 1A). The free energy landscape reveals an identical barrier of 40 kcal/mol for these two deamination results (Figures 2a, 3a, 3b, and Table S1 which shows a summary of the simulated reactions free energy barriers in kcal/mol). However, the two possible end states have different stabilities, with the charged species being lower in free energy by 5 kcal/mol, as expected from the pKa of glycolic acid (3.83 at 300 K) and from the pKb of ammonia (4.7). The configuration of the transition state was identified by committor analysis, and a representative structure is shown in Figure 4a. The transition state is characterized by the partial breaking of the C–N bond with simultaneous splitting of a water molecule and hydroxide (OH–) nucleophilic attack at the Cα (with partial carbocation character; Figures 4a and 5a).
The free energy landscape for glycine decarboxylation is depicted in Figure 3c. The transition state (Figures 4b and 5b) is characterized by the simultaneous i) partial breaking of the C–C bond, ii) partial linearization of the CO2 group, and iii) a half-split water molecule, whose proton moves towards the Cα atom (with partial carbanion character). We estimated a free energy barrier of 43 kcal/mol, very similar to that found for deamination. The product state appears 12 kcal/mol less stable than reactants; however, we note that the further reaction [CO2 + OH– → HCO3–] is expected to decrease the free energy by 10 kcal/mol,51 bringing glycine to a similar stability with respect to [CH3NH3+ + HCO3–] (Figure 2a, Table S1).
Compared to glycine, isovaline (5) displays a similar decarboxylation pathway, leading to the formation of sec-butylamine (9), but a different deamination pathway resulting in products (6 and 7) which in turn may decarboxylate to yield 2-butene (8; Figures 1b and 2b). Similar to the deamination of glycine, no presence of the unsaturated deaminated analog, 2-methyl butanoate (7’) was observed as a deamination product of isovaline (the same argument observed above for glycine can be invoked here). The deamination of isovaline yields two products as the possible outcomes of the same transition state, corresponding to a barrier of 36 kcal/mol (Figures 2b, 3d, 4c, 5c). The first product is 2,2-ethyl-methyl-α-acetolactone (6) (albeit with a C–O–C bridge that is intermittently formed and broken on the picosecond time scale), structurally simple to reach from the transition state by reducing the C–C–O angle. This species is, however, quite unstable, with a free energy 32 kcal/mol higher than isovaline (Figures 2b, 3d). A more stable product, lying at 20 kcal/mol above isovaline, is cis-2-methyl-2-butenoic acid (commonly known as angelic acid, 7) in its carboxylate anionic form, obtained after deprotonation (operated by the leaving ammonia molecule) of the methylene group with concomitant formation of a C=C double bond. We remark that a single transition state (labeled TS5(6,7) in Figures 2–5) leads to compounds 5, 6, 7 with equal probability, as we explicitly tested with committor analysis. Note that the trans isomer of 7, known as tiglic acid, could also be obtained by a similar mechanism, however we did not explicitly perform the latter simulation. Experimentally, pure angelic acid tends to irreversibly convert to tiglic acid, which is more stable by 8.5 kcal/mol than angelic acid as deduced by heats of combustion.52 Therefore, at 100 °C, our simulations suggest that an isovaline decomposition to angelic/tiglic acid will result in an equilibrium ratio of about 10−7 between products and reactants.
We extended our metadynamics simulations to explore possible further evolution of angelic acid (7) toward decarboxylation, by explicitly targeting this reaction with extensive umbrella sampling simulations. Starting from 7, a barrier of 55 kcal/mol leads to the cleavage of the C–COO– bond and protonation of the Cα from a water molecule, eventually resulting in the formation of carbon dioxide and 2-butene (Figures 2b and 3e,). The high barrier may be explained by the relative inaccessibility of the Cα to solvent molecules, due to surrounding bulky groups, until a significant elongation of the C–COO– bond is achieved (Figures 4d and 5d). We remark that if, instead, decarboxylation is attempted starting from 2,2-ethyl-methyl-α-acetolactone (6), the system rapidly evolves first into angelic acid, confirming that this lactone is an unlikely deviation, apparently of scarce relevance in the reconstructed reaction network. Our results suggest that decarboxylation of angelic acid entails a very large free energy barrier, and results in products of very low stability compared to isovaline; thus, it is not expected that 2-butene derived from isovaline would significantly contribute to the observed pool of meteoritic organic molecules.
