Abstract
This study examined associations and changes overtime in low back kinematics and disability, pain, pain catastrophizing, and depression and assessed whether associations and changes overtime varied between individuals who meet the classification criteria for chronic low back pain at 6 months and those who do not. Findings suggested that those persons with a higher ratio of lumbar contribution to thorax motion and smaller pelvic tilt during forward bending had higher scores on measures of disability, pain and pain catastrophizing. This same association was found in those who met classification criteria for chronic low back pain at 6 months. Opposing associations were found in the group not meeting classification criteria for chronic low back pain, specifically, increased pelvic tilt was positively associated with higher pain catastrophizing scores.
Keywords: Low Back Pain, Kinematics, Pain Catastrophizing, Depression, Disability
Practitioner Summary:
This study examined associations and changes overtime in low back kinematics and psychosocial and clinical factors and whether associations and changes overtime varied between individuals who meet the classification criteria for chronic low back pain at 6 months and those who do not, Results suggest that associations exist between psychological factors and kinematic changes during the time between an acute low back pain episode to meeting classification for chronic low back pain at 6 months.
1.1. Introduction
Low back pain (LBP) is a medical disorder commonly characterized by symptom duration (National Institute of Neurological Disorders and Stroke 2018). Acute LBP is defined as pain lasting a short duration- days to weeks. Whereas, sub-acute LBP is defined as pain lasting 4 weeks to 3 months (National Institute of Neurological Disorders and Stroke 2018). In contrast, “chronic LBP is defined as a back pain problem that has persisted at least 3 months, and has resulted in pain on at least half the days in the past 6 months”(Deyo et al. 2014). Understanding the transition from the acute and sub-acute stages to the chronic stage is critically important to address the overwhelming prevalence of this condition; specifically, an estimated 31% of the world’s population reports experiencing LBP over the past month and 38% report LBP over the past year (Hoy et al. 2012). It is clear from the literature that disability is a result of this chronic pain state as evidenced by LBP being the leading cause of years lived with disability in North America (Hoy et al. 2014, Global Burden of Disease Study 2013 Collaborators 2015). Significant costs are another result of LBP; the annual direct (i.e., treatment) costs of LBP are estimated at $90 billion (Luo et al. 2004, Dagenais, Caro, and Haldeman 2008).
In efforts to thwart the disability rates and cost that occur when LBP transitions to a chronic state, it is imperative that mechanisms, such as the biomechanics of movement and psychological factors, associated with LBP symptoms during the acute and sub-acute time period are well understood. Understanding these mechanisms and their relationship with each other could provide direction to targeted treatments.
Biomechanical movement patterns of the lumbar spine during activities of daily living have the potential to contribute to LBP symptoms. Initial cross-sectional studies have shown discrepancies in lumbo-pelvic kinematics for people with acute and sub-acute LBP compared to back healthy individuals. Specifically, during a forwarding bending clinical test, it has been reported that people with acute or sub-acute LBP exhibit more pelvic tilt and less lumbar flexion when compared to those without LBP (Shojaei, Salt, et al. 2017). In addition, it has been reported that people with acute or subacute LBP have less contribution from the lumbar spine at specific time points during forward bending compared to healthy individuals (Shojaei, Vazirian, et al. 2017). These findings suggest people in an episode of acute LBP have altered lumbo-pelvic kinematics or lumbo-pelvic coordination (LPC) compared to those without LBP (Shojaei, Salt, et al. 2017, Shojaei, Vazirian, et al. 2017).
The Cognitive Behavioral Model of Fear of Movement/(Re)injury describes the relationship between biopsychosocial factors and disability.(Vlaeyen et al. 1995) This model suggests pain catastrophizing, the exaggerated and negative orientation of pain, can lead to disuse, disability, and depression, in turn creating a vicious circle of continued pain. There is evidence to support the role of the variables included in the Cognitive Behavioral Model of Fear of Movement/(Re)injury model in predicting disability among persons with acute and chronic LBP (Buer and Linton 2002, Crombez et al. 1999, Fourney et al. 2011, Riley, Ahern, and Follick 1988, Swinkels-Meewisse et al. 2006, Vlaeyen et al. 1995, Sieben et al. 2002). Studies exist describing the relationship between low back kinematics, fear of movement, and disability; yet these studies are limited by the duration of follow up and the psychosocial factors evaluated (Thomas et al. 2008, Thomas and France 2008, 2007).
Because it is plausible that changes in LPC described in the LBP biomechanical literature could be related to biopsychosocial factors described in the Cognitive Behavioral Model of Fear of Movement/(Re)injury model, the aims of this study are to: 1) examine the associations and changes overtime of LPC and disability, pain, pain catastrophizing, and depression; and 2) assess whether associations and changes overtime vary between individuals who meet the classification criteria for chronic LBP at 6 months and those who do not. LPC was characterized by two biomechanical measures: 1) lumbar contribution to thorax motion (i.e., lumbo-thoracic ratio); and 2) pelvic tilt.
1.2. Methods and Materials
1.2.1. Design
Prospective data from participants in a randomized clinical trial were used to address the study aims.
1.2.2. Sample
This secondary analysis included a subsample of people who participated in a randomized clinical trial. In the clinical trial, the 44 participants were: 1) 18 years of age or older; 2) being treated for health care provider diagnosed acute LBP (≤ 3 months); and 3) had access to a telephone. Persons who were found to have a cognitive impairment, intention to harm themselves or another, or were currently abusing substances were excluded. Participants were recruited from ambulatory care clinics (Women’s Health, Family Medicine, Internal Medicine, and Urgent Treatment) and health registries (Kentucky Women’s Health Registry and ResearchMatch©) at a large university health care system (University of Kentucky 2017, Vanderbilt University 2017). The 29 participants in this study were retained because they completed biomechanical assessments at baseline. A demographic description of the sample is provided in Table 1.
Table 1.
Descriptive summary of study variables at baseline (N =29)
| Mean (SD); range or n (%) | |
|---|---|
| Demographic characteristics | |
| Age | 53.5 (10.2); 30–75 |
| Sex | |
| Female | 24 (82.8%) |
| Male | 5 (17.2%) |
| Working full-time | |
| Yes | 15 (51.7%) |
| No | 14 (48.3%) |
| Classification | |
| Chronic low back pain | 13 (50.0%) |
| Non-chronic low back pain | 13 (50.0%) |
| Psychosocial characteristics | |
| Disability | 6.5 (4.7); 0–18 |
| Pain catastrophizing | 11.0 (8.2); 2–41 |
| Pain | 4.2 (2.1); 0–8 |
| Depression | 7.1 (4.9); 1–19 |
| Lumbo-pelvic coordination | |
| Lumbo thoracic ratio (fast-paced) | 0.4 (0.1); 0.3–0.7 |
| Lumbo thoracic ratio (slow-paced) | 0.5 (0.1); 0.3–0.7 |
| Pelvic rotation* (fast-paced) | 62.6 (15.8); 25.2–99.8 |
| Pelvic rotation* (slow-paced) | 54.4 (15.5); 17.9–86.4 |
(Scales of measure: Disability- Roland-Morris Disability Scale; Pain- Wisconsin Brief Pain Inventory; Pain catastrophizing- Pain Catastrophizing Scale; Depression- Center for Epidemiological Studies-Depression)
Measured in degrees
1.2.3. Human Subjects Protection
This study was approved by the medical institutional review board at a large academic institution. Participants signed informed consent documents and all research personnel completed human subject’s protection training. All data were collected and stored securely to ensure participant confidentiality.
