Abstract
Quantification and detection of various contaminants in the ecosystem have become critically important in the past few decades due to their exhaustive use in soil and aquatic ecosystems. The contamination by both organic and inorganic contaminants in the ecosystem has drawn attention due to their persistence, biological accumulation, and toxicity. Organic contaminants reach the air, water, food, soil, and other systems through drift mechanism and have detrimental effect on various life systems after entering the food chain, thus interfering the normal biological process of the ecosystem. Inorganic contaminants have less solubility, primarily get adsorbed, and accumulate on lower sediments. The sources of both organic and inorganic contaminants include anthropogenic activities which dispose industrial and sewage effluent directly into water bodies. Most of the contaminants are very much toxic and have tumorigenic, carcinogenic, and mutagenic effect on various life-forms. Biosensors have various prospective and existing applications in the detection of these compounds in the environment by transducing a signal. It also has immense applications in the detection of different contaminants in the food industry, environmental monitoring, disease diagnosis, etc. where reliable and precise analyses are required. This chapter points out a comprehensive glimpse on different biosensors and their characteristics, operating principles, and their designs, based on transduction types and biological components. Efforts have been made to summarize various applications of biosensors in food industry, environmental monitoring, drug delivery systems, and clinical diagnostics etc.
Keywords: Biosensors, environment monitoring; Disease diagnosis; Drug delivery
Introduction
Biosensors are devices comprising of a biological and physicochemical component to detect an analyte by producing a signal which can be measured (Mishra et al. 2019; Rovira and Domingo 2019; Mehrotra 2016). The first biosensor was invented by American biochemist “L.L Clark” in the year 1950 and the term “biosensor” was first introduced by “Cammann” in 1977 (Bhalla et al. 2016; Krishnaperumal and Lakshmanan 2013; Tchounwou et al. 2012). According to IUPAC nomenclature, biosensors are integrated receptor–transducer devices, which are able to provide selective quantitative or semiquantitative analytical information using a biological recognition element (Tangahu et al. 2011; Thévenot et al. 2001). A typical biosensor usually comprises of a biosensing element and a transducer. It has various biological applications and is used for the detection of several components such as pollutants, microbial load, metabolites, control parameters, and various other substances (Neethirajan et al. 2018). It also has immense applications in food industry, clinical diagnostics, and various other areas where reliable and precise analyses are required (Rasheed et al. 2019; Mishra et al. 2018). During the last few decades, numerous biosensing elements and devices have been developed (Grieshaber et al. 2008). Biosensors have numerous applications in various fields such as bio-monitoring of pollutants, disease diagnostics etc. The most common used biosensor is blood glucose biosensor which is used to check blood glucose levels (Nilsen et al. 2019; Yoo and Lee 2010).
Detection of various contaminants such as chemical and hazardous pollutants, drug detection, and detection of toxins in food, water, and soil ecosystems are some applications where biosensors are regularly used (Kimmel et al. 2007). Recent advancement in recombinant DNA technology led to the development of DNA-based or aptamer-based biosensors which act as a diagnostic tool in clinical assessment (Zhu et al. 2015). Incorporation of nanoparticles in biosensors helps in improving its parameters such as reliability, validity, lower detection limit, residence time, stability, sensitivity, etc. (Malekzad et al. 2017) (Fig. 14.1).
Fig. 14.1.
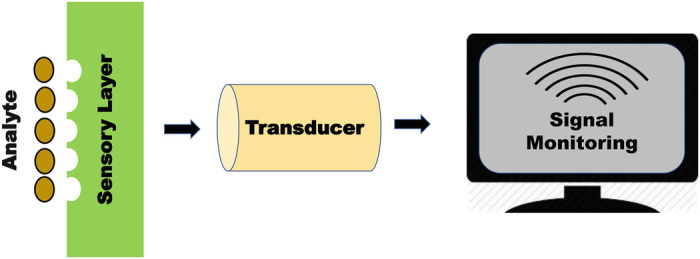
General components of a biosensor
Types of Biosensors
The biological sensing material can be DNA probes, antibodies and enzymes, and cell receptors which interact with the analyte. The transducer may be optical, physicochemical, or piezoelectric material that translates the biological signals to optical and electrical signals (Nguyen et al. 2019). Biosensors are divided into different groups depending on signal transductions (Rocchitta et al. 2016). The electrochemical sensors were first introduced in 1962 by Leland C. Clark during the NASS symposium (Bhalla et al. 2016). In this sensor, molecules interact with the reactants and transduce an electrical signal which is directly proportional to the concentration of the analyte (Grieshaber et al. 2008). Based on this principle, it employs impedimetric, amperometric, and potentiometric sensors converting sensing information into a measurable signal (Malhotra et al. 2017).
Voltammetric Biosensors
Voltammetric biosensors measure the current produced during reduction or oxidation of electro-active reactant or product (Hung Tzang et al. 2001). In voltammetric biosensors, the electroactive species reduction or oxidation current is measured by producing the potential drop (Goyal et al. 2010). Here, the reference electrode and constant potential is applied to the working electrode which measures the current which is either directly proportional to the formation of a biocatalytic layer or to the volume concentration of electroactive species (Sangamithirai et al. 2018).
Potentiometric Biosensors
They usually measure the potential of the biosensor electrode with respect to a reference electrode (Bundschuh et al. 2018; Pisoschi 2016). The potential drop carries the analytical signal between the two reference electrodes or between the reference electrode and the analytical electrode separated by the membrane. The transducer used in potentiometric transducers is the ion-selective electrode (ISE) (Cuartero et al. 2019).
Conductometric Biosensors
These biosensors involve the change in conductance arising due to the biochemical reaction (Jaffrezic-Renault and Dzyadevych 2008). These biosensors measure the electrical conductivity of the solution during a biochemical reaction. The sensing element is an enzyme and less discounted for the detection of affine interactions (Velychko et al. 2016).
Optical Biosensors
It measures light absorbed or emitted during a biochemical reaction (Damborský et al. 2016). In these biosensors, the optical fibers detect the analytes on the basis of light scattering fluorescence or absorption (Long et al. 2013). These biosensors measure both the affinity and catalytic reactions. The sensing element causes a change in absorbance or fluorescence which changes the refractive index between two media having different densities (Pospíšilová et al. 2015). They are superior to nonelectrical biosensors as they allow multiple analyte detection using various monitoring wavelengths (Dey and Goswami 2011). The adaptability of using optic probes is because of their ability to transmit signals that account on changes in polarity, time, wavelength, wave propagation, distribution of the spectrum, or intensity of the light (Peltomaa et al. 2018). They are solely based on the principle of light scattering absorption, internal reflection, fluorescence, surface plasmon resonance, or luminescence spectroscopy (Fan et al. 2008).