Direct isovaline decarboxylation proceeds through a simple mechanism (Figure 1), yielding sec-butylamine after crossing a barrier of 48 kcal/mol (Figures 2b, Table S1). This decomposition channel is potentially relevant: compared to deamination, decarboxylation encompasses a much higher barrier (with half-life of 107 years at 100 °C or 104 years at 150 °C, as estimated from Eyring’s transition state theory formula) but, in turn, it leads to a much more stable product (only 2 kcal/mol above isovaline; Figures 2b and 3f). Similar to the reaction connecting angelic acid (7) with 2-butene, the mechanism involves the cleavage of the C–COO– bond and the protonation of the Cα atom from a water molecule (Figures 4e and 5e). At the transition state, the Cα keeps a tetrahedral sp3 character, enabling access to solvent only on the same side as the leaving CO2 moiety and protecting the opposite side by steric hindrance. This is confirmed by inspection of all the available trajectories in the transition state region, including umbrella sampling and committor analysis. Thus, the solvent proton replaces the carboxylate functional group without changing the chirality of the Cα. We conclude that the decarboxylation pathway itself does not lead to sec-butylamine racemization. We note, instead, that the presence of the α-hydrogen on sec-butylamine is generally accepted to strongly increase its hydrothermal racemization rate. In our specific case, this indicates that sec-butylamine derived from isovaline would racemize at a faster rate than its parent amino acid inside the asteroid parent body. Preliminary metadynamics simulations suggested other possible decarboxylation mechanisms, such as simultaneous isovaline decarboxylation and deamination, or the formation of formic acid instead of carbon dioxide; however, such pathways turned out to be unlikely under the scrutiny of umbrella sampling.
DISCUSSION
Comparison to previous computational modeling and experimental results
The current understanding of amino acid decomposition processes in solution is mostly based on experimental information. The most relevant theoretical study to date addressed glycine and alanine decomposition based on a quantum mechanics / molecular mechanics (QM/MM) approach, with the amino acid and up to two water molecules described with a semi-empirical molecular orbital method, immersed in a bath of classical water molecules.53 The previous work yielded a glycine decarboxylation barrier of 46 kcal/mol, close to ours. In our simulations, based on a more accurate quantum description and on more general collective variables, we find that, with respect to the previous work, the products are significantly less stable than the reactants. Concerning glycine deamination, Alexandrova and Jorgensen (2011) reported a free energy barrier of 51 kcal/mol for the formation of an α-lactone, and > 90 kcal/mol for the formation of glycolic acid. Instead, we predict glycolic acid formation as the only viable reaction, with a much lower barrier of only 40 kcal/mol, in good agreement with experiments as discussed below, thus pointing to a more accurate description of the reaction pathway in our framework.
The interpretation of the available experimental results in the literature is controversial. As pointed out by Cox and Seward (2007), various reports using different experimental conditions and reaction vessels often presented contradictory conclusions, so that it is not clear whether glycine and other amino acids preferentially decarboxylate or deaminate, and through which mechanism. In several cases, decarboxylation was a priori assumed to happen, and deamination was not explicitly tested. A decarboxylation enthalpy barrier of 39 kcal/mol above 170 °C was inferred from NMR analyses,54 and similarly from chromatography above 200 °C.51 Snider and Wolfenden (2000) also reported a slow-down of the reaction with increasing ionic strength, suggestive of a transition state less polarized than the reactants, which is consistent with the decomposition mechanisms found in this work. An enthalpy barrier of 33 kcal/mol was inferred from an in situ infrared spectrometer flow reactor above 310 °C.56 The free energy barriers we observed for deamination and decarboxylation are similar within statistical errors (about 2 kcal/mol), and using Eyring formula (k = kBT/h e−ΔG*/kBT) the corresponding decomposition rate is between 10−11 and 10−13 s−1 at 100 °C, equivalent to half-lives between one thousand and several tens of thousands of years; the upper rate is in agreement with experimental measurements.54–56 Given the survival of amino acids in meteorites to the present day, these half-lives would be consistent with suggestions that although there may have been available liquid water for several millions of years inside the parent body, the alteration processes may have only been episodic and not continuous throughout that period of time (i.e. episodes of aqueous alteration may have been triggered by short-term events like impacts).42
Similar to chromatographic experiments,55 and based on our findings, we conclude that deamination and decarboxylation decomposition pathways could be simultaneously available in a hydrothermal glycine solution. The simulations, however, predict the formation of ammonium glycolate and methylammonium bicarbonate ions at a similar pace, with the latter products being much more stable than the former (if the exothermic hydroxylation of CO2 occurs starting from methylammonium) and in similar equilibrium proportions as the glycine reactants. From the kinetic point of view, deamination appears to be moderately favored over decarboxylation, even if the free energy difference of 3 kcal/mol does not appear very significant considering statistical uncertainties as well as the intrinsic accuracy of DFT calculations.57 Therefore, following long-term aqueous conditions on asteroid parent bodies, methylamine is expected as the dominant meteoritic glycine decomposition product (leaving aside the possibility of products themselves entering further reaction pathways). Indeed, the abundance of amines is greater than that of amino acids in meteorites experiencing extended increasing aqueous processing, while the opposite is true in minimally aqueously altered carbonaceous chondrites.23–26 It should be noted that in situ spectrophotometric measurements pointed to sizable glycine dimerization in addition to decomposition.35 However, investigating such additional multi-molecular process is beyond the scope of this work.
