Abstract
Objective:
To compare the diagnostic and prognostic performance of native T1 mapping (T1), extracellular volume (ECV) mapping, and late gadolinium enhancement (LGE) imaging for evaluating cardiac amyloidosis (CA).
Background:
CA is a progressive infiltrative process in the extracellular space that is often underdiagnosed and holds a poor prognosis. Cardiac magnetic resonance (CMR) offers novel techniques for detecting and quantifying the disease burden of CA.
Methods:
We searched PubMed for published studies using native T1, ECV, or LGE to diagnose and prognosticate CA. A total of 18 diagnostic (2015 subjects) and 13 prognostic studies (1483 subjects) were included for analysis. Pooled sensitivities, specificities, diagnostic odds ratios (DOR) of all diagnostic tests were assessed by bivariate analysis. Pooled hazard ratios (HR) for mortality for the three techniques were determined.
Results:
Bivariate comparison showed that ECV (DOR: 84.6; 95% confidence interval [CI]: 30.3 to 236.2) had a significantly higher DOR for CA than LGE (DOR: 20.1; 95% CI: 9.1 to 44.1, p = 0.03 vs ECV). There was no significant difference between LGE and native T1 for sensitivity, specificity, and DOR. HR was significantly higher for ECV (HR: 4.27; 95% CI: 2.87 to 6.37) compared to LGE (HR: 2.60; 95% CI: 1.90 to 3.56; p = 0.03 vs ECV) and native T1 (HR: 2.04; 95% CI: 1.24 to 3.37; p = 0.01 vs ECV).
Conclusions:
ECV demonstrates a higher diagnostic odds ratio for assessing cardiac amyloid than LGE and a higher hazard ratio for adverse events as compared to LGE and native T1. Additionally, native T1 showed similar sensitivity and specificity as ECV and LGE without requiring contrast. Though limited by study heterogeneity, this meta-analysis suggests that ECV provides high diagnostic and prognostic utility for the assessment of cardiac amyloidosis.
Keywords: Cardiac, amyloid, magnetic resonance, mapping, native T1, extracellular volume, late gadolinium enhancement
Introduction
Cardiac amyloidosis (CA) is an infiltrative disease in which fibrillary proteins such as immunoglobulin light chains and transthyretin are deposited in the extracellular space and lead to eventual heart failure (1). The two most common types of CA are light chain amyloidosis (AL) and transthyretin amyloidosis (ATTR). Despite the major differences in precursor proteins, patient demographics, and clinical course, both types of CA benefit from early diagnosis and prognostication in order to initiate treatment and slow disease progression (1).
The method for detection and characterization of CA include the gold standard of endomyocardial biopsy (EMB), serum protein electrophoresis and urine protein electrophoresis for characterizing light-chains, and 99m technetium (Tc)-labeled 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD), 99mTc-labeled pyrophosphate (PYP), or 99mTc-labeled hydroxymethylene diphosphonate (HMDP) for the diagnosis of ATTR amyloid. A multi-society guideline published in 2019 also recommends the use of advanced imaging in the diagnosis of CA (2). Cardiac magnetic resonance (CMR) with gadolinium has gained clinical acceptance for evaluating CA due to its high spatial resolution and ability to identify pathology in the extracellular space. Specifically, late gadolinium enhancement (LGE) demonstrates excellent diagnostic accuracy when compared to EMB and is an independent predictor of mortality (3). More recently, novel imaging techniques such as extracellular volume (ECV) and native T1 mapping (T1) have been suggested as alternative modalities for providing incremental diagnostic and prognostic information (4). With the development of new targeted therapies such as tafamidis, non-invasive imaging has garnered significant interest for early risk stratification and treatment response monitoring (1).
In this study, we conducted a meta-analysis to compare the diagnostic and prognostic performance of LGE, ECV and T1. Our goal is to understand the role of ECV and T1 in CA evaluation.
Methods
Search Strategy and Selection
This meta-analysis was conducted according to standard guidelines from the Meta-analysis of Observational Studies in Epidemiology (5), the Preferred Reporting Items for Systematic Reviews and Meta-analyses documents (6), and the Methodological Standards for Meta-Analyses and Qualitative Systematic Reviews of Cardiac Prevention and Treatment Studies (7). We performed a systematic search for published studies evaluating LGE, native T1 mapping, and ECV diagnostic and prognostic performance for cardiac amyloidosis using PubMed (search last updated August 2019).
Key words used were (“cardiac” OR “cardiomyopathy”) AND (“amyloid” OR “AL” OR “ATTR” OR “amyloidosis” OR “TTR”) AND (“MRI” OR “CMR” OR “MR” OR “T1” OR “ECV” OR “LGE”). Abstracts were independently reviewed and selected by two investigators (JAP, MJK) based on the following eligibility criteria: a) pertaining to LGE, native T1 mapping, or ECV, b) investigating diagnosis or prognosis of cardiac amyloidosis in human adults, c) complete analytic study in English.
Cardiac amyloidosis was suspected or diagnosed based on a confirmed history of systemic amyloidosis and evidence of cardiac involvement by clinical history, biomarkers, electrocardiogram (ECG), echocardiography (Echo), CMR, nuclear imaging such as scintigraphy, or EMB. Only complete analytic studies published in peer-reviewed journals were included. Abstracts from meetings, case reports, editorials, and reviews were excluded.
Data Extraction
Data from each study was extracted by two investigators (JAP, MJK). Studies were excluded if they contained: a) complete overlap of subjects with other studies, b) incomplete data, c) unconventional methods. Any uncertainties regarding a study were resolved by consultation with the senior reviewer (MS). In the case of partially overlapping studies, non-overlapping subgroups could be included in the analysis. Complete data consisted of sufficient information to determine the sensitivity and specificity for diagnosis of CA or unadjusted hazard ratio (HR) for mortality from CA. The reference test for diagnosis of CA could be based on either clinical criteria, ECG, Echo, nuclear imaging, or EMB. CMR with LGE could be included as additional diagnostic criteria if combined with another reference test for the purpose of analyzing a non-LGE CMR parameter. Qualitative and quantitative parametric tests were considered appropriate if they included a criteria or cut-off for a positive result, respectively.
