Abstract
Despite over a half-century of recognizing fibrinolytic abnormalities after trauma, we remain in our infancy in understanding the underlying mechanisms causing these changes, resulting in ineffective treatment strategies. With the increased utilization of viscoelastic hemostatic assays (VHAs) to measure fibrinolysis in trauma, more questions than answers are emerging. Although it seems certain that low fibrinolytic activity measured by VHA is common after injury and associated with increased mortality, we now recognize subphenotypes within this population and that specific cohorts arise depending on the specific time from injury when samples are collected. Future studies should focus on these subtleties and distinctions, as hypofibrinolysis, acute shutdown, and persistent shutdown appear to represent distinct, unique clinical phenotypes, with different pathophysiology, and warranting different treatment strategies.
Trauma-induced coagulopathy (TIC) encompasses a spectrum of coagulation changes after severe injury. There are multiple TIC phenotypes categorized by changes in thrombin generation, platelet function, and fibrinolysis, measured by coagulation protein levels1 and functional viscoelastic assays.2,3 In this multifactorial disease process, inhibiting systemic hyperfibrinolysis has become a focus of early resuscitation efforts due to the reported survival benefit of antifibrinolytics in trauma.4 Consistent with the findings in trauma, several large randomized clinical trials (RCTs) have shown a reduction in blood product administration with empiric antifibrinolytics in cardiac5 and orthopedic surgery.6 However, the benefits of these agents appeared to be limited in trauma patients in profound shock.7,8
A proposed mechanism for the limited efficacy of antifibrinolytics in mature trauma centers has been attributed to the large incidence of low fibrinolytic activity after severe injury.9 Low fibrinolytic activity, as measured by thromboelastography, has been associated with increased mortality.10–12 This has been termed “fibrinolysis shutdown,” but the definition can be further refined by whether this is a genuine inhibition of the fibrinolytic system after being initially activated, or if the fibrinolysis had never been initiated (hypofibrinolysis). While, intuitively, low systemic fibrinolysis levels measured by viscoelastic hemostatic assays (VHAs) would be associated with a hypercoagulable state, a cohort of these patients can also have elevated fibrin degradation products and bleeding complications,13,14 indicative of a hidden fibrinolytic activity. This phenomenon has been termed “occult” hyperfibrinolysis, and it is speculated that pathologic active fibrinolysis at a local injury level fails to extend into the circulation, remaining undetectable by VHA. However, this data interpretation is questionable because fibrinolysis quantification is based on circulating D-dimer and plasmin–antiplasmin (PAP) complexes, which have a half-life exceeding 12 hours.15
Despite the repeatedly demonstrated association between VHA-measured low fibrinolysis and increased mortality, ongoing confusion exists on the terminology, physiology, and clinical significance of impaired fibrinolysis in trauma. The purpose of this review is to provide an historical perspective on clinical studies that described and tested therapies for fibrinolysis shutdown, as well as appraise and synthesize the existing literature on impaired postinjury fibrinolysis to define future directions in managing these coagulation changes and considerations for using antifibrinolytics in this patient population.
HISTORY OF FIBRINOLYSIS SHUTDOWN AND TERMINOLOGY
Fibrinolysis Shutdown
The term fibrinolysis shutdown was first used in 196916 in a description of the effects of electroplexy, myocardial infarction, and elective surgery on fibrinolysis. This study documented a commonality of an acute stress event activating the fibrinolytic system, followed by an endogenous inhibition of the fibrinolytic system that lasted for days to weeks depending on the clinical scenario. This study was stimulated by a previous report by Innes and Sevitt17 who described a progressive prolongation of euglobulin lysis time (ELT) from admission to 6 hours after injury. Prior work by Hardaway et al18 in the 1950s suggested that trauma patients develop early hypercoagulability, resulting in disseminated intravascular coagulation (DIC) in the microvasculature, which triggered a subsequent endogenous autoheparinization and fibrinolysis to prevent progression to irreversible shock. Pathologic fibrinolysis shutdown was demonstrated in animals recovering from hemorrhagic shock that failed to clear microthrombi in small visceral vessels, resulting in organ failure,19 reversible by profibrinolytic agents after resuscitation.20 Cafferata et al21 in 1969 provided the most compelling evidence of fibrinolytic system failure in 12 patients with uncontrolled bleeding after surgical hemostasis in trauma. Eight of these patients had thrombi in their lungs; in 1 nonsurvivor treated with antifibrinolytic, the bleeding rate did not change. The authors proposed heparin should be used in this clinical scenario but cautioned that “courage to administer” this therapy was needed in the setting of unclear surgical hemostasis.
Fibrinolysis Shutdown Versus Hypofibrinolysis
Investigations of coagulation in elective surgery patients in the 1970s identified an increased risk of deep vein thrombosis (DVT) with low fibrinolytic activity after surgery,22 although this was not reproduced in other studies.23,24 Variable definitions and assays to define fibrinolysis shutdown were likely responsible for these inconsistencies. The ELT to definition of fibrinolysis shutdown was commonly used in coagulation research, but was known to have limitations.25 Griffith26 and Knight et al27 both demonstrated that prolonging of ELTs postoperatively successfully predicts postoperative thrombotic complications. To add further confusion, the term hypofibrinolysis was introduced in 1974.28 This new type of impaired fibrinolysis was diagnosed by a lack of ELT shortening or persistently elevated plasminogen activator inhibitor activity in blood samples obtained after venous occlusion of the upper extremity.29–32 Hypofibrinolysis represents an impaired activation of the fibrinolytic system, whereas fibrinolysis shutdown is activation of the fibrinolytic system with subsequent inhibition beyond a physiologic level.
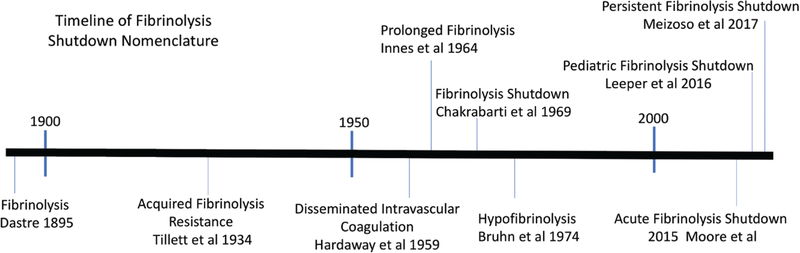
Plasminogen activator inhibitor 1 (PAI-1) identification in the 1980’s as a key regulator of tissue plasminogen activator (t-PA) was a critical advance in understanding fibrinolysis,33 although evidence of its existence dated back to the 1960s.34 This fast-acting t-PA inhibitor was subsequently identified as a culprit in postoperative DVT.35 The first study to associate PAI-1 with fibrinolysis shutdown in trauma was published in 198536 based on a review of the acute phase response by Kushner37; it was speculated that PAI-1 was an acute phase protein produced as a response to injury.36 A decade later, it was demonstrated that endothelial cells synthesized and released PAI-1 in response to an unknown plasma mediator in patients undergoing orthopedic surgery.38 Of numerous antigen activity levels measured pre- and postvenous occlusion, residual PAI-1 activity was associated with the highest DVT risk.29 Hypofibrinolysis and fibrinolysis shutdown have been demonstrated in other conditions such as diabetic vascular disease,39,40 and active inflammatory bowel disease.41 There is also a genetic autosomal dominant inheritance of impaired t-PA release, which is associated with DVTs.31 Additional work has implicated impaired urokinase release as a potential cause of hypofibrinolysis and risk for DVT.42 An important limitation in the current literature is assuming that hypofibrinolysis shutdown and fibrinolysis shutdown represent the same pathophysiology. Many clinic studies citing low fibrinolytic activity only include 1 measurement of fibrinolysis over time, which cannot effectively differentiate the 2 pathologies. A history of nomenclature on low fibrinolytic activity is depicted in Figure 1.
Figure 1.
Timeline of fibrinolysis nomenclature.
EARLY CLINICAL TRIALS OF TREATMENTS FOR FIBRINOLYSIS RESISTANCE
Early Clinical Studies Using Fibrinolytic Activators to Lyse Acute Thromboses
The treatment of acute thrombosis through activation of the fibrinolytic system preceded clinical trials aimed to treat fibrinolysis shutdown. Yamakawa43 in 1918 demonstrated activation of proteolytic enzymes, resulting in fibrin clot degradation, by removal of unidentified fibrinolytic inhibitors through chemical modification of plasma. Chloroform-incubated blood promoted fibrinolysis through removing a yet to be defined fibrinolytic inhibitor.44 The major breakthrough in treating acute thrombosis via fibrinolysis was the isolation of fibrinolysin (streptokinase) from hemolytic Streptococcus in the 1930s by Tillett and Garner.45 This group also provided one of the first descriptions of acquired fibrinolysis resistance in humans.46 Despite demonstration of fibrinolysin causing clot degradation in vitro,47 the mechanism for fibrin degradation remained unclear for over a decade. Christensen and Macleod48 in 1945 demonstrated that fibrin degradation occurred indirectly via activation of a circulating zymogen.48 This key identification of this abundant zymogen, plasminogen, would lead to the future of lytic therapy for acute thrombotic complications.
