Abstract
Objectives:
We sought to describe the presentation, course, and outcomes of hospitalized pediatric coronavirus disease 2019 patients, with detailed description of those requiring mechanical ventilation, and comparisons between critically ill and noncritical hospitalized pediatric patients.
Design:
Observational cohort study.
Setting:
Riley Hospital for Children at Indiana University Health in Indianapolis in the early weeks of the coronavirus disease 2019 pandemic.
Patients:
All hospitalized pediatric patients with confirmed coronavirus disease 2019 as of May 4, 2020, were included.
Interventions:
Patients received therapies including hydroxychloroquine, remdesivir, tocilizumab, and convalescent serum and were managed according to an institutional algorithm based on evidence available at the time of presentation.
Measurements and Main Results:
Of 407 children tested for severe acute respiratory syndrome-coronavirus 2 at our hospital, 24 were positive, and 19 required hospitalization. Seven (36.8%) were critically ill in ICU, and four (21%) required mechanical ventilation. Hospitalized children were predominantly male (14, 74%) and African-American or Hispanic (14, 74%), with a bimodal distribution of ages among young children less than or equal to 2 years old (8, 42%) and older adolescents ages 15–18 (6, 32%). Five of seven (71.4%) of critically ill patients were African-American (n = 3) or Hispanic (n = 2). Critical illness was associated with older age (p = 0.017), longer duration of symptoms (p = 0.036), and lower oxygen saturation on presentation (p = 0.016); with more thrombocytopenia (p = 0.015); higher C-reactive protein (p = 0.031); and lower WBC count (p = 0.039). Duration of mechanical ventilation averaged 14.1 days. One patient died.
Conclusions:
Severe, protracted coronavirus disease 2019 is seen in pediatric patients, including those without significant comorbidities. We observed a greater proportion of hospitalized children requiring mechanical ventilation than has been reported to date. Older children, African-American or Hispanic children, and males may be at risk for severe coronavirus disease 2019 requiring hospitalization. Hypoxia, thrombocytopenia, and elevated C-reactive protein may be useful markers of critical illness. Data regarding optimal management and therapies for pediatric coronavirus disease 2019 are urgently needed.
Keywords: adolescents, coronavirus disease 2019, critical care, health disparities, pediatrics, severe acute respiratory syndrome coronavirus 2
While children are at lower risk for severe disease, they can experience morbidity and mortality due to coronavirus disease 2019 (COVID-19) (1). Models project between 3.7 and 37 million children could develop COVID-19 by the end of 2020 (2). There are limited pediatric clinical data. The objective of this observational study is to describe the demographics, clinical presentation, and outcomes to date of hospitalized pediatric COVID-19 patients in the earliest weeks of the pandemic. The hospital courses of those mechanically ventilated are described in detail. We then sought to compare demographics and clinical presentation of critically ill children to children who were hospitalized but not critically ill.
MATERIALS AND METHODS
This report includes all patients with confirmed COVID-19 hospitalized at Riley Hospital for Children at Indiana University Health in Indianapolis, as of May 4, 2020. Testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Indiana began February 26, and the first confirmed case was March 6 (3). Critical illness was defined as requiring admission to the PICU. All COVID-19 patients had an infectious disease consultation. Institutional treatment guidelines at the time considered hydroxychloroquine, and remdesivir if available via compassionate use. Tocilizumab was available for patients with cytokine storm. Convalescent serum was available for some. Standardized laboratories were obtained. Oxygenation saturation index (OSI) was calculated with the following formula (4): (mean airway pressure × Fio2 × 100) ÷ peripheral oxygen saturation. Data were abstracted from medical records after the study was deemed exempt from review by the Institutional Review Board at Indiana University.
Statistical analyses included Fisher exact test for categorical variables, Mann-Whitney U test for continuous nonparametric variables, and t test for continuous normally distributed variables. p value of less than 0.05 was considered significant. Analysis was completed using StataSE 14.2 (StataCorp, College Station, TX).
RESULTS
At the time of writing, 24 out of 407 children tested positive for SARS-CoV-2 at our hospital. Nineteen pediatric COVID-19 patients required hospitalization. Seven were critically ill, with four requiring intubation (Fig. 1). Demographics, comorbidities, and presenting symptoms stratified by critical illness are presented (Table 1). Comorbidities were present in two intubated patients: one had cerebral palsy and restrictive lung disease and the other had an elevated body mass index and new-onset diabetic ketoacidosis.
Figure 1.
