Abstract
Purpose of Review:
Extracorporeal cardiopulmonary resuscitation (ECPR) is a contemporary resuscitation approach that employs veno-arterial extracorporeal membrane oxygenation (VA-ECMO). This approach is increasingly used worldwide to mitigate the widespread hemodynamic and multi-organ dysfunction that accompanies cardiac arrest.
Recent Findings:
In this review, the physiology of VA-ECMO and ECPR, the role of ECPR in contemporary resuscitation care, the complications associated with ECPR and VA-ECMO usage, and intensive care considerations for this population are discussed.
Summary:
ECPR offers a promising mechanism to mitigate multi-organ injury and allow time for the institution of supportive interventions required to effectively treat cardiac arrest. More prospective data in the context of extensive pre-hospital and hospital collaboration is needed to promote its successful use.
Keywords: Extracorporeal CPR, ECPR, Extracorporeal Membrane Oxygenation
Introduction
Cardiac arrest is a state of systemic hypoperfusion that can lead to devastating multi-organ injury and death if left unmitigated. The central physiologic aberration is the lack of perfusion to the vital organs. Advanced cardiac life support (ACLS) protocols employ the combination of early and high-quality chest compressions, rapid defibrillation, and vasoactive medications to maintain perfusion of the heart and brain while the etiology of the cardiac arrest is diagnosed and reversed. Despite widespread implementation of these ACLS protocols and considerable public health efforts, the rates of neurologically favorable survival in North America have improved only minimally over the past two decades.1
More recently, the usage of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) in association with cardiopulmonary resuscitation, together termed extracorporeal cardiopulmonary resuscitation (ECPR), has been associated with promising improvements in the rate of neurologically intact survival compared to traditional ACLS-based approaches.2 In this review, we detail the physiologic principles of VA-ECMO and ECPR, the role of VA-ECMO in clinical care, potential complications associated with the use of ECPR in the adult population, and intensive care considerations for this patient population.
Physiology of VA-ECMO and ECPR
Cardiac arrest induces systemic circulatory and cardiac pump dysfunction.3 Chest compressions provide one-fourth of the normal cardiac output.4–6 Together, the hypoperfusion caused by cardiac arrest and the suboptimal replacement provided by chest compressions lead to ongoing hypoperfusion throughout the course of CPR. Lactic acid production increases resulting in acidemia which worsens vasodilation and cardiac dysfunction.7–11 The central role of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) systems is to provide circulatory and ventilatory support to replace native cardiorespiratory function.
Blood is retrieved from the systemic venous circulation, pumped through the VA-ECMO oxygenator for oxygenation and carbon dioxide extraction, and then returned to a central artery, much like cardiopulmonary bypass.12 However, unlike conventional cardiopulmonary bypass which requires a midline sternotomy and direct cannulation of the great vessels, modern VA-ECMO support can be rapidly instituted in the cardiac catheterization laboratory, at the patient’s bedside, or in the field via percutaneous cannulation of peripheral vessels.13 The cardiac catheterization laboratory offers the advantage of direct visualization with fluoroscopy and ultrasound, thus minimizing cannulation-related complications. Further, many patients have an ischemic etiology to their cardiac arrest, particularly in ventricular fibrillation, where coronary artery disease has been implicated in up to 85% of cases.14 Therefore, cannulation in the cardiac catheterization laboratory also offers rapid access for urgent coronary angiography. Cannulation is typically performed under sterile conditions with the insertion of large cannulae via the common femoral artery and vein. The tip of the venous cannula typically lies in the superior vena cava or right atrium and the tip of the arterial cannula typically lies in the descending aorta or common iliac artery. Blood is removed through the venous cannula by the centrifugal pump which then pushes the blood through the oxygenator. After oxygenation, the blood is returned back through the arterial cannula to perfuse the arterial tree in retrograde fashion. The degree of oxygenation provided to the blood is determined by the fraction of oxygen provided in the sweep gas which flows through the oxygenator. Carbon dioxide removal is simultaneously performed by the rate of sweep gas flowing through the oxygenator. These two processes are similar to FiO2 and minute ventilation provided by ventilators. Therefore, the key measures of therapy intensity are the circuit flow (measured in liters per minute), fraction of oxygen delivery (percentage), and the carbon dioxide removal sweep (measured in liters per minute).
Once a patient has been cannulated, oxygenated blood is infused retrogradely via the arterial cannula into the aorta. The blood is then circulated around the body using the power generated by the VA-ECMO pump until it reaches the vena cava. It is expected that the retrograde blood flow from the VA-ECMO arterial cannula may collide with antegrade blood flow from the native cardiac function. However, in many cases, the native cardiac function is unable to produce enough antegrade blood flow to produce any significant hemodynamic effects.