1.2.4. Data Collection
A questionnaire battery containing the measures described below were self-reported using the REDCap® application. Baseline data including biomechanical data was collected in a gait laboratory on campus by a trained graduate student from August 2015 to August 2017, and two additional follow-up surveys along with biomechanical measurements were administered 2.5 and 6 months following the baseline assessment. Participants were also asked to complete weekly diaries using the REDCap® application, summarizing current pain levels and location of pain, depression, pain catastrophizing, and perceived functional disability. Participants who did not complete at least one pain diary monthly were not able to be classified as having chronic or non-chronic lower back pain.
1.2.5. Measures
Perceived functional disability was defined as an individual’s perception of their activity limitations caused by their pain and was measured with the 24-item Roland-Morris Disability Scale. A strong correlation has been reported between the 11-item and 24-item versions (r = .93; Cronbach’s α = .84). Scale items are summed and a higher total score indicates higher disability (scale score range: 0–24) (Stroud, McKnight, and Jensen 2004). The scale has high internal consistency as supported by the Cronbach’s alpha for this sample, which is 0.86; this is compared with the maximum possible Cronbach’s alpha of 1 which would indicate perfect association among items, suggesting consistency in what is being measured across all items.
Pain severity was measured using the Wisconsin Brief Pain Inventory (BPI; Cronbach α = .85)(Daut, Cleeland, and Flanery 1983) which uses an 11-point Likert-type scale (pain severity: 0 = no pain - 10 = pain as bad as you can imagine). The BPI item stating Please rate your pain by selecting the one number that best describes your pain on average was used as the indicator for pain. There is evidence to support the concurrent validity of the BPI with other pain instruments (Daut, Cleeland, and Flanery 1983, Zalon 1999). The items on the scale were closely related as evidenced by the Cronbach’s alpha for the complete scale in this sample of 0.90.
Pain catastrophizing was defined as “an exaggerated negative mental set brought to bear by an actual/anticipated painful experience”. This construct was measured using the Pain Catastrophizing Scale (PCS) (Sullivan, Bishop, and Pivik 1995), a 13-item scale which uses a 5-point Likert scale (0 = not at all - 4 = all the time; Cronbach’s α = .94; test-retest reliability = .78) which has evidence supporting concurrent and discriminant validity. Scale items were summed and a higher total score indicated higher pain catastrophizing (scale score range: 0–52) (Osman et al. 1997). Cronbach’s alpha for this sample was 0.92, indicating the items on the scale are closely associated with each other.
Depressive symptoms score was measured using the Center for Epidemiological Studies-Depression (CES-D),(Radloff 1977) a 20-item scale using a 4-point Likert response set to measure weekly frequency (0 = rarely or less than one day to 3 = most of the time or 5–7 days; scale score range: 0 – 60; Cronbach’s α = .89). Scale items 4, 8, 12 and 16 were reverse scored and the scale items were summed. A higher total score suggests more depressive symptoms (Radloff 1977, Thorn et al. 2011).
Demographic information on age, gender, and work status was collected at baseline. Work status was classified as working full-time or not working full-time.
Biomechanical measurement involved quantification of LPC during a trunk forward bending and backward return activity similar to our earlier works (Vazirian et al. 2017, Shojaei, Salt, et al. 2017, Shojaei, Vazirian, et al. 2017). Briefly, participants completed two sets of a trunk forward bending task at self-selected slow and fast paces in the sagittal plane. During these tasks, trunk kinematics were tracked using wireless Inertial Measurement Units (IMUs; Xsens Technologies, Enschede, Netherlands) attached to straps superficial to the T10 and the S1 spinous processes. During the self-selected slow pace task participants were instructed to stand in an upright posture, bend forward using a self-selected slow pace to reach their maximum comfortable trunk tilt, hold their bending posture for 5 seconds, and extend back up to the original upright position. For the self-selected fast pace task participants performed the same task as fast as possible but without a pause at the maximum trunk rotation. Before conducting these tasks, the desired method of performing them (i.e., with extended knees and arms being hanging in front at the fully flexed posture) was demonstrated to participants by one of the research personnel. Each task was repeated three times and the slow pace task was always performed prior to the fast pace task. The sampling rate of the IMUs was 50 Hz. The IMU kinematic raw data were filtered at 6Hz using a fourth order, bidirectional, Butterworth filter (Kristianslund, Krosshaug, and van den Bogert 2012, Wong and Lee 2004, Winter 1984). IMU have reported intratest repeatability and test-retest reliability, ranging from 0.669 to 0.997 (ICCs) and there is evidence to support concurrent validity of IMU measurements (Yun et al. 2015, Al-Amri et al. 2018)
Lumbo-Pelvic Coordination (LPC) was characterized by the amount of pelvic tilt along with the percent contribution of lumbar flexion to total thorax rotation, denoted as lumbo-thoracic ratio (LTR). This was calculated at maximum forward flexion (i.e., when participant was fully flexed forward) (Vazirian et al. 2017). Thoracic and pelvic tilt in the sagittal plane relative to the initial standing posture, were calculated using the extracted rotation matrices from IMUS (Shojaei, Salt, et al. 2017, Shojaei, Vazirian, et al. 2017). At each time, lumbar flexion was calculated as the difference between the thoracic and pelvic tilt.
Classification of Chronic and Non-chronic.
For the purposes of this study, acute and subacute were grouped together and called non-chronic LBP. The NIH Task Force of Research Standards for Chronic LBP’s definition of chronic LBP was used to classify participants as chronic or non-chronic. The task force specified that “chronic LBP is defined as a back pain problem that has persisted at least 3 months, and has resulted in pain on at least half the days in the past 6 months”(Deyo et al. 2014). Participants were classified as chronic LBP when they met two criteria. First, they specified pain in the low back to be a source of pain. Second, they responded with pain greater than a 0 on a scale of 0–10 to the item Please rate your pain by selecting the one number that best describes your pain at its least in the last week for one data point for three out of the six months of data collected. Those who did not meet the criteria above were classified as non-chronic.