Study Quality
The quality of included studies was assessed by two investigators (JAP, MSK) using the Revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) instrument (8) for diagnostic studies and Quality in Prognostic Factor Studies (QUIPS) tool (9). Publication bias was assessed by visual analysis of funnel plots and using the Peter’s and Egger’s methods (10,11).
Statistical Analysis
Dichotomous variables are presented as percentages and continuous variables as mean ± standard deviation or median [interquartile range]. Pooled values are presented as point estimates [95% confidence interval].
Both univariate and bivariate pooling was performed for diagnostic studies. Univariate method was performed using the “meta” package in R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). Pooled estimates of sensitivity, specificity, positive predictive value, negative predictive value, likelihood ratios and diagnostic odds ratio (DOR) were pooled using a random-effects model with the DerSimonanian-Laird method based on inverse variance weighting (12). Individual estimates of 95% confidence intervals were calculated with the Clopper-Pearson interval exact method for binomial confidence intervals (13). Bivariate analysis of pooled sensitivity, specificity, and DOR estimates between the diagnostic techniques was performed as described by Reitsma et al (14) and Van Houwelingen et al (15) using SAS/STAT software, version 9.4, of the SAS System for Windows (SAS Institute Inc., Cary, North Carolina).
Unadjusted HR were also univariately pooled using the random-effects model with the DerSimonanian-Laird method based on inverse variance weighting (12). HR for studies with continuous variables (i.e. ECV and T1) that reported the HR as incremental change per unit increase were converted to a binary HR and standard deviation based on high and low CA burden groups as pre-specified by the studies. Statistical significance for hypothesis testing was set at α < 0.05, 2-tailed level.
Heterogeneity between studies was assessed visually from Forest plots of the individual parameters and using the Cochran’s Q index and the inconsistency index (I2). Significant statistical heterogeneity was defined based on having both a significant Cochran’s Q (p < 0.05) and I2 > 50%. Meta-regression and sensitivity analyses (including exclusion of 1 study at a time) were conducted to explore heterogeneity. Subgroup analysis was performed for: 1) studies that included EMB as the reference test, 2.) studies that excluded CMR as the reference test, 3.) studies that excluded clinical criteria or Echo as the reference test, 4.) studies that only included AL CA.
Results
Search Results
Our literature search identified 1323 relevant abstracts; of these, 24 abstracts for diagnostic studies and 25 abstracts for prognostic studies were considered eligible for data extraction. Six diagnostic and 12 prognostic studies were excluded. Figure 1 shows the summary of our literature search. A total of 18 diagnostic and 13 prognostic studies were included for analysis (Table 1 & 2). Additional information regarding study design can be found in Supplemental Table 1 & 2.
Figure 1. Flow Diagram of Review and Selection Process.
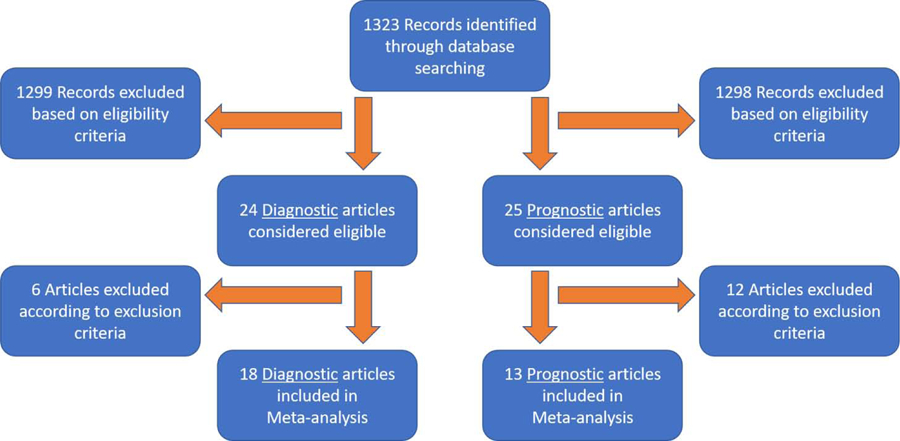
Overview of study review process based on eligibility and exclusion criteria.
Table 1.
Diagnostic Study Characteristics
| Author Year | Cardiac Amyloid (n) | Control (n) | Amyloid Type | Control Type | Cardiac Validation | Modality | LGE Criteria | T1 Cutoff (ms) | ECV Cutoff |
|---|---|---|---|---|---|---|---|---|---|
| Baggiano1 2019 | 441 | 427 | AL, ATTR | Non-CA | Biopsy, CMR, Nuclear | T1 ECV | - | 1091 | 0.370 |
| Nam2 2018 | 46 | 30 | AL | HCM, Healthy | Biopsy | T1, ECV | - | 1131 | 0.401 |
| Martinez-Naharro3 2018 | 215 | 56 | ATTR | Non-CA | Biopsy, Echo, Nuclear | T1, ECV | - | 1048 | 0.469 |
| Pandey4 2017 | 28 | 35 | - | Healthy | Biopsy | LGE | Diffuse | - | - |
| Gallego-Delgado5 2016 | 5 | 26 | ATTR | Non-CA | CMR, Nuclear | ECV | - | - | 0.357 |
| Bhatti6 2016 | 22 | 20 | AL | Non-PCD | Biopsy | LGE | Typical | - | - |
| Barison7 2014 | 36 | 30 | AL, ATTR | Suspected Amyloid | Echo | ECV | - | - | 0.316 |
| Aquaro8 2014 | 52 (30)* | 27 | AL | Healthy, Suspected CA | Biopsy, Echo, EKG | LGE, ECV | Typical | - | 0.300 |
| White9 2014 | 15 | 10 | AL, AA, ATTR | Non-CA | Biopsy | LGE | Diffuse | - | - |
| Takeda10 2013 | 6 | 20 | AL | HCM, HHD | Biopsy, Clinical | LGE | Diffuse | - | - |
| Karamitsos11 2013 | 39 | 67 | AL | Healthy, AS, Non-CA | Clinical, Echo | LGE, T1 | Presence | 1020 | - |
| Dickerson12 2012 | 6 | 13 | - | RCM | Biopsy | LGE | Typical | - | - |
| Wassmuth13 2011 | 31 | 48 | AL, AA, ATTR | Healthy | Biopsy, Echo, EKG | LGE | Presence | - | - |
| Syed14 2010 | 84 | 36 | AL, ATTR | Non-CA | Biopsy, Echo | LGE | Diffuse | - | - |
| Austin15 2010 | 17 | 21 | AL, ATTR | Suspected Non-CA | Biopsy | LGE | Typical | - | - |
| Ruberg16 2009 | 21 | 7 | AL | Non-CA | Biopsy, Clinical | LGE | Presence | - | - |
| Vogelsberg17 2008 | 15 | 18 | - | HF & RCM | Biopsy | LGE | Typical | - | - |
| Maceira18 2005 | 29 | 16 | AL, ATTR | HTN | Echo | LGE | Presence | - | - |
Only 30 patients were included for ECV.