The Role of ECPR in Clinical Care
The use of ECPR as part of OHCA treatment was described as early as the 1980s.15 ECPR was largely employed in the post-operative setting until percutaneous VA-ECMO emerged in the last decade. This increased the portability and rapidity with which ECPR could be instituted in the acute setting. ECPR strategies are now most commonly employed for patients that do not have sustained return of spontaneous circulation (ROSC) after initial ACLS efforts.16 A recently published population-based registry from Europe noted that 4% of out-of-hospital cardiac arrests of presumed cardiac etiology with attempted resuscitation were treated with ECPR between May 2011 and January 2018.17 There were no differences in survival between the subjects treated with ECPR and conventional CPR. Lunz et al noted that the use of ECPR for refractory cardiac arrest at five expert European centers was associated with a 19% rate of favorable neurologic outcomes among allcomers at three months. The authors noted the potential for a greater prevalence of favorable neurologic outcomes when more strict inclusion criteria for ECPR criteria are employed.18 Multiple other centers have published observational data describing formal ECPR protocols and outcomes for patients in whom ECPR has been deployed.19–23 To date, there are no published randomized controlled trials comparing ECPR to conventional ACLS approaches. However, multiple randomized trials are presently underway,24–27 the majority of which have more strict inclusion criteria than the aforementioned registry data.17 The inclusion criteria for the Minnesota Resuscitation Consortium’s ECPR protocol and the four ongoing clinical trials are outlined in Table 1.25,28
Table 1.
Eligibility Criteria for ECPR in the Ongoing Clinical Trials.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Minnesota Resuscitation Protocol28/ARREST Trial25 | |
|
|
| EROCA Trial26 | |
|
|
| INCEPTION Trial24 | |
|
|
| SUB30 Trial27 | |
|
|
CPR: Cardiopulmonary resuscitation; OHCA: Out-of-Hospital Cardiac Arrest; ROSC: Return of Spontaneous Circulation; VT/VF: Ventricular Tachycardia/Ventricular Fibrillation.
In contemporary practice, ECPR has been employed for patients suffering from the spectrum of cardiac arrest. Reported data have evaluated outcomes after both in-hospital and out-of-hospital cardiac arrest with both ventricular tachycardia/ventricular fibrillation (VT/VF; together termed ‘shockable rhythms’) and pulseless electrical activity/asystole (together termed ‘non-shockable rhythms’). Improved survival outcomes have been reported in patients with shockable rhythms who were treated with ECPR compared to patients with non-shockable rhythms.2 The duration of arrest preceding initiation of ECPR is directly related to the severity of metabolic insult that can be expected in a patient with cardiac arrest.29 ECPR offers a platform for stabilization of the patient’s hemodynamic and respiratory status such that the metabolic derangement can be treated appropriately and reversed. This has been associated with significantly increased neurologically intact survival when compared to standard ACLS approaches.
VA-ECMO alone is not sufficient for a patient’s survival. Once a patient is cannulated with VA-ECMO, a large multi-disciplinary team is responsible for the care of the patient. This team typically involves physicians from cardiology, cardiac surgery, critical care, and neurology collaborating with nurses, perfusionists, and therapists. The focus of the VA-ECMO therapy is to provide continuous hemodynamic and respiratory support while the underlying causes for the index cardiac arrest are addressed. The vast and resource-intensive nature of this multi-disciplinary effort suggests that patients may be best served in expert tertiary care centers.30 In the state of Minnesota, the need for a tertiary center has led to the creation of the Minnesota Resuscitation Consortium (MRC), which is a collaboration between emergency medical services and the hospital systems. In 2012, the MRC developed a protocol for all patients from the Minneapolis-St. Paul metro area with ROSC after VT/VF arrest to proceed to the cardiac catheterization for coronary evaluation and hemodynamic support as needed. This was associated with improvements in neurologically intact survival.28 In 2015, the MRC began a protocol for patients with refractory VT/VF OHCA. These patients were rapidly transported from the scene of arrest with ongoing CPR to the University of Minnesota where ECPR was initiated in the cardiac catheterization laboratory.19 Coronary angiography was then performed demonstrating a high level of complex coronary artery disease; PCI was performed as needed. This protocolized strategy involving ECPR and coronary angiography has markedly improved both survival and neurological outcomes.
More recently, the concept of mobile ECPR has evolved to allow for more rapid ECPR initiation.30 With this approach, the Minnesota Mobile Resuscitation Consortium has developed a team and protocol for the decentralization of ECPR with VA-ECMO initiation in centers closer to the patient followed by transport to a resuscitation center with centralization of the complicated critical care and VA-ECMO management. The participating health care systems use a common team of physicians and flight crew medics/nurses with extensive training in the initiation of VA ECMO and resuscitation of these patients. The team operates 24/7 using a hub and spoke model. EMS personnel activate the team immediately upon identifying a potential patient in the field. The MRC mobile ECMO resuscitation team rendezvous with the EMS personnel at the closest participating emergency department. Upon arrival to the emergency department, patients that need ECPR are directly cannulated for VA-ECMO by the specialized team. Patients are subsequently taken to the local hospital’s cardiac catheterization laboratory and, after coronary angiography/PCI, are transported to the Resuscitation Center/VA-ECMO center for further management and care. This approach has been employed to minimize the time where ACLS is the primary method of perfusion, since the odds of a favorable neurologic outcome decline rapidly with prolonged out-of-hospital resuscitation.