1.2.6. Data Analysis
Descriptive statistics, including means and standard deviations or frequency distributions were used to summarize study variables at baseline. At each of the three time points, Pearson’s correlation coefficient was calculated to examine associations between indicators of LPC (i.e., LTR and pelvic tilt) at each pace (fast-paced, slow paced) and disability, pain, pain catastrophizing and depression, individually. We also used Pearson’s correlation coefficients to describe the relationship between indicators of LPC, which was quantified by LTR and pelvic tilt (fast-paced, slow paced), and disability, pain, pain catastrophizing and depression individually by chronic/non-chronic LBP status. We were able to use the parametric test of correlation since all of the outcome variables were approximately normally distributed, with bell-shaped distributions and little difference between the corresponding means and medians. All data analysis was conducted using SAS version 9.4, with an alpha level of .05 throughout. Of note, the analysis plan provided did not account for intervention effects because none were identified during the conduct of the unpublished randomized clinical trial.
1.3. Results
Biomechanical measurements and survey data were available for 29, 25 and 24 participants at baseline, 2.5- and 6-month assessments, respectively. The average age of the 29 participants was 53.5 years (SD = 10.2; range = 30–75; see Table 1) and the majority were female (83%) and working full-time (52%). Half of the participants met the guideline for being classified as chronic LBP (50%). At the baseline assessment, the average disability scale score was 6.5 (SD = 4.7 out of a range from 0 to 24), and the average pain catastrophizing scale score was 11.5 (SD = 8.5; out of a potential score of 0–52). Participants rated their average level of pain as 4.2 (SD = 2.1), on a scale of 0–10, and reported relatively low depressive symptoms scores, on average (M = 7.1, SD = 4.9, out of potential range from 0–60).
Associations between LPC variables and disability, pain, pain catastrophizing and depression are reported for each data collection point (baseline, 2.5 and 6 months). At baseline there was a positive association between LTR and pain catastrophizing at a fast pace; those with higher LTR at the end point of the fast-paced forward bending task also had higher pain catastrophizing scores (Pearson’s r = .44; p = .016). Those with higher disability scores used less pelvic tilt at the end point of the fast-paced and slow-paced forward bending task [Pearson’s r = −.45, p = .014 (fast-paced), Pearson’s r = −.43, p = .021 (slow-paced)]. There were no significant associations between pain or depression and LTR or pelvic tilt at baseline.
At 2.5 months, those with higher pain catastrophizing scores had higher LTR during forward bending at both a fast and slow pace [Table 2, Figures 1 & 2; Pearson’s r = .49, p = .013 (fast-paced), Pearson’s r = .46, p = .014 (slow-paced)]. There was a negative association between pelvic tilt and pain catastrophizing scores; those with higher pain catastrophizing scores used less pelvic tilt at the end point of the fast-paced and slow-paced forward bending task [Pearson’s r = −.52, p = .008 (fast-paced), Pearson’s r = −.47, p = .011 (slow-paced)].
Table 2.
Pearson product-moment correlations with p-values (parentheses) among psychosocial measures and lumbo-pelvic coordination at each assessment.
| Disability | Pain | Pain catastrophizing | Depression | |
|---|---|---|---|---|
| Baseline (n = 29) | ||||
| Lumbo thoracic ratio (fast) | 0.24 (.21) | 0.15 (.44) | 0.44 (.016) | 0.06 (.77) |
| Lumbo thoracic ratio (slow) | 0.18 (.36) | 0.14 (.47) | 0.17 (.37) | −0.07 (.73) |
| Pelvic tilt* (fast) | −0.45 (.014) | −0.35 (.063) | −0.34 (.074) | 0.14 (.48) |
| Pelvic tilt* (slow) | −0.43 (.021) | −0.29 (.12) | −0.23 (.23) | 0.23 (.22) |
| 2.5 Month (n = 25) | ||||
| Lumbo thoracic ratio (fast) | 0.41 (.041) | 0.05 (.83) | 0.49 (.013) | 0.51 (.037) |
| Lumbo thoracic ratio (slow) | 0.48 (.010) | 0.16 (.43) | 0.46 (.014) | 0.56 (.017) |
| Pelvic tilt* (fast) | −0.50 (.010) | −0.16 (.45) | −0.52 (.008) | −0.49 (.041) |
| Pelvic tilt* (slow) | −0.53 (.004) | −0.25 (.21) | −0.47 (.011) | −0.51 (.029) |
| 6 Month (n = 24) | ||||
| Lumbo thoracic ratio (fast) | 0.30 (.15) | 0.31 (.15) | 0.43 (.035) | 0.35 (.33) |
| Lumbo thoracic ratio (slow) | 0.41 (.044) | 0.42 (.041) | 0.46 (.020) | 0.04 (.92) |
| Pelvic tilt* (fast) | −0.54 (.007) | −0.58 (.004) | −0.53 (.008) | −0.06 (.88) |
| Pelvic tilt* (slow) | −0.53 (.006) | −0.59 (.002) | −0.49 (.014) | 0.17 (.64) |
Measured in degree
Figure 1.
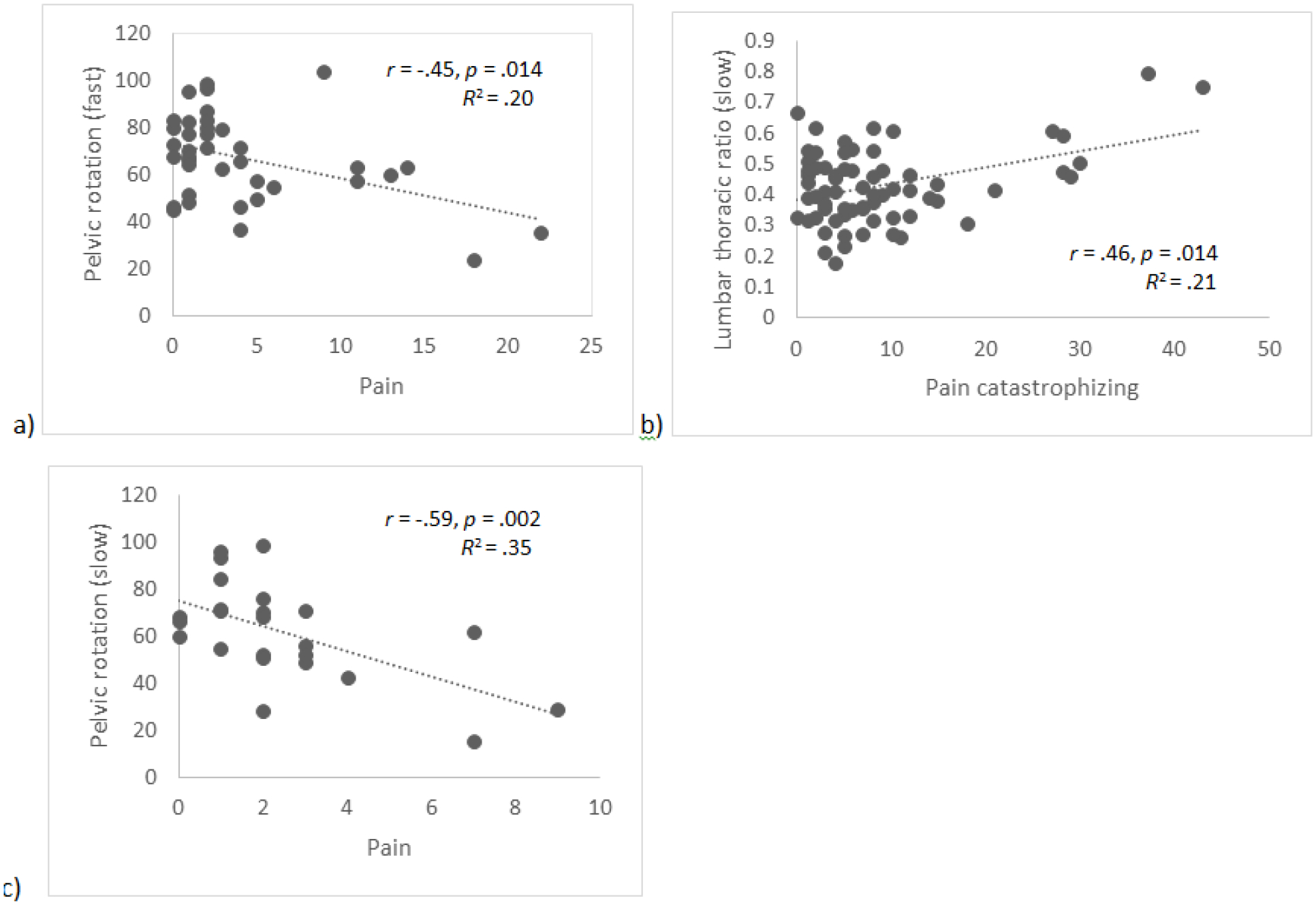
Selected scatterplots to provide a visual representation of the relationships between biomechanical and psychosocial variables.