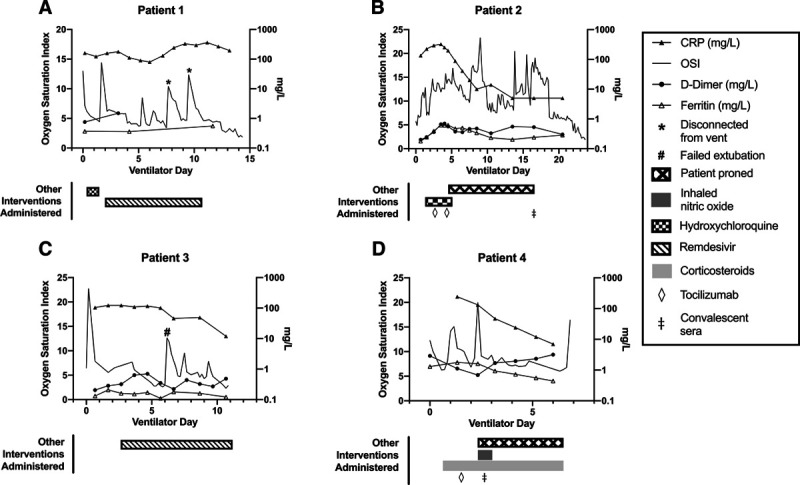
PICU course for mechanically ventilated pediatric coronavirus disease 2019 patients. Summary of additional details specific to intubated patients: A, Adolescent African-American male with no past medical history. Required mechanical ventilation for 15 d and hospitalized for a total of 20 d. Initial interleukin (IL)-6 level 9 pg/mL (normal < 5 pg/mL). B, Adolescent white male with history of cerebral palsy and restrictive lung disease. Required mechanical ventilation for 24 d and hospitalized for a total of 36 d. Initial IL-6 level 32 pg/mL. C, Infant African-American male with no past medical history. Required mechanical ventilation for 11 d and hospitalized for a total of 20 d. Initial IL-6 level 11 pg/mL. D, Adolescent African-American male with elevated body mass index presenting with new-onset diabetic ketoacidosis. Required mechanical ventilation for 7 d and hospitalized for a total of 11 d. Initial IL-6 level less than 5 pg/mL. This patient died after sudden cardiac arrest. CRP = C-reactive protein, OSI = oxygenation saturation index.
Table 1.
Demographic, Clinical and Laboratory Characteristics, and Clinical Course for Hospitalized Patients to Date
| Characteristics | Critically Ill, n = 7 | General Ward, n = 12 | Total, n = 19 | p |
|---|---|---|---|---|
| Demographics and clinical characteristics on presentation, n (%) | ||||
| Age, yr, median (IQR) | 16 (7–17) | 1.8 (0.4–5) | 5 (0.8–16) | 0.017 |
| ≤ 2 | 1 (14) | 7 (58) | 8 (42) | |
| 3–14 | 2 (29) | 3 (25) | 5 (26) | |
| 15–18 | 4 (57) | 2 (17) | 6 (32) | |
| Male sex | 7 (100) | 7 (58) | 14 (74) | 0.11 |
| Race/ethnicity | 0.75 | |||
| White | 2 (29) | 2 (17) | 4 (21) | |
| African-American | 3 (43) | 4 (33) | 7 (37) | |
| Hispanic | 2 (29) | 5 (42) | 7 (37) | |
| Asian | 0 (0) | 1 (8) | 1 (5) | |
| Racial/ethnic minority | 5 (71) | 10 (83) | 15 (79) | 0.60 |
| Comorbiditiesa | 3 (43) | 5 (42) | 8 (42) | 0.999 |
| Presenting symptoms | ||||
| Fever | 5 (71) | 7 (58) | 12 (63) | 0.66 |
| Dyspnea | 5 (71) | 7 (58) | 12 (63) | 0.66 |
| Chest pain | 2 (29) | 2 (17) | 4 (21) | 0.60 |
| Cough | 3 (43) | 2 (17) | 5 (26) | 0.27 |
| Emesis | 1 (14) | 2 (17) | 2 (16) | 0.999 |
| Sore throat | 1 (14) | 1 (8) | 2 (11) | 0.999 |
| Dysgeusia and/or anosmia | 2 (29) | 1 (8) | 3 (16) | 0.52 |
| Days of symptoms before presentation, median (IQR) | 4 (3–14) | 1 (0.5–3.5) | 3 (1–4) | 0.036 |
| Peripheral oxygen saturation on room air within 24 hr of time of SARS-CoV-2 testing, median (IQR) | 86% (78–96) | 98% (95.5–99) | 96% (88–99) | 0.016 |
| Critically Illbn = 6 | General Wardn = 8 | Totalbn = 14 | p | |
| Initial laboratories and imaging results within 24 hr of time of SARS-CoV-2 testing, median (IQR) | ||||
| WBC (K/cumm) | 5.7 (4.7–7.1) | 8.5 (7.3–12.1) | 7.4 (5.8–9.6) | 0.039 |
| Platelets (K/cumm) | 133 (94–224) | 339 (216–356) | 246 (137–339) | 0.014 |
| Thrombocytopenia, n (%) | 4 (66) | 0 (0) | 4 (29) | 0.015 |
| n = 6 | n = 7 | n = 13 | ||
| ANC | 4 (3.1–4.8) | 4.9 (4.5–11.9) | 4.7 (3.9–5.2) | 0.15 |
| Neutropenia by ANC, n (%) | 0 (0) | 3 (43) | 3 (23) | 0.12 |
| ALC | 0.75 (0.4–1.2) | 1.7 (1.5–2.7) | 1.5 (0.8–2.4) | 0.085 |
| Lymphopenia by ALC, n (%) | 3 (50) | 1 (14) | 4 (29) | 0.27 |
| C-reactive protein (mg/dL) | 11.5 (8.1–16) | 3.6 (0.5–6) | 5.7 (1.1–9.7) | 0.031 |
| Ferritin (ng/mL) | 193 (142–367) | 219 (36–327) | 205 (139–327) | 0.57 |