Superscript number references the study listed in the Appendix 1.
AA=autoimmune amyloidosis, AL=light chain amyloidosis, AS=aortic stenosis, ATTR=transthyretin amyloidosis, CA=cardiac amyloidosis, CMR=cardiac magnetic resonance, ECG=electrocardiogram, Echo=echocardiography, ECV=extracellular volume mapping, HCM=hypertrophic cardiomyopathy, HF=heart failure, HHD=hypertensive heart disease, HTN=hypertension, LGE=late gadolinium enhancement, n=number, ms=milliseconds, PCD=plasma cell dyscrasias, RCM=restricted cardiomyopathy, T1=native T1 mapping.
Table 2.
Prognostic Study Characteristics
| Author Year | Number (n) | Deaths (n) | Follow-up (mo) | Amyloid Type | Cardiac Validati on | Age | Male (%) | EF (%) | NYH A Class >2 (%) | Modality | LGE Criteria | T1 Cutoff (ms) | ECV Cutoff |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wan19 2019 | 77 | 46 | 28[14–38] | AL | CMR, Echo | 50±10 | 66 | 48±14 | 45 | T1, ECV | - | 1394* | 0.442* |
| Ridouani20 2018 | 42 | 18 | 27[14–40] | AL, ATTR | Biopsy, Nuclear | 69±13 | 70 | 54±15 | 50 | ECV | - | - | 0.590 |
| Kotecha21 2018 | 100 | 28 | 23±15 | AL | CMR | 64±11 | 61 | 65±11 | 15 | ECV | - | - | 0.45* |
| Illman22 2018 | 76 | 52 | 20[NP] | AL | CMR | 60±10 | 67 | 58±13 | 29 | LGE | Global | - | - |
| Wan23 2018 | 78 | 54 | 38[27–46] | AL | CMR, Echo | 59±11 | 60 | 50±12 | - | LGE | Transmural | - | - |
| Martinez-Naharro3 2018 | 227 | 95 | 32±17 | ATTR | Biopsy, Clinical, Nuclear | 72±11 | 82 | 56±1 4 | - | T1, ECV | - | 1065* | 0.525* |
| Lin24 2018 | 82 | 21 | 8[NP] | AL | - | 56±9 | 63 | 63±15 | 34 | LGE, ECV | Global | - | 0.440 |
| Ochs25 2017 | 68 | 44† | 14[4–49] | AL | Biopsy | 58±10 | 59 | 57±12 | >50 | LGE | Transmural | - | - |
| Baroni26 2017 | 42 | 31 | 37[10–50] | AL, ATTR | Suspected | 57±12 | 74 | 56±11 | - | LGE | Typical | - | - |
| Bhatti6 2016 | 251 | 97 | 28[5–56] | AL | Suspected | 63[56–69] | 64 | 61[51–68] | - | LGE | Typical | - | - |
| Fontana27 2015 | 250 | 67 | 24±13 | AL,ATTR | Biopsy, Nuclear | 67±12 | 68 | 60±14 | - | LGE | Transmural | - | - |
| Banypersad28 2014 | 100 | 25 | 23 [NP] | AL | - | 62±10 | 67 | 66±11 | 15 | T1, ECV | - | 1044 | 0.450 |
| White9 2014 | 90 | 50 | 29[12–44] | AL, ATTR | Suspected | 62±13 | 58 | 56[47–65] | >50 | LGE | Global | - | - |
Dichotomous variables are presented as percentages and continuous variables as mean ± standard deviation or median [interquartile range].
Cutoff was calculated based on high and low cardiac disease groups as prespecified by the study.
Reported as heart transplantation or death.
Superscript number references the study as listed in the Appendix 1.
AL=light chain amyloidosis, ATTR=transthyretin amyloidosis, CMR=cardiac magnetic resonance, Echo=echocardiography, ECV=extracellular volume mapping, LGE=late gadolinium enhancement, n=number, NP=not provided, mo=months, ms=milliseconds, T1=native T1 mapping.
Clinical Characteristics
The 18 diagnostic studies included a total of 2015 subjects. The characteristics of the CA and control groups are shown in Supplemental Table 3 & 4. Of the subjects with CA, 69% were AL, 21% were ATTR, and 10% were another type or unspecified. The CA group included 1108 subjects with a weighted mean age of 68 and 71% male. The control group included 907 subjects with a weighted mean age of 59 and 61% male. The CA group had a lower weighted mean left ventricular ejection fraction (LVEF) (58 vs 61% for CA and control respectively) and left ventricular end-diastolic volume index (LVEDVI) (67 vs 72 ml/m2 for CA and control respectively). The cut-offs for ECV and T1 ranged from 0.30 to 0.47 and 1020 to 1131 milliseconds respectively. LGE diagnostic criteria for CA included typical, diffuse, or presence of enhancement.