Note: a) Baseline disability and pelvic rotation (fast), b) 2.5-month pain catastrophizing and lumbar thoracic ratio (slow), c) 6-month pain and pelvic rotation (slow)
Figure 2.

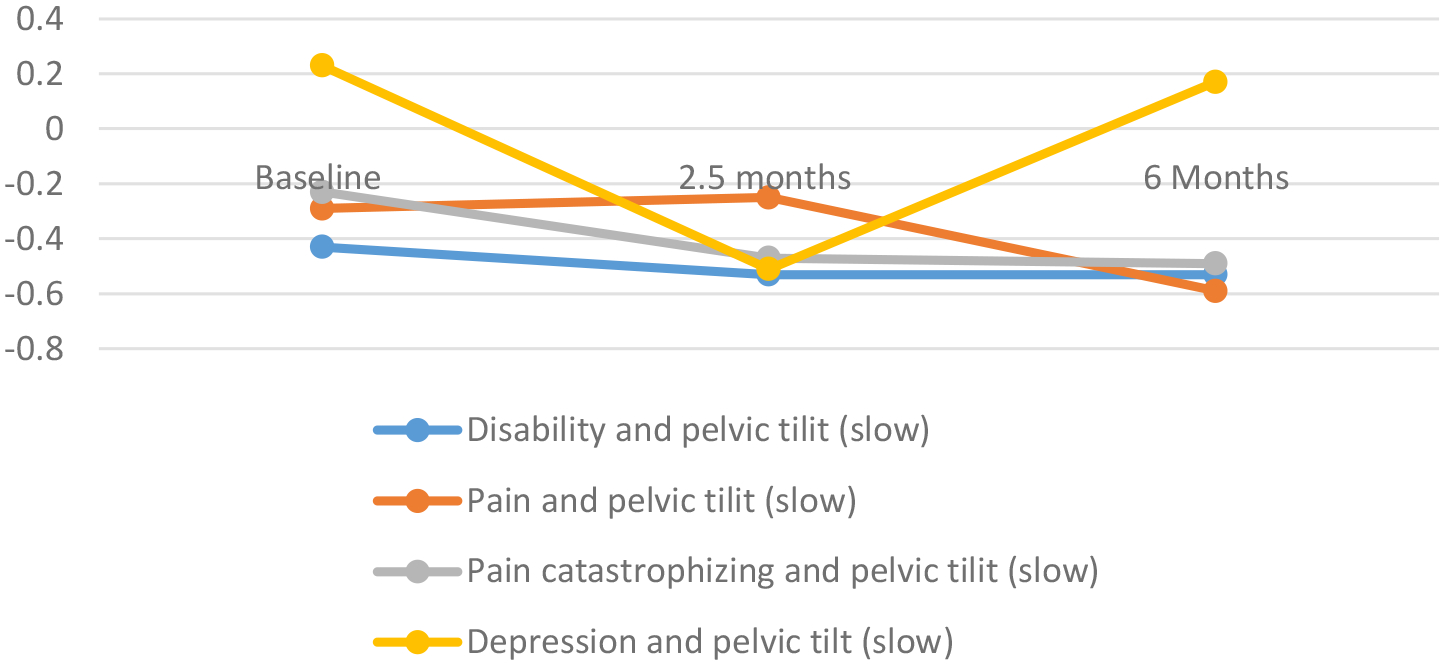


a. Correlations between psychosocial and clinical variables and pelvic tilt (fast) overtime.
b. Correlations between psychosocial and clinic variables and pelvic tilt (slow) overtime.
c. Correlations between psychosocial and clinical variables and lumbo thoracic ratio (fast) over time.
d. Correlations between psychosocial and clinical variables and lumbo thoracic ratio (slow) over time.
We identified significant associations between LTR and pelvic tilt and disability at 2.5 months (Table 2, Figures 1 & 2). Those with a higher LTR at the end point of the fast-paced and slow-paced forward bending task had significantly higher disability score [Pearson’s r = .41, p = .041 (fast-paced), Pearson’s r = .48, p = .010 (slow-paced)]. Similar to the pain catastrophizing, those with higher disability scores also used less pelvic tilt at the end point of the fast-paced and slow-paced forward bending task forward bending at the fast and slow pace [Pearson’s r = −.50, p = .010 (fast-paced), Pearson’s r = −.53, p = .004 (slow-paced)]. The associations of LTR and pelvic tilt with depressive symptoms followed the same pattern: there were positive correlations between depression and LTR [Pearson’s r = .51, p = .037 (fast-paced), Pearson’s r = .56, p = .017) and negative correlations between depressive symptoms and pelvic tilt [Pearson’s r = −.49, p = .041 (fast-paced), Pearson’s r = −.51, p = .029 (slow-paced)]. No significant associations were found between pain and LTR or pelvic tilt at 2.5 months.