The first successful use of a plasminogen activator (streptokinase) to treat human disease occurred in 1949 to clear complex parapneumonic infections.49 Systemic streptokinase was used nearly a decade later to treat myocardial infarctions,50 which changed to t-PA in the 1980s because of increased clot lysis efficacy with fewer adverse events.51 Hesitance to use plasminogen activators in trauma has stemmed from its use to treat pulmonary embolism, in which urokinase (u-PA) was effective in clearing clots and reduced mortality, but led to a 40% bleeding complication rate.52
Stanozolol to Treat Postoperative Fibrinolysis Shutdown
In the 1970s, intramuscular anabolic steroids (stanozolol) were found to increase fibrinolysis and were efficacious in treating Raynaud disease, by increasing blood flow with concurrent reduction in fibrinogen.53 However, subsequent double-blinded studies in elective surgery,54 vascular surgery,55 and intensive care unit56 failed to demonstrate a clinical benefit. Enthusiasm for treating fibrinolysis resistance weaned with these repeatedly negative trials. The RCTs treating fibrinolysis shutdown are summarized in Table 1.
Table 1.
Clinical Trials to Treat Fibrinolysis Shutdown
| Author | n | Population | Treatment | Thrombotic Outcome | Intervention Versus Control | Fibrinolysis Quantification |
|---|---|---|---|---|---|---|
| Knight et al27 | 128 | General surgery | Arm SCD | DVT | 32% vs 14%a | ELTa POD 3 |
| Blamey et al54 | 60 | General surgery | Stanozolol IM 50 mg preoperative | DVT | 41% vs 35% | Fibrin plate lysisa POD 1 |
| Cuschieri et al56 | 50 | General surgery | Stanozolol IM 50 mg preoperative | Pulmonary complication | 67% vs 65% | Fibrin plate lysisa POD 1 |
| Berridge et al55 | 27 | Vascular surgery | Stanozolol IM 50 mg preoperative and 5 mg PO 6 wk | Early graft thrombosis | 8% vs 14% | ELTa POD 7 |
| Cahan et al68 | 48 | General surgery | Leg SCD and heparin | DVT | 0% vs 0% | PAI-1 activity |
| Killewich et al69 | 44 | General surgery | Leg SCD and heparin | DVT | 0% vs 0% | PAI-1 activity |
Abbreviations: DVT, deep vein thrombosis; ELT, euglobulin lysis time; IM, intramuscular; PAI-1, plasminogen activator inhibitor 1; PO, per os; POD, postoperative day; SCD, sequential compression device.
Statistical difference.
Plasminogen Activators to Treat Postinjury Fibrinolysis Shutdown
Treatment of fibrinolysis shutdown with a fibrinolytic agent was investigated in the 1960s.20 Hardaway et al57 translated these findings to humans and infused u-PA in patients with severe lung injury, markedly reducing oxygen requirements without bleeding complications. This mixed-population phase I trial included trauma patients within 48 hours postinjury. Despite the encouraging results, no other human trials to treat organ failure with a plasminogen activator have been completed. However, continued animal work supporting plasminogen activators to treat acute lung injury (ALI)58,59 is encouraging.
Using plasminogen activators as prophylaxis for patients at elevated risk of pulmonary embolism reduced hemodynamic collapse and death, but increased bleeding.60 The risk of increasing intracranial hemorrhage after blunt trauma makes plasminogen activators a less appealing target, as roughly a quarter of severely injured patients have intracranial bleeding.61 To our knowledge, there are no current RCTs to treat posttrauma or surgical fibrinolysis shutdown with plasminogen activators.
CURRENT UNDERSTANDING OF FIBRINOLYSIS SHUTDOWN AND FUTURE DIRECTION IN TRAUMA
Fibrinolysis Shutdown: A Target for Reducing Postinjury Thrombotic Complications and Organ Failure
Trauma patients are prone to thrombotic events, with DVT rates approaching 60% with surveillance.62 There is evidence for thrombosis in the pulmonary vasculature in nearly 25% of severely injured patients within 48 hours of their injury.63 Furthermore, microvascular clots in organs other than the lungs have been implicated in nonlung organ dysfunction.64,65 It is intuitive that even in the setting of injury with hemorrhage, fibrinolysis is required for overall homeostasis to clear the microvasculature of excessive fibrin deposition. ALI models implicated PAI-1, in addition to other antifibrinolytics (α−2 antiplasmin and thrombin activatable fibrinolysis inhibitor [TAFI]), as culprits in progression to pulmonary failure.66 In 1991, Enderson et al67 observed that most multisystem trauma patients had low fibrinolytic activity and elevated D-dimer. Moore et al9 13 years later reported that 65% of severely injured patients had low VHA-measured fibrinolytic activity within 12 hours of injury. This low VHA-measured fibrinolysis (identified at the time as fibrinolysis shutdown) was associated with increased mortality (due to organ failure) compared to patients with physiologic levels of fibrinolytic activity. VHA-detected fibrinolysis shutdown in children was subsequently demonstrated to be associated with both increased mortality and VTE.12 This VHA-defined shutdown was also associated with a high number of traumatic brain injury deaths.10
Subsequently, numerous studies have identified a consistent trend in which a low rate of clot degradation, as measured by VHA, is associated with increased mortality (Table 2). The definitions of shutdown were based on the thrombelastography (TEG) variable lysis at 30 minutes (LY30) prediction of mortality in adult9 and pediatric trauma patients,12 with a threshold of <0.9%. This same cutoff has been validated to identify increased mortality in trauma with both kaolin14 and rapid activated TEG.10 A recent analysis suggests that an LY30 <0.5% in a rapid TEG may have improved specificity for identify patients with low fibrinolytic activity and increased mortality.70 Rotational thrombelastography (ROTEM) detection of fibrinolysis shutdown has also been defined as maximum lysis (ML) l <5%,13 <3%,71 and clot lysis index (CLI) >97%.70
Table 2.
Clinical Studies Using VHA to Identify Shutdown and Associated Outcomes
| First Author, Year | Adult | Population | No. of Centers | N | Assay | Definition | SD Prevalence | 95% CI for SD Prevalence | Mortality SD Versus PY |
|---|---|---|---|---|---|---|---|---|---|
| Moore et al,9 2014 | Yes | Within 12 h ISS >15 |
1 | 180 | Kaolin TEG | LY30 <0.9% | 64% | 57%–71% | 17% vs 3% |
| Moore et al,10 2016 | Yes | Within 1 h ISS >15 |
2 | 2540 | Rapid TEG | LY30 <0.9% | 46% | 44%–48%a | 22% vs 14% |
| Leeper et al,77 2017 | No | Trauma activation | 1 | 130 | Kaolin TEG | LY30 <0.9% | 38% | 30%–46% | 15.7% vs 1.7% |
| Meizoso et al,78 2017 | Yes | ICU admission | 1 | 181 | Rapid TEG | LY30 <0.9% | 58% | 51%–65% | 17% vs 9% |
| Moore et al,79 2017 | Yes | Trauma activation | 1 | 398 | Rapid TEG | LY30 <0.9% | 22% | 18%–26% | 18% vs 7% |
| Gall et al,13 2018 | Yes | ACIT study | 5 | 914 | EXTEM ROTEM | ML <5% | 59% | 56%–62%a | 15% vs 7.1% |
| Gomez-Builes et al,71 2018 | Yes | Trauma AIS >2 | 1 | 550 | EXTEM ROTEM | ML <3.5% | 26% | 22%–30% | 12.8% vs 6.4% |
| Roberts,80 2018 | Yes | Trauma admissions | 3 | 795 | Rapid TEG | LY30 <0.9% | 44% | 41%–47%a | 10% vs 7% |
| Stettler et al,70 2019 | Yes | Trauma activations | 1 | 216 | EXTEM ROTEM | CLI60 >97% | 20% | 15%–25% | 25% vs 4% |
| Stettler et al,70 2019a | Yes | Trauma activations | 1 | 216 | Rapid TEG | LY30 <0.5% | 20% | 15%–25% | 24% vs 5% |
| Cardenas et al,14 2019 | Yes | PROPPR study | 12 | 547 | Kaolin TEG | LY30 <0.9% | 61% | 57%–65%a | 17.4% vs 11.6% |
Abbreviations: ACIT, Activation of Coagulation and Inflammation in Trauma; AIS, abbreviated injury severity score; CI, confidence interval; CLI, clot lysis index; ICU, intensive care unit; ISS, injury severity score; LY30, lysis at 30 minutes; ML, maximal lysis; PROPPR, Pragmatic, Randomized Optimal Platelet and Plasma Ratio; PY physiologic fibrinolysis; ROTEM, rotational thrombelastography; SD, fibrinolysis shutdown; TEG, thrombelastography; VHA, viscoelastic hemostatic assay.
Not accounting for intracenter correlation (same study but used different assay).
