Abstract
Background:
Stereotactic body radiotherapy (SBRT) is a non-invasive ablative treatment for hepatocellular carcinoma (HCC). This report aimed to address the limited availability of long-term outcomes post SBRT for HCC from North America.
Methods:
Localized HCC patients without vascular invasion, who were ineligible for other liver-directed therapies and treated with SBRT at the University of Toronto or University of Michigan were pooled to determine overall survival (OS), cumulative recurrence rates, and ≥ grade 3 toxicity. Multivariable analysis determined factors affecting OS and local recurrence rates.
Results:
In 297 patients with 436 HCCs (42% > 3 cm) one, three & five- year OS was 77·3%, 39·0%, and 24·1%, respectively. On Cox proportional hazards regression analysis, liver transplant post SBRT, Child Pugh (CP) A liver function, alpha-feto protein (AFP) ≤ 10 ng/ml , and Eastern Co-operative Oncology Group (ECOG) performance status 0 significantly improved OS.(hazard ratio {HR}=0·06, 95%CI- 0·02–0·25; p<0·001; {HR}=0·42, 95% CI-0·29–0·60, p<0·001; HR=0·61, 95% CI- 0·44–0·83; p=0·002 and HR=0·71, 95% CI= 0·51–0·97, p=0·034 respectively).
Cumulative
local recurrence was 6·3% (95% CI= 0.03–0.09) and 13·3% (95% CI=0.06–0.21) at one and three years respectively. Using Cox regression modelling, local control was significantly higher using breath-hold motion management and in HCC smaller than 3 cm (HR=0.52, 95% CI=0.58–0.98; p=0.042 and HR=−0.53, 95%CI=0.26–0.98; p=0.042, respectively). Worsening of CP score by ≥2 points three months after SBRT was seen in 15·9%.
Conclusions:
SBRT confers high local control and long-term survival in a substantial proportion of HCC patients unsuitable for, or refractory to standard local/regional treatments. Liver transplant should be considered if appropriate downsizing occurs following SBRT.
Keywords: Liver, cancer, hepatocellular carcinoma, stereotactic, radiation
INTRODUCTION
Curative treatments for hepatocellular carcinoma (HCC) include liver transplant, surgical resection, and local ablative therapies.1 While transplant and surgical resection are associated with five-year survival rates of approximately 66%,2 a minority of patients are eligible for liver transplant and resection is feasible only in patients with good liver function and sufficient liver remnant. Radiofrequency ablation (RFA) and microwave ablation are alternative local therapies for small HCCs; however, risk of recurrence increases as the HCC diameter increases above 3 cm, and some patients recur post local ablation or are not well-suited for ablative treatments due to tumor location, size, multifocality, impaired liver function and/or medical contraindications.3 Transarterial chemo embolization (TACE) improves overall survival (OS) but is not curative4 and is associated with an 80% recurrence rate over two years. Overall, a substantial proportion of patients are ineligible for, or recur following standard local-regional therapies, and thus have inherently worse prognosis.
SBRT is a non-invasive ablative treatment established as a curative treatment for lung cancer and for ablation of oligo-metastases from a variety of primary cancers.5,6 For HCC, SBRT has generally been reserved for patients who are either high-risk, ineligible, or have progressed despite other liver-directed therapies.7 Several studies, majorly from Asia, have shown its potential role in all stages of HCC.8 There are no published randomized trials comparing SBRT with other liver directed therapies. We report pooled long-term outcomes of patients with early, intermediate and advanced Barcelona Clinic Liver Cancer (BCLC) stage HCC, generally unsuitable for or refractory to standard local therapies, treated in two North American centres. We hypothesize that SBRT of HCC in patients ineligible for standard liver- directed therapies, or with recurrence following such therapies, would result in a high rate of durable long-term local control and a substantial proportion of patients alive three to five years post SBRT.
MATERIALS AND METHODS
Patients
This included HCC patients without vascular invasion, treated with SBRT at the Princess Margaret Cancer Centre (PM) and the University of Michigan (UM). Patients from UM were part of a prospectively maintained database, while data was retrieved retrospectively at PM. Eligibility criteria included patients with a histological or radiological diagnosis of T1, T2 or T3a HCC (AJCC TNM 7th edition) who were planned for SBRT from 1st June 2003 to 31st December 2016, and received biologically effective doses (BED) of at least 45 Gy10, which is equivalent to 30Gy in six fractions, the lowest dose fractionation schedule used at PM as per the Phase I/II trial.9 Exclusion criteria included vascular invasion, extrahepatic spread, ruptured HCC, ≥ five HCCs and patients who received planned systemic therapy after SBRT.10,11 Tumors treated with SBRT as a bridging therapy to planned liver transplant and patients with no imaging or clinical follow up after treatment completion were also excluded (Fig 1). There was no upper limit to HCC size, and bland vascular thrombosis was permitted, as long as there was no definite HCC vascular invasion, as reviewed by an experienced hepatobiliary radiologist. Patients at both institutions were evaluated within a multi-disciplinary framework and generally deemed ineligible for other standard therapies due to co-morbidities or technical reasons, or as failure of prior therapies (e.g. RFA, TACE) before being offered SBRT.
Figure 1. CONSORT Diagram.