Constraints of our asteroid-like processing model
Our work provides detailed quantitative insight about amino acid decomposition reactions in simulated asteroidal hydrothermal conditions; however, discussions of the interpretation and relevance of these results from the point of view of asteroid parent body processes and meteorite organics must consider the following factors. First, we chose a specific temperature and two amino acids particularly representative of meteoritic chemistry: the simplest and most common one (glycine), and the one with the most reports of L-enantiomeric excess in meteorites (isovaline). Temperatures above 100 °C and a higher concentration of protons or catalytic transition metal ions, which may have occurred for extensively altered chondrites, may strongly alter the amino acid decomposition kinetics. Second, the reaction network we reconstructed (Figure 1) is part of a larger scheme in which amino acid decomposition products can take part in further reactions with other species not explicitly considered here. In this sense, even a relatively evanescent intermediate, of apparent low relevance, could increase the available molecular inventory, and possibly play a key role toward a stable product in the more global prebiotic network in the parent body. Third, the reaction rates and equilibrium populations we inferred are pertinent to bulk, neutral water solutions. As such, they provide a reference reaction network that can be used as a starting point to address various questions about the viability of different prebiotic scenarios, including: what is the optimal range of temperature for a given reaction?; would an acidic or alkaline pH favor a synthetic/decomposing pathway?; under what conditions would mineral surfaces play significant role in stabilizing transition states, intermediates, and products? These are questions that future experimental and theoretical efforts may be able to answer, incrementing our understanding about the origins of prebiotic meteoritic organic matter.
Implications for the origins of some meteoritic soluble organic compounds
Although meteoritic organic compounds formed in distinct stages through the birth and evolution of the solar system, we can constrain their origin to two main environments and regimes: (1) pre-parent body, dominated by gas-and ice-grain chemistry that occurred in the molecular cloud, the solar nebula, or the protoplanetary disk, and (2) parent body, dominated by hydrothermal processes. Given the unknown concentration of the molecular species available for chemical development, the unsettled and changing physical conditions such as temperature and pressure, and the uncertain level of processing that occurred in each parent body, it may prove challenging to assess the dominant synthetic routes leading to the origins of meteoritic amino acids.27 However, it is useful to note that (a) the detection of the simplest amino acid (glycine) in the interstellar medium remains controversial,58,59 (b) the observed abundances of meteoritic amino acids and those synthesized after hydrolysis of UV-irradiated interstellar ice analogs decreases with increasing carbon,60,61 and (c) only aminoacetonitrile, but no glycine, has been observed before the hydrolysis of interstellar ice analogs irradiated at temperatures ranging from 20 to 300 K.62 From these observations, it can be suggested that some degree of processing inside the parent body may favor augmenting the molecular abundance and diversity of meteoritic amino acids, and that prolonged processing could result in the amino acid decomposition pathways studied here.
From our computational analyses, we have found that the deamination and decarboxylation of glycine occurs with similar barriers leading to the production of the hydroxylated acid (glycolic acid) and methylamine. In contrast, the deamination and decarboxylation of isovaline occurs at different rates, and it is not the hydroxylated acid, but rather the α-β-unsaturated acid (7) that would be the fastest decomposition product over sec-butylamine, although the amine is expected to dominate at equilibrium by virtue of its higher stability. The difference in decomposition products may originate from the relative inaccessibility of the Cα to solvent molecules in isovaline relative to glycine (see Scheme 1 and electron localization function – ELF-analysis shown in Figure 5). It should be noted, furthermore, that neither the decarboxylation of glycine nor that of isovaline result in the formation of formic acid. This observation suggests that meteoritic formic acid must have formed before the accretion of the parent body, or through reaction mechanisms that are not related to the decomposition of amino acids inside the parent body, e.g., catalytic hydrogenation of CO2, or the hydrolysis of the methyl formate produced by the reaction of methanol and carbon monoxide.
Our results do not provide evidence for the formation of saturated aliphatic monocarboxylic acids, namely, acetic acid and 2-methylbutanoic acid, as deamination products of glycine and isovaline respectively. These carboxylic acids have been detected in various carbonaceous chondrites and constitute some of the most abundant water-soluble organic compounds in meteorites,22,24,25,63 but our calculations suggest that deamination of amino acids does not contribute to their reported abundances. This contrasts with methylamine and sec-butylamine, the corresponding decarboxylation products of glycine and isovaline. This observation suggests that either aliphatic acids originated in cold interstellar pre-solar regions and were later incorporated inside the parent body, or that they formed from organic species that underwent synthetic processes other than glycine and isovaline decomposition, e.g., oxidation of aliphatic aldehydes or hydrolysis of alkyl nitriles inside the parent body.