Defining Fibrinolysis Shutdown: Discrepancies in Whole Blood Versus Plasma Analysis
Defining fibrinolysis shutdown in trauma remains challenging. This would be ideally accomplished by measuring all the fibrinolytic regulators (plasma and cellular based), paired with a functional fibrinolytic assay. The historic gold standard for measuring fibrinolysis activity is the ELT. As previously mentioned, prolonged ELT in trauma patients has been associated with thrombotic morbidity.17 However, this assay is time and resource intensive, lacks sensitivity for detecting fibrinolysis inhibition,25 and does not account for the confounding effect of platelets and antiplasmin on fibrinolysis because it is not whole blood based. The importance of platelet functionality on clot generation has been well established,72 and the local microenvironment promotes a stable clot structure that is fibrinolysis resistant.73 Platelets provide the highest concentration of circulating PAI-1,74 which can effectively be released by thrombin activation, successfully blocking t-PA.75 While an association of increased platelet function with fibrinolysis shutdown has not been described, sensitivity to t-PA–mediated fibrinolysis correlates with platelet dysfunction in trauma patients.76
Other techniques to assess fibrinolytic activity include measurements of fibrinolysis degradation products (D-dimer) or complexes, such as the PAP complex. Because the fibrinolytic system is dynamic, and these biomarkers have extended plasma half-lives (D-dimer: 16 hours, PAP: 12 hours),15 isolated measurements do not represent the patient’s current systemic fibrinolytic activity. The time course of these fibrinolysis products has not been fully elucidated in injured patients, whose fibrinolytic system could have been activated before the first blood draw. This highlights the importance of functional, “real time,” whole blood assays to quantify fibrinolysis activity in trauma patients. The clinical laboratory measurements of pathologic fibrinolysis phenotypes are summarized in Table 3.
Table 3.
Fibrinolysis Phenotypes
| Phenotype | VHA Clot Degradation (LY30, ML) | PAP | d-Dimer | t-PA Antigen | t-PA Activity | PAI-1 Antigen | PAI-1 Activity |
|---|---|---|---|---|---|---|---|
| Hyperfibrinolysis | ⇑ | ⇑⇑ | ⇑⇑ | ⇑⇑ | ⇑⇑ | ⇔⇓ | ⇓⇓ |
| Acute shutdown | ⇓ | ⇑⇑ | ⇑⇑ | ⇑ | ⇔⇓ | ⇔ | ⇓ |
| Hypofibrinolysis | ⇓ | ⇔ | ⇔ | ⇔ | ⇓ | ⇔ | ⇑ |
| Physiologic | ⇔ | ⇑ | ⇑ | ⇔⇑ | ⇔ | ⇔ | ⇔ |
| Acquired shutdown | ⇓ | ⇑ | ⇑ | ⇓ | ⇓ | ⇑⇑ | ⇑⇑ |
| Persistent shutdowna | ⇓ | ⇔ | ⇔ | ⇓ | ⇓ | ⇑ | ⇑ |
Abbreviations: LY30, lysis at 30 min; ML, maximal lysis; PAI-1, plasminogen activator inhibitor 1; PAP plasmin–antiplasmin levels; t-PA, tissue plasminogen activator; VHA, viscoelastic hemostatic assays.
Greater than or equal to 24 h from injury.
Local Versus Systemic Fibrinolysis
The measurement of systemic fibrinolysis does not necessarily reflect local fibrinolytic activity. Discrepancies in the biomarkers of fibrinolysis activation and low systemic functional fibrinolytic activity have been appreciated for over 25 years.67 Raza et al81 described this discordance between VHA and plasma bases assays when reporting that 57% of acutely injured trauma patients had low fibrinolysis and high PAP complexes. Low levels of VHA-measured fibrinolysis activity with simultaneous high levels of D-dimer and PAP14,81 have recently been reported. It remains unclear if these patients have ongoing local fibrinolysis at the injury site not reflective of systemic circulation or if they had prior activation of their fibrinolytic system and have now shut it down.
There are no prospective trauma studies confirming a reduction in D-dimer and PAP levels over time in patients treated with antifibrinolytics presenting to the hospital with low fibrinolytic activity. However, antifibrinolytics can effectively reduce elevated fibrinolytic activity measured by VHAs on subsequent blood draws.8 PAP and D-dimer levels in severe injury would be expected to be markedly elevated as the clot forms at the site of injury, creating the potent cofactor fibrin,82 for generating plasmin in a t-PA–mediated fashion. Despite the limitations of D-dimer and PAP to quantify fibrinolysis, their elevated levels on presentation to the hospital are biomarkers of injury severity and poor prognosis.13,14,81,83
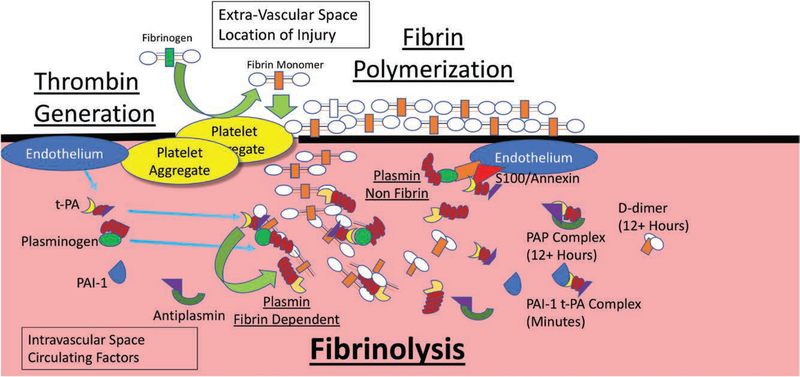
Factors that line the endothelium, such as S100A10 on Annexin A-2, can augment plasmin generation.84 In a large prospective multicenter study from Europe, circulating S100A10 was associated with elevated PAP and D-dimer levels, but 2 distinct cohorts existed with elevated S100A10.13 The cohort with low ML on ROTEM (presumed fibrinolysis shutdown) had a 30% mortality rate within several days after injury, compared to patients with an elevated ML (presumed hyperfibrinolysis) who died predominantly within the first 24 hours. The high S100A10 group with low ML (presumed shutdown) had a lower rate of massive transfusion and increased rate of thrombotic complication. While the low ML cohort may have “occult” hyperfibrinolysis at the site of injury, the data are not consistent with this proposal, because the low ML high PAP patients did not die early from bleeding. These laboratory derangements would be expected in patients with prior activation of their fibrinolytic system, who have shut it down by the time of blood draw. Plasmin generation via the S100A10 Annexin pathway occurs in a non–fibrin-dependent mechanism,84 which does not necessarily represent active fibrinolysis. The local regulation of fibrinolysis and plasmin generation is summarized in Figure 2.
Figure 2.
Local regulation of fibrinolysis. The different levels of regulation of fibrinolysis and clot generation at a local level. The vascular wall at the site of injury promotes platelet aggregation and thrombin generation. This results in fibrin polymerization. At the same time, the endothelium is activated to release tissue plasminogen activator (t-PA) via a yet to be defined mechanism. This results in local plasmin activation via t-PA binding to the growing fibrin chain and colocalization of plasminogen. The resulting plasmin cleaves the fibrin chain, exposing more sites for plasminogen and t-PA to bind increasing fibrinolytic activity promoting clot degradation. Plasmin can also be generated in a non–fibrin-mediated fashion away from the site of injury with endothelial surface receptors such as annexin and s100, which colocalize t-PA and plasminogen promoting plasmin generation. This plasmin generation and fibrinolysis is kept in check with circulating proteins that bind and complex t-PA and plasmin (plasminogen activator inhibitor 1 [PAI-1] and α2 antiplasmin). Platelets can also locally release these fibrinolytic inhibitors. Fibrin clot degradation is also regulated by intrinsic clot properties such as fibrin cross-linking and cleavage of lysine residues which were not incorporated due to size. The laboratory assessment of fibrinolysis measures the efferent blood from the site of injury that has mixed with the systemic circulation. D-dimer and plasmin–antiplasmin (PAP) complexes will remain in the circulation for hours after injury while t-PA PAI-1 complexes are cleared in minutes from the liver (although in states of shock duration remains unknown). Measurement of total antigen of these proteins does reflect the activity, as the complex, which cannot generate plasmin, is included in the total antigen measurement. Viscoelastic hemostatic assays (VHA) assessment of blood contains components of the proteases and inhibitors from the site of injury and other remote ischemic organ beds, but not the local injury milieu which has been diluted and altered after it has been through circulation. Neither laboratory technique depicts the local endothelial contribution to clot strength and fibrinolysis.
In the study by Gall et al,13 patients with low ML and high D-dimer levels had additional coagulation abnormalities and increased blood transfusions compared to patients with low D-dimer levels. Recently, Cardenas et al14 reported that bleeding trauma patients with low LY30 and elevated D-dimer levels had prolonged international normalize ratio of prothrombin time (INR), partial thromoplastin time (PTT), and platelet inhibition. They also described a pattern of trauma patients with plasma-based detection of fibrinolysis with elevated fibrin degradation products and PAP levels, but no evidence of systemic fibrinolysis activity at the time of blood draw. This laboratory assessment fits the clinical phenotype of fibrinolysis shutdown. A recent publication from Toronto71 also implicated fibrinolysis shutdown with an independent increased risk of blood transfusions. These investigators found no difference in mortality in patients with fibrinolysis shutdown compared to moderate levels of fibrinolysis after adjusting for confounders. They suggested that fibrinolysis shutdown was a protective mechanism after severe injury to reduce bleeding. However, this study was limited to 58 deaths and used 7 covariates, raising questions about statistical power and model overfitting.