At 6 months, significant associations were found between LTR and pelvic tilt and disability, pain and pain catastrophizing (Table 2, Figures 1 & 2). LTR and pelvic tilt were not associated with depressive symptoms. Positive associations were identified between LTR and disability, pain and pain catastrophizing. Those with larger LTR at the end point of the fast-paced and slow-paced forward bending task forward bending also had higher levels of disability, pain and pain catastrophizing [Disability: Pearson’s r = .41, p = .044 (slow-paced); Pain: Pearson’s r = .42; p = .041 (slow-paced); Pain catastrophizing: Pearson’s r = .43; p = .035 (fast-paced), Pearson’s r = .46, p = .020 (slow-paced)]. Additionally, those with higher levels of disability, pain and pain catastrophizing used less pelvic tilt during forward bending at a fast and slow pace [Disability: Pearson’s r = −.54, p = .007 (fast-paced), Pearson’s r = −.53, p = .006 (slow-paced); Pain: Pearson’s r = −.58; p = .004 (fast-paced), Pearson’s r = −.59, p = .002 (slow-paced); Pain catastrophizing: Pearson’s r = −.53; p = .008 (fast-paced), Pearson’s r = −.49, p = .014 (slow-paced)]. No significant associations were found between depression and LTR or pelvic tilt at 6 months.
We also evaluated associations between LTR and pelvic tilt and disability, pain, pain catastrophizing and depression in those who met classification criteria for chronic LBP at 6 months and those that did not (Table 3). In those who met classification criteria for chronic LBP, persons with a higher pain catastrophizing had higher LTR (Pearson’s r = .63; p = .020) and used less pelvic tilt (Pearson’s r = −.57, p = .043) at the end point of the fast-paced forward bending task. This same relationship between pelvic tilt and pain catastrophizing during fast paced forward bending was found at 2.5 and 6 months (2.5 month, Pearson’s r = −.70, p = .037; 6 month Pearson’s r = −.65, p = .029). In contrast, in those who didn’t meet classification criteria for chronic LBP at 6 months, persons who had higher pain catastrophizing used more pelvic tilt during fast paced forward bending at 2.5 and 6-months (2.5 month, Pearson’s r = .54, p = .046; 6 month Pearson’s r = .56, p = .048). The only additional significant association was between disability and pelvic tilt at 6 months among those who met classification criteria for chronic LBP at 6 months. Specifically, those with higher disability scores used less pelvic tilt (Pearson’s r = −.61, p = .045).
Table 3.
Pearson product-moment correlations with p-values (parentheses) among psychosocial measures and lumbo-pelvic coordination at each assessment for those with and without chronic LBP.
| Non-chronic | Chronic | |||||||
|---|---|---|---|---|---|---|---|---|
| Disability | Pain | Pain catastrophizing | Depression | Disability | Pain | Pain catastrophizing | Depression | |
| Baseline (n = 13) | Baseline (n = 13) | |||||||
| Lumbo thoracic ratio (fast) | −0.07 (.85) | −0.47 (.11) | 0.07 (.83) | 0.04 (.89) | 0.18 (.56) | 0.26 (.39) | 0.63 (.020) | 0.24 (.42) |
| Lumbo thoracic ratio (slow) | −0.27 (.37) | −0.07 (.81) | −0.28 (.35) | −0.07 (.83) | 0.38 (.21) | 0.34 (.25) | 0.36 (.23) | −0.01 (.98) |
| Pelvic tilt (fast) | −0.31 (.30) | 0.11 (.72) | 0.17 (.59) | 0.30 (.32) | −0.27 (.38) | −0.16 (.60) | −0.57 (.043) | −0.14 (.65) |
| Pelvic tilt (slow) | −0.25 (.40) | 0.11 (.71) | 0.13 (.68) | 0.09 (.77) | −0.46 (.11) | −0.48 (.096) | −0.29 (.33) | 0.28 (.35) |
| 2.5 Month (n = 14) | 2.5 Month (n = 9) | |||||||
| Lumbo thoracic ratio (fast) | 0.22 (.46) | 0.18 (.55) | −0.43 (.13) | −0.01 (.97) | 0.38 (.31) | −0.15 (.73) | 0.67 (.051) | 0.42 (.30) |
| Lumbo thoracic ratio (slow) | 0.27 (.35) | 0.14 (.64) | −0.40 (.15) | 0.05 (.88) | 0.43 (.25) | −0.08 (.86) | 0.57 (.11) | 0.49 (.18) |
| Pelvic tilt (fast) | −0.28 (.33) | −0.09 (.33) | 0.54 (.046) | 0.28 (.36) | −0.48 (.19) | −0.03 (.95) | −0.70 (.037) | −0.34 (.40) |
| Pelvic tilt (slow) | −0.41 (.14) | −0.16 (.59) | 0.52 (.056) | 0.30 (.32) | −0.46 (.21) | −0.04 (.93) | −0.61 (.084) | −0.46 (.21) |
| 6 Month (n = 13) | 6 Month (n = 11) | |||||||
| Lumbo thoracic ratio (fast) | 0.02 (.96) | 0.06 (.85) | −0.41 (.16) | 0.74 (.26) | 0.27 (.42) | 0.16 (.65) | 0.48 (.14) | 0.17 (.75) |
| Lumbo thoracic ratio (slow) | −0.01 (.98) | −0.01 (.98) | −0.37 (.21) | 0.44 (.56) | 0.40 (.22) | 0.28 (.40) | 0.49 (.13) | −0.25 (.64) |
| Pelvic tilt* (fast) | −0.12 (.70) | −0.31 (.33) | 0.56 (.048) | 0.67 (.33) | −0.61 (.045) | −0.54 (.084) | −0.65 (.029) | 0.11 (.83) |
| Pelvic tilt* (slow) | −0.10 (.75) | −0.28 (.38) | 0.44 (.13) | 0.39 (.61) | −0.59 (.057) | −0.53 (.093) | −0.56 (.073) | 0.41 (.42) |
Measured in degrees
1.4. Discussion
In our sample of individuals presenting with acute/subacute LBP, those persons with higher LTR and smaller pelvic tilt at the end point of the forward bending task had higher scores on measures of disability, pain and pain catastrophizing at 6 months. This same association was found in those who met classification criteria for chronic LBP at 6 months. Opposing associations were found in the group not meeting the classification criteria for chronic LBP at 6 months, specifically, increased pelvic tilt was positively associated with higher pain catastrophizing scores suggesting pain catastrophizing has an opposite relationship with biomechanics measurements in those who meet the classification criteria for chronic LBP and those that do not at 6 months. These findings, which suggest that differences in the relationship between psychological factors and biomechanical measurements exist in those who meet criteria for chronic LBP and those who do not, have important clinical implications; specifically, if this relationship is validated in future research, identification of psychological risk factors could be used a prognostic indicator and prevention strategies could be identified targeting the psychological risks. Similarly, low back pain treatments might target, not only the biomechanical behavior, but also include psychological approaches to care for individuals at higher risk for chronicity and disability. Similarly, the use of the biomechanical measurements to predict outcomes of LBP offers a unique objective quantifiable tool to enhance clinical management.