Our calculations show an α-β-unsaturated compound (angelic acid, 7) as the deamination product of isovaline, with a further decarboxylation leading to 2-butene. The abundance of 7 (or any other α-β-unsaturated monocarboxylic acid) has not been evaluated in analyses of meteoritic organics. Although 2butene has been reported in analyses of extracts from the Murchison meteorite in a mix of isomers,24 this decomposition pathway exhibits a very high barrier that suggests that the meteoritic origins of 2-butene would not be necessarily related to the decomposition of the amino acid, except in cases of extended hydrothermal processing at higher temperatures. Another possibility is that 7 could react to form β-amino acids inside the parent body, considering that α-β-unsaturated carboxylic species readily undergo 1,4nucleophilic additions.64–65 Indeed, the concentration of β-amino acids is higher than that of α-amino acids in more extensively aqueously altered carbonaceous chondrites.34,66 Our computational results of the deamination of isovaline (an α-alkyl-α-amino acid) would support a parent body scenario in which the concentration of β-amino acids increases at the expense of the decomposition of α-amino acids through aqueous alteration. However, the possibility that the higher abundance of β-amino acids may be an inherent characteristic of the parent body’s molecular budget might not be discarded.
The more stable decarboxylation product of isovaline, sec-butylamine, has been found in carbonaceous chondrites exhibiting varying levels of aqueous and thermal processing.28,67 The stability of sec-butylamine is consistent with the idea that at least a portion of this meteoritic amine may be produced upon decomposition of the amino acid either in the parent body stage or through amino acid extraction (the extraction of amino acids from meteoritic samples is typically done using hot water at 100 °C) and sample processing during analyses.
In addition to the detailed decomposition pathways shown here and reading the reactions in Figure 1 in reverse order, it can be predicted that glycine could be obtained from methylamine or from glycolic acid, and isovaline from sec-butylamine or from angelic acid, through relatively-low free energy barriers that range from 15 to 45 kcal/mol. These results suggest that the synthesis of meteoritic amino acids under hydrothermal conditions appear feasible even without invoking the aid of catalyzers or synthetic routes such as the Strecker-cyanohydrin synthesis, similar to recent experimental results.15 Our predictions could be tested in future experiments, helping to inform both terrestrial and extraterrestrial amino acid formation processes.
Enantiomeric excess at the dawn of the solar system
The enantiomeric excess (ee) of L-isovaline has been observed in meteorites with various processing histories,8,9,33,34 and a direct relationship has been suggested between the degree of aqueous alteration and the percentage of L-ee found in carbonaceous chondrites.8 The enantiomeric composition of meteoritic sec-butylamine has shown contradictory results. Aponte et al. (2015, 2016), reported racemic mixtures of sec-butylamine in carbonaceous chondrites from which enantiomeric excesses of L-isovaline was found ranging from 0 to 18%. In contrast, enantiomeric enrichments of (S)-sec-butylamine ranging from 0 to 66% in moderately and minimally aqueously altered CR2 and CR3 chondrites in which isovaline was racemic (L-ee from 0 to 3 ± 4%34,68) was also reported.69 One notable piece of information obtained from our models is the enantioselective decomposition of isovaline into sec-butylamine. According to this result, any ee present in L-isovaline, would be conserved when decarboxylated into (S)sec-butylamine, indicating that an observed (S)-ee of sec-butylamine could be a potential indicator of Lisovaline ee. Additionally, our results suggest that sec-butylamine will racemize at a faster rate than isovaline given the presence of the α-hydrogen and the lower steric hindrance; in fact, isovaline is more stable against racemization relative to α-H-α-amino acids.70
The lack of observed ee of sec-butylamine in meteorites across varying degrees of alteration and in meteorites with a significant L-isovaline ee may suggest that, despite our theoretical results, the decarboxylation of enantioenriched isovaline is not a major reaction in the parent body. However, alternate explanations exist, including that: (a) enantioenriched sec-butylamine is not observed because of the expected faster racemization of the amine relative to that of isovaline, or (b) a small L-ee of isovaline existed that created an ee in sec-butylamine formed upon decomposition that is below current detection limits, but that parent-body conditions subsequently amplified the isovaline ee,71,72 without having a significant effect on the enantiomeric composition of sec-butylamine. The (S)-sec-butylamine ee reported by Pizzarello and Yarnes (2016) would suggest that sec-butylamine and isovaline may be synthetically linked through the decomposition of the amino acid in those less-aqueously-altered carbonaceous chondrites.