Calorimetric Biosensors
Also known as thermal biosensors, they work on the change in enthalpy during a reaction (Y. Zhang and Tadigadapa 2004). These are developed by integrating biosensor components into a physical transducer. It is used for the detection of a pathogen in water and food by measuring the change in optical density or color of the test sample upon a chemical reaction (Park et al. 2007).
Enzymatic Sensors
These sensors include biological material having certain antibiological activities (Hwang et al. 2018). The simplest form of enzymatic biosensors is capable of reversible reduction or oxidation on the electrode upon application of electrochemically active potential (Bernards et al. 2008). Enzymatic sensors were further categorized into inhibitor and substrate sensors. Inhibitor sensors often tend to determine the reducing activity of the enzyme or substances, while substrate biosensors tend to determine selected substrates and its enzymatic reactions (Campanella et al. 2000).
Impedimetric Biosensors
It measures the variation impedance of an electrochemical cell with AC frequency (Guan et al. 2004). These involve ion-sensitive silicon field-based sensors which enhance the resolving power of the transducer by raising its sensitivity. Mostly, biologically modified impedimetric biosensors are used to determine small proteins and peptides on the basis of a net charge on it (Kim et al. 2019).
Piezoelectric Biosensors
It involves measurement of mass change during biomolecular interaction. They are also considered as mass-based biosensors and are based on the principle of sound vibrations and also called as acoustic biosensors (Tombelli 2012). They produce an electrical signal when mechanical force is applied (Pohanka 2017). Sensing molecules are directly attached to a piezoelectric surface which is piezoelectric in nature. Hence, mechanical vibrations arise from the interaction between the sensing molecules and analyte and translate them to electrical signals (Pohanka 2018).
Immunosensors
They are widely used to detect the immunochemical reaction which occurs between antigens and antibodies (Piro and Reisberg 2017). Hence, they are employed to detect the presence of antibodies and used as a diagnostic indication for toxic substances. They determine the antigens in both media (biological liquids and natural environment) (Balahura et al. 2019). They detect any compound having high selectivity and specificity against specific antibodies (Wen et al. 2017).
DNA Sensors
The major component of DNA sensors is nucleic acids, mostly DNA. These sensing materials are the fragments commonly called DNA primers or DNA probes which reflect specificity of the whole DNA structure. These probes or primers are synthesized by amplification of DNA by PCR (polymerase chain reaction) (Campàs i Homs 2002). They are modified to increase the stability or to facilitate introduction of probes into biosensors. This type of biosensors helps in revealing non-macromolecular and protein compounds which interact with specific DNA fragments (Diculescu et al. 2005). On the basis of the type of biorecognition unit used, they are classified as nucleic acid-based, enzymatic, whole cell-based, antibody-based, or aptamer-based biosensors (Rasheed and Sandhyarani 2017) (Table 14.1).
Table 14.1.
Different types of biosensors, their transducers, specifications, and applications
| Biosensor | Transducer type | Specifications | Applications | References |
|---|---|---|---|---|
| Amperometric | Electrochemical | Usually redox reaction which brings change in current between reference and working electrode | Quantification and detection of alcohols, cholesterol, urea, amino acids, and glucose | Goriushkina et al. (2009) |
| Baroxymeter | Others | Measures respiration of bacterial cells by pressure measurements | Detection of toxic components in wastewater | Tzoris et al. (2002) |
| Bioluminescent | Optical | Light emission principle in which viable bacteria responds to any physical chemical or biological change | Quantification and detection of heavy metal, food toxicants, and environmental monitoring | Alloush et al. (2006) |
| Colorimetric | Optical | Monitors the change in optical density of a sample during a reaction | Water- and foodborne pathogens | Radhakrishnan et al. (2014) |
| Conductometric | Electrochemical | Measures change in the electrical conductivity of the medium upon entry of any analyte that changes the concentration of ionic species by measuring electrical conductivity when analyte reacts with medium | Detection of protein markers, chemicals, heavy metals | Jaffrezic-Renault and Dzyadevych (2008) |
| DNA sensors | DNA primers or DNA probes | Synthesized by amplification of DNA by PCR (polymerase chain reaction) | Measures non-macromolecular and protein compounds | Rasheed and Sandhyarani (2017) |
| Fluorescence | Optical | Emit fluorescence during immobilization of fluorescent-tagged biomolecules during reaction with analyte | Water or iron availability in plants or cell populations, BOD measurement, water in microbial habitat | Lei et al. (2006) |
| FET-based biosensor | Others | Measures change in conductance of field effect biosensor | IV blood pH recording, clinical investigation | Xu et al. (2005) |
| Impedance | Electrochemical | Measures the changes in conductivity or resistivity due to biorecognition event in medium | Polychlorinated biphenyls, milk toxins, PCBs, endocrine-disrupting hormones | Jaffrezic-Renault and Dzyadevych (2008) |
| Immunosensors | Based on ELISA technique | Amplifies and detect an antigen–antibody reaction | Clinical chemistry, antigen–antibody reaction | Balahura et al. (2019) |
| Piezoelectric | Others | Monitors changes in resonating frequency due to absorption or desorption of analyte results in current generation in piezoelectric material | Cellular studies, nucleic acid sensing | Skládal (2016) |
| Potentiometric | Electrochemical | Measures charge or potential accumulation between a reference and an ion-selective electrode | Determination of carbon dioxide, urea, pesticides, neurotransmitters, sugars, etc. | Pisoschi (2016) |
| Pyroelectric | Measurement of change in current induced by an analyte upon a temperature difference | Diagnostics | Spain and Venkatanarayanan (2014) | |
| Supramolecular | Entities composed of lipid membranes, poly-enzyme complexes, cell organelles, immobilization of organic thin film | Inhabit intermediate between DNA enzyme and sensor | Wajs et al. (2016) |
Biosensors Based on Supramolecular Structures of a Cell
These biosensors inhabit intermediate between DNA enzyme and sensors, since they have a hierarchical structure. Sometimes these entities are composed of lipid membranes, poly-enzyme complexes, and cell organelles (chloroplasts and mitochondria) (Duan et al. 2013). These biosensors are less stable as they are derived from their natural component and cannot maintain operating parameters during procedures that are time consuming (Wajs et al. 2016).
Biosensor Design and Operation
It is an analytical device that employs certain biological compounds to recognize some molecules by providing their presence and concentration as a signal for processing and recording (Vigneshvar et al. 2016). Usually, biosensors contain three components: the first one is the recognition element which is a membrane having various biological structures. The second one is the transducer and the last one is the electronic system which amplifies the signal and records the signal for data presentation (Kozitsina et al. 2018).