| Characteristics | Critically Illb, n = 6 | General Ward, n = 6 | Total, n = 12 | p |
| Lactate dehydrogenase (U/L) | 329 (247–432) | 359 (275–472) | 353 (261–452) | 0.63 |
| d-dimer (ng/mL) | 212 (200–755) | 278 (200–390) | 212 (200–418) | 0.80 |
| n = 6 | n = 9 | n = 15 | ||
| Presence of coinfection by respiratory viral panelc, n (%) | 3 (50) | 2 (22) | 5 (33) | 0.33 |
| Abnormal chest radiograph within 24 hr of SARS-CoV-2 testingd, n (%) | 6 (100) | 5 (56) | 11 (73) | 0.10 |
ALC = absolute lymphocyte count, ANC = absolute neutrophil count, IQR = interquartile range, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Presence of clinical comorbidities such as asthma (n = 3), obesity (n = 2), hematological disorder (n = 2), and restrictive lung disease (n = 1).
Patient with new diagnosis of T-cell ALL in blast crisis excluded from laboratory, radiological, and hospital course analysis.
Presence of respiratory coinfections by respiratory viral panel polymerase chain reaction testing: rhino/enterovirus (n = 2), coronavirus NL63 (n = 1), respiratory syncytial virus (n = 1), and parainfluenza (n = 1).
Presence of chest radiograph findings such as opacities with bilateral opacities (n = 7) or unilateral opacities (n = 4).
Boldface entries are those with a statistical significance (p < 0.05).
As compared to other hospitalized patients with pediatric COVID-19, those who developed critical illness had longer duration of symptoms (p = 0.036) and significantly lower oxygen saturation on room air (p = 0.016) on presentation. The critically ill also had significantly more thrombocytopenia (p = 0.015), and higher C-reactive protein (CRP) (p = 0.031). WBC was significantly lower in critically ill patients (p = 0.039), although interquartile ranges (IQRs) were within normal range for both groups. Five patients had coinfections with other respiratory viruses, including rhinovirus/enterovirus (n = 2), human coronavirus NL63 (n = 1), respiratory syncytial virus (n = 1), and parainfluenza virus (n = 1). These included three critically ill (3/6, 50%) and two noncritical patients (2/9, 22%; p = 0.33). All critically ill patients had abnormal chest radiograph findings compared with 56% of noncritical patients; however, there was no statistical significance.
Of the critically ill, three patients were treated with high flow nasal cannula and had an uneventful PICU course. Four patients required intubation (Fig. 1), with three intubated within 24 hours of presentation due to hypoxia. The fourth presented in new-onset diabetic ketoacidosis/hyperosmolar hyperglycemia. He initially tested negative for SARS-CoV-2, was intubated on hospital day 4, and tested positive for SARS-CoV-2 at that time. Duration of mechanical ventilation averaged 14.1 days. All critically ill patients were initially treated with broad-spectrum antibiotics until diagnosis of COVID-19 was confirmed. Cultures from blood and other specimens were obtained at admission to rule out bacterial infection as appropriate.
Therapies, including pharmacologic and supportive care, are outlined in Figure 1. Remdesivir was considered for all mechanically ventilated patients; two received it. One patient did not qualify, and the other was not given remdesivir due to acute kidney injury and mild rhabdomyolysis from hyperosmolar hyperglycemia.