CONCLUSIONS
In this work, we modeled the effects of aqueous processing leading to the decomposition of the amino acids glycine and isovaline through deamination and decarboxylation. We have been mindful of not being contradicting or confusing towards the broader scientific community in our mechanistic assessments. Our work aimed to be a stepping point for future work that may link computational and experimental analyses to the origins of organic matter in the solar system; no changes were made to the text. Our results have several implications for the origins of soluble meteoritic organic compounds such as amino acids, carboxylic acids and amines. We found that the deamination and decarboxylation of glycine occur through similar barriers, suggesting that both processes would occur in parallel inside the parent body. In contrast, we found that the deamination of isovaline occurs at a faster rate than decarboxylation, and that this process results in the formation of an α-β-unsaturated acid. This may help to explain the observed increase in relative abundance of β-amino acids compared to α-amino acids in meteorites with increasing levels of aqueous processing. Additionally, although aliphatic amines were found as decarboxylation products of glycine and isovaline, our computations did not find evidence for the formation of analogous aliphatic carboxylic acids after deamination, suggesting that these compounds may have synthetic origins that are not related to the decomposition of amino acids inside the parent body. Finally, we found that the decarboxylation of isovaline occurs in an enantioselective fashion, meaning that the chiral configuration of isovaline would be conserved in the sec-butylamine produced. The decomposition of isovaline in meteorites exhibiting L-ee of this amino acid would produce (S)enantioenriched sec-butylamine, although the enantioenriched amine could then racemize more quickly than its amino acid precursor.
Supplementary Material
Acknowledgments
Computational resources supporting this work were provided by the NASA High-End Computing (HEC) Program through the NASA Advanced Supercomputing (NAS) Division at Ames Research Center via support from the Goddard Center for Astrobiology, and by GENCI French National Supercomputing Facility, through allocations 2016–091387 and 2017–091387 and GENCI-TGCC allocations t201609s042 and A0010910143. This research was supported by French state funds managed by the ANR within the Investissements d’Avenir programme under reference ANR-11-IDEX-0004–02, within the framework of the cluster of excellence MATériaux Interfaces Surfaces Environnement (MATISSE) led by Sorbonne Universités, NASA Astrobiology Institute through award 13–13NAI7–0032 to the Goddard Center for Astrobiology, and a grant from the Simons Foundation (SCOL award 302497 to J.P.D.).
Footnotes
(S)-and (R)-notations are more appropriate, however, the amino acid literature uses L-and D-notations based on analogy with glyceraldehyde; for consistency to the nomenclature used in previous meteoritic amino acid studies, we equate (S)-to L-and (R)-to D-based on their structures.
References
- (1).Cronin JR; and Chang S. (1993) Organic matter in meteorites: molecular and isotopic analyses of the Murchison meteorite In The Chemistry of Life’s Origins (eds. Greenberg JM, Mendoza-Gόmez CX and Pirronello V.), pp. 209–258. Kluwer Academic Publishers. [Google Scholar]
- (2).Pizzarello S.; Cooper GW; and Flynn GJ (2006) The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles In Meteorites and the Early Solar System II (eds. Lauretta DS and McSween HY), pp 625–651. University of Arizona Press: Tucson, AZ. [Google Scholar]
- (3).Burton AS; Stern JC; Elsila JE; Glavin DP; Dworkin JP Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 2012, 41, 5459–5472. [DOI] [PubMed] [Google Scholar]
- (4).Cobb AK; Pudritz RE Nature’s starships. I. Observed abundances and relative frequencies of amino acids in meteorites. Astrophys. J. 2014, 783, 140 (12pp). [Google Scholar]
- (5).Elsila JE; Aponte JC; Blackmond DG; Burton AS; Dworkin JP; Glavin DP Meteoritic amino acids: Diversity in compositions reflects parent body histories. ACS Cent. Sci. 2016a, 2, 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Gherase Dragos, Hazen Robert M., Krishnamurthy Ramanarayanan, Blackmond Donna G.. MineralInduced Enantioenrichment of Tartaric Acid. Synlett 2017; 28(01): 89–92. [Google Scholar]