The basic part of any sensor is the recognition element which responds to one or many analytes among various substances (Luka et al. 2015). The commonly used recognition elements include biological structures such as living cells, nucleic acids, receptors, antibodies, and enzymes (Rahaie and Kazemi 2010). Transducers convert the variations into optical or electric signal when a reaction occurs between the analytes and the selective biological layer. This signal is further measured using an electronic or light-sensitive device (Rahaie and Kazemi 2010). The biological component is immobilized by membrane or physical entrapment, covalent binding, or noncovalent interactions. Analytes bind with biological material resulting in the generation of an electronic response. Sometimes these reactions may be exogenous or release oxygen, hydrogen, or electrons ions (Nguyen et al. 2019). The transducer amplifies the changes in the product linked into the signal. Signal processing involves minimizing the reference signal arising from a similar transducer without any biological component and also smoothens the unwanted signal noise (Semenova et al. 2019).
Biosensors for Monitoring and Diagnostic Purposes
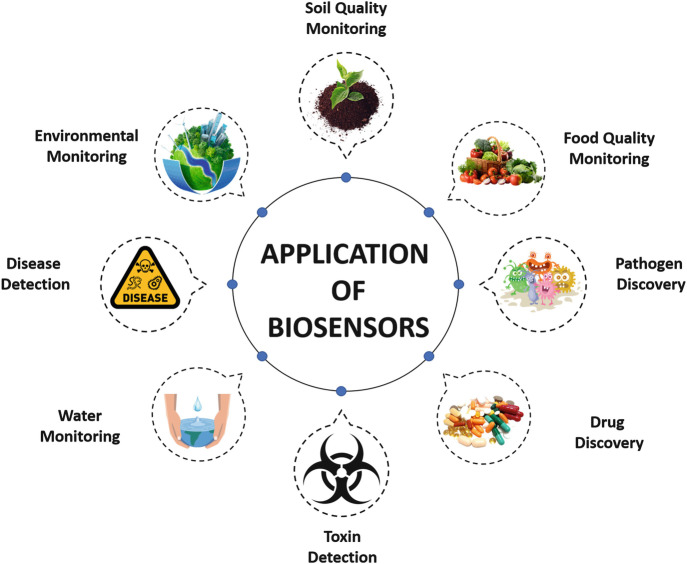
Biosensors exhibit numerous promising applications in various fields such as environmental monitoring, molecular diagnostics, pathogen detection, food industries, etc. (Mehrotra 2016). Biosensors monitor the presence of various contaminants in order to ensure the quality of drinking water, food, and soil. Biosensors detect contaminants at a low concentration which is a matter of priority for environmental protection and disease prevention as well (Rodriguez-Mozaz et al. 2006) (Fig. 14.2).
Fig. 14.2.
Different applications of biosensors
Biosensors for Monitoring Water Quality
Presently, water quality monitoring has become a primary environmental concern due to anthropogenic activities that are deteriorating the quality of water (Bi et al. 2018). To improve it, different preventive measures need to be taken like enhancing the methods for sustaining natural resources, controlling the release of toxic compounds into the environment, and treating the localized sources of contamination (Ezeonu et al. 2012), as different contaminants like insecticides, pesticides, and surface-active molecules (SAM) enter the water sources and affect the life of marine organisms. These interventions disturb the water equilibrium and reduce the natural ability of self-purification. As a result, it induces harmful effects on humans upon consuming the contaminated water (Agrawal et al. 2010). Hence, there is need for the monitoring of toxic compounds in water. Regrettably, conventional methods like chromatography, spectrometry, etc. have their limitations as they work on chemical and physical principles. Moreover, these approaches are high-priced and labor-intensive, have narrow range of sensitivity, and do not robustly provide an effective result of the ecological situation (Starodub et al. 2005). Therefore, sensors involving a biological component/organism have gained attention and can serve as reliable and effective analytical techniques for assessing environmental contamination (Kovalchuk and Kovalchuk 2008).
Animals, culture of different tissues, microbes, protozoa, and water plants can function as biological indicators for checking toxicity (Parmar et al. 2016). At the same instance, it may also aid in determining group-specific substances that are highly sensitive to specific groups present on the contaminant (Parmar et al. 2016). From different living organisms, Daphnia magna St. is an effective candidate for determining the total toxicity level of ecological contaminants as it has chemiluminescence (ChL) property. In an experiment, 5 organisms of Daphnia in 10 mL were found to be optimum for producing ChL response in the presence of H2O2. Using this idea, a Daphnia-based ChL biosensor has been created, which is aided with a computer controlling device for automatic detection of ChL signal. This biosensor especially detects the presence of SAM and is able to give measurement in the range of 20 mgL−1 or less (Starodub 2009). Another biosensor has been developed using bioluminescent bacteria. For this, bacteria were isolated from the Azov Sea and Black Sea according to their tolerance to SAM and other toxic entities to assess the toxicity of water samples. To develop this computer-controlled biosensor, bioluminescent bacteria like Photobacterium phosphoreum K3 and Vibrio fischeri F1/Sh1 are used. This biosensor works on bioluminescence inhibition as majority of SAM and toxic compounds inhibit bioluminescence activity. This inhibition response determines the presence of toxic compounds (Kuznetsov et al. 2002). Various other biosensors that possess the ability to determine specific toxic substances like chlororganics, cyanides, and phosphororganics have also been developed working on the principle of the electrolyte–insulator–semiconductor (EIS) system (Starodub et al. 2012). One such system is surface plasmon resonance (SPR)-based biosensor, which involves ethoxylated nonylphenol (NphEO) and immunoglobulin gamma (IgG) antibodies for detection. This approach measures the response of an SPR sensor against the NphEO concentration in the test sample (Bakhmachuk et al. 2017). Another powerful system using same component is ISFET biosensor, which has five times higher sensitivity than SPR-based biosensor and is the preferred biosensor for water assessment (Grieshaber et al. 2008).
Biosensor for Infectious Disease Detection
The spread of various infectious diseases like avian influenza, Hendra, Nipah, and SARS has become a global threat, which demands considerable effort to regulate their proliferation (Karesh et al. 2012). As there are various challenges associated with these infectious diseases, there is need for the development of diagnostic tools for eradicating/minimizing the chances of virus outbreak beforehand (Reyes et al. 2013). Biosensors have emerged as an attractive tool for providing robust information on these diseases. Usually, biosensors are characterized on the basis of their biological component and nature of the process like biocatalytic agent (such as enzyme), immunological agent (such as antibody), and nucleic acid material (such as DNA) (Mehrotra 2016). Majority of the biosensors developed for detecting pathogens involved in causing infectious disease are based on the principle of electrochemical reaction. This type of biosensor holds the majority as they are cost effective, independent of solution turbidity, low power requirement, high sensitivity, and simple instrumentation (Srinivasan and Tung 2015).