There is ongoing debate on the most reliable method for measuring fibrinolysis after trauma, and whether we should treat patients with presumed fibrinolysis abnormalities based on VHA or plasma-based laboratory tests (D-dimer, Clauss fibrinogen). These questions will need RCTs. To date, the only RCT contrasting these 2 techniques in trauma was a single-center study that demonstrated nearly a 50% mortality reduction with goal-directed resuscitation guided by VHA over conventional assays85; however, the specific impact of using LY30 over D-dimer levels to guide tranexamic acid (TXA) use was not explicitly evaluated in this study for its impact on mortality. Recent analysis demonstrated that both of the commonly used clinical VHA (TEG and ROTEM) have good agreement in identifying the same patients with low fibrinolysis activity after injury that has elevated mortality.70
Subphenotypes of Low Fibrinolysis Activity on Presentation to the Hospital
Recent studies from different countries13,14,71 suggested that trauma patients presenting to the hospital with low fibrinolytic activity may be paradoxically at risk of bleeding. Indeed, there is a subphenotype of fibrinolysis shutdown that has increased susceptibility to t-PA–mediated fibrinolysis and platelet dysfunction.86 This paradoxical coagulation phenotype likely happens in patients who had previously activated their fibrinolytic system, but had shut the system down by the time of their first blood draw, given the evidence of depletion of fibrinolytic inhibitors. Based on the original description of fibrinolysis shutdown in trauma,17 this is likely true fibrinolysis shutdown, and it remains unclear if this early inhibition of fibrinolysis is a protective or pathologic event after injury.
The other subphenotype of fibrinolysis shutdown, described in a Denver study,86 lacked t-PA sensitivity with retention of fibrinolytic inhibitors and had a 5-fold elevation in mortality compared to patients lacking t-PA sensitivity without fibrinolysis shutdown. This patient population may have hypofibrinolysis rather than fibrinolysis shutdown, because there was no clear evidence that they had activated their fibrinolytic system. Definitive proof of hypofibrinolysis by using isolated extremity venous occlusion31 would be clinically challenging in the setting of trauma. An alternative laboratory measurement to differentiate hypofibrinolysis from fibrinolysis shutdown is needed; potential strategies include measuring PAI-1 activity32 and documenting minimal elevation of PAP or D-dimer after injury. Differentiating hypofibrinolysis from fibrinolysis shutdown in trauma is critical because both types of patients present with low fibrinolytic activity but represent different pathophysiology. Clustering all patients who present with a low TEG LY30 into the fibrinolysis shutdown category has been an oversight. Studies are needed to differentiate the fibrinolysis subphenotypes to define the spectrum of postinjury fibrinolysis pathology and develop targeted treatment modalities.
Timing of Fibrinolysis Changes
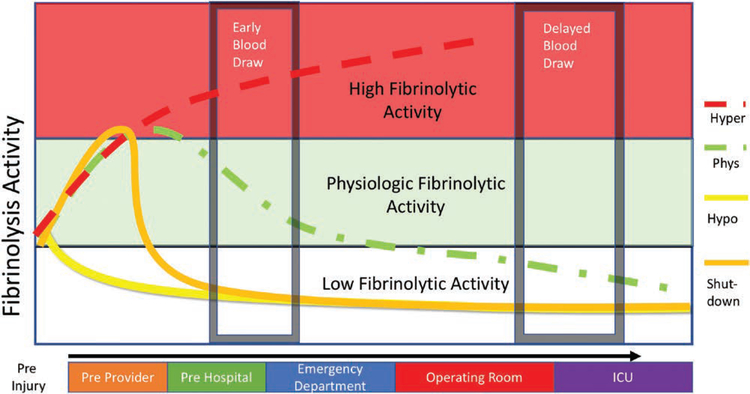
Perhaps the most critical component of treating postinjury fibrinolysis is understanding the relationship between time from injury and its measurement in systemic blood samples. The transition from a wide range of fibrinolysis activity to fibrinolysis resistance within hours of injury has been documented for over 50 years,17 reaffirmed in the 1980s,36 and further verified within the past 5 years.87 These transitions make research and treatment challenging, as patients can shift between different states of fibrinolytic activity from injury to resuscitation. Single blood draws can capture ephemeral fibrinolytic states. The longer the delay from injury to blood sampling, the higher the probability that the patient has low fibrinolytic activity measured by VHA. This is evident in several studies. Blood samples obtained from patients obtained with a median of 2 hours from injury had a prevalence of 58% of shutdown.13 This was comparable to the prevalence of shutdown in patients with bloods samples obtained on admission to the intensive care unit,11 while 65% of severely injured patients with blood samples within 12 hours of injury had fibrinolysis shutdown.9 This contrasts to early field blood draws in trauma activations (not all severely injured patients), among which the prevalence of shutdown may be as low as 22%.86 A schematic of how trauma patients can be misclassified by fibrinolysis phenotype is depicted in Figure 3. Contrasting similar patient populations at comparable times from injury is essential for advancing our understanding of fibrinolysis after injury. It is likely that a significant number of patients in the reports by Moore et al,9 Meizoso et al,11 and Gall et al13 were misclassified as being in acute fibrinolysis shutdown due to the timing from injury to blood draw. Blood samples obtained within an hour of injury indicate that the acute fibrinolysis shutdown prevalence is 20%–40%.10,12,71
Figure 3.
Timing of blood draw and fibrinolysis phenotypes. The theoretical time course of fibrinolysis changes of the various phenotypes of fibrinolysis after severe injury. With severe injury and shock, the expected response is activation of the fibrinolytic system to counterbalance early hypercoagulability. This occurs early after injury and often before prehospital providers arrive on the scene. After initial prehospital resuscitation, the phenotypes of postinjury fibrinolysis emerge. Patients who develop acute fibrinolysis shutdown will have a rapid transition to a low fibrinolytic state, while patients with physiologic fibrinolysis will have a more gradual decline in fibrinolytic activity. The hypofibrinolytic phenotype will have a blunted response to trauma and retain low fibrinolysis activity early after injury. Early blood draws (within hour of injury) can stratify patients into respective phenotypes except for hypofibrinolysis and fibrinolysis shutdown. After resuscitation, all phenotypes converge into a low fibrinolytic state due to a postresuscitation acquired fibrinolysis resistance from plasminogen activator inhibitor 1 (PAI-1) elevation. Patients who have sustained hyperfibrinolytic after initial in-hospital resuscitation efforts are unlikely to be alive several hours after injury. Obtaining blood samples several hours after provides a feedback on successful resuscitation efforts, but differentiating a patient’s initial fibrinolytic phenotype based on viscoelastic hemostatic assays (VHA) is not possible as all prior phenotypes have converged to a fibrinolytic resistant state. This postresuscitation fibrinolytic resistant state is not associated with increased mortality, but the duration that these patients remain in fibrinolysis shutdown predicts adverse outcomes. ICU indicates intensive care unit.
Decades of research have demonstrated a transition to fibrinolysis resistance several hours after injury or surgery,16,17,24,36,38 which is different than acute fibrinolysis shutdown. The transition to fibrinolysis resistance has been proposed to be an acute phase response via PAI-1 upregulation.36 In a small study of hyperfibrinolytic trauma patients who did not receive antifibrinolytics, fibrinolysis was significantly inhibited within 4 hours of injury, correlating to a several thousand fold PAI-1 upregulation during this time frame.87 With an endogenous transition to increased fibrinolysis resistance with PAI-1 production after resuscitation, differentiating pathologic early fibrinolysis shutdown, from a physiologic acquired shutdown is difficult. Elevated PAI-1 activity is not characteristic of acute fibrinolysis shutdown,86 and the mediator of this early low fibrinolytic activity is yet to be identified. Tissue injury appears to play a role in impairing fibrinolysis in animal models,88–90 which is consistent with the association of blunt injuries with acute fibrinolysis shutdown.10 This endogenous impairment of fibrinolysis followed by a PAI-1 surge after resuscitation could be responsible for persistent fibrinolysis shutdown.