Initial cross sectional studies suggested that those in an acute episode of LBP use more pelvic tilt and less lumbar flexion than healthy controls (Shojaei, Vazirian, et al. 2017, Shojaei, Salt, et al. 2017). These findings are partially supported by research conducted from other groups; Shum and colleagues found that persons with sub-acute LBP have decreased lumbar flexion when bending in a seated position in an additional cross sectional study (Shum, Crosbie, and Lee 2007). Higher disability scores in the group meeting criteria for chronic LBP at 6 months were associated with less pelvic tilt. It is plausible that some persons who initially present with acute/subacute LBP will use adaptive biomechanics, specifically more pelvic tilt, which is unlike healthy controls; yet this adaptive mechanism is protective against meeting criteria for chronic LBP. Future studies should be conducted comparing healthy back controls, acute/subacute LBP and chronic LBP with consideration of personal and family histories of pain including LBP prospectively collecting psychosocial, clinical and biomechanical data.
Few prospective studies have been conducted in non-chronic LBP populations investigating the variables included in this study. With this being said, conflicting findings have been reported in the chronic LBP literature (Ebrahimi et al. 2018, Pranata et al. 2018, Jung et al. 2018). Some studies have identified positive associations between altered chronic LBP kinematics and disability (Ebrahimi et al. 2018, Pranata et al. 2018, Jung et al. 2018). For example, Pranata and colleagues reported increased lumbar-hip coordination in patients with higher disability scores (Pranata et al. 2018). Yet, Jung and colleagues did not find a significant association between disability and trunk flexion ratios (i.e., the range of motion of lumbar flexion divided by the pelvic anterior tilting) (Jung et al. 2018). Variations in kinematic measurements and samples are possible causes for conflicting findings in the literature. Our findings offer new insights into the role of disability in lumbar and pelvic kinematics in acute and sub-acute LBP; yet many questions remain.
Similarly, conflicting findings have been reported in the literature in regards to the relationship of level of pain with kinematic changes in persons with LBP and our current understanding is largely from studies of chronic LBP patients(Jung et al. 2018, Pranata et al. 2018). No relationship was identified between trunk flexion ratios and pain in the study by Jung and colleagues (Jung et al. 2018). In contrast, the relationship between pain level and disability has been described and individuals with high disability have been found to have higher lumbar-hip mean absolute relative phase angles than those with lower disability and back healthy controls suggesting pain plays a role in lumbo-pelvic kinematics (Pranata et al. 2018).
Relationships between kinematic changes and pain catastrophizing were identified at each time point. In support of our findings, the association between pain catastrophizing and acute, sub-acute, chronic LBP and acute-to-chronic LBP transition has been described in the literature (Pincus et al. 2002, Wertli et al. 2014). Although scant, the literature describing the relationship between kinematic changes and pain catastrophizing in LBP also supports our study findings. Ross and colleagues conducted a study in back healthy individuals (N = 16) and an induced LBP condition. They then measured pain catastrophizing and lumbar kinematics. They found that persons with higher pain catastrophizing scores were more likely to be “stabilizers” or to use tightened neuromuscular control of the spinal movements which was the root mean square of three spinal angles during a lumbar flexion and extension task; thus a similar measurement to our LTR (Ross et al. 2017). Vaisy and colleagues conducted a cross-sectional study with findings that are in conflict with Ross et al. and support our study findings. Our study found associations between pain catastrophizing and increased LTR, which was supported in the Vaisy and colleagues study; yet they also found increased pelvic tilt, where we found that those with increased pain catastrophizing used decreased pelvic tilt (Vaisy et al. 2015). An additional cross-sectional study investigated patients with chronic LBP (N =22) and found increased myoelectric activity of lumbar muscles during flexion and neuro-mechanical changes in persons with higher pain catastrophizing scores (Henchoz et al. 2013). Psychological factors including pain catastrophizing have been identified as independent predictors of functional disability in a cross-sectional study of 52 patients with chronic pain (Dubois et al. 2014). Similarly, individuals with greater pain-related fears have been found to use more postural adaptions (enhanced use of the knee) than those with less pain-related fears (Jacobs et al. 2016). Based on study findings and prior literature, pain catastrophizing is associated with kinematic changes in the lumbar spine. Our findings suggest that in those that presented with acute/subacute LBP who meet the classification criteria for chronic LBP at 6 months, associations exist between kinematic changes and pain catastrophizing. These findings suggest that the interaction between biomechanical changes and psychosocial factors may play a key role in LBP chronicity. Thus, it is plausible that biomechanical measurements may be effective as a guide to psychological treatments and, in turn, psychological treatments may exert an effect on biomechanical changes. In the only identified longitudinal study, Dubois and colleagues conducted an 18-month study of workers with a history of at least one disabling LBP episode (N = 100). Seventy-seven completed the 6 month data collection. Interestingly, this study did not identify any associations between disability and psychological factors including pain catastrophizing (Dubois et al. 2016). Our findings suggest there is a relationship between disability, pain and pain catastrophizing and changes in lumbo-pelvic kinematics at 6 months. The differences in samples, disabled workers versus persons with acute/subacute LBP, could account for the conflicting findings.
There is little evidence from from the literature that depression is associated with kinematic changes (Jung et al. 2018). Consistent with the literature, our study did not identify significant relationships with kinematic changes and depressive symptoms at 6 months. Yet, at 2.5 months, the associations between depression and kinematic changes were significant and mimicked those between kinematic variables and pain catastrophizing. It is plausible that variability over time has not been well-studied in current literature. With this being said, there are few studies and the inconsistent metrics and samples should limit conclusions from the literature; the results of this study provide evidence that further investigation is needed.
1.4.1. Strengths and Limitations
The primary strengths of this study are the longitudinal nature of the design, the length of follow up, and the relatively robust retention of participants. The primary limitation, particularly for the assessment of associations among the subgroups defined by chronic/non-chronic pain, is sample size. While the number of subjects included in this exploratory study is similar to other studies in this area (Henchoz et al. 2013, Vaisy et al. 2015), further investigation of these relationships are needed, particularly when considering subgroup analysis. A larger sample size in future research will allow a more rigorous evaluation of the normality assumption as it pertains to study variables, with the potential for transformations to be applied if needed. An additional limitation is the lack of detailed LBP history prior to the acute/subacute LBP episode, which impacts our ability to describe the development of chronic LBP in relationship to kinematics and psychosocial variables. We used a simulated controlled movement during our biomechanical measurement. Although collecting slow-paced task data prior to fast-paced task data allowed for consistency in data collection, it also created the potential for order effect. Our study only included kinematic data; the collection of electromyography (EMG) and electroencephalogram (EEG) data will be considered for future studies.