Discontinuation of VA-ECMO Therapy
The patient’s goals of care are critically important in planning discontinuation of VA-ECMO. For most patients receiving ECPR, the goals of care cannot be assessed adequately prior to initiation. Patients with DNR status are typically excluded from ECPR; however, the variations in goals for patients who are otherwise characterized as ‘full code’ make these discussions very important. In cases of ECPR, these discussions are often had with family members as soon as possible after resuscitation. However, family members are often unable to discuss the topic fully while grappling with the shock of the days events. Therefore, repeated empathic discussions are had to explore the wishes of the patient as the surrogate medical decision makers are able.
In most cases of ECPR, VA-ECMO is considered a bridge to recovery. However, treatment with VA-ECMO may also be a bridge to durable mechanical support or transplantation in other settings. These options will depend on the wishes of the patient as articulated by the surrogate decision makers. When possible, if durable mechanical support or transplant would be necessary, the patient is allowed to recover as fully as possible to participate in the discussion where possible. Patients may be extubated while on ECMO to facilitate these discussions, though this is not always possible. The destination options available should determine the strategy employed and criteria required before VA ECMO can be decannulated.
Decannulation from VA-ECMO is necessary to allow progression of clinical care and limit the complications associated with the invasive VA-ECMO therapy. Discontinuation of VA-ECMO therapy requires a complex interplay of decisions that are guided by a combination of clinical, hemodynamic, and imaging data. Fundamental to this process is addressing the underlying etiology of the cardiac arrest wherever possible. This is important in an effort to improve hemodynamic recovery while also preventing future re-arrest.3 In patients with refractory VT/VF OHCA, significant coronary artery disease has been previously demonstrated as the inciting factor for these cardiac arrest in the majority of patients.14 Thus, cardiac recovery is often the central criterion which facilitates decannulation from VA-ECMO.14
In the MRC protocol, cardiorespiratory recovery is systematically evaluated through the use of VA-ECMO turndown examinations. These examinations are done to evaluate the cardiorespiratory response to progressive decreases in VA-ECMO circuit flow. Patients generally have their VA-ECMO flow decreased by 0.5 L/min every three minutes while their hemodynamic response is evaluated through clinical and echocardiographic parameters. The hemodynamic response is judged by evaluation of heart rate, mean arterial pressure, arterial oxygen saturation, and mixed venous oxygen saturation at each level of circuit flow. The echocardiographic examination evaluates left ventricular function, right ventricular function, left ventricular end-diastolic diameter, and left ventricular end-systolic diameter at each level of circuit flow. The patient is deemed to be suitable for decannulation is they are tolerating ≤2 L/minute of circuit flow and the following three criteria: 1) LVEF >25% with low-moderate levels of inotropic support 2) mean arterial pressure >55 mmHg, and 3) arterial oxygen saturation was >92% with <10 mmHg of positive end-expiratory pressure and <50% fraction of inspired oxygen concentration on the patient’s ventilator.
The physical removal of the VA-ECMO cannulae and closure of the vascular access sites may be done percutaneously or via open surgical repair.31 Percutaneous closure currently involves removal of the cannulae with manual pressure. Surgical closure is usually done via a traditional cut-down approach in the operating room using direct surgical visualization and repair of the vessels. In the MRC protocol, decannulation is uniformly sought in the operating room. Operative repair provides rapid and durable vascular repair and the potential for rapid reinstitution of VA-ECMO support in the event that the patient becomes hemodynamically unstable.
Complications of ECPR Therapy
While ECPR offers a promising approach to contemporary cardiac arrest management, its use may also be associated with significant morbidity. The complications can be broadly divided into access site complications, bleeding complications, thrombotic complications, and the North-South syndrome (Figure 1).
Figure 1: Complications of ECPR Therapy Specific to VA-ECMO.