These critically ill patients had complicated courses. One patient, treated with remdesivir, developed transaminitis (alanine transaminase 290 U/L and aspartate transaminase 1,271 U/L) and significant rhabdomyolysis (creatine kinase 55,360 U/L) with peak values the day after completing remdesivir. Two patients developed secondary bacterial infections. One patient developed increased hypoxia and hypothermia after 14 days of ventilation; respiratory culture demonstrated Pseudomonas aeruginosa. He was treated with cefepime and nebulized tobramycin. Another patient failed extubation after 8 days of ventilation; respiratory culture demonstrated Haemophilus influenzae and Moraxella catarrhalis. He was treated with amoxicillin-clavulanate. One patient died after sudden cardiac arrest. His CRP and ferritin improved after receiving tocilizumab and convalescent sera, however, on day 6 of mechanical ventilation (hospital day 11), he developed a nonperfusing junctional rhythm, then ventricular tachycardia, and was unable to be resuscitated. Cause of death remains unclear and a postmortem examination was not performed.
DISCUSSION
We describe characteristics, critical care courses, and comparisons among hospitalized pediatric COVID-19 patients, including seven with critical illness, during the early weeks of the pandemic at a U.S. children’s hospital. These initial findings deserve further exploration in larger studies. We noted severe COVID-19 disease for some patients, with a greater proportion of hospitalized children requiring mechanical ventilation (21.5%) than has been reported to date. These included patients without comorbidities. We also experienced a pediatric death related to COVID-19.
At the time of diagnosis with SARS-CoV-2, critically ill patients had symptoms for a longer period of time and were significantly more hypoxic, thrombocytopenic, and inflamed. Per international reports, mechanical ventilation for children with COVID-19 is rare with only one in the Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) study (1%), two in Lombardy, Italy (0.1%), and 13 out of 2,141 in China (5–7). U.S. hospitals have reported lower proportions of intubated critically ill pediatric COVID-19 patients and fewer ventilated days (8, 9). Thrombocytopenia was associated with critical illness and has been described as an early finding in patients with COVID-19 (10). Thrombocytopenia is suspected to be multifactorial and could be due to cytokine storm or direct effects of SARS-CoV-2 on bone marrow, host immunity, or lung parenchyma (11). Consistent with a Chinese study, CRP was elevated in 10 of 13 children (12), and in our cohort, higher CRP was associated with critical illness. WBC was lower in critically ill patients, although the IQRs for both groups were within normal. Our center appears to have a greater proportion of pediatric cases requiring mechanical ventilation than is reported elsewhere (5–9). Further research is needed to understand factors associated with critical illness and mechanical ventilation for pediatric COVID-19.
We note that the clinical and laboratory findings in critically ill children with COVID-19 in this study (decreased oxygen saturation, thrombocytopenia, and elevated CRP) are also seen in children with bacterial sepsis (13, 14). Thus, the present study does not provide clinical findings or biomarkers that might distinguish critical COVID-19 from sepsis. Typical management for these critical cases includes broad-spectrum antimicrobials while SARS-CoV-2 testing and bacterial cultures are pending.
The CRP and OSI of the four intubated patients improved with various therapies. The most striking improvements were noted in patients 2 and 4 following tocilizumab, convalescent sera, and prone positioning. The OSI for patients 1 and 3 did improve after starting remdesivir, although CRP did not. This difference, of unclear clinical significance, could be due to the tocilizumab targeting the IL-6 receptor and causing mitigation of the cytokine cascade, while remdesivir and convalescent plasma target SARS-CoV-2. With a small number of ventilated patients and three of four having received a combination of therapies, it is challenging to investigate the role of any single therapy.
The death of patient 4 occurred unexpectedly. His CRP and ferritin improved after receiving tocilizumab and convalescent sera, however, his d-dimer gradually rose compared with the other three patients. Although there has been a concern for thrombotic events in patients with COVID-19, this patient was on anticoagulation at the time with a therapeutic anti-Xa of 0.5 international units/mL.
Certain demographics appear to be associated with critical illness in pediatric COVID-19. Although there was one critically ill infant in our cohort, older children appear more likely to have critical illness. Most patients in our report were male (73.7%), and all critically ill patients were male. A slight male predominance among children with COVID-19 has been reported out of Italy (57%, CONFIDENCE study), the United States (52–57%), and China (56.6%) (6–9, 15). By contrast, eight of 10 infants with COVID-19 were female in another Chinese report (16). Larger pediatric studies could better define whether risk of severe COVID-19 in the United States is increased in male children.