1.5. Conclusion
Our study findings suggest that in our sample of people who initially presented with acute/subacute LBP, patients with a higher LTR and smaller pelvic tilt at the end point of the forward bending task had higher scores on measures of disability, pain and pain catastrophizing. In those who met criteria for chronic LBP at 6 months, higher LTR and smaller pelvic tilt was associated with increased pain catastrophizing at baseline. Similarly, smaller pelvic tilt was associated with increased pain catastrophizing at 2.5 months and smaller pelvic tilt was associated with higher disability and pain catastrophizing at 6 months in those who met criteria for chronic LBP at 6 months. Objective evidence is provided linking psychological factors and kinematic changes during the time between an acute/subacute LBP episode to meeting classification for chronic LBP at 6 months. A furthered understanding of modifiable factors contributing to low back pain chronicity is critically important to health care providers offering care to this population. Characterizing the relationship between psychological factors and kinematic changes, as we have done in this study, could facilitate tailored holistic approaches to treatment.
Acknowledgements:
Dr. Salt would also like to acknowledge Drs. Suzanne Segerstrom and Leslie Crofford for their mentorship during the conduct of this study.
Funding Statement: This work was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health [UL1TR000117]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosure Statement: None related to this work
Data Availability Statement: Data are available at the request of the first author, Elzabeth Salt, PhD, APRN at egsalt0@uky.edu
Declaration of Interests Statement: There are no competing interests in regards to this research by any authors.
Contributor Information
Elizabeth Salt, University of Kentucky, College of Nursing, 315 College of Nursing Building, 751 Rose Street, Lexington, KY 40536-0232; United States..
Amanda T. Wiggins, University of Kentucky, College of Nursing, 315 College of Nursing Building, 751 Rose Street, Lexington, KY 40536-0232; United States..
Mary Kay Rayens, University of Kentucky, College of Nursing, 315 College of Nursing Building, 751 Rose Street, Lexington, KY 40536-0232; United States..
Quenten Hooker, Washington University, School of Medicine, Campus Box 8502, 4444 Forest Park Ave., Suite 1101, St. Louis, MO 63108-2212; United States..
Iman Shojaei, University of Kentucky, College of Engineering, 351 Ralph G. Anderson Building, Lexington KY 40506-0503; United States.
Babak Bazrgari, University of Kentucky, College of Engineering, 351 Ralph G. Anderson Building, Lexington KY 40506-0503; United States..
References
- Al-Amri M, Nicholas K, Button K, Sparkes V, Sheeran L, and Davies JL. 2018. “Inertial Measurement Units for Clinical Movement Analysis: Reliability and Concurrent Validity.” Sensors (Basel) 18 (3). doi: 10.3390/s18030719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer Nina, and Linton Steven J. 2002. “Fear-avoidance beliefs and catastrophizing: occurrence and risk factor in back pain and ADL in the general population.” Pain 99 (3):485–491. [DOI] [PubMed] [Google Scholar]
- Crombez Geert, Vlaeyen Johan WS, Heuts Peter HTG, and Lysens Roland. 1999. “Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability.” Pain 80 (1):329–339. [DOI] [PubMed] [Google Scholar]
- Dagenais S, Caro J, and Haldeman S. 2008. “A systematic review of low back pain cost of illness studies in the United States and internationally.” Spine J 8 (1):8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Daut Randall L, Cleeland Charles S, and Flanery Randall C. 1983. “Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases.” Pain 17 (2):197–210. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, Carrino J, Chou R, Cook K, DeLitto A, Goertz C, Khalsa P, Loeser J, Mackey S, Panagis J, Rainville J, Tosteson T, Turk D, Von Korff M, and Weiner DK. 2014. “Report of the NIH Task Force on research standards for chronic low back pain.” J Pain 15 (6):569–85. doi: 10.1016/j.jpain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois JD, Abboud J, St-Pierre C, Piche M, and Descarreaux M. 2014. “Neuromuscular adaptations predict functional disability independently of clinical pain and psychological factors in patients with chronic non-specific low back pain.” J Electromyogr Kinesiol 24 (4):550–7. doi: 10.1016/j.jelekin.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Dubois JD, Cantin V, Piche M, and Descarreaux M. 2016. “Physiological and Psychological Predictors of Short-Term Disability in Workers with a History of Low Back Pain: A Longitudinal Study.” PLoS One 11 (10):e0165478. doi: 10.1371/journal.pone.0165478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi S, Kamali F, Razeghi M, and Haghpanah SA. 2018. “Correlation between Trunk-Pelvis Inter-Segmental Coordination Parameters during Walking and Disability Level in Chronic Low Back Pain Patients.” J Biomed Phys Eng 8 (2):193–202. [PMC free article] [PubMed] [Google Scholar]
- Fourney Daryl R, Gunnar Andersson, Arnold Paul M, Dettori Joseph, Cahana Alex, Fehlings Michael G, Norvell Dan, Samartzis Dino, and Chapman Jens R. 2011. “Chronic low back pain: a heterogeneous condition with challenges for an evidence-based approach.” Spine 36:S1–S9. [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Study 2013 Collaborators. 2015. “Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013.” Lancet 386 (9995):743–800. doi: 10.1016/s0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchoz Y, Tetreau C, Abboud J, Piche M, and Descarreaux M. 2013. “Effects of noxious stimulation and pain expectations on neuromuscular control of the spine in patients with chronic low back pain.” Spine J 13 (10):1263–72. doi: 10.1016/j.spinee.2013.07.452. [DOI] [PubMed] [Google Scholar]
- Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, Woolf A, Vos T, and Buchbinder R. 2012. “A systematic review of the global prevalence of low back pain.” Arthritis Rheum 64 (6):2028–37. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, Williams G, Smith E, Vos T, Barendregt J, Murray C, Burstein R, and Buchbinder R. 2014. “The global burden of low back pain: estimates from the Global Burden of Disease 2010 study.” Ann Rheum Dis 73 (6):968–74. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Roy CL, Hitt JR, Popov RE, and Henry SM. 2016. “Neural mechanisms and functional correlates of altered postural responses to perturbed standing balance with chronic low back pain.” Neuroscience 339:511–524. doi: 10.1016/j.neuroscience.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Kwon OY, Yi CH, Cho SH, Jeon HS, Weon JH, and Hwang UJ. 2018. “Predictors of dysfunction and health-related quality of life in the flexion pattern subgroup of patients with chronic lower back pain: The STROBE study.” Medicine (Baltimore) 97 (29):e11363. doi: 10.1097/md.0000000000011363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristianslund E, Krosshaug T, and van den Bogert AJ. 2012. “Effect of low pass filtering on joint moments from inverse dynamics: implications for injury prevention.” J Biomech 45 (4):666–71. doi: 10.1016/j.jbiomech.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Luo X, Pietrobon R, Sun SX, Liu GG, and Hey L. 2004. “Estimates and patterns of direct health care expenditures among individuals with back pain in the United States.” Spine (Phila Pa 1976) 29 (1):79–86. doi: 10.1097/01.Brs.0000105527.13866.0f. [DOI] [PubMed] [Google Scholar]
- National Institute of Neurological Disorders and Stroke. 2018. “Low Back Pain”. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Low-Back-Pain-Fact-Sheet.