The duration of fibrinolysis shutdown after postinjury resuscitation may be the critical insult leading to organ injury and thrombotic complications. Acute phase reactants are proposed to play both protective and destructive roles.37 Specifically, fibrinolysis activation in trauma followed by a delayed upregulation of fibrinolytic inhibitors would be an expected physiologic response to prevent excessive bleeding, but at the cost of increased thrombotic risks. The upregulation of fibrinolysis inhibitors after fibrinolysis activation is appreciated in thrombolytic therapy, whereas in the postlytic period, PAI-1 levels become upregulated.91 This PAI-1 elevation after reperfusion is presumably a physiologic response, because the prevalence of low fibrinolytic activity between 4 and 6 hours after injury exceeds 80%, yet only patients with persistently low fibrinolysis at 24 hours from injury have adverse outcomes.87 A Miami study suggests an 8-fold mortality increase in trauma patients that retain low fibrinolytic activity for 7 days postinjury, compared to those who recover from shutdown.11 A similar observation was also appreciated from data combined from multiple trauma centers, in which patients who remained in fibrinolysis shutdown at 24 hours had a 3-fold increased rate in mortality.92 Similarly, persistent fibrinolysis shutdown after resuscitation has also been associated with adverse outcomes in pediatric trauma.93 The mechanism for persistent fibrinolysis shutdown is presumed to be related to PAI-1. While data are lacking in trauma, prospective observation data in patients with sepsis showed improved survival with recovery of fibrinolytic activity associated with decreases in PAI-1.94
DISCUSSION AND FUTURE DIRECTION
Low Fibrinolysis Activity at Hospital Arrival: Fibrinolysis Shutdown Versus Hypofibrinolysis
Low fibrinolytic activity measured by VHA is common in severely injured trauma patients on hospital presentation. These patients have increased mortality compared to patients with moderate levels of fibrinolysis. Subphenotypes of low fibrinolysis early after injury are apparent. One subphenotype has laboratory measurements suggesting prior activation of their fibrinolytic system with current low systemic fibrinolytic activity, which can be appropriately defined as fibrinolysis shutdown (Figure 3). Patients with early fibrinolysis shutdown can have associated prolonged INR, low fibrinogen, and platelet dysfunction, with ongoing blood transfusion requirements. These patients require treatment of their coagulation abnormalities, which includes goal-directed plasma, cryoprecipitate, and platelet-based resuscitation. In the setting of uncontrolled bleeding, empiric blood component low-ratio driven resuscitation is appropriate until coagulation assay results can be obtained.95 While this patient population may have local fibrinolysis at the site of injury, the role of antifibrinolytics in this population remains unclear.
The other phenotype of low fibrinolytic activity on presentation to the hospital can be termed hypofibrinolysis (Figure 3). While a laboratory definition of hypofibrinolysis is yet to be determined (VHA, D-dimer level, PAP level, PAI-1 activity, or other), these patients do not manifest prior evidence of a large activation of the fibrinolytic system and retain normal or even elevated levels of fibrinolysis inhibitors. These patients are less likely to have bleeding complications, but have increased mortality compared to other trauma patients with higher VHA-measured systemic fibrinolytic activity without evidence of depletion of systemic fibrinolytic inhibitors. Potential therapeutic targets for patients with hypofibrinolysis would include early anticoagulation versus profibrinolytic agents such as t-PA. Genetic linkage of hypofibrinolysis has previously been appreciated in patients with DVT due to impaired t-PA release from venous occlusion.96 However, the safety of anticoagulating of hypofibrinolysis in this patient population after trauma has not been studied. This patient population would be unlikely to respond to antifibrinolytic agents given that they have a weakened endogenous capacity to generate plasmin and attempts to reduce this further would be essentially pointless. The key clinic implication is differentiating hypofibrinolysis from fibrinolysis shutdown as they represent different pathophysiology. Assuming that all trauma patients with low systemic fibrinolytic activity measured with viscoelastic assays are the same will result in a failure to develop effective resuscitation strategies and lead to continued confusion in the trauma literature.
Acquired Fibrinolysis Resistance and Persistent Fibrinolysis Shutdown
After resuscitation, an acute phase response generates PAI-1 upregulation of causing fibrinolysis resistance in most trauma patients. Acquired fibrinolysis resistance between 4 and 12 hours after injury is not associated with increased morbidity or mortality and likely represents a physiologic response to injury and recovery from hemorrhagic shock. Therefore, when evaluating trauma patients’ fibrinolytic status, results from blood samples early after injury should not be considered the same as those from samples obtained 2–4 hours after resuscitation. Pooling results of these temporally distinct samples will misclassify pathologic and physiologic fibrinolysis. The transition from physiologic to pathologic acquired fibrinolysis resistance appears to become evident at 24 hours after injury, presumably due to persistently elevated PAI-1. Recovery from fibrinolysis resistance is associated with improved survival in sepsis and trauma.
Attenuation of PAI-1 level activity 24 hours after injury is an appealing therapeutic target to treat fibrinolysis shutdown in trauma, because identification of these patients and initiation of therapy can be done in a logistically feasible time frame. The question remains whether the optimal treatment strategy in these patients is to reduce fibrinolysis inhibitors such as PAI-1, or whether a plasminogen activator can be used without increased bleeding, particularly in the setting of traumatic brain injury. There is currently a phase II randomized control trial, Statin and Aspirin in Trauma (STAT), using these medications to treat persistent fibrinolysis shutdown underway. (NCT02901067).
Relevance of Antifibrinolytics
The benefits of antifibrinolytics in treating pathologic hyperfibrinolysis detected by VHA were proposed by Dr Stazl’s liver transplant team in 1966.97 However, their enthusiasm for utilization of aminocaproic acid was diminished after they had experienced a series of lethal thrombotic complications with empiric use several years later. This prompted their recommendation to use antifibrinolytics only when there was laboratory evidence of excessive fibrinolysis and the patient was actively bleeding.98 In trauma, the antifibrinolytic tranexamic acid gained popularity after the Effects of Tranexamic Acid on Death, Vascular Occlusive Events, and Blood Transfusions in Trauma Patients with Significant Haemorrhage (CRASH)-2 trial which showed a modest reduction in mortality in a 20,000-patient multicenter study performed in predominantly resource-limited countries.4 Subsequent observational studies in mature trauma systems have failed to identify an overall survival benefit in all trauma patients who receive TXA.7,99–102 However, in a subgroup analysis, patients in hemorrhagic shock appear to have a survival benefit99,102 which is consistent with the CRASH-2 trial that patients with extremely low systolic blood pressures had the greatest survival benefit.4 This has fueled an active debate in trauma if patients receive TXA selectively based on their fibrinolytic status103 or if VHA measurement is irrelevant for guiding TXA use and any bleeding patient should receive this medication.104
Post hoc analysis of the CRASH-2 data identified the danger associated with TXA use was related to delivery 3 hours after injury, which was associated with increased mortality.105 While these deaths were attributed to bleeding, it is unclear why they occurred. An animal model suggests that this is related to increased plasmin generation through an alternative pathway of urokinase generation via TXA conformational changes to plasminogen.106 It is important to consider that patients with microvascular thrombosis can be bleeding to death as described by Cafferata et al21 in 1969. However, there were not increased thrombotic complications in the TXA arm appreciated in subgroup populations of CRASH-2.107 Combining the CRASH-2 with postpartum hemorrhage with 40,000 patients supports a survival benefit in TXA when combining these large studies with an emphasis on early administration.108 However, these large analyses are not reflective of advanced trauma systems, particularly in regional trauma centers with immediately available blood components or whole blood. Recent evidence in mature trauma systems has identified TXA as a risk factor for increased thrombotic complications.109,110 This was supported by a randomized control trial for TXA use in traumatic brain injury.111 Interestingly, a 2-g dosing of tranexamic acid over 1 g has been reported at a national meeting to reduce mortality in traumatic brain injury, but results are yet to be published. Beyond thrombotic complications, there is a concern that TXA is associated with increased mortality when given to patients with physiologic levels in fibrinolysis and no benefit in patients in fibrinolysis shutdown.112 While the mechanism and causality remain unclear, TXA use is associated with a persistent fibrinolysis shutdown113 which is associated with increased mortality.11,92,93 Based on animal work,20 prolonged fibrinolysis shutdown would be a risk factor for mortality due to microvascular thrombosis leading to organ failure because of failure to clear these clots, but the clinical translation has not been validated.
The literature supporting TXA-guided therapy based on hyperfibrinolysis measure by VHA also remains unclear. Even goal-directed TXA has not been associated with improved overall survival114 but has been associated with an early reduction in mortality.115 This is consistent with a recent meta-analysis of TXA use associated with a reduction in 24-hour mortality but not 30-day mortality.116 This can be interpreted in 2 ways, one in which is TXA reduced early mortality from bleeding, but increases the risk of delayed death from subsequent prolongation of fibrinolysis shutdown rendering the overall survival negated; or 2, VHA are insensitive to accurately identify which patients are hyperfibrinolytic. Continued research is needed in this field, and the answer will likely not ultimately be obtained until a randomized control trial is created to address this clinical question.
From these conflicting data, several considerations can be made in regard to the use of TXA in trauma and the relevance to hyperfibrinolysis. The first is that the survival benefit of TXA in a mature trauma system is limited to patients in profound hemorrhagic shock or severe head injury, within 3 hours of injury. On the other hand, the administration of TXA prolongs the time a patient remains in fibrinolysis shutdown, which is a risk factor for delayed mortality. There is now concern that TXA use in mature trauma systems is associated with thrombotic complications. The treating teams of these severely injured trauma patients should be aware of the patients who received TXA, which may help with future decision making on anticoagulation or imaging to rule out thrombotic complications. The decision to give trauma patients TXA should be an informed and collaborative team approach. Emergency medicine, anesthesiologists, and surgeons should all agree when it is appropriate to deliver the medication and should anticipate the sequelae of medically inducing fibrinolysis shutdown. It is essential to remember that hyperfibrinolysis after trauma is an early event that has long been successfully corrected by resuscitation without antifibrinolytics due to endogenous fibrinolysis shutdown.17 The question remains who will optimally benefit from pharmacologic intervention of this process.