- (7).Alexander J. Wagner, Dmitry Yu. Zubarev, Aspuru-Guzik Alán, and Donna G Blackmond Chiral Sugars Drive Enantioenrichment in Prebiotic Amino Acid Synthesis. ACS Cent. Sci 2017, 3, 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Glavin DP, and Dworkin JP (2009) Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proceedings of the National Academy of Sciences 106, 5487–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Burton AS, Elsila JE, Hein JE, Glavin DP and Dworkin JP (2013) Extraterrestrial amino acids identified in metal-rich CH and CB carbonaceous chondrites from Antarctica. Meteorit. Planet. Sci. 48, 390–402. [Google Scholar]
- (10).Grimm RE, and McSween HY Jr. (1989) Water and the thermal evolution of carbonaceous chondrite parent bodies. Icarus 82, 244–280. [Google Scholar]
- (11).Shukolyukov A., Lugmair GW (1993) Fe-60 in eucrites. Earth and Planetary Science Letters 119, 159–166. [Google Scholar]
- (12).Rosenberg ND et al. (2001) Modeling aqueous alteration of CM carbonaceous chondrites. Meteor. Planet. Sci 36, 239–244. [Google Scholar]
- (13).Peltzer ET, Bada JL, Schlesinger G. and Miller SL (1984) The chemical conditions on the parent body of the Murchison meteorite: some conclusions based on amino, hydroxy and dicarboxylic acids. Adv. Space Res 4, 69–74. [DOI] [PubMed] [Google Scholar]
- (14).Cooper G. & Cronin JR Linear and cyclic aliphatic carboxamides of the Murchison meteorite: Hydrolyzable derivatives of amino acids and other carboxylic acids. Geochim. Cosmochim. Acta 59, 1003–1015 (1995). [DOI] [PubMed] [Google Scholar]
- (15).Koga and Naraoka (2017) A new family of extraterrestrial amino acids in the Murchison meteorite. Scientific Reports 7, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Zolensky ME, Bourcier WL, Gooding JL, 1989. Aqueous alteration on the hydrous asteroids: Results of EQ3/6 computer simulations. Icarus 78, 411–425. [Google Scholar]
- (17).Zolotov MY (2012) Aqueous fluid composition in CI chondritic materials: Chemical equilibrium assessments in closed systems. Icarus 220, 713–729. [Google Scholar]
- (18).Rodante F., Thermodynamics and kinetics of decomposition processes for standard α-amino acids and some of their dipeptides in the solid state. Thermochim. Acta 1992, 200, 47–61. [Google Scholar]
- (19).Cohen BA, and Coker RF (2000) Modeling of Liquid Water on CM Meteorite Parent Bodies and Implications for Amino Acid Racemization. Icarus 145, 369–381. [Google Scholar]
- (20).Kebukawa Y., Chan QHS, Tachibana S., Kobayashi K., Zolensky ME (2017) One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity. Science Advances 3: e1602093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Pizzarello S., Feng X., Epstein S., and Cronin JR (1994) Isotopic analyses of nitrogenous compounds from the Murchison meteorite: ammonia, amines, amino acids, and polar hydrocarbons. Geochim. Cosmochim. Acta 58, 5579–5587. [DOI] [PubMed] [Google Scholar]
- (22).Aponte JC, Alexandre MR, Wang Y., Brearley AJ, Alexander CO’D, and Huang Y. (2011) Effects of secondary alteration on the composition of free and IOM-derived monocarboxylic acids in carbonaceous chondrites. Geomchim. Cosmochim. Acta 75, 2309–2323. [Google Scholar]
- (23).Aponte JC, Dworkin JP, and Elsila JE (2015) Indigenous aliphatic amines in the aqueously altered Orgueil meteorite. Meteoritics & Planetary Science 50, 1733–1749. [Google Scholar]
- (24).Yuen G., Blair N., Des Marais DJ, and Chang S. (1984) Carbon isotopic composition of individual, low molecular weight hydrocarbons and monocarboxylic acids from the Murchison meteorite. Nature 307, 252–254. [DOI] [PubMed] [Google Scholar]
- (25).Huang Y., Wang Y., Alexandre MR, Lee T., Rose-Petruck C., Fuller M. and Pizzarello S. (2005) Molecular and compound-specific isotopic characterization of monocarboxylic acids in carbonaceous meteorites. Geochim. Cosmochim. Acta 69, 1073–1084. [Google Scholar]
- (26).Aponte JC, McLain HL, Dworkin JP, and Elsila JE (2016) Aliphatic amines in Antarctic CR2, CM2 and CM1/2 carbonaceous chondrites. Geomchim. Cosmochim. Acta 189, 296–311. [Google Scholar]
- (27).Aponte JC, Elsila JE, Glavin DP, Milam SN, Charnley SB, Dworkin JP 2017. Pathways to meteoritic glycine and methylamine. ACS Earth & Space Chemistry 1, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Glavin DP; Dworkin JP; Sandford SA Detection of cometary amines in samples returned by Stardust. Meteorit. Planet. Sci 2008, 43, 399–413. [Google Scholar]