The 13 prognostic studies included a total of 1483 subjects. The characteristics of included subjects are shown in Supplemental table 5. Among the subjects, 72% were AL, 26% were ATTR, and 2% were another type or unspecified. The weighted mean age was 63 with 67% male. The weighted mean LVEF was 59% and LVEDVI was 65 ml/m2. The weighted mean follow-up period was 25 months.
Diagnostic Performance
The univariate and bivariate diagnostic meta-analysis results are included in Table 3 and Table 4, respectively. The Forest plots of the univariate sensitivity, specificity, and DOR estimates are presented in Figure 2 & 3. Bivariate comparison showed that ECV had higher DOR for CA than LGE (84.6 vs 20.1, p = 0.03). Otherwise, there was no significant difference between LGE and T1 for sensitivity, specificity, and DOR. LGE had significant heterogeneity for all parameters. ECV had significant heterogeneity for sensitivity and DOR. Diagnostic subgroup analysis can be seen in Supplemental Table 6. Exclusion of clinical criteria or Echo as reference tests resulted in significant improvement in heterogeneity.
Table 3.
Univariate Pooled Diagnostic Performance
| Modality | Studies | Number | Sensitivity | Specificity | Positive PV | Negative PV | Positive LR | Negative LR | Odds Ratio |
|---|---|---|---|---|---|---|---|---|---|
| LGE | 13 | 703 | 0.78 [0.68–0.85] | 0.87 [0.73–0.94] | 0.86 [0.75–0.93] | 0.80 [0.68–0.88] | 5.48 [3.03–9.91] | 0.25 [0.17–0.36] | 38.5 [11.6–128.4] |
| T1 | 4 | 1321 | 0.85 [0.81–0.88] | 0.86 [0.83;0.89] | 0.89 [0.84–0.93] | 0.80 [0.63–0.91] | 6.12 [4.43–8.42] | 0.18 [0.13–0.24] | 34.1 [19.8–58.7] |
| ECV | 6 | 1369 | 0.89 [0.79–0.95] | 0.89 [0.86–0.92] | 0.92 [0.90–0.94] | 0.87 [0.73–0.94] | 8.42 [6.22–11.41] | 0.12 [0.05–0.25] | 85.8 [38.4–192.0] |
Pooled values are presented as point estimates [95% confidence interval].
ECV=extracellular volume mapping, LGE=late gadolinium enhancement, LR=likelihood ratio, PV=predictive value, T1=native T1 mapping.
Table 4.
Bivariate Pooled Diagnostic Performance
| Modality | Studies | Number | Sensitivity | Specificity | Odds Ratio |
|---|---|---|---|---|---|
| LGE | 13 | 703 | 0.84 [0.74–0.90] | 0.80 [0.68–0.88] | 20.1 [9.1–44.1] |
| T1 | 4 | 1321 | 0.89 [0.80–0.95] | 0.80 [0.61–0.91] | 34.6 [11.4–105.1] |
| ECV | 6 | 1369 | 0.93 [0.86–0.96] | 0.87 [0.74–0.94] | 84.6 [30.3–236.2]* |
Pooled values are presented as point estimates [95% confidence interval].
p < 0.05 vs LGE.
ECV = extracellular volume mapping, LGE = late gadolinium enhancement, T1 = native T1 mapping.
Figure 2. Forest Plots of Sensitivities and Specificities.
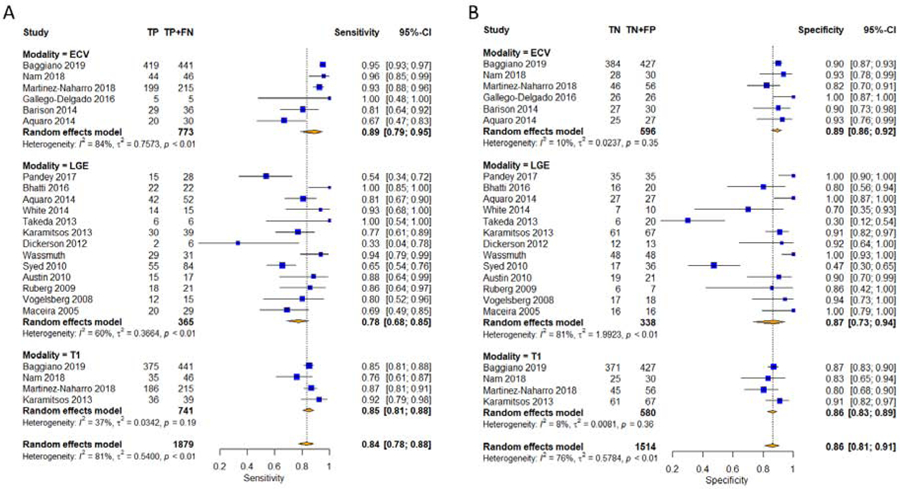
Univariate pooled sensitivities (A) and specificities (B) for diagnosing cardiac amyloidosis with native T1 mapping, ECV, and LGE. CI=confidence interval, ECV=extracellular volume mapping, FN=false negative, FP=false positive, LGE=late gadolinium enhancement, T1=native T1 mapping, TN=true negative, TP=true positive.
Figure 3. Forest Plots of Diagnostic Odds Ratios.