CONCLUSIONS
In conclusion, despite over a half-century of recognition of fibrinolytic abnormalities postinjury, our understanding of the underlying mechanisms causing these changes remains in infancy, resulting in ineffective treatment strategies. With the increased utilization of VHA to measure fibrinolysis in trauma, more questions than answers are emerging. Although it seems certain that low fibrinolytic activity measured by VHA is common after injury and associated with increased mortality, we now recognize subphenotypes within this population. To understand why trauma patients manifest with specific coagulation abnormalities, it is important to describe the specific patient cohort and the specific time from injury when samples are collected. Ideally, this would include having a relatively homogeneous group (similar injury pattern and degree of shock) and sequential sample collection within short intervals between. These studies will provide crucial information to advance TIC research, as hypofibrinolysis, acute fibrinolysis shutdown, and persistent fibrinolysis shutdown appear to represent distinct, unique clinical phenotypes, with different pathophysiology, and warranting different treatment strategies.
ACKNOWLEDGMENTS
This study was supported in part by National Institute of General Medical Sciences grants: T32-GM008315, National Heart Lung and Blood Institute grant UM 1HL120877, National Institute of Health/National Center for Research Resources (NIH/NCRR) Colorado Clinical and Translational Sciences Institute (CCTSI) grant number UL1 RR025780, and Department of Defense Contract Number United States Medical Research Acquistion Activity (USAMRAA), W81XWH-12-2-0028. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences (NIGMS), National Heart Lung and Blood Institute (NHLBI), National Institutes of Health, or the Department of Defense. H.B.M. and E.E.M. have shared intellectual property with Haemonetics.
Footnotes
The authors declare no conflicts of interest.
DISCLOSURES
Name: Hunter B. Moore, MD, PhD.
Contribution: This author helped conceptualize and design the study; acquire, analyze, and interpret the data; and draft and critically revise the manuscript.
Name: Ernest E. Moore, MD.
Contribution: This author helped conceptualize and design the study, analyze and interpret the data, and critically revise the manuscript.
Name: Matthew D. Neal, MD.
Contribution: This author helped conceptualize and design the study, analyze and interpret the data, and draft and critically revise the manuscript.
Name: Forest R. Sheppard, MD.
Contribution: This author helped acquire, analyze, and interpret the data and draft and critically revise the manuscript.
Name: Lucy Z. Kornblith, MD.
Contribution: This author helped acquire, analyze, and interpret the data and draft and critically revise the manuscript.
Name: Dominik F. Draxler, MD, PhD.
Contribution: This author helped acquire the data, analyze, and interpret the data and draft the manuscript.
Name: Mark Walsh, MD.
Contribution: This author helped acquire the data, analyze and interpret the data, and draft and critically revise the manuscript.
Name: Robert L. Medcalf, PhD.
Contribution: This author helped analyze and interpret the data and draft and critically revise the manuscript.
Name: Mitch J. Cohen, MD.
Contribution: This author helped analyze and interpret the data and draft and critically revise the manuscript.
Name: Bryan A. Cotton, MD.
Contribution: This author helped analyze and interpret the data and draft and critically revise the manuscript.
Name: Scott G. Thomas, MD.
Contribution: This author helped analyze and interpret the data and critically revise the manuscript.
Name: Christine M. Leeper, MD.
Contribution: This author helped analyze and interpret the data and draft the manuscript.
Name: Barbara A. Gaines, MD.
Contribution: This author helped analyze and interpret the data and critically revise the manuscript.
Name: Angela Sauaia, MD, PhD.
Contribution: This author helped conceptualize and design the study, analyze and interpret the data, and draft and critically revise the manuscript.
This manuscript was handled by: Richard P. Dutton, MD.
Reprints will not be available from the authors.
REFERENCES
- 1.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg 2013;74:1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin TL, Moore EE, Moore HB, et al. A principal component analysis of postinjury viscoelastic assays: clotting factor depletion versus fibrinolysis. Surgery. 2014;156:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White NJ, Contaifer D Jr, Martin EJ, et al. Early hemostatic responses to trauma identified with hierarchical clustering analysis. J Thromb Haemost. 2015;13:978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shakur H, Roberts I, Bautista R, et al. ; CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. [DOI] [PubMed] [Google Scholar]
- 5.Myles PS, Smith JA, Forbes A, et al. ; ATACAS Investigators of the ANZCA Clinical Trials Network. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376:136–148. [DOI] [PubMed] [Google Scholar]
- 6.Barrachina B, Lopez-Picado A, Remon M, et al. Tranexamic acid compared with placebo for reducing total blood loss in hip replacement surgery: a randomized clinical trial. Anesth Analg. 2016;122:986–995. [DOI] [PubMed] [Google Scholar]
- 7.Valle EJ, Allen CJ, Van Haren RM, et al. Do all trauma patients benefit from tranexamic acid? J Trauma Acute Care Surg. 2014;76:1373–1378. [DOI] [PubMed] [Google Scholar]
- 8.Harvin JA, Peirce CA, Mims MM, et al. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. J Trauma Acute Care Surg. 2015;78:905–909. [DOI] [PubMed] [Google Scholar]
- 9.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore HB, Moore EE, Liras IN, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg 2016;222:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. J Am Coll Surg. 2017;224:575–582. [DOI] [PubMed] [Google Scholar]
- 12.Leeper CM, Neal MD, McKenna C, Sperry J, Gaines BA. Abnormalities in fibrinolysis at the time of admission are associated with DVT, mortality and disability in a pediatric trauma population. J Trauma Acute Care Surg. 2017;82:27–34. [DOI] [PubMed] [Google Scholar]
- 13.Gall LS, Vulliamy P, Gillespie S, et al. The S100A10 pathway mediates an occult hyperfibrinolytic subtype in trauma patients. Ann Surg. 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Cardenas JC, Wade CE, Cotton BA, et al. Teg Lysis shutdown represents coagulopathy in bleeding trauma patients: analysis of the proppr cohort. Shock. 2019;51:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rühl H, Berens C, Winterhagen A, Müller J, Oldenburg J, Pötzsch B. Label-free kinetic studies of hemostasis-related biomarkers including D-Dimer using autologous serum transfusion. PLoS One. 2015;10:e0145012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti R, Hocking ED, Fearnley GR. Reaction pattern to three stresses--electroplexy, surgery, and myocardial infarction--of fibrinolysis and plasma fibrinogen. J Clin Pathol. 1969;22:659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Innes D, Sevitt S. Coagulation and fibrinolysis in injured patients. J Clin Pathol. 1964;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardaway RM, James PM Jr, Anderson RW, Bredenberg CE, West RL. Intensive study and treatment of shock in man. JAMA. 1967;199:779–790. [PubMed] [Google Scholar]
- 19.Hardaway RM, Brune WH, Geever EF, Burns JW, Mock HP. Studies on the role of intravascular coagulation in irreversible hemorrhagic shock. Ann Surg. 1962;155:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardaway RM, Drake DC. Prevention of “irreversible” hemorrhagic shock with fibrinolysin. Ann Surg. 1963;157:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cafferata HT, Aggeler PM, Robinson AJ, Blaisdell FW. Intravascular coagulation in the surgical patient: its significance and diagnosis. Am J Surg. 1969;118:281–291. [DOI] [PubMed] [Google Scholar]
- 22.Mansfield AO. Alteration in fibrinolysis associated with surgery and venous thrombosis. Br J Surg. 1972;59:754–757. [DOI] [PubMed] [Google Scholar]
- 23.Gallus AS, Hirsh J, Gent M. Relevance of preoperative and postoperative blood tests to postoperative leg-vein thrombosis. Lancet. 1973;2:805–809. [DOI] [PubMed] [Google Scholar]
- 24.Macintyre IM, Webber RG, Crispin JR, et al. Plasma fibrinolysis and postoperative deep vein thrombosis. Br J Surg. 1976;63:694–697. [DOI] [PubMed] [Google Scholar]
- 25.Katz J, Lurie A, Becker D, Metz J. The euglobulin lysis time test: an ineffectual monitor of the therapeutic inhibition of fibrinolysis. J Clin Pathol. 1970;23:529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffiths NJ. Factors affecting the fibrinolytic response to surgery. Ann R Coll Surg Engl. 1979;61:12–16. [PMC free article] [PubMed] [Google Scholar]