- Osman Augustine, Barrios Francisco X, Kopper Beverly A, Hauptmann Wendy, Jones Jewel, and O’neill Elizabeth. 1997. “Factor structure, reliability, and validity of the Pain Catastrophizing Scale.” Journal of behavioral medicine 20 (6):589–605. [DOI] [PubMed] [Google Scholar]
- Pincus T, Burton AK, Vogel S, and Field AP. 2002. “A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain.” Spine (Phila Pa 1976) 27 (5):E109–20. [DOI] [PubMed] [Google Scholar]
- Pranata A, Perraton L, El-Ansary D, Clark R, Mentiplay B, Fortin K, Long B, Brandham R, and Bryant AL. 2018. “Trunk and lower limb coordination during lifting in people with and without chronic low back pain.” J Biomech 71:257–263. doi: 10.1016/j.jbiomech.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Radloff LS. 1977. “The CES-D Scale: a self-report depression scale for research in the general population.” Appl Psychol Measurement. 1 (3):385–401. [Google Scholar]
- Riley JF, Ahern DK, and Follick MJ. 1988. “Chronic pain and functional impairment: assessing beliefs about their relationship.” Archives of physical medicine and rehabilitation 69 (8):579–582. [PubMed] [Google Scholar]
- Ross GB, Sheahan PJ, Mahoney B, Gurd BJ, Hodges PW, and Graham RB. 2017. “Pain catastrophizing moderates changes in spinal control in response to noxiously induced low back pain.” J Biomech 58:64–70. doi: 10.1016/j.jbiomech.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Shojaei I, Salt EG, Hooker Q, Van Dillen LR, and Bazrgari B. 2017. “Comparison of lumbo-pelvic kinematics during trunk forward bending and backward return between patients with acute low back pain and asymptomatic controls.” Clin Biomech (Bristol, Avon) 41:66–71. doi: 10.1016/j.clinbiomech.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei I, Vazirian M, Salt EG, Van Dillen LR, and Bazrgari B. 2017. “Timing and magnitude of lumbar spine contribution to trunk forward bending and backward return in patients with acute low back pain.” J Biomech 53:71–77. doi: 10.1016/j.jbiomech.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum GL, Crosbie J, and Lee RY. 2007. “Movement coordination of the lumbar spine and hip during a picking up activity in low back pain subjects.” Eur Spine J 16 (6):749–58. doi: 10.1007/s00586-006-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieben Judith M, Vlaeyen Johan WS, Tuerlinckx Sandrine, and Portegijs Piet JM. 2002. “Pain‐related fear in acute low back pain: the first two weeks of a new episode.” European Journal of Pain 6 (3):229–237. [DOI] [PubMed] [Google Scholar]
- Stroud Michael W, McKnight Patrick E, and Jensen Mark P. 2004. “Assessment of self-reported physical activity in patients with chronic pain: Development of an abbreviated Roland-Morris Disability Scale.” The Journal of Pain 5 (5):257–263. [DOI] [PubMed] [Google Scholar]
- Sullivan Michael JL, Bishop Scott R, and Pivik Jayne. 1995. “The pain catastrophizing scale: development and validation.” Psychological assessment 7 (4):524. [Google Scholar]
- Swinkels-Meewisse Ilse EJ, Roelofs Jeffrey, Schouten Erik GW, Verbeek André LM, Oostendorp Rob AB, and Vlaeyen Johan WS. 2006. “Fear of movement/(re) injury predicting chronic disabling low back pain: a prospective inception cohort study.” Spine 31 (6):658–664. [DOI] [PubMed] [Google Scholar]
- Thomas JS, and France CR. 2007. “Pain-related fear is associated with avoidance of spinal motion during recovery from low back pain.” Spine (Phila Pa 1976) 32 (16):E460–6. doi: 10.1097/BRS.0b013e3180bc1f7b. [DOI] [PubMed] [Google Scholar]
- Thomas JS, and France CR. 2008. “The relationship between pain-related fear and lumbar flexion during natural recovery from low back pain.” Eur Spine J 17 (1):97–103. doi: 10.1007/s00586-007-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JS, France CR, Lavender SA, and Johnson MR. 2008. “Effects of fear of movement on spine velocity and acceleration after recovery from low back pain.” Spine (Phila Pa 1976) 33 (5):564–70. doi: 10.1097/BRS.0b013e3181657f1a. [DOI] [PubMed] [Google Scholar]
- Thorn BE, Day MA, Burns J, Kuhajda MC, Gaskins SW, Sweeney K, McConley R, Ward LC, and Cabbil C. 2011. “Randomized trial of group cognitive behavioral therapy compared with a pain education control for low-literacy rural people with chronic pain.” Pain 152 (12):2710–20. doi: 10.1016/j.pain.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Kentucky. 2017. “Kentucky Women’s Health Registry.” http://www.ccts.uky.edu/ccts/kwhrbiospecimens. [Google Scholar]
- Vaisy M, Gizzi L, Petzke F, Consmuller T, Pfingsten M, and Falla D. 2015. “Measurement of Lumbar Spine Functional Movement in Low Back Pain.” Clin J Pain 31 (10):876–85. doi: 10.1097/ajp.0000000000000190. [DOI] [PubMed] [Google Scholar]
- University Vanderbilt. 2017. Research Match. Online [Google Scholar]
- Vazirian M, Shojaei I, Agarwal A, and Bazrgari B. 2017. “Lumbar contribution to the trunk forward bending and backward return; age-related differences.” Ergonomics 60 (7):967–976. doi: 10.1080/00140139.2016.1237676. [DOI] [PubMed] [Google Scholar]
- Vlaeyen Johan WS, Kole-Snijders Ank MJ, Boeren Ruben GB, and Van Eek H. 1995. “Fear of movement/(re) injury in chronic low back pain and its relation to behavioral performance.” Pain 62 (3):363–372. [DOI] [PubMed] [Google Scholar]
- Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, and Weiser S. 2014. “Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review.” Spine J 14 (11):2639–57. doi: 10.1016/j.spinee.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Winter DA 1984. “Biomechanics of human movement with applications to the study of human locomotion.” Crit Rev Biomed Eng 9 (4):287–314. [PubMed] [Google Scholar]
- Wong TK, and Lee RY. 2004. “Effects of low back pain on the relationship between the movements of the lumbar spine and hip.” Hum Mov Sci 23 (1):21–34. doi: 10.1016/j.humov.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Yun WS, Kim H, Ahn JH, Park YB, and Park YJ. 2015. “Individual characteristics of reliable lumbar coupling motions.” Eur Spine J 24 (9):1917–25. doi: 10.1007/s00586-015-4081-0. [DOI] [PubMed] [Google Scholar]
- Zalon Margarete L. 1999. “Comparison of pain measures in surgical patients.” Journal of Nursing Measurement 7 (2):135–152. [PubMed] [Google Scholar]