- (29).Elsila JE, Glavin DP, & Dworkin JP (2009) Cometary glycine detected in samples returned by Stardust. Meteoritics and Planetary Science 44(9):1323–1330. [Google Scholar]
- (30).Elsila JE, Callahan MP, Dworkin JP, Glavin DP, McLain HL, Noble SK, and Gibson EK Jr. (2016b) The origin of amino acids in lunar regolith samples. Geomchim. Cosmochim. Acta 172, 357–369. [Google Scholar]
- (31).Altwegg K.; Balsiger H.; Bar-Nun A.; Berthelier J-J; Bieler A.; Bochsler P.; Briois C.; Calmonte U.; Combi MR; Cottin H.; De Keyser J.; Dhooghe F.; Fiethe B.; Fuselier SA; Gasc S.; Gombosi TI; Hansen KC; Haessig M.; Jäckel A.; Kopp E.; Korth A.; Le Roy L.; Mall U.; Marty B.; Mousis O.; Owen T.; Rème H.; Rubin M.; Sémon T.; Tzou C-Y; Waite JH; Wurz P. (2016) Prebiotic chemicals -amino acid and phosphorus-in the coma of comet 67P/Churyumov-Gerasimenko. Sci. Adv 2, e1600285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cronin JR and Pizzarello S. 1997. Enantiomeric excesses in meteoritic amino acids. Science 275, 951–955. [DOI] [PubMed] [Google Scholar]
- (33).Pizzarello S., Zolensky M. and Turk KA (2003) Nonracemic isovaline in the Murchison meteorite: Chiral distribution and mineral association. Geochim. Cosmochim. Acta 67, 1589–1595. [Google Scholar]
- (34).Glavin DP, Callahan MP, Dworkin JP, and Elsila JE (2010) The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci 45, 1948–1972. [Google Scholar]
- (35).Cox JS, and Seward TM (2007) The reaction kinetics of alanine and glycine under hydrothermal conditions. Geochim. Cosmochim. Acta, 71, 2264–2284. [Google Scholar]
- (36).Pietrucci F., and Saitta AM (2015) Formamide reaction network in gas phase and solution via a unified theoretical approach: Toward a reconciliation of different prebiotic scenarios. Proceedings of the National Academy of Sciences 112, 15030–15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Pietrucci F. (2017) Strategies for the exploration of free energy landscapes: Unity in diversity and challenges ahead. Rev. Phys 2, 32–45. [Google Scholar]
- (38).Pérez-Villa A., Georgelin T., Lambert JF, Maurel MC, Guyot F., Saitta AM, Pietrucci F. (2017) A common precursor to both prebiotic and biological pathways to RNA nucleotides. ChemRxiv DOI: 10.26434/chemrxiv.5519041. [DOI] [Google Scholar]
- (39).Saitta AM and Saija F. (2014) Miller experiments in atomistic computer simulations. Proceedings of the National Academy of Sciences 111, 13768–13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Clayton RN, and Mayeda TK (1999) Oxygen isotope studies of carbonaceous chondrites. Geochimica et Cosmochimica Acta 63, 2089–2104. [Google Scholar]
- (41).Keil. Thermal alteration of asteroids: evidence from meteorites. Planet. Space Sci 2000, 48, 887–903. [Google Scholar]
- (42).Brearley AJ 2006. The action of water In Meteorites and the Early Solar System II (eds. Lauretta DS, Leshin LA and McSween HY), pp. 587–624. University of Arizona Press: Tucson, AZ. [Google Scholar]
- (43).Guo and Eiler. Temperatures of aqueous alteration and evidence for methane generation on the parent bodies of the CM chondrites. Geochim. Cosmochim. Acta 2007, 71, 5565–5575. [Google Scholar]
- (44).Pizzarello S., Williams LB, Lehman J., Holland GP and Yarger JL (2011) Abundant ammonia in primitive asteroids and the case for a possible exobiology. Proc. Natl. Acad. Sci. USA 108, 4303–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Perdew JP, Burke K., Ernzerhof M. (1996) Generalized gradient approximation made simple. Phys. Rev. Lett 77, 3865–3868. [DOI] [PubMed] [Google Scholar]
- (46).Grimme S. (2006) Semiempirical gga-type density functional constructed with a long-range dispersion correction. J. Comput. Chem 27, 1787–1799. [DOI] [PubMed] [Google Scholar]
- (47).CPMD Copyright IBM Corp. and by Max Planck Institute Stuttgart. www.cpmd.org, 2000-2017. [Google Scholar]
- (48).Laio A., Parrinello M. (2002) Escaping free-energy minima. Proc. Natl. Acad. Sci. U.S.A, 99, 1256212566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Torrie GM, Valleau JP (1977) Nonphysical sampling distributions in monte carlo free-energy estimation: Umbrella sampling. J. Comput. Phys 23, 187–199. [Google Scholar]
- (50).Bonomi M. et al. (2009) PLUMED: a portable plugin for free-energy calculations with molecular dynamics. Comput. Phys. Commun 180, 1961–1972. [Google Scholar]