Different electrochemical methods like amperometric, impedance, and potentiometric are used to examine the changes which take place during disease detection (Hammond et al. 2016). The amperometric biosensor generally involves biosensor marker, antibody–antigen, and DNA hybridization reactions with an electrochemical transducer which amplifies the signal to a significant level for detection (Belluzo et al. 2008). One of the most common amperometric sensors is a glucometer (Yoo and Lee 2010). Previously, Gong and his colleagues have developed an amperometric-based immunosensor for detecting Newcastle disease (Gong et al. 2003). Another amperometric-based immunosensor has also been developed to diagnose forest-spring encephalitis with great precision (Brainina et al. 2003). Biosensors based on label-free amperometric immunosensor have been developed for Japanese B encephalitis vaccine (Yuan et al. 2005). Another group of researchers developed an optical biosensor to check the presence of Newcastle disease virus with sensitivity to 10 ng/ml (Lee and Thompson 1996). SPR-associated immunosensors have also been developed to detect the coronavirus responsible for severe acute respiratory syndrome (SARS) (Huang et al. 2009). Another type of biosensor, i.e., piezoelectric biosensors, has also been developed by the research group to assess the food and mouth disease virus (Gajendragad et al. 2001). Moreover, piezoelectric-associated DNA biosensor has also been developed by a group of researchers to detect hepatitis B virus infection with limited concentration range of about 0.02–0.14 μg/ml (Yao and Fu 2014).
Biosensor for Pathogen Detection
Foodborne illnesses have become a major health issue worldwide and raised the health safety concern in both food industries and regulatory bodies (Fung et al. 2018). Microbes have also been found to perform a function that is beneficial to food and its production, whereas some of them have been found to be associated with food spoilage (Rawat 2015). Few of food pathogen microbes are Aeromonas spp., Bacillus anthracis, Bacillus subtilis, Brucella spp., Campylobacter spp., Clostridium spp., Escherichia coli, Listeria monocytogenes, Salmonella spp., Shigella spp., Staphylococcus aureus, and Yersinia enterocolitica (Mody and Griffin 2015). These microbes have been found to produce cell metabolites and toxins which can cause severe diseases. These issues have prompted the researchers to develop a cheap, portable, and robust detector for pathogens (Abbasian et al. 2018). They developed different biosensors to detect these pathogenic microbes in food sources. Therefore, robust detection approaches for assessing foodborne pathogens are classified into antigen–antibody-based, bacteriophage-based, and nucleic acid-based biosensors (Mehrotra 2016). The rapid detection of foodborne pathogens has increased the popularity of these biosensors in the food sector, as these biosensors are effective in examining the food quality and microbial contamination in food products (Law et al. 2014).
The different types of immunosensors have been developed which measure the signal induced on the interaction of specific antibodies with a targeted antigen. An immunosensor has been found effective in detecting two species of Salmonella (i.e., S. gallinarum and S. pullorum) in chicken meat and eggs (Cinti et al. 2017). Another immunosensor based on a screen-printed interdigitated microelectrode has been developed to detect E. coli O157:H7 as well as S. typhimurium in chicken and milk sample within the range of 103–106 CFU/mL (Xu et al. 2016). An enzyme-based biosensor is another category, in which an enzyme works as a bioreceptor for detection. One of the most common enzyme-based biosensors is a glucometer which measures the level of glucose with the help of the immobilized glucose oxidase enzyme (Yoo and Lee 2010). Hesari and his colleagues developed an enzyme-based biosensor to robustly detect the E. coli contamination in drinking water (Hesari et al. 2016). Another category of biosensor, i.e., optical biosensor, has also been developed in which the toxins or pathogens are labeled with fluorescent compound, which on interaction with surface biosensor gets triggered by laser wave and induces a signal for detection (Bosch et al. 2007). Based on this principle, Adak and his group have developed an optical biosensor for S. aureus detection with detection limit in the range of 102–103 CFU/mL (Adak et al. 2013). Fluorescence resonance energy transfer (FRET)-based biosensors are also a type of optical biosensor. This type of biosensor has been found to be effective in detecting S. aureus in spilled milk and buffer (He et al. 2014). Moreover, Zhang with his colleagues developed a surface plasmon resonance (SPR) biosensor for the rapid detection of E. coli O157:H7, L. monocytogenes, and S. enteritidis in food products (Zhang et al. 2014). A colorimetric biosensor, another type of optical biosensor method, has allowed us to robustly detect pathogens like Listeria spp. and S. aureus in food products as well as the environment (Oluwaseun et al. 2018).
On the other hand, different electrochemical biosensors have also been developed to detect the microbes in contaminated food. Recently, amperometric biosensors with high sensitivity have been developed for identifying different foodborne pathogens like C. jejuni, E. coli O157:H7, L. monocytogenes, and Salmonella species (Arora et al. 2018), although a field effect transistor-based potentiometric biosensor has been developed to detect the presence of E. coli with detection limit as low as 10 cells/mL (Moran et al. 2016). Another electrochemical impedimetric biosensor based on aptasensors has been developed by Sheikhzadeh and his colleagues to detect the presence of Salmonella typhi in apple juice having a detectable range of 102–108 CFU/mL (Sheikhzadeh et al. 2016), whereas Zhang and his team developed aptasensors based on surface-enhanced Raman spectroscopy to detect Staphylococcus aureus and Salmonella typhimurium in pork with a working range of 102–107 CFU/mL (Zhang et al. 2015). Moreover, magnetoelastic sensors have also been developed to detect the foodborne pathogens like Staphylococcus aureus and Salmonella typhimurium in food items like spinach and tomato (Byeon et al. 2015; Li et al. 2010).
Conclusion
Biosensors have various applications in different fields such as disease diagnosis, environment monitoring, food control, drug discovery, biomedical research, forensics, etc. These devices need the interaction of various disciplines and are dependent on very special features like interaction of biomolecular analytes with recognition elements, device fabrication and design, on-chip electronics, sampling techniques, microfluidics, etc. Incorporation of nanoparticles in biosensors provides an opportunity to build a new generation of sensing technologies. Nanoparticles improve the magnetic, optical, electrochemical, and mechanical properties of the biosensors. No studies have been reported on understanding the mechanism of interaction between biomolecules and nanomaterials on nanofilms or surface of electrodes for fabrication of new-generation biosensors. However, nanoparticle-based biosensors show attractive prospects which will be applied in process control, food analysis, environmental monitoring, and clinical diagnosis in the near future.
Contributor Information
Joginder Singh, Email: joginder.15005@lpu.co.in.
Ashish Vyas, Email: ashish.vyas@lpu.co.in.
Shanquan Wang, Email: wangshanquan@sysu.edu.cn.
Ram Prasad, Email: rpjnu2001@gmail.com.
Joginder Singh, Email: joginder.15005@lpu.co.in.