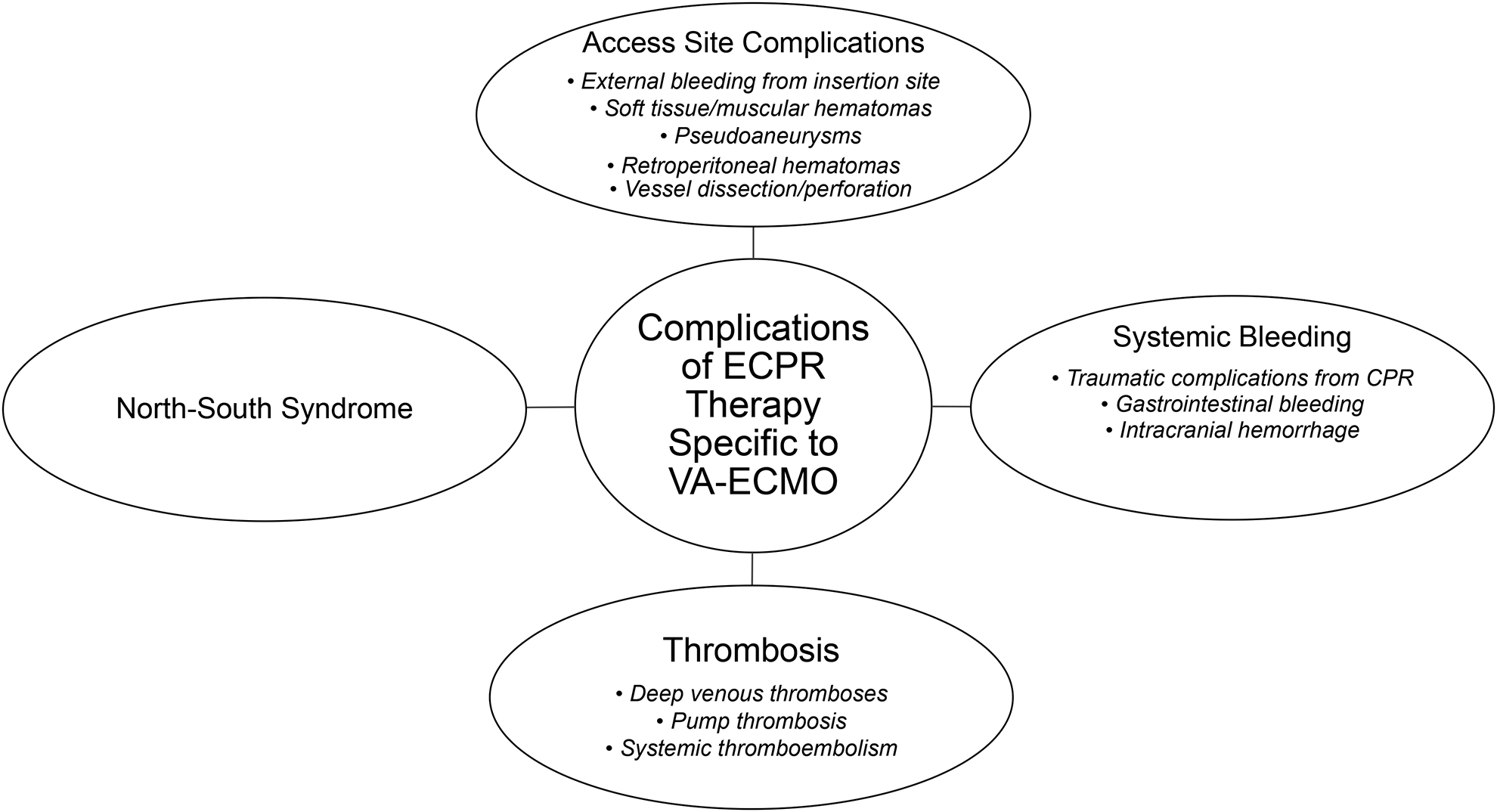
CPR: cardiopulmonary resuscitation, ECPR: extracorporeal-assisted cardiopulmonary resuscitation, VA-ECMO: veno-arterial extracorporeal membrane oxygenation.
Access site complications are the most common reported complication of ECPR and may emerge from the need for urgent institution of large-bore peripheral arterial and venous access. The spectrum of access site complications may include soft tissue/muscular hematomas, retroperitoneal hematomas, pseudoaneurysms, vessel perforation, vessel dissection, and acute limb ischemia from embolism and/or thrombosis. Access site complications have been reported to occur in up to 20% of patients from small observational series.32–34 Prior data suggests a higher incidence of access site complications in patients with the peripheral artery disease and diabetic vasculopathy.32 Avoidance of access site and vascular complications is vital in this population since vascular complications have been associated with increased mortality in patients cannulated for ECPR.34 Ultrasound guided vascular access in the cardiac catheterization laboratory offers a technique for precise and rapid visualization of vessels during cannula placement, and thus potentially limits access site complications.19 The prophylactic use of a distal limb reperfusion catheter in the limb with the femoral arterial cannula may additionally limit the incidence of acute limb ischemia.34,35 Frequent monitoring of the cannulated limb with near infrared spectroscopy might facilitate the early diagnosis and reversal of ischemia.36
In addition to access site bleeding complications, there is an inherent risk of systemic bleeding complications with ECPR due to the need for systemic anticoagulation. Moreover, ECPR patients have been reported to have increased consumption of the intrinsic coagulation factors as well as constant activation of the fibrinolytic system.37 Bartos et al have previously reported an incidence of 5–10% of major bleeding complications in the refractory VT/VF population that was treated with ECPR.38 The reported complications include spontaneous bleeding as well as CPR-induced bleeding. Examples include spontaneous upper and lower gastrointestinal bleeding, hemothorax, hemopericardium, intraperitoneal bleeding, retroperitoneal bleeding, and intracranial hemorrhage. All patients in this cohort received non-contrast CT scans of the head, chest, abdomen, and pelvis on admission to assess for bleeding complications. While anticoagulation is required to maintain the efficacy of the VA ECMO circuit and reduce thrombotic complications, coagulation status must be frequently assessed and tightly controlled to balance the risks of bleeding and thrombosis. Various laboratory tests are used at different centers including activated clotting time, PTT, heparin anti-Xa level.