Limited data currently exist on racial/ethnic disparities among hospitalized pediatric COVID-19 patients. In this report, 74% of hospitalized pediatric COVID-19 patients and five of seven critically ill children (71%) were African-American or Hispanic. Indiana’s population is 85.1% Caucasian, 9.8% African-American, and 7.1% Hispanic (17). Thus, both moderate and severe COVID-19 disproportionately affected African-American and Hispanic children. Given the few significant comorbidities in our report, disparities may point to greater risks for acquiring SARS-CoV-2 among African-American and Hispanic children. Structural racism underlies wide health inequities in the United States (18) and must be addressed in the COVID-19 pandemic, including impacts of the pandemic on children of color.
Limitations of this report include the small number of patients, which limits the conclusions that may be drawn. Additionally, some mild cases did not have laboratories completed due to a combination of an average 12-hour turnaround in SARS-CoV-2 testing and rapid clinical improvement, leading to same-day discharges from the hospital. We note, however, key observations that may inform other pediatric centers and should prompt investigation in larger studies. Although these data come from review of medical records, an institutional algorithm and infectious disease consultation on each case supported consistent documentation.
CONCLUSIONS
Severe, protracted COVID-19 disease is seen in pediatric patients, including those without significant comorbidities. Older children, African-American or Hispanic children, and males may be at risk for severe COVID-19 requiring hospitalization. Hypoxia, thrombocytopenia, and elevated CRP may be useful markers of critical illness. Larger studies are needed to examine associations with severe COVID-19 in pediatrics. Data regarding optimal management and therapies for pediatric COVID-19 are urgently needed.
ACKNOWLEDGMENTS
We acknowledge the physicians, nurses, and staff caring for these and all patients with coronavirus disease 2019, as well as the families and communities coping with the severe impacts of this disease.
Footnotes
*See also p. 921.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (grant numbers K23 HD095778, T32 HD069047); and by the National Institute of Allergy and Infectious at the National Institutes of Health Diseases (grant number T32 AI07637).
Drs. Bhumbra, Malin, Khaitan, John, Rowan, and Enane disclosed off-label use of remdesivir, convalescent plasma, hydroxychloroquine, and tocilizumab. Drs. Kirkpatrick and Enane are supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (grant numbers K23 HD095778, T32 HD069047). Dr. Bhumbra is supported by the National Institute of Allergy and Infectious at the National Institutes of Health Diseases (grant number T32 AI07637).
REFERENCES
- 1.CDC Covid Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020. 69:343–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pathak EB, Salemi JL, Sobers N, et al. COVID-19 in children in the United States: Intensive care admissions, estimated total infected, and projected numbers of severe pediatric cases in 2020. J Public Health Manag Pract. 2020. 26:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Indiana State Department of Health. Novel Coronavirus (COVID-19). 20192020. Updated May 5, 2020. Available at: https://coronavirus.in.gov/. Accessed May 5, 2020
- 4.Muniraman HK, Song AY, Ramanathan R, et al. Evaluation of oxygen saturation index compared with oxygenation index in neonates with hypoxemic respiratory failure. JAMA Netw Open. 2019. 2:e191179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020. 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parri N, Lenge M, Buonsenso D. Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group: Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020. doi: 10.1542/peds.2020-0702 [Google Scholar]
- 8.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. ; International COVID-19 PICU Collaborative. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBiasi RL, Song X, Delaney M, et al. Severe COVID-19 in children and young adults in the Washington, DC metropolitan region. J Pediatr. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed MZ, Khakwani M, Venkatadasari I, et al. Thrombocytopenia as an initial manifestation of COVID-19; case series and literature review. Br J Haematol. 2020. 189:1057–1058 [DOI] [PubMed] [Google Scholar]
- 11.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020. 99:1205–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: Clinical and epidemiological features. Clin Infect Dis. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prusakowski MK, Chen AP. Pediatric sepsis. Emerg Med Clin North Am. 2017. 35:123–138 [DOI] [PubMed] [Google Scholar]
- 14.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005. 6:2–8 [DOI] [PubMed] [Google Scholar]
- 15.CDC Covid Response Team. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020. 69:422–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei M, Yuan J, Liu Y, et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020. 323:1313–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United States Census Bureau QuickFacts Indiana. 2020 Available at: https://www.census.gov/quickfacts/IN?. Accessed April 17, 2020.
- 18.Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: Evidence and interventions. Lancet. 2017. 389:1453–1463 [DOI] [PubMed] [Google Scholar]