References
- Abbasian F, Ghafar-Zadeh E, Magierowski S. Microbiological sensing technologies: a review. Bioengineering. 2018;5(1):1–33. doi: 10.3390/bioengineering5010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adak AK, Boley JW, Lyvers DP, Chiu GT, Low PS, Reifenberger R, Wei A. Label-free detection of Staphylococcus aureus captured on immutable ligand arrays. ACS Appl Mater Interfaces. 2013;5(13):6404–6411. doi: 10.1021/am4016236. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Pandey RS, Sharma B. Water pollution with special reference to pesticide contamination in India. J Water Resour Prot. 2010;02(05):432–448. doi: 10.4236/jwarp.2010.25050. [DOI] [Google Scholar]
- Alloush HM, Lewis RJ, Salisbury VC. Bacterial bioluminescent biosensors: applications in food and environmental monitoring. Anal Lett. 2006;39(8):1517–1526. doi: 10.1080/00032710600713172. [DOI] [Google Scholar]
- Arora S, Ahmed N, Siddiqui S. Detecting food borne pathogens using electrochemical biosensors: an overview. Int J Chem Stud. 2018;6(1):1031–1039. [Google Scholar]
- Bakhmachuk A, Gorbatiuk O, Rachkov A, Dons’koi B, Khristosenko R, Ushenin I et al (2017) Surface Plasmon resonance investigations of bioselective element based on the recombinant protein a for immunoglobulin detection. Nanoscale Res Lett 12(1). 10.1186/s11671-017-1903-5 [DOI] [PMC free article] [PubMed]
- Balahura LR, Stefan-Van Staden RI, Van Staden JF, Aboul-Enein HY. Advances in immunosensors for clinical applications. J Immunoass Immunochem. 2019;40(1):40–51. doi: 10.1080/15321819.2018.1543704. [DOI] [PubMed] [Google Scholar]
- Belluzo MS, Ribone MÉ, Lagier CM. Assembling amperometric biosensors for clinical diagnostics. Sensors. 2008;8(3):1366–1399. doi: 10.3390/s8031366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards DA, MacAya DJ, Nikolou M, Defranco JA, Takamatsu S, Malliaras GG. Enzymatic sensing with organic electrochemical transistors. J Mater Chem. 2008;18(1):116–120. doi: 10.1039/b713122d. [DOI] [Google Scholar]
- Bhalla N, Jolly P, Formisano N, Estrela P. Introduction to biosensors. Essays Biochem. 2016;60(1):1–8. doi: 10.1042/EBC20150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W, Weng B, Yuan Z, Ye M, Zhang C, Zhao Y, et al. Evolution characteristics of surface water quality due to climate change and LUCC under scenario simulations: a case study in the Luanhe River Basin. Int J Environ Res Public Health. 2018;15(8):1724. doi: 10.3390/ijerph15081724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch ME, Sánchez AJR, Rojas FS, Ojeda CB. Recent development in optical fiber biosensors. Sensors. 2007;7(6):797–859. doi: 10.3390/s7060797. [DOI] [Google Scholar]
- Brainina K, Kozitsina A, Beikin J. Electrochemical immunosensor for forest-spring encephalitis based on protein a labeled with colloidal gold. Anal Bioanal Chem. 2003;376(4):481–485. doi: 10.1007/s00216-003-1912-3. [DOI] [PubMed] [Google Scholar]
- Bundschuh M, Filser J, Lüderwald S, McKee MS, Metreveli G, Schaumann GE et al (2018) Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Eur 30(1). 10.1186/s12302-018-0132-6 [DOI] [PMC free article] [PubMed]
- Byeon HM, Vodyanoy VJ, Oh JH, Kwon JH, Park MK. Lytic phage-based magnetoelastic biosensors for on-site detection of methicillin-resistant Staphylococcus aureus on spinach leaves. J Electrochem Soc. 2015;162(8):B230–B235. doi: 10.1149/2.0681508jes. [DOI] [Google Scholar]
- Campanella L, Persi L, Tomassetti M. New tool for superoxide and nitric oxide radicals determination using suitable enzymatic sensors. Sensors Actuators B Chem. 2000;68(1):351–359. doi: 10.1016/S0925-4005(00)00415-9. [DOI] [Google Scholar]
- Campàs i Homs M. DNA sensors. Anal Lett. 2002;35(12):1875–1894. doi: 10.1081/AL-120014280. [DOI] [Google Scholar]
- Cinti S, Volpe G, Piermarini S, Delibato E, Palleschi G (2017) Electrochemical biosensors for rapid detection of foodborne salmonella: a critical overview. Sensors 17(8). 10.3390/s17081910 [DOI] [PMC free article] [PubMed]
- Cuartero M, Parrilla M, Crespo GA. Wearable potentiometric sensors for medical applications. Sensors (Switzerland) 2019;19(2):1–24. doi: 10.3390/s19020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damborský P, Švitel J, Katrlík J. Optical biosensors. Essays Biochem. 2016;60(1):91–100. doi: 10.1042/EBC20150010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey D, Goswami T (2011) Optical biosensors: a revolution towards quantum nanoscale electronics device fabrication. J Biomed Biotechnol 2011. 10.1155/2011/348218 [DOI] [PMC free article] [PubMed]
- Diculescu VC, Paquim AMC, Brett AMO. Electrochemical DNA sensors for detection of DNA damage. Sensors. 2005;5(6–10):377–393. doi: 10.3390/s5060377. [DOI] [Google Scholar]
- Duan X, Rajan NK, Routenberg DA, Huskens J, Reed MA. Regenerative electronic biosensors using supramolecular approaches. ACS Nano. 2013;7(5):4014–4021. doi: 10.1021/nn306034f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeonu CS, Tagbo R, Anike EN, Oje OA, Onwurah INE. Biotechnological tools for environmental sustainability: prospects and challenges for environments in Nigeria—a standard review. Biotechnol Res Int. 2012;2012:1–26. doi: 10.1155/2012/450802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, White IM, Shopova SI, Zhu H, Suter JD, Sun Y. Sensitive optical biosensors for unlabeled targets: a review. Anal Chim Acta. 2008;620(1–2):8–26. doi: 10.1016/j.aca.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung F, Wang H-S, Menon S. Food safety in the 21st century. Biom J. 2018;41(2):88–95. doi: 10.1016/j.bj.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajendragad MR, Kamath KNY, Anil PY, Prabhudas K, Natarajan C. Development and standardization of a piezo electric immunobiosensor for foot and mouth disease virus typing. Vet Microbiol. 2001;78(4):319–330. doi: 10.1016/S0378-1135(00)00307-2. [DOI] [PubMed] [Google Scholar]
- Gong JL, Gong FC, Zeng GM, Shen GL, Yu RQ. An amperometric immunosensor for the Newcastle disease antibody assay. Anal Lett. 2003;36(2):287–302. doi: 10.1081/AL-120017691. [DOI] [Google Scholar]