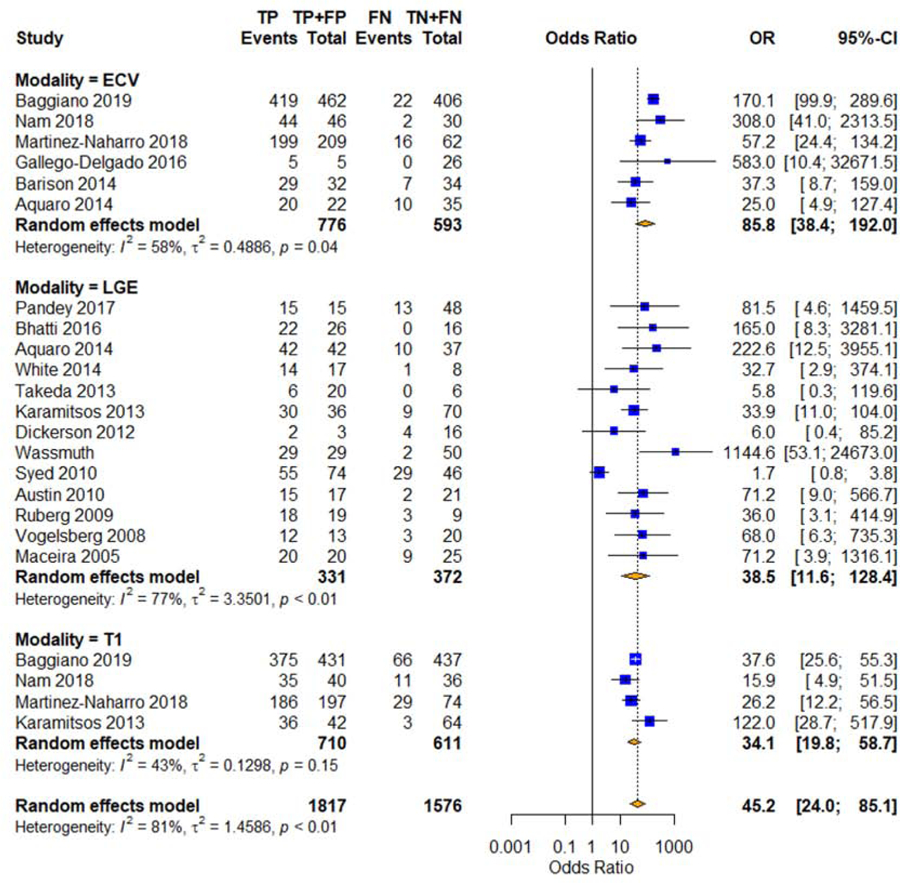
Univariate pooled diagnostic odds ratios for cardiac amyloidosis with native T1 mapping, ECV, and LGE. CI=confidence interval, ECV=extracellular volume mapping, FN=false negative, FP=false positive, LGE=late gadolinium enhancement, OR=odds ratio, T1=native T1 mapping, TN=true negative, TP=true positive.
For the meta-regression, we used publication year, age, gender, LVEF, LVEDVI, and LGE pattern as the covariates for DOR. ECV was significantly correlated to publication year (p = 0.03) and LVEDVI (p = 0.001). Native T1 was significantly correlated to LVEF (p = 0.038). Sensitivity analysis for DOR showed that the exclusion of Syed et al (16) significantly reduced the LGE heterogeneity (Cochran’s Q p = 0.44, I2 = 0%) and the exclusion of Baggiano et al (17) significantly reduced the ECV heterogeneity (Cochran’s Q p = 0.26, I2 = 24%). The DOR of ECV remained significantly higher than that of LGE with the exclusion of Syed et al (16) (p = 0.02), but not with the exclusion of Baggiano et al (17) (p = 0.40).
Prognostic Performance
The prognostic meta-analysis results are included in Table 5. The Forest plots of the HR are presented in Figure 4. The HR was significantly higher for ECV (4.27) compared to LGE (2.60, p = 0.03 vs ECV) and T1 (2.04, p = 0.01 vs ECV). There was no significant difference between T1 and LGE (p = 0.50). There was significant heterogeneity for LGE and native T1. Prognostic subgroup analysis can be seen in Supplemental Table 7.
Table 5.
Pooled Prognostic Performance
| Modality | Studies | Number | Hazard Ratio |
|---|---|---|---|
| LGE | 7 | 855 | 2.60 [1.90–3.56] |
| T1 | 3 | 404 | 2.04 [1.24–3.37] |
| ECV | 6 | 628 | 4.27 [2.87–6.37]*† |
Pooled values are presented as point estimates [95% confidence interval].
p < 0.05 vs LGE.
p < 0.05 vs T1.
ECV = extracellular volume mapping, LGE = late gadolinium enhancement, T1 = native T1 mapping.
Figure 4. Forest Plots of Hazard Ratios.
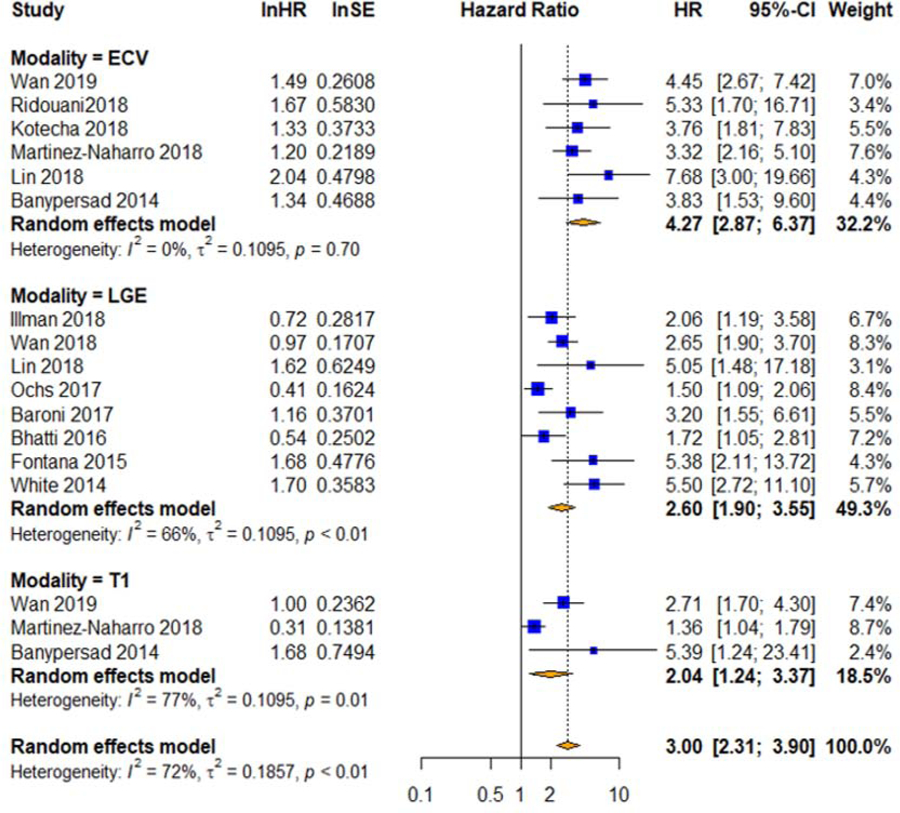
Pooled unadjusted hazard ratios for mortality with native T1 mapping, ECV, and LGE. CI=confidence interval, ECV=extracellular volume mapping, HR=hazard ratio, LGE=late gadolinium enhancement, ln=natural log, T1=native T1 mapping, SE=standard error of the hazard ratio.