- (51).Wang X., Conway W., Burns R., McCann N., Maeder M. (2010) Comprehensive Study of the Hydration and Dehydration Reactions of Carbon Dioxide in Aqueous Solution. J. Phys. Chem. A 114, 1734–1740. [DOI] [PubMed] [Google Scholar]
- (52).Buckles RE, Mock GV, Locatell L. (1955) Tiglic and angelic acids. Chem. Rev 55, 659–677. [Google Scholar]
- (53).Alexandrova ANN, and Jorgensen WL (2011) On the mechanism and rate of spontaneous decomposition of amino acids. J. Phys. Chem. B 115, 13624–13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Snider MJ, and Wolfenden R. (2000) The rate of spontaneous decarboxylation of amino acids. J. Am. Chem. Soc 122, 11507–11508. [Google Scholar]
- (55).Sato N., Quitain AT, Kang K., Daimon H., and Fujie K. (2004) Reaction kinetics of amino acid decomposition in high-temperature and high-pressure water. Ind. Eng. Chem. Res 43, 3217–3222. [Google Scholar]
- (56).Li J., and Brill TB (2003) Spectroscopy of hydrothermal reactions. Int. J. Chem. Kinet 35, 602–610. [Google Scholar]
- (57).Grimme S., Antony J., Ehrlich S., Krieg H. (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys 132, 154104. [DOI] [PubMed] [Google Scholar]
- (58).Kuan Y-J; Charnley SB; Huang H-C; Tseng W-L; Kisiel Z. (2003) Interstellar glycine. Astrophys. J 593, 848–867. [Google Scholar]
- (59).Snyder LE; Lovas FJ; Hollis JM; Friedel DN; Jewell PR; Remijan A.; Ilyushin VV; Alekseev EA; Dyubko SF (2005) A rigorous attempt to verify interstellar glycine. Astrophys. J 619, 914–930. [Google Scholar]
- (60).Bernstein MP; Dworkin JP; Sandford SA; Cooper GW; Allamandola LJ Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [DOI] [PubMed] [Google Scholar]
- (61).Muñoz-Caro GM; Meierhenrich UJ; Schutte WA; Barbier B.; Segovia AA; Rosenbauer H.; Thiemann WH-P; Brack A.; Greenberg JM Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [DOI] [PubMed] [Google Scholar]
- (62).Danger G.; Borget F.; Chomat M.; Duvernay F.; Theulé P.; Guillemin J-C; d’Hendecourt LLS; Chiavassa T. Experimental investigation of aminoacetonitrile formation through the Strecker synthesis in astrophysical-like conditions: Reactivity of methanimine (CH2NH), ammonia (NH3), and hydrogen cyanide (HCN). Astron. Astrophys 2011a, 535, A47 (9pp). [Google Scholar]
- (63).Yuen GU; Kvenvolden KA (1973). Monocarboxylic acids in Murray and Murchison carbonaceous chondrites. Nature 246, 301–302. [Google Scholar]
- (64).Hawkins JM and Fu GC (1986) Asymmetric Michael reactions of 3,5-dihydro-4H-dinaphth[2,1c:1’,2’-e]azepine with methyl crotonate. J. Org. Che 51, 2820–2822. [Google Scholar]
- (65).Pardo L., Osman R., Weinstein, Rabinowitz JR (1993) Mechanisms of Nucleophilic Addition to Activated Double Bonds: 1,2-and 1,4-Michael Addition of Ammonia. J. Am. Chem. Soc 115, 8263–8269. [Google Scholar]
- (66).Ehrenfreund P.; Glavin DP; Botta O.; Cooper G.; Bada JL (2001) Extraterrestrial amino acids in Orgueil and Ivuna: Tracing the parent body of CI type carbonaceous chondrites. Proc. Natl. Acad. Sci. U.S.A 98, 2138–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Aponte JC; Abreu NM; Dworkin JP; Elsila JE (2017) Distribution of Aliphatic Amines in CO, CV and CK Carbonaceous Chondrites and Relation to Mineralogy and Processing History. Meteoritics & Planetary Science 52, 2632–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Pizzarello S., Huang Y., and Alexandre MR (2008) Proceedings of the National Academy of Sciences 105, 3700–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Pizzarello S. and Yarnes T. (2016) Enantiomeric excesses of chiral amines in ammonia-rich carbonaceous meteorites. Earth Planet. Sci. Lett 443, 176–184. [Google Scholar]
- (70).Pollock GE, Cheng C-N, Cronin SE, Kvenvolden KA (1975) Stereoisomers of isovaline in the Murchison meteorite. Geochim. Cosmochim. Acta 39, 1571–1573. [Google Scholar]
- (71).Soai K., Shibata T., Morioka H., and Choji K. 1995. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 378, 767–768. [Google Scholar]
- (72).Klussmann M., Iwamura H., Mathew SP, Wells DH, Pandya U., Armstrong A., and Blackmond DG 2006. Thermodynamic control of asymmetric amplification in amino acid catalysis. Nature 441, 621–623. [DOI] [PubMed] [Google Scholar]
- (73).Savin A., Nesper R., Wengert S. and Fässler TF (1997) ELF: The Electron Localization Function. Angew. Chem. Int. Ed. Engl 36, 1808–1832. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.