- Goriushkina TB, Soldatkin AP, Dzyadevych SV. Application of amperometric biosensors for analysis of ethanol, glucose, and lactate in wine. J Agric Food Chem. 2009;57(15):6528–6535. doi: 10.1021/jf9009087. [DOI] [PubMed] [Google Scholar]
- Goyal RN, Gupta VK, Chatterjee S. Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. Sensors Actuators B Chem. 2010;149(1):252–258. doi: 10.1016/j.snb.2010.05.019. [DOI] [Google Scholar]
- Grieshaber D, MacKenzie R, Vörös J, Reimhult E. Electrochemical biosensors - sensor principles and architectures. Sensors. 2008;8:1400–1458. doi: 10.3390/s80314000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JG, Miao YQ, Zhang QJ. Impedimetric biosensors. J Biosci Bioeng. 2004;97(4):219–226. doi: 10.1263/jbb.97.219. [DOI] [PubMed] [Google Scholar]
- Hammond JL, Formisano N, Estrela P, Carrara S, Tkac J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016;60(1):69–80. doi: 10.1042/EBC20150008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Li Y, He D, Wang K, Shangguan J, Shi H. Aptamer-fluorescent silica nanoparticles bioconjugates based dual-color flow cytometry for specific detection of Staphylococcus aureus. J Biomed Nanotechnol. 2014;10(7):1359–1368. doi: 10.1166/jbn.2014.1828. [DOI] [PubMed] [Google Scholar]
- Hesari N, Alum A, Elzein M, Abbaszadegan M. A biosensor platform for rapid detection of E. coli in drinking water. Enzym Microb Technol. 2016;83:22–28. doi: 10.1016/j.enzmictec.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Huang JC, Chang Y-F, Chen K-H, Su L-C, Lee C-W, Chen C-C, et al. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in human serum using a localized surface plasmon coupled fluorescence fiber-optic biosensor. Biosens Bioelectron. 2009;25(2):320–325. doi: 10.1016/j.bios.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Tzang C, Yuan R, Yang M. Voltammetric biosensors for the determination of formate and glucose-6-phosphate based on the measurement of dehydrogenase-generated NADH and NADPH. Biosens Bioelectron. 2001;16(3):211–219. doi: 10.1016/S0956-5663(00)00143-3. [DOI] [PubMed] [Google Scholar]
- Hwang DW, Lee S, Seo M, Chung TD. Recent advances in electrochemical non-enzymatic glucose sensors – a review. Anal Chim Acta. 2018;1033:1–34. doi: 10.1016/j.aca.2018.05.051. [DOI] [PubMed] [Google Scholar]
- Jaffrezic-Renault N, Dzyadevych SV. Conductometric microbiosensors for environmental monitoring. Sensors. 2008;8(4):2569–2588. doi: 10.3390/s8042569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karesh WB, Dobson A, Lloyd-Smith JO, Lubroth J, Dixon MA, Bennett M, et al. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380(9857):1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Iezzi R, Shim BS, Martin DC. Impedimetric biosensors for detecting vascular endothelial growth factor (VEGF) based on poly(3,4-ethylene dioxythiophene) (PEDOT)/gold nanoparticle (Au NP) composites. Front Chem. 2019;7:1–11. doi: 10.3389/fchem.2019.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel DW, LeBlanc G, Meschievitz ME, Cliffel DE. Chemical sensors and biosensors. Anal Chem. 2007;84(2):685–707. doi: 10.1021/ac202878q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk I, Kovalchuk O. Transgenic plants as sensors of environmental pollution genotoxicity. Sensors. 2008;8(3):1539–1558. doi: 10.3390/s8031539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozitsina AN, Svalova TS, Malysheva NN, Okhokhonin AV, Vidrevich MB, Brainina KZ. Sensors based on bio and biomimetic receptors in medical diagnostic, environment, and food analysis. Biosensors. 2018;8(2):1–34. doi: 10.3390/bios8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaperumal I, Lakshmanan R. An approximate analytical method for the evaluation of the concentrations and current for hybrid enzyme biosensor. ISRN Phys Chem. 2013;2013:1–12. doi: 10.1155/2013/202781. [DOI] [Google Scholar]
- Kuznetsov AM, Rodicheva EK, Medvedeva SE, Gitelson JI. Bioluminescent bioassays based on luminous bacteria marker system. Biolumin Chemilumin. 2002;4:323–326. doi: 10.1142/9789812776624_0072. [DOI] [Google Scholar]
- Law JWF, Mutalib NSA, Chan KG, Lee LH. Rapid metho ds for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol. 2014;5:1–19. doi: 10.3389/fmicb.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WE, Thompson HG. Detection of newcastle disease virus using an evanescent wave immune-based biosensor. Can J Chem. 1996;74(5):707–712. doi: 10.1139/v96-076. [DOI] [Google Scholar]
- Lei Y, Chen W, Mulchandani A. Microbial biosensors. Anal Chim Acta. 2006;568(1–2):200–210. doi: 10.1016/j.aca.2005.11.065. [DOI] [PubMed] [Google Scholar]
- Li S, Li Y, Chen H, Horikawa S, Shen W, Simonian A, Chin BA. Direct detection of Salmonella typhimurium on fresh produce using phage-based magnetoelastic biosensors. Biosens Bioelectron. 2010;26(4):1313–1319. doi: 10.1016/j.bios.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Long F, Zhu A, Shi H. Recent advances in optical biosensors for environmental monitoring and early warning. Sensors (Switzerland) 2013;13(10):13928–13948. doi: 10.3390/s131013928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka G, Ahmadi A, Najjaran H, Alocilja E, Derosa M, Wolthers K, et al. Microfluidics integrated biosensors: a leading technology towards lab-on-A-chip and sensing applications. Sensors (Switzerland) 2015;15(12):30011–30031. doi: 10.3390/s151229783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekzad H, Sahandi Zangabad P, Mirshekari H, Karimi M, Hamblin MR. Noble metal nanoparticles in biosensors: recent studies and applications. Nanotechnol Rev. 2017;6(3):301–329. doi: 10.1515/ntrev-2016-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S, Verma A, Tyagi N, Kumar V. Biosensors: principle, types and applications. Int J Adv Res Innov Ideas Edu. 2017;3(2):3639–3644. [Google Scholar]