For the meta-regression, we used publication year, follow-up duration, age, gender, NT-proBNP, E/e’, LVEF, and LVEDVI as the covariates. We found that only gender and follow-up duration significantly correlated with the HR of native T1 (p < 0.01). Sensitivity analysis showed that exclusion of Martinez-Naharro et al (4) significantly reduced the heterogeneity for T1 (Cochran’s Q p = 0.28, I2 = 0%) and ECV HR was no longer significantly higher than that of T1 (p = 0.05). The exclusion of Ochs et al (18) significantly reduced the heterogeneity for LGE (Cochran’s Q p = 0.08, I2 = 47%) and ECV HR was no longer significantly higher than that of LGE (p = 0.06).
Quality and Bias Assessment
The selected studies had an overall low risk of bias based on the 7 items of the QUADAS-2 questionnaire and the 6 items from QUIPS (Supplemental Table 8 & 9). Egger’s test suggested the presence of publication bias for LGE in the diagnostic meta-analysis, but Peter’s test did not demonstrate evidence of significant publication bias for any of the parameters for either meta-analysis.
Discussion
In this study we demonstrate that T1 and ECV are comparable to LGE for evaluating CA. Only ECV was found to have a significantly better diagnostic and prognostic performance than that of LGE. Given the inherent differences between each modality, quantitative imaging parameters may have specific roles in evaluating CA, depending on the clinical situation.
Late Gadolinium Enhancement
LGE is one of the first histologically-proven methods for non-invasively detecting CA (19). The diagnosis of CA with LGE is based upon the pattern of enhancement seen visually by the reader. LGE occurs when gadolinium contrast distributes into the extracellular space without entering intact myocardial cells. This results in a relative decrease in T1 relaxation in focal areas of scarring or infiltration. The definition of “typical” amyloid pattern can vary between readers, but generally refers to a global subendocardial pattern that does not match a coronary artery territory distribution. However, patterns can also include patchy, diffuse, or transmural involvement. In cases of diffuse symmetrical disease, LGE may be inadequate for detecting CA due to the lack of normal myocardium as a visual reference. As a result, quantitative parameters such as ECV and T1 have been proposed as an alternative method for evaluating CA in patients with diffuse disease. Nevertheless, LGE remains as an independent predictor of mortality (20), with transmural involvement having a worse outcome than only subendocardial involvement (21).
Extracellular Volume Mapping
Although ECV has become a well-established surrogate for diffuse fibrosis, the mechanism of extracellular expansion in CA is different from other myocardial disease processes. The deposition of amyloid occurs only in the interstitial space between cells and is independent of cardiomyocyte necrosis. Consequently, the degree of extracellular expansion in CA is substantial, with an average ECV of 54% in ATTR and 51% in AL (17,22). In comparison, ECV in healthy subjects is about 20 to 26% and elevated to 27 to 31% in diffuse fibrotic conditions such as aortic stenosis, heart failure, and hypertrophic cardiomyopathy, rarely going beyond 40% (23–25). Given that ECV can quantify myocardial changes not visible on LGE (26), it could be used for early detection of CA. In a study by Banysperad et al (27), a stepwise increase in ECV was shown between healthy, suspected CA, and definitive CA groups. This supports the high sensitivity estimate we found with ECV. Furthermore, the ECV level has been shown to correlate with disease severity, which includes both markers of systolic and diastolic dysfunction (28). As a result, ECV has been suggested for tracking cardiac disease response to treatment (29).
Native T1 Mapping
Elevations of native T1 can occur with fibrosis, infiltrative processes, or edema (30). However, it should be noted that native T1, unlike ECV, is influenced by both intracellular and extracellular/interstitial factors (31). Although native T1 mapping would seem less specific to interstitial changes, a study by Karamitsos et al (32) showed that subjects with CA still had significantly higher native T1 compared to those with aortic stenosis. Even patients with systemic amyloidosis without evidence of cardiac involvement had significantly higher native T1 compared to healthy controls (32). Given that native T1 mapping obviates the need for gadolinium, it could serve as an alternative evaluation for CA in patients with renal dysfunction. This is supported in our analysis with native T1 mapping having comparable diagnostic performance to that of LGE. However, in patients with only mildly elevated T1, there may be a “gray zone” where LGE or ECV is required for a more definitive diagnosis. For prognostication, our analysis showed that native T1 had the lowest point estimate HR, though only a small number of studies were included. Unlike ECV and LGE (20), no single study has demonstrated that native T1 is an independent predictor of mortality in CA (4,33).
Limitations
One of the main limitations of this meta-analysis was the heterogeneity between studies, specifically concerning the diagnostic criteria for cardiac amyloidosis, and varying techniques and cut-offs for defining abnormal ECV or native T1. However, this is indicative of the current state of the rapidly evolving non-invasive diagnostic criteria for cardiac amyloidosis, and the maturing CMR tools of T1 mapping and ECV. Recent consensus documents (2) concerning the imaging diagnosis of CA, and standardization of parametric mapping techniques (34) will hopefully mitigate these issues in future studies.