- 27.Knight MT, Dawson R, Melrose DG. Fibrinolytic response to surgery. Labile and stable patterns and their relevance to postoperative deep venous thrombosis. Lancet. 1977;2:370–373. [DOI] [PubMed] [Google Scholar]
- 28.Bruhn HD, Jipp P, Okoye S, Oltmann A. [Hypofibrinolysis in acute myocardial infarction (author’s transl)]. Med Klin. 1974;69:1951–1955. [PubMed] [Google Scholar]
- 29.Stegnar M, Peternel P, Keber D, Vene N. Poor fibrinolytic response to venous occlusion by different criteria in patients with deep vein thrombosis. Thromb Res. 1991;64:445–453. [DOI] [PubMed] [Google Scholar]
- 30.Booth NA, Walker E, Maughan R, Bennett B. Plasminogen activator in normal subjects after exercise and venous occlusion: t-PA circulates as complexes with C1-inhibitor and PAI-1. Blood. 1987;69:1600–1604. [PubMed] [Google Scholar]
- 31.Petäjä J, Rasi V, Myllylä G, Vahtera E, Hallman H. Familial hypofibrinolysis and venous thrombosis. Br J Haematol. 1989;71:393–398. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen G, Horellou MH, Kruithof EK, Conard J, Samama MM. Residual plasminogen activator inhibitor activity after venous stasis as a criterion for hypofibrinolysis: a study in 83 patients with confirmed deep vein thrombosis. Blood. 1988;72:601–605. [PubMed] [Google Scholar]
- 33.Dellas C, Loskutoff DJ. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost. 2005;93:631–640. [DOI] [PubMed] [Google Scholar]
- 34.Brakman P, Mohler ER Jr, Astrup T. A group of patients with impaired plasma fibrinolytic system and selective inhibition of tissue activator-induced fibrinolysis. Scand J Haematol. 1966;3:389–398. [DOI] [PubMed] [Google Scholar]
- 35.Páramo JA, Alfaro MJ, Rocha E. Postoperative changes in the plasmatic levels of tissue-type plasminogen activator and its fast-acting inhibitor–relationship to deep vein thrombosis and influence of prophylaxis. Thromb Haemost. 1985;54:713–716. [PubMed] [Google Scholar]
- 36.Kluft C, Verheijen JH, Jie AF, et al. The postoperative fibrinolytic shutdown: a rapidly reverting acute phase pattern for the fast-acting inhibitor of tissue-type plasminogen activator after trauma. Scand J Clin Lab Invest. 1985;45:605–610. [DOI] [PubMed] [Google Scholar]
- 37.Kushner I The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. [DOI] [PubMed] [Google Scholar]
- 38.Kassis J, Hirsh J, Podor TJ. Evidence that postoperative fibrinolytic shutdown is mediated by plasma factors that stimulate endothelial cell type I plasminogen activator inhibitor biosynthesis. Blood. 1992;80:1758–1764. [PubMed] [Google Scholar]
- 39.García Frade LJ, de la Calle H, Torrado MC, Lara JI, Cuellar L, García Avello A. Hypofibrinolysis associated with vasculopathy in non insulin dependent diabetes mellitus. Thromb Res. 1990;59:51–59. [DOI] [PubMed] [Google Scholar]
- 40.Juhan-Vague I, Roul C, Alessi MC, Ardissone JP, Heim M, Vague P. Increased plasminogen activator inhibitor activity in non insulin dependent diabetic patients–relationship with plasma insulin. Thromb Haemost. 1989;61:370–373. [PubMed] [Google Scholar]
- 41.Gris JC, Schved JF, Raffanel C, et al. Impaired fibrinolytic capacity in patients with inflammatory bowel disease. Thromb Haemost. 1990;63:472–475. [PubMed] [Google Scholar]
- 42.Levi M, Lensing AW, Büller HR, et al. Deep vein thrombosis and fibrinolysis. Defective urokinase type plasminogen activator release. Thromb Haemost. 1991;66:426–429. [PubMed] [Google Scholar]
- 43.Yamakawa S The autodigestion of normal serum through the action of certain chemical agents. II. J Exp Med. 1918;27:711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christensen LR. The activation of plasminogen by chloroform. J Gen Physiol. 1946;30:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tillett WS, Garner RL. The fibrinolytic activity of hemolytic streptococci. J Exp Med. 1933;58:485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tillett WS, Edwards LB, Garner RL. Fibrinolytic activity of hemolytic streptococci. the development of resistance to fibrinolysis following acute hemolytic streptococcus infections. J Clin Invest. 1934;13:47–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garner RL, Tillett WS. Biochemical studies on the fibrinolytic activity of hemolytic streptococci: ii. nature of the reaction. J Exp Med. 1934;60:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christensen LR, Macleod CM. A proteolytic enzyme of serum: characterization, activation, and reaction with inhibitors. J Gen Physiol. 1945;28:559–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tillett WS, Sherry S. The effect in patients of streptococcal fibrinolysin and streptococcal desoxyribonuclease on fibrinous, purulent, and sanguinous pleural exudations. J Clin Invest. 1949;28:173–190. [PubMed] [Google Scholar]
- 50.Fletcher AP, Alkjaersig N, Smyrniotis FE, Sherry S. The treatment of patients suffering from early myocardial infarction with massive and prolonged streptokinase therapy. Trans Assoc Am Physicians. 1958;71:287–296. [PubMed] [Google Scholar]
- 51.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76:142–154. [DOI] [PubMed] [Google Scholar]
- 52.Urokinase pulmonary embolism trial. Phase 1 results: a cooperative study. JAMA. 1970;214:2163–2172. [PubMed] [Google Scholar]
- 53.Cooper RG, Mitchell WS, Illingworth KJ, Jayson MI. Fibrinolytic enhancement with stanozolol fails to improve symptoms and signs in patients with post-surgical back pain. Scand J Rheumatol. 1991;20:414–418. [DOI] [PubMed] [Google Scholar]
- 54.Blamey SL, McArdle BM, Burns P, Carter DC, Lowe GD, Forbes CD. A double-blind trial of intramuscular stanozolol in the prevention of postoperative deep vein thrombosis following elective abdominal surgery. Thromb Haemost. 1984;51:71–74. [PubMed] [Google Scholar]
- 55.Berridge DC, Frier M, Westby JC, Hopkinson BR, Makin GS. Double-blind randomized trial of perioperative fibrinolytic enhancement for femoropopliteal bypass. Br J Surg. 1991;78:101–104. [DOI] [PubMed] [Google Scholar]
- 56.Cuschieri RJ, Morran CG, Lowe GD, Blamey SL, Forbes CD, McArdle CS. The effect of fibrinolytic stimulation by stanozolol on postoperative pulmonary complications. Haemostasis. 1985;15:353–356. [DOI] [PubMed] [Google Scholar]
- 57.Hardaway RM, Harke H, Tyroch AH, Williams CH, Vazquez Y, Krause GF. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg 2001;67:377–382. [PubMed] [Google Scholar]
- 58.Conhaim RL, Watson KE, Dovi WF, Bates ML. Inhaled thrombolytics reduce lung microclot and leukocyte infiltration after acute blood loss. Shock. 2014;41:528–536. [DOI] [PubMed] [Google Scholar]
- 59.Enkhbaatar P, Murakami K, Cox R, et al. Aerosolized tissue plasminogen inhibitor improves pulmonary function in sheep with burn and smoke inhalation. Shock. 2004;22:70–75. [DOI] [PubMed] [Google Scholar]
- 60.Meyer G, Vicaut E, Danays T, et al. ; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402–1411. [DOI] [PubMed] [Google Scholar]
- 61.Khorgami Z, Fleischer WJ, Chen YA, Mushtaq N, Charles MS, Howard CA. Ten-year trends in traumatic injury mechanisms and outcomes: a trauma registry analysis. Am J Surg. 2018;215:727–734. [DOI] [PubMed] [Google Scholar]
- 62.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601–1606. [DOI] [PubMed] [Google Scholar]
- 63.Schultz DJ, Brasel KJ, Washington L, et al. Incidence of asymptomatic pulmonary embolism in moderately to severely injured trauma patients. J Trauma. 2004;56:727–731. [DOI] [PubMed] [Google Scholar]
- 64.Kwaan HC. Microvascular thrombosis: a serious and deadly pathologic process in multiple diseases. Semin Thromb Hemost. 2011;37:961–978. [DOI] [PubMed] [Google Scholar]
- 65.Gando S Microvascular thrombosis and multiple organ dysfunction syndrome. Crit Care Med. 2010;38:S35–S42. [DOI] [PubMed] [Google Scholar]
- 66.Rancourt RC, Ahmad A, Veress LA, Rioux JS, Garlick RB, White CW. Antifibrinolytic mechanisms in acute airway injury after sulfur mustard analog inhalation. Am J Respir Cell Mol Biol. 2014;51:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Enderson BL, Chen JP, Robinson R, Maull KI. Fibrinolysis in multisystem trauma patients. J Trauma. 1991;31:1240–1246. [DOI] [PubMed] [Google Scholar]
- 68.Cahan MA, Hanna DJ, Wiley LA, Cox DK, Killewich LA. External pneumatic compression and fibrinolysis in abdominal surgery. J Vasc Surg. 2000;32:537–543. [DOI] [PubMed] [Google Scholar]
- 69.Killewich LA, Cahan MA, Hanna DJ, et al. The effect of external pneumatic compression on regional fibrinolysis in a prospective randomized trial. J Vasc Surg. 2002;36:953–958. [DOI] [PubMed] [Google Scholar]
- 70.Stettler GR, Moore EE, Moore HB, et al. Redefining post injury fibrinolysis phenotypes using two viscoelastic assays. J Trauma Acute Care Surg. 2019;86:679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez-Builes JC, Acuna SA, Nascimento B, Madotto F, Rizoli SB. Harmful or physiologic: diagnosing fibrinolysis shutdown in a trauma cohort with rotational thromboelastometry. Anesth Analg. 2018;127:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoffman M, Monroe DM 3rd. A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–965. [PubMed] [Google Scholar]