- Mehrotra P. Biosensors and their applications - a review. J Oral Biol Craniofac Res. 2016;6(2):153–159. doi: 10.1016/j.jobcr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra GK, Barfidokht A, Tehrani F, Mishra RK (2018) Food safety analysis using electrochemical biosensors. Foods 7(9). 10.3390/foods7090141 [DOI] [PMC free article] [PubMed]
- Mishra S, Bharagava RN, More N, Yadav A, Zainith S, Mani S, Chowdhary P (2019) Heavy metal contamination: an alarming threat to environment and human health. Environ Biotechnol Sustain Future:103–125. 10.1007/978-981-10-7284-0_5
- Mody RK, Griffin PM (2015) 103 - Foodborne disease. In: Bennett JE, Dolin R, Blaser MJ (eds) Mandell, douglas, and Bennett’s principles and practice of infectious diseases, 8th edn. Content Repository Only!, Philadelphia, pp 1283–1296.e3. 10.1016/B978-1-4557-4801-3.00103-X
- Moran KLM, Fitzgerald J, McPartlin DA, Loftus JH, O’Kennedy R (2016) Biosensor-based technologies for the detection of pathogens and toxins. In: Scognamiglio V, Rea G, Arduini F, Palleschi G (eds) Biosensors for sustainable food - new opportunities and technical challenges, vol 74. Elsevier, pp 93–120. 10.1016/bs.coac.2016.04.002
- Neethirajan S, Ragavan V, Weng X, Chand R (2018) Biosensors for sustainable food engineering: challenges and perspectives. Biosensors 8(1). 10.3390/bios8010023 [DOI] [PMC free article] [PubMed]
- Nguyen HH, Lee SH, Lee UJ, Fermin CD, Kim M. Immobilized enzymes in biosensor applications. Materials. 2019;12(1):1–34. doi: 10.3390/ma12010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen E, Smalling KL, Ahrens L, Gros M, Miglioranza KSB, Picó Y, Schoenfuss HL. Critical review: grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs. Environ Toxicol Chem. 2019;38(1):46–60. doi: 10.1002/etc.4290. [DOI] [PubMed] [Google Scholar]
- Oluwaseun AC, Phazang P, Sarin NB (2018) Biosensors: a fast-growing technology for pathogen detection in agriculture and food sector. In: Rinken T, Kivirand K (eds) Biosensing technologies for the detection of pathogens - a prospective way for rapid analysis, 1st edn. INTECH, pp 37–52. 10.5772/57353
- Park SC, Cho EJ, Moon SY, Yoon SI, Kim YJ, Kim DH, Suh JS. A calorimetric biosensor and its application for detecting a cancer cell with optical imaging. IFMBE Proc. 2007;14(1):637–640. doi: 10.1007/978-3-540-36841-0_147. [DOI] [Google Scholar]
- Parmar TK, Rawtani D, Agrawal YK. Bioindicators: the natural indicator of environmental pollution. Front Life Sci. 2016;9(2):110–118. doi: 10.1080/21553769.2016.1162753. [DOI] [Google Scholar]
- Peltomaa R, Glahn-Martínez B, Benito-Peña E, Moreno-Bondi MC (2018) Optical biosensors for label-free detection of small molecules. Sensors (Basel, Switzerland) 18(12). 10.3390/s18124126 [DOI] [PMC free article] [PubMed]
- Piro B, Reisberg S (2017) Recent advances in electrochemical immunosensors. Sensors (Switzerland) 17(4). 10.3390/s17040794 [DOI] [PMC free article] [PubMed]
- Pisoschi AM. Potentiometric biosensors: concept and analytical applications-an editorial. Biochem Anal Biochem. 2016;5(3):19–20. doi: 10.4172/2161-1009.1000e164. [DOI] [Google Scholar]
- Pohanka M. The piezoelectric biosensors: principles and applications, a review. Int J Electrochem Sci. 2017;12(1):496–506. doi: 10.20964/2017.01.44. [DOI] [Google Scholar]
- Pohanka M (2018) Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Materials 11(3). 10.3390/ma11030448 [DOI] [PMC free article] [PubMed]
- Pospíšilová M, Kuncová G, Trögl J. Fiber-optic chemical sensors and fiber-optic bio-sensors. Sensors (Switzerland) 2015;15(10):25208–25259. doi: 10.3390/s151025208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R, Suni II, Bever CS, Hammock BD. Impedance biosensors: applications to sustainability and remaining technical challenges. ACS Sustain Chem Eng. 2014;2(7):1649–1655. doi: 10.1021/sc500106y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaie M, Kazemi SS. Lectin-based biosensors: as powerful tools in bioanalytical applications. Biotechnology. 2010;9(4):428–443. doi: 10.3923/biotech.2010.428.443. [DOI] [Google Scholar]
- Rasheed PA, Sandhyarani N. Electrochemical DNA sensors based on the use of gold nanoparticles: a review on recent developments. Microchim Acta. 2017;184(4):981–1000. doi: 10.1007/s00604-017-2143-1. [DOI] [Google Scholar]
- Rasheed T, Bilal M, Nabeel F, Adeel M, Iqbal HMN. Environmentally-related contaminants of high concern: potential sources and analytical modalities for detection, quantification, and treatment. Environ Int. 2019;122:52–66. doi: 10.1016/j.envint.2018.11.038. [DOI] [PubMed] [Google Scholar]
- Rawat S. Food spoilage: microorganisms and their prevention. Pelagia Res Libr Asian J Plant Sci Res. 2015;5(4):47–56. [Google Scholar]
- Reyes R, Ahn R, Thurber K, Burke TF. Urbanization and infectious diseases: general principles, historical perspectives, and contemporary challenges. In: Fong IW, editor. Challenges in infectious diseases. New York: Springer; 2013. pp. 123–146. [Google Scholar]
- Rocchitta G, Spanu A, Babudieri S, Latte G, Madeddu G, Galleri G et al (2016) Enzyme biosensors for biomedical applications: strategies for safeguarding analytical performances in biological fluids. Sensors (Switzerland) 16(6). 10.3390/s16060780 [DOI] [PMC free article] [PubMed]
- Rodriguez-Mozaz S, Lopez De Alda MJ, Barceló D. Biosensors as useful tools for environmental analysis and monitoring. Anal Bioanal Chem. 2006;386(4):1025–1041. doi: 10.1007/s00216-006-0574-3. [DOI] [PubMed] [Google Scholar]
- Rovira J, Domingo JL. Human health risks due to exposure to inorganic and organic chemicals from textiles: a review. Environ Res. 2019;168:62–69. doi: 10.1016/j.envres.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Sangamithirai D, Munusamy S, Narayanan V, Stephen A. A voltammetric biosensor based on poly(o-methoxyaniline)-gold nanocomposite modified electrode for the simultaneous determination of dopamine and folic acid. Mater Sci Eng C. 2018;91:512–523. doi: 10.1016/j.msec.2018.05.070. [DOI] [PubMed] [Google Scholar]
- Semenova D, Silina YE, Koch M, Micheli L, Zubov A, Gernaey KV. Sensors for biosensors: a novel tandem monitoring in a droplet towards efficient screening of robust design and optimal operating conditions. Analyst. 2019;144(8):2511–2522. doi: 10.1039/c8an02261e. [DOI] [PubMed] [Google Scholar]