The studies included in this meta-analysis had significant variability in the type of validation test. Several studies used EMB, which continues to be the histological gold-standard for diagnosing CA. More recent studies used LGE with CMR or 99mTc-PYP/DPD/HMDP scintigraphy for diagnosing ATTR, which have been well-validated. Earlier studies, however, tended to use more clinical diagnostic criteria, which included history of presentation, biomarkers, EKG, and Echo. Because these methods are not specific to CA, they can lead to misclassification. In a recent meta-analysis by Brownrigg et al (35) that only used EMB as the reference test, LGE was found to have a higher DOR of 69.0 with a 95% confidence interval of 18.1 to 263.0. Ideally, all included studies would have used EMB as a reference test, but this has become a less common due to advances in imaging techniques.
Another major limitation is the generalizability of our analysis. There was a lack of uniformity in study designs, recruitment strategies, control groups, and amyloid centers. The meta-analysis included both prospectively and retrospectively designed studies of varying population sizes. In the diagnostic studies, control groups included healthy, non-cardiac systemic amyloidosis, or non-amyloid cardiomyopathy subjects. We also included both AL and ATTR patients. Given the differences in disease progression including the degree of myocardial edema and necrosis (36), our meta-analysis would have benefited from more T1 and T2 studies. Variability in LGE patterns, cut-off values, field strengths, vendors, and scanners could have also influenced our results. Additionally, our meta-analysis was conducted using PubMed and does not include studies that may be exclusively found in other databases such as Web of Science, EMBASE, Scopus, and The Cochrane Library.
Finally, we expected typical sources of bias such as small-study effects, decline effect, and early-extreme bias to influence our results (37). Small-study effects, which we assessed using Egger’s and Peter’s test, was most likely the largest potential source of bias in our meta-analysis given the large number of single center trials. Regarding decline effect and early-extreme bias, the meta-regression showed that publication year was a significant covariate of diagnostic performance for ECV. However, the trend was opposite of what we would expect from these biases, with more extreme values in later studies.
Conclusion
ECV may have better diagnostic and prognostic performance than that of LGE. Additionally, T1 mapping was comparable to other modalities in terms of sensitivity and specificity for diagnosing CA. Though limited by the heterogeneity of the included studies, our results suggest that the use of ECV for CA may improve accuracy, provide additional disease characterization, and help guide treatment. Furthermore, only needing to assess native T1 could simplify the diagnosis of CA in many patients with suspected amyloid and avoid the use of gadolinium. Further research is needed to investigate multimodal CMR assessment of CA.
Supplementary Material
Central Illustration. Diagnostic and Prognostic Performance of LGE, Native T1, and ECV.
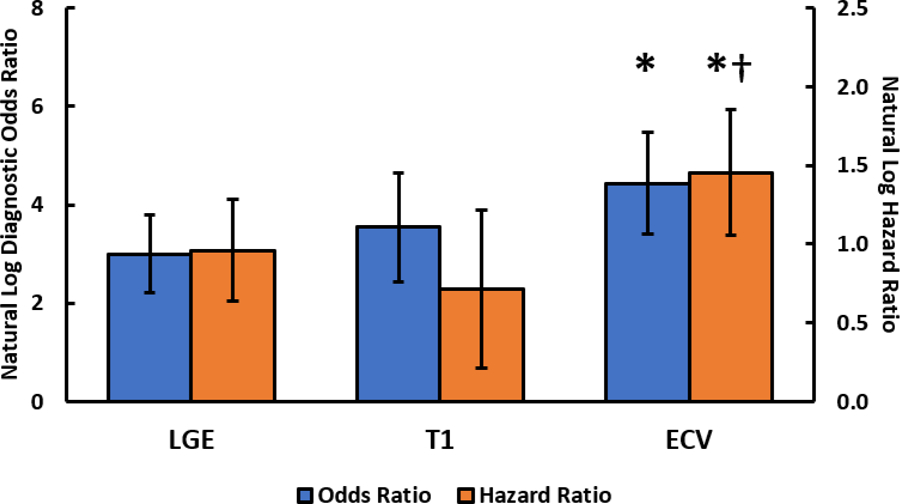
Natural log of the bivariate diagnostic odds ratio (blue) and unadjusted hazard ratio (orange) for LGE, native T1, and ECV. The whiskers represent the 95% confidence interval. The asterisk (*) indicates that ECV had a significantly higher diagnostic odds ratio (p = 0.03) and hazard ratio (p = 0.03) than that of LGE. The dagger (†) indicates that ECV had a significantly higher hazard ratio than that of native T1 (p = 0.01). ECV=extracellular volume mapping, LGE=late gadolinium enhancement, T1=native T1 mapping.
Perspectives.
Competency in medical knowledge:
Comprehensive evaluation for CA may include LGE, ECV, and native T1 mapping for diagnosis, prognosis, and disease progression.
Translational Outlook:
The inclusion of ECV to standard CMR evaluation may improve detection and risk stratification of CA.
Sources of Funding:
Dr. Salerno receives grant support from NIH R01 HL131919 and research support from Siemens Healthcare.