Thrombotic complications are also commonly encountered among patients being treated with VA-ECMO. Systemic and pump thromboses may occur due to the contact of blood and non-endothelialized surfaces in the VA-ECMO circuit. In addition, patients may be pro-coagulable due to the activation of the inflammatory system in their critically ill state.39 Anticoagulation therapy, primarily with heparin or bivalirudin, is therefore, employed routinely in patients on VA-ECMO to prevent these thromboses. Pump thrombosis may occur at the pump head. This is a rare, but often catastrophic, event that may lead to significant hemolysis or loss of hemodynamic support and death. Any thrombosis on the arterial side of the circuit may also lead to systemic thromboembolism. The discovery of arterial clot may require replacement of the affected circuit component.
North-south syndrome is a complication unique to peripheral VA-ECMO therapy.40 This syndrome requires both native cardiac function and severely impaired lung function. In this case, poorly oxygenated blood from significant volumes of native antegrade cardiac contractile flow is pumped forward to the point where it meets the retrograde peripheral VA ECMO flow. Any organs perfused by the antegrade blood are at risk of poor oxygenation. The highest risk is present for the coronary arteries and tissue perfused by the brachiocephalic artery including the right brain; however, all great vessels can be perfused with the antegrade blood if cardiac function is sufficient. North-south syndrome typically occurs in the setting of retrograde blood flow from the VA-ECMO circuit when native cardiac contractility is improving. The oxygen saturation should be monitored closely with either pulse oximetry or arterial blood gas measurement from the territory perfused by the brachiocephalic artery, such as the right hand, arm, or face. In most cases, North-South syndrome is remedied by adjusting the ventilator to enhance oxygenation of the antegrade blood. If this is not possible, a separate venous perfusion cannula may be added to create a veno-arterio-venous ECMO circuit where oxygenated blood is perfused in both the pre-existing femoral arterial cannula and also into the right internal jugular venous perfusion cannula. This oxygenates blood in the right atrium thereby limiting the need for lung function.
Intensive Care Considerations in ECPR Patients
Although rapid ECPR initiation is of the utmost importance, the majority of patients develop multi-organ failure requiring prolonged intensive care unit (ICU) stays. Competing pathophysiologic therapeutic targets such as bleeding, traumatic injuries, and ischemic vital organ manifestations make the management of these patients extremely complex (Figure 2). These issues, combined with the management of significant neurological injury, call for complex team collaborations and social support structures that are currently very limited.41 In the MRC experience, patients who survived to discharge typically received VA-ECMO support for a mean of 3.5 days.38 They were extubated at a mean time of 7 days with 16 days in the ICU and 21 days before hospital discharge.
Figure 2: Critical Care Associated with ECPR.
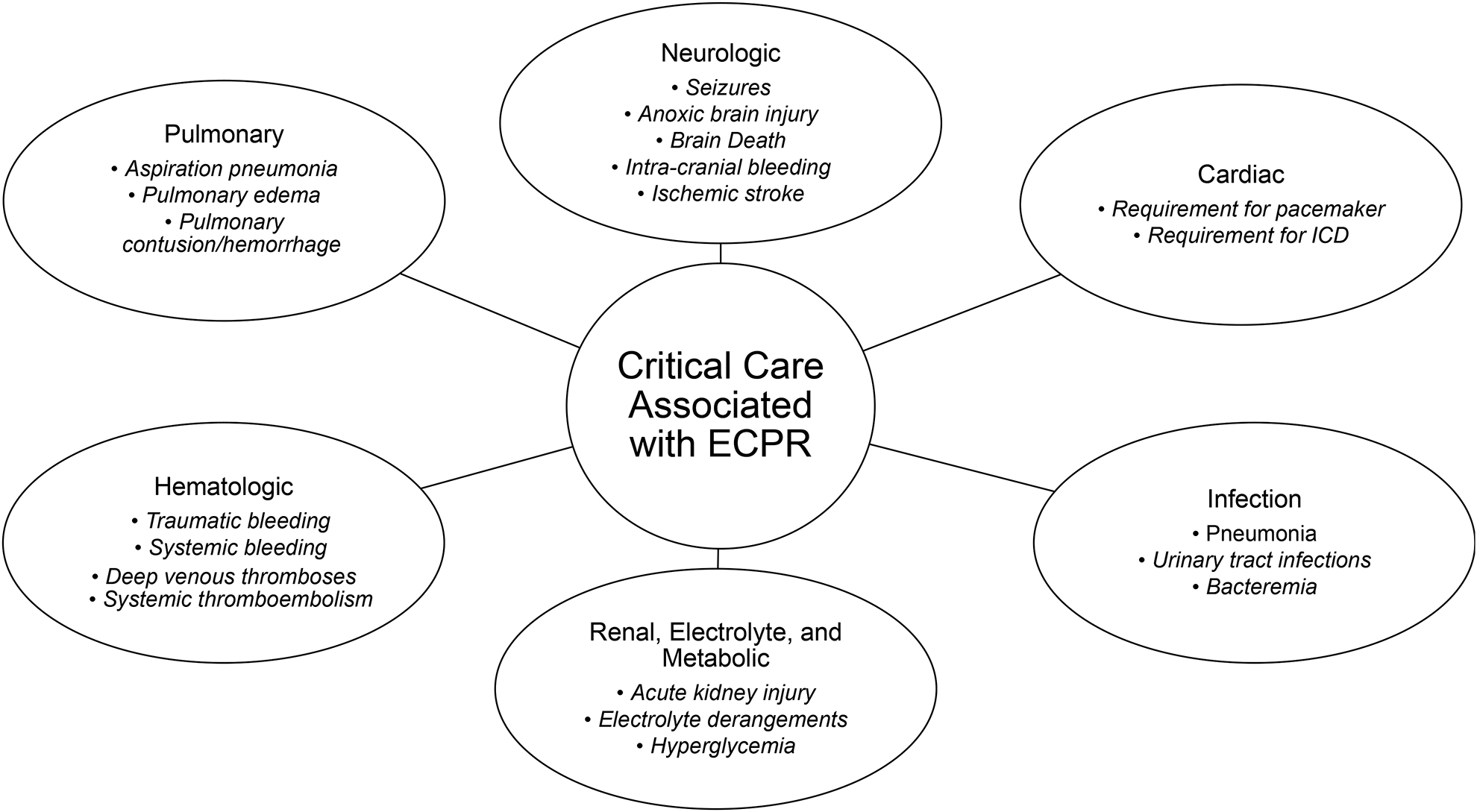
ECPR: extracorporeal-assisted cardiopulmonary resuscitation, ICD: implantable cardioverter-defibrillators.