- Sheikhzadeh E, Chamsaz M, Turner APF, Jager EWH, Beni V. Label-free impedimetric biosensor for salmonella Typhimurium detection based on poly [pyrrole-co-3-carboxyl-pyrrole] copolymer supported aptamer. Biosens Bioelectron. 2016;80:194–200. doi: 10.1016/j.bios.2016.01.057. [DOI] [PubMed] [Google Scholar]
- Skládal P. Piezoelectric biosensors. TrAC Trends Anal Chem. 2016;79:127–133. doi: 10.1016/j.trac.2015.12.009. [DOI] [Google Scholar]
- Spain E, Venkatanarayanan A (2014) Review of physical principles of sensing and types of sensing materials. Comprehensive materials processing, vol 13. Elsevier. 10.1016/B978-0-08-096532-1.01302-9
- Srinivasan B, Tung S. Development and applications of portable biosensors. J Lab Autom. 2015;20(4):365–389. doi: 10.1177/2211068215581349. [DOI] [PubMed] [Google Scholar]
- Starodub N (2009) Biosensors in the system of express control of chemicals, regularly used as terrorist means, to prevent non-desirable consequences. In: Bahadir AM, Duca G (eds) The role of ecological chemistry in pollution research and sustainable development, 1st edn. Springer, pp 275–284. 10.1007/978-90-481-2903-4_27
- Starodub NF, Katzev AM, Starodub VM, Levkovetz IA, Goncharuk VV, Klimenko NA, et al. Biosensors for water quality monitoring. In: Alexander Omelchenko AAPWJS, et al., editors. Modern tools and methods of water treatment for improving living standards. Netherlands: Springer; 2005. pp. 51–70. [Google Scholar]
- Starodub NF, Ogorodniichuk YA, Sitnik YA, Slishik NF (2012) Biosensors for the control of some toxins, viral and microbial infections to prevent actions of bioterrorists. In: Nikolelis D (ed) Portable chemical sensors: weapons against bioterrorism, 1st edn. Springer, pp 95–117. 10.1007/978-94-007-2872-1
- Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng 2011. 10.1155/2011/939161
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Molecular, clinical and environmental toxicicology volume 3: environmental toxicology. Mol Clin Environ Toxicol. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4. [DOI] [Google Scholar]
- Thévenot DR, Toth K, Durst RA, Wilson GS. Electrochemical biosensors: recommended definitions and classification1International Union of Pure and Applied Chemistry: Physical Chemistry Division, Commission I.7 (Biophysical Chemistry); Analytical Chemistry Division, Commission V.5 (Electroanalytical) Biosens Bioelectron. 2001;16(1–2):121–131. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- Tombelli S (2012) Piezoelectric biosensors for medical applications. Biosens Med Appl. Elsevier Masson SAS. 10.1016/B978-1-84569-935-2.50002-4
- Tzoris A, Fearnside D, Lovell M, Hall EAH. Direct toxicity assessment of wastewater: Baroxymeter, a portable rapid toxicity device and the industry perspective. Environ Toxicol. 2002;17(3):284–290. doi: 10.1002/tox.10059. [DOI] [PubMed] [Google Scholar]
- Velychko TP, Soldatkin OO, Melnyk VG, Marchenko SV, Kirdeciler SK, Akata B, et al. A novel conductometric urea biosensor with improved analytical characteristic based on recombinant urease adsorbed on nanoparticle of silicalite. Nanoscale Res Lett. 2016;11(1):1–6. doi: 10.1186/s11671-016-1310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneshvar S, Sudhakumari CC, Senthilkumaran B, Prakash H. Recent advances in biosensor technology for potential applications - an overview. Front Bioeng Biotechnol. 2016;4:1–9. doi: 10.3389/fbioe.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajs E, Fernández N, Fragoso A. Supramolecular biosensors based on electropolymerised pyrrole-cyclodextrin modified surfaces for antibody detection. Analyst. 2016;141(11):3274–3279. doi: 10.1039/c6an00532b. [DOI] [PubMed] [Google Scholar]
- Wen W, Yan X, Zhu C, Du D, Lin Y. Recent advances on electrochemical Immunosensors. Anal Chem. 2017;89:138–156. doi: 10.1021/acs.analchem.6b04281. [DOI] [PubMed] [Google Scholar]
- Xu J, Luo X, Chen H. Analytical aspects of FET-based biosensors. Front Biosci. 2005;10:420–430. doi: 10.2741/1538. [DOI] [PubMed] [Google Scholar]
- Xu M, Wang R, Li Y. Rapid detection of Escherichia coli O157:H7 and Salmonella typhimurium in foods using an electrochemical immunosensor based on screen-printed interdigitated microelectrode and immunomagnetic separation. Talanta. 2016;148:200–208. doi: 10.1016/j.talanta.2015.10.082. [DOI] [PubMed] [Google Scholar]
- Yao CY, Fu WL. Biosensors for hepatitis B virus detection. World J Gastroenterol. 2014;20(35):12485–12492. doi: 10.3748/wjg.v20.i35.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo EH, Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors. 2010;10(5):4558–4576. doi: 10.3390/s100504558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Zhang L, Li Q, Chai Y, Cao S. A label-free amperometric immunosenor based on multi-layer assembly of polymerized o-phenylenediamine and gold nanoparticles for determination of Japanese B encephalitis vaccine. Anal Chim Acta. 2005;531(1):1–5. doi: 10.1016/j.aca.2004.10.072. [DOI] [Google Scholar]
- Zhang H, Ma X, Liu Y, Duan N, Wu S, Wang Z, Xu B. Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens Bioelectron. 2015;74:872–877. doi: 10.1016/j.bios.2015.07.033. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kitaoka H, Tsuji S, Tamai M, Kobayashi H, Honjoh KI, Miyamoto T. Development of a simultaneous detection method for foodborne pathogens using surface plasmon resonance biosensors. Food Sci Technol Res. 2014;20(2):317–325. doi: 10.3136/fstr.20.317. [DOI] [Google Scholar]
- Zhang Y, Tadigadapa S. Calorimetric biosensors with integrated microfluidic channels. Biosens Bioelectron. 2004;19(12):1733–1743. doi: 10.1016/j.bios.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Liu G, Kai M, Miller AOA. DNA aptamers in the diagnosis and treatment of human diseases. Molecules. 2015;20(12):20979–90997. doi: 10.3390/molecules201219739. [DOI] [PMC free article] [PubMed] [Google Scholar]