- 73.Brass LF, Zhu L, Stalker TJ. Minding the gaps to promote thrombus growth and stability. J Clin Invest. 2005;115:3385–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Booth NA, Simpson AJ, Croll A, Bennett B, MacGregor IR. Plasminogen activator inhibitor (PAI-1) in plasma and platelets. Br J Haematol. 1988;70:327–333. [DOI] [PubMed] [Google Scholar]
- 75.Huebner BR, Moore EE, Moore HB, et al. Thrombin provokes degranulation of platelet α-granules leading to the release of active Plasminogen Activator Inhibitor-1 (PAI-1). Shock. 2018;50:671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moore HB, Moore EE, Chapman MP, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost. 2015;13:1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leeper CM, Neal MD, McKenna C, Sperry JL, Gaines BA. Abnormalities in fibrinolysis at the time of admission are associated with deep vein thrombosis, mortality, and disability in a pediatric trauma population. J Trauma Acute Care Surg. 2017;82:27–34. [DOI] [PubMed] [Google Scholar]
- 78.Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. J Am Coll Surg. 2017;224:575–582. [DOI] [PubMed] [Google Scholar]
- 79.Moore HB, Moore EE, Huebner BR, et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg. 2017;83:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roberts DJ, Kalkwarf KJ, Moore HB, et al. Time course and outcomes associated with transient versus persistent fibrinolytic phenotypes after injury: a nested, prospective, multicenter cohort study. J Trauma Acute Care Surg. 2019;86:206–213. [DOI] [PubMed] [Google Scholar]
- 81.Raza I, Davenport R, Rourke C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11:307–314. [DOI] [PubMed] [Google Scholar]
- 82.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257:2912–2919. [PubMed] [Google Scholar]
- 83.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Surette AP, Madureira PA, Phipps KD, Miller VA, Svenningsson P, Waisman DM. Regulation of fibrinolysis by S100A10 in vivo. Blood. 2011;118:3172–3181. [DOI] [PubMed] [Google Scholar]
- 85.Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore HB, Moore EE, Huebner BR, et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg. 2017;83:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore HB, Moore EE, Gonzalez E, et al. Reperfusion shutdown: delayed onset of fibrinolysis resistance after resuscitation from hemorrhagic shock is associated with increased circulating levels of plasminogen activator inhibitor-1 and postinjury complications. Blood. 2016;128:206. [Google Scholar]
- 88.Moore HB, Moore EE, Lawson PJ, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery. 2015;158:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Macko AR, Moore HB, Cap AP, Meledeo MA, Moore EE, Sheppard FR. Tissue injury suppresses fibrinolysis after hemorrhagic shock in nonhuman primates (rhesus macaque). J Trauma Acute Care Surg. 2017;82:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prat NJ, Montgomery R, Cap AP, et al. Comprehensive evaluation of coagulation in swine subjected to isolated primary blast injury. Shock. 2015;43:598–603. [DOI] [PubMed] [Google Scholar]
- 91.Astedt B, Casslén B, Lecander I, Nilsson L, Norgren L. Rebound increase of PAI-1 following local intra-arterial rt-PA infusion, a possible cause of reocclusion. Blood Coagul Fibrinolysis. 1993;4:563–567. [DOI] [PubMed] [Google Scholar]
- 92.Roberts DJ, Kalkwarf KJ, Moore HB, et al. Time course and outcomes associated with transient versus persistent fibrinolytic phenotypes after injury: a nested, prospective, multicenter cohort study. J Trauma Acute Care Surg. 2019;86:206–213. [DOI] [PubMed] [Google Scholar]
- 93.Leeper CM, Neal MD, McKenna CJ, Gaines BA. Trending fibrinolytic dysregulation: fibrinolysis shutdown in the days after injury is associated with poor outcome in severely injured children. Ann Surg. 2017;266:508–515. [DOI] [PubMed] [Google Scholar]
- 94.Prakash S, Verghese S, Roxby D, Dixon D, Bihari S, Bersten A. Changes in fibrinolysis and severity of organ failure in sepsis: a prospective observational study using point-of-care test–ROTEM. J Crit Care. 2015;30:264–270. [DOI] [PubMed] [Google Scholar]
- 95.Nunns GR, Moore EE, Stettler GR, et al. Empiric transfusion strategies during life-threatening hemorrhage. Surgery. 2018;164:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petäjä J, Rasi V, Vahtera E, Myllylä G. Familial clustering of defective release of t-PA. Br J Haematol. 1991;79:291–295. [DOI] [PubMed] [Google Scholar]
- 97.Von Kaulla KN, Kaye H, von Kaulla E, Marchioro TL, Starzl TE. Changes in blood coagulation. Arch Surg. 1966;92:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Groth CG, Pechet L, Starzl TE. Coagulation during and after orthotopic transplantation of the human liver. Arch Surg. 1969;98:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Ann Surg. 2015;261:390–394. [DOI] [PubMed] [Google Scholar]
- 100.Wafaisade A, Lefering R, Bouillon B, Böhmer AB, Gäßler M, Ruppert M; TraumaRegister DGU. Prehospital administration of tranexamic acid in trauma patients. Crit Care 2016;20:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Howard JT, Stockinger ZT, Cap AP, Bailey JA, Gross KR. Military use of tranexamic acid in combat trauma: does it matter? J Trauma Acute Care Surg. 2017;83:579–588. [DOI] [PubMed] [Google Scholar]
- 102.Boutonnet M, Abback P, Le Saché F, et al. ; Traumabase Group. Tranexamic acid in severe trauma patients managed in a mature trauma care system. J Trauma Acute Care Surg. 2018;84:S54–S62. [DOI] [PubMed] [Google Scholar]
- 103.Moore EE, Moore HB, Gonzalez E, Sauaia A, Banerjee A, Silliman CC. Rationale for the selective administration of tranexamic acid to inhibit fibrinolysis in the severely injured patient. Transfusion. 2016;56(suppl 2):S110–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roberts I Fibrinolytic shutdown: fascinating theory but randomized controlled trial data are needed. Transfusion. 2016;56(suppl 2):S115–S118. [DOI] [PubMed] [Google Scholar]
- 105.Napolitano LM, Cohen MJ, Cotton BA, Schreiber MA, Moore EE. Tranexamic acid in trauma: how should we use it? J Trauma Acute Care Surg. 2013;74:1575–1586. [DOI] [PubMed] [Google Scholar]
- 106.Hijazi N, Abu Fanne R, Abramovitch R, et al. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood. 2015;125:2558–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roberts I, Edwards P, Prieto D, et al. Tranexamic acid in bleeding trauma patients: an exploration of benefits and harms. Trials. 2017;18:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gayet-Ageron A, Prieto-Merino D, Ker K, Shakur H, Ageron FX, Roberts I; Antifibrinolytic Trials Collaboration. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40 138 bleeding patients. Lancet. 2018;391:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boudreau RM, Deshpande KK, Day GM, et al. Prehospital tranexamic acid administration during aeromedical transport after injury. J Surg Res. 2019;233:132–138. [DOI] [PubMed] [Google Scholar]
- 110.Myers SP, Kutcher ME, Rosengart MR, et al. Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism. J Trauma Acute Care Surg. 2019;86:20–27. [DOI] [PubMed] [Google Scholar]
- 111.Chakroun-Walha O, Samet A, Jerbi M, et al. Benefits of the tranexamic acid in head trauma with no extracranial bleeding: a prospective follow-up of 180 patients. Eur J Trauma Emerg Surg. 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 112.Moore HB, Moore EE, Huebner BR, et al. Tranexamic acid is associated with increased mortality in patients with physiological fibrinolysis. J Surg Res. 2017;220:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meizoso JP, Dudaryk R, Mulder MB, et al. Increased risk of fibrinolysis shutdown among severely injured trauma patients receiving tranexamic acid. J Trauma Acute Care Surg. 2018;84:426–432. [DOI] [PubMed] [Google Scholar]
- 114.Liras IN, Cotton BA, Cardenas JC, Harting MT. Prevalence and impact of admission hyperfibrinolysis in severely injured pediatric trauma patients. Surgery. 2015;158:812–818. [DOI] [PubMed] [Google Scholar]
- 115.Khan M, Jehan F, Bulger EM, et al. ; PROPPR Study Group. Severely injured trauma patients with admission hyperfibrinolysis: is there a role of tranexamic acid? Findings from the PROPPR trial. J Trauma Acute Care Surg. 2018; 85:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El-Menyar A, Sathian B, Asim M, Latifi R, Al-Thani H. Efficacy of prehospital administration of tranexamic acid in trauma patients: a meta-analysis of the randomized controlled trials. Am J Emerg Med. 2018;36:1079–1087. [DOI] [PubMed] [Google Scholar]