The lengthy hospitalization course of ECPR patients is due to the multitude of critical illnesses that result from the index cardiac arrest (Figure 2). Anoxic brain injury and brain death are the leading causes of death in patients that die during the index admission.38 Approximately 30–40% of patients suffer acute kidney injury and accompanying hyperglycemia, metabolic, and electrolyte derangements. Thus, ~15% of patients require hemodialysis during the course of the hospitalization.38,42 Culture-positive infections occur in ~50% of patients during their ICU stay. The most common etiologies are pneumonias related to aspiration during the initial phases of CPR; however, urinary tract infections and bacteremias also occur.38 Transfusions are required in >70% of ECPR patients. A significant amount of left ventricular recovery occurs during the first two weeks. Around 30% of survivors will have an indication for implantable cardioverter-defibrillator placement.38
Conclusion
In summary, ECPR offers a promising mechanism to mitigate multi-organ injury and allow time for the institution of supportive interventions required to effectively treat cardiac arrest. The current experience supports the notion that extensive prehospital and hospital collaboration is necessary for successful implementation of ECPR programs. While VA ECMO is a critical aspect of any ECPR program, the post-ECMO care is also critical and complex. Multi-disciplinary teams are necessary within the structure of a resuscitation center to provide the complex care to the patients and their families. Prospective randomized trials are currently comparing ECPR to conventional ACLS protocols for the management of cardiac arrest.
Key Points.
ECPR provides significant hemodynamic support for the systemic circulatory derangement and cardiac pump dysfunction induced by cardiac arrest.
Identifying and addressing the underlying cause of cardiac arrest is of paramount importance in deciding when to decannulate from VA-ECMO support – in many cases, this is significant coronary artery disease.
ECPR patients may suffer from access site complications, systemic bleeding, thrombotic complications, or North-South syndrome as a result of ECPR treatment.
Patients that are treated with ECPR often have significant multi-organ dysfunction that requires a highly coordinated multi-disciplinary intensive care approach to address their comorbidities.
Acknowledgements:
Extracorporeal membrane oxygenation is currently only licensed for use in experimental settings.
Financial Support: Dr. Yannopoulos has received NIH funds related to basic and clinical CPR research from the National Heart, Lung, and Blood Institute (award numbers 5U01HL133818-03 and 4R33HL142696-02) and a grant from the Helmsley Philanthropic Trust for the broad community implementation of ECPR and mobile ECPR in the Minneapolis St Paul metropolitan area.
Footnotes
Conflicts of Interest: None of the authors had any conflicts of interest to declare.
References
- 1.American Heart Association. Heart disease and stroke statistics—2019 update. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- *2.Yannopoulos D, Bartos JA, Aufderheide TP, et al. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest. Circulation. 2019;139(12):e530–e552. [DOI] [PubMed] [Google Scholar]; This scientific statement outlines the role of VA-ECMO as a hemodynamic support mechanism in cardiac arrest.