Abbreviations:
- AL
light chain amyloidosis
- ATTR
transthyretin amyloidosis
- CA
cardiac amyloidosis
- CMR
cardiac magnetic resonance
- DOR
diagnostic odds ratio
- ECG
electrocardiogram
- Echo
echocardiography
- ECV
extracellular volume mapping
- EMB
endomyocardial biopsy
- HR
hazard ratio
- LGE
late gadolinium enhancement
- T1
native T1 mapping
Footnotes
Disclosures: There are no conflicts of interest regarding the content of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maurer Mathew S, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation 2017;135:1357–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorbala S, Ando Y, Bokhari S et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 2 of 2-Diagnostic criteria and appropriate utilization. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology 2019. [DOI] [PubMed]
- 3.Bhatti S, Watts E, Syed F et al. Clinical and prognostic utility of cardiovascular magnetic resonance imaging in myeloma patients with suspected cardiac amyloidosis. European heart journal cardiovascular Imaging 2016;17:970–7. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Naharro A, Kotecha T, Norrington K et al. Native T1 and Extracellular Volume in Transthyretin Amyloidosis. JACC Cardiovascular imaging 2019;12:810–819. [DOI] [PubMed] [Google Scholar]
- 5.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao G, Lopez-Jimenez F, Boyd J et al. Methodological Standards for Meta-Analyses and Qualitative Systematic Reviews of Cardiac Prevention and Treatment Studies: A Scientific Statement From the American Heart Association. Circulation 2017;136:e172–e194. [DOI] [PubMed] [Google Scholar]
- 8.Whiting PF, Rutjes AWS, Westwood ME et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Annals of Internal Medicine 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 9.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing Bias in Studies of Prognostic Factors. Annals of Internal Medicine 2013;158:280–286. [DOI] [PubMed] [Google Scholar]
- 10.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of Two Methods to Detect Publication Bias in Meta-analysis. Jama 2006;295:676–680. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 13.Clopper CJ, Pearson ES. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika 1934;26:404–413. [Google Scholar]
- 14.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of clinical epidemiology 2005;58:982–90. [DOI] [PubMed] [Google Scholar]
- 15.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Statistics in medicine 2002;21:589–624. [DOI] [PubMed] [Google Scholar]
- 16.Syed IS, Glockner JF, Feng D et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovascular imaging 2010;3:155–64. [DOI] [PubMed] [Google Scholar]
- 17.Baggiano A, Boldrini M, Martinez-Naharro A et al. Noncontrast Magnetic Resonance for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovascular imaging 2019. [DOI] [PubMed]
- 18.Ochs MM, Fritz T, Arenja N et al. Regional differences in prognostic value of cardiac valve plane displacement in systemic light-chain amyloidosis. Journal of Cardiovascular Magnetic Resonance 2017;19:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogelsberg H, Mahrholdt H, Deluigi CC et al. Cardiovascular Magnetic Resonance in Clinically Suspected Cardiac Amyloidosis: Noninvasive Imaging Compared to Endomyocardial Biopsy. Journal of the American College of Cardiology 2008;51:1022–1030. [DOI] [PubMed] [Google Scholar]
- 20.Lin L, Li X, Feng J et al. The prognostic value of T1 mapping and late gadolinium enhancement cardiovascular magnetic resonance imaging in patients with light chain amyloidosis. Journal of Cardiovascular Magnetic Resonance 2018;20:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontana M, Pica S, Reant P et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 2015;132:1570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks J, Kramer CM, Salerno M. Markedly increased volume of distribution of gadolinium in cardiac amyloidosis demonstrated by T1 mapping. Journal of magnetic resonance imaging : JMRI 2013;38:1591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puntmann VO, Peker E, Chandrashekhar Y, Nagel E. T1 Mapping in Characterizing Myocardial Disease: A Comprehensive Review. Circulation research 2016;119:277–99. [DOI] [PubMed] [Google Scholar]
- 24.Gottbrecht M, Kramer CM, Salerno M. Native T1 and Extracellular Volume Measurements by Cardiac MRI in Healthy Adults: A Meta-Analysis. Radiology 2019;290:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan JA, Michaelsson E, Shaw PW et al. Extracellular volume by cardiac magnetic resonance is associated with biomarkers of inflammation in hypertensive heart disease. Journal of hypertension 2019;37:65–72. [DOI] [PubMed] [Google Scholar]
- 26.Wong Timothy C, Piehler K, Meier Christopher G et al. Association Between Extracellular Matrix Expansion Quantified by Cardiovascular Magnetic Resonance and Short-Term Mortality. Circulation 2012;126:1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banypersad SM, Sado DM, Flett AS et al. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circulation Cardiovascular imaging 2013;6:34–9. [DOI] [PubMed] [Google Scholar]
- 28.Barison A, Aquaro GD, Pugliese NR et al. Measurement of myocardial amyloid deposition in systemic amyloidosis: insights from cardiovascular magnetic resonance imaging. Journal of internal medicine 2015;277:605–14. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Naharro A, Abdel-Gadir A, Treibel TA et al. CMR-Verified Regression of Cardiac AL Amyloid After Chemotherapy. JACC: Cardiovascular Imaging 2018;11:152–154. [DOI] [PubMed] [Google Scholar]
- 30.Robinson AA, Chow K, Salerno M. Myocardial T1 and ECV Measurement. Underlying Concepts and Technical Considerations 2019;12:2332–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovascular imaging 2016;9:67–81. [DOI] [PubMed] [Google Scholar]
- 32.Karamitsos TD, Piechnik SK, Banypersad SM et al. Noncontrast T1 Mapping for the Diagnosis of Cardiac Amyloidosis. JACC: Cardiovascular Imaging 2013;6:488–497. [DOI] [PubMed] [Google Scholar]
- 33.Banypersad SM, Fontana M, Maestrini V et al. T1 mapping and survival in systemic light-chain amyloidosis. European heart journal 2015;36:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messroghli DR, Moon JC, Ferreira VM et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). Journal of Cardiovascular Magnetic Resonance 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brownrigg J, Lorenzini M, Lumley M, Elliott P. Diagnostic performance of imaging investigations in detecting and differentiating cardiac amyloidosis: a systematic review and meta-analysis. ESC heart failure 2019;6:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotecha T, Martinez-Naharro A, Treibel TA et al. Myocardial Edema and Prognosis in Amyloidosis. J Am Coll Cardiol 2018;71:2919–2931. [DOI] [PubMed] [Google Scholar]
- 37.Fanelli D, Costas R, Ioannidis JPA. Meta-assessment of bias in science. Proceedings of the National Academy of Sciences 2017;114:3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