- 3.Kosmopoulos M, Kalra R, Bartos JA, Raveendran G, Yannopoulos D. Contemporary approaches to cardiopulmonary resuscitation: physiology-guided approaches. Journal of Emergency and Critical Care Medicine. 2019. [Google Scholar]
- 4.Duggal C, Weil MH, Gazmuri RJ, et al. Regional blood flow during closed-chest cardiac resuscitation in rats. Journal of applied physiology (Bethesda, Md : 1985). 1993;74(1):147–152. [DOI] [PubMed] [Google Scholar]
- 5.Lurie KG, Mulligan KA, McKnite S, Detloff B, Lindstrom P, Lindner KH. Optimizing standard cardiopulmonary resuscitation with an inspiratory impedance threshold valve. Chest. 1998;113(4):1084–1090. [DOI] [PubMed] [Google Scholar]
- 6.Lurie K, Voelckel W, Plaisance P, et al. Use of an inspiratory impedance threshold valve during cardiopulmonary resuscitation: a progress report. Resuscitation. 2000;44(3):219–230. [DOI] [PubMed] [Google Scholar]
- 7.Marsh JD, Margolis TI, Kim D. Mechanism of diminished contractile response to catecholamines during acidosis. The American journal of physiology. 1988;254(1 Pt 2):H20–27. [DOI] [PubMed] [Google Scholar]
- 8.Toller W, Wolkart G, Stranz C, Metzler H, Brunner F. Contractile action of levosimendan and epinephrine during acidosis. European journal of pharmacology. 2005;507(1–3):199–209. [DOI] [PubMed] [Google Scholar]
- 9.Levy B, Collin S, Sennoun N, et al. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Med. 2010;36(12):2019–2029. [DOI] [PubMed] [Google Scholar]
- 10.Schotola H, Toischer K, Popov AF, et al. Mild metabolic acidosis impairs the β-adrenergic response in isolated human failing myocardium. Critical care (London, England). 2012;16(4):R153–R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ives SJ, Andtbacka RH, Noyes RD, et al. alpha1-Adrenergic responsiveness in human skeletal muscle feed arteries: the impact of reducing extracellular pH. Experimental physiology. 2013;98(1):256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrams D, Combes A, Brodie D. Extracorporeal Membrane Oxygenation in Cardiopulmonary Disease in Adults. Journal of the American College of Cardiology. 2014;63(25, Part A):2769–2778. [DOI] [PubMed] [Google Scholar]
- 13.Voicu S, Henry P, Malissin I, et al. Improving cannulation time for extracorporeal life support in refractory cardiac arrest of presumed cardiac cause - Comparison of two percutaneous cannulation techniques in the catheterization laboratory in a center without on-site cardiovascular surgery. Resuscitation. 2018;122:69–75. [DOI] [PubMed] [Google Scholar]
- *14.Yannopoulos D, Bartos JA, Raveendran G, et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. Journal of the American College of Cardiology. 2017;70(9):1109–1117. [DOI] [PubMed] [Google Scholar]; This study describes the prominent role of coronary artery disease in the pathogenesis of refractory OHCA.
- 15.Grunau B, Hornby L, Singal RK, et al. Extracorporeal Cardiopulmonary Resuscitation for Refractory Out-of-Hospital Cardiac Arrest: The State of the Evidence and Framework for Application. The Canadian journal of cardiology. 2018;34(2):146–155. [DOI] [PubMed] [Google Scholar]
- 16.Richardson AS, Schmidt M, Bailey M, Pellegrino VA, Rycus PT, Pilcher DV. ECMO Cardio-Pulmonary Resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation. 2017;112:34–40. [DOI] [PubMed] [Google Scholar]
- *17.Bougouin W, Dumas F, Lamhaut L, et al. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. European heart journal. 2019. [DOI] [PubMed] [Google Scholar]; This study details real-world usage patterns of ECPR in the European population.
- 18.Lunz D, Calabrò L, Belliato M, et al. Extracorporeal membrane oxygenation for refractory cardiac arrest: a retrospective multicenter study. Intensive Care Medicine. 2020. [DOI] [PubMed] [Google Scholar]
- *19.Yannopoulos D, Bartos JA, Martin C, et al. Minnesota Resuscitation Consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5(6):e003732. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the ECPR network of the Minnesota Resuscitation Consortium.
- 20.Sakamoto T, Morimura N, Nagao K, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85(6):762–768. [DOI] [PubMed] [Google Scholar]
- 21.Johnson NJ, Acker M, Hsu CH, et al. Extracorporeal life support as rescue strategy for out-of-hospital and emergency department cardiac arrest. Resuscitation. 2014;85(11):1527–1532. [DOI] [PubMed] [Google Scholar]
- 22.Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88–94. [DOI] [PubMed] [Google Scholar]
- 23.Wang CH, Chou NK, Becker LB, et al. Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest--a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation. 2014;85(9):1219–1224. [DOI] [PubMed] [Google Scholar]
- 24.Bol ME, Suverein MM, Lorusso R, et al. Early initiation of extracorporeal life support in refractory out-of-hospital cardiac arrest: Design and rationale of the INCEPTION trial. American heart journal. 2019;210:58–68. [DOI] [PubMed] [Google Scholar]
- 25.ClinicalTrials.gov. Advanced Reperfusion Strategies for Refractory Cardiac Arrest (ARREST). 2019; https://clinicaltrials.gov/ct2/show/NCT03880565. Accessed November 21, 2019.
- 26.ClinicalTrials.gov. ECPR for Refractory Out-Of-Hospital Cardiac Arrest (EROCA). 2017; https://clinicaltrials.gov/ct2/show/NCT03065647. Accessed January 4, 2020.
- 27.ClinicalTrials.gov. Pre-hospital ECMO in Advanced Resuscitation in Patients With Refractory Cardiac Arrest. (SUB30). 2019; https://clinicaltrials.gov/ct2/show/NCT03700125. Accessed January 4, 2020.
- *28.Garcia S, Drexel T, Bekwelem, et al. Early access to the cardiac catheterization laboratory for patients resuscitated from cardiac arrest due to a shockable rhythm: The Minnesota Resuscitation Consortium Twin Cities Unified Protocol. J Am Heart Assoc. 2016;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the ECPR pathway in the Minnesota Resuscitation Consortium.
- *29.Bartos JA, Grunau B, Carlson C, et al. Improved Survival with Extracorporeal Cardiopulmonary Resuscitation Despite Progressive Metabolic Derangement Associated with Prolonged Resuscitation. Circulation. 2020;0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes how the onset of metabolic derangements are associated with the time to VA-ECMO initiation in VA-ECMO patients.
- 30.Abrams D, Garan AR, Abdelbary A, et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Medicine. 2018;44(6):717–729. [DOI] [PubMed] [Google Scholar]
- 31.Majunke N, Mangner N, Linke A, et al. Comparison of Percutaneous Closure Versus Surgical Femoral Cutdown for Decannulation of Large-Sized Arterial and Venous Access Sites in Adults After Successful Weaning of Veno-Arterial Extracorporeal Membrane Oxygenation. The Journal of invasive cardiology. 2016;28(10):415–419. [PubMed] [Google Scholar]
- 32.Bisdas T, Beutel G, Warnecke G, et al. Vascular Complications in Patients Undergoing Femoral Cannulation for Extracorporeal Membrane Oxygenation Support. The Annals of Thoracic Surgery. 2011;92(2):626–631. [DOI] [PubMed] [Google Scholar]
- 33.Rupprecht L, Lunz D, Philipp A, Lubnow M, Schmid C. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel. 2015;7(4):320–326. [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka D, Hirose H, Cavarocchi N, Entwistle JW. The Impact of Vascular Complications on Survival of Patients on Venoarterial Extracorporeal Membrane Oxygenation. Ann Thorac Surg. 2016;101(5):1729–1734. [DOI] [PubMed] [Google Scholar]
- 35.Lamb KM, DiMuzio PJ, Johnson A, et al. Arterial protocol including prophylactic distal perfusion catheter decreases limb ischemia complications in patients undergoing extracorporeal membrane oxygenation. Journal of Vascular Surgery. 2017;65(4):1074–1079. [DOI] [PubMed] [Google Scholar]
- 36.Patton-Rivera K, Beck J, Fung K, et al. Using near-infrared reflectance spectroscopy (NIRS) to assess distal-limb perfusion on venoarterial (V-A) extracorporeal membrane oxygenation (ECMO) patients with femoral cannulation. Perfusion. 2018;33(8):618–623. [DOI] [PubMed] [Google Scholar]
- 37.Ruggeri L, Franco A, Alba AC, et al. Coagulation Derangements in Patients With Refractory Cardiac Arrest Treated With Extracorporeal Cardiopulmonary Resuscitation. Journal of cardiothoracic and vascular anesthesia. 2019;33(7):1877–1882. [DOI] [PubMed] [Google Scholar]
- *38.Bartos JA, Carlson K, Carlson C, et al. Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: Critical care and extracorporeal membrane oxygenation management. Resuscitation. 2018;132:47–55. [DOI] [PubMed] [Google Scholar]; This study describes intensive care considerations for ECPR patients.
- 39.Murphy DA, Hockings LE, Andrews RK, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfusion medicine reviews. 2015;29(2):90–101. [DOI] [PubMed] [Google Scholar]
- 40.Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circulation Heart failure. 2018;11(9):e004905. [DOI] [PubMed] [Google Scholar]
- 41.Dalia AA, Ortoleva J, Fiedler A, Villavicencio M, Shelton K, Cudemus GD. Extracorporeal Membrane Oxygenation Is a Team Sport: Institutional Survival Benefits of a Formalized ECMO Team. Journal of cardiothoracic and vascular anesthesia. 2019;33(4):902–907. [DOI] [PubMed] [Google Scholar]
- 42.Haas NL, Coute RA, Hsu CH, Cranford JA, Neumar RW. Descriptive analysis of extracorporeal cardiopulmonary resuscitation following out-of-hospital cardiac arrest-An ELSO registry study. Resuscitation. 2017;119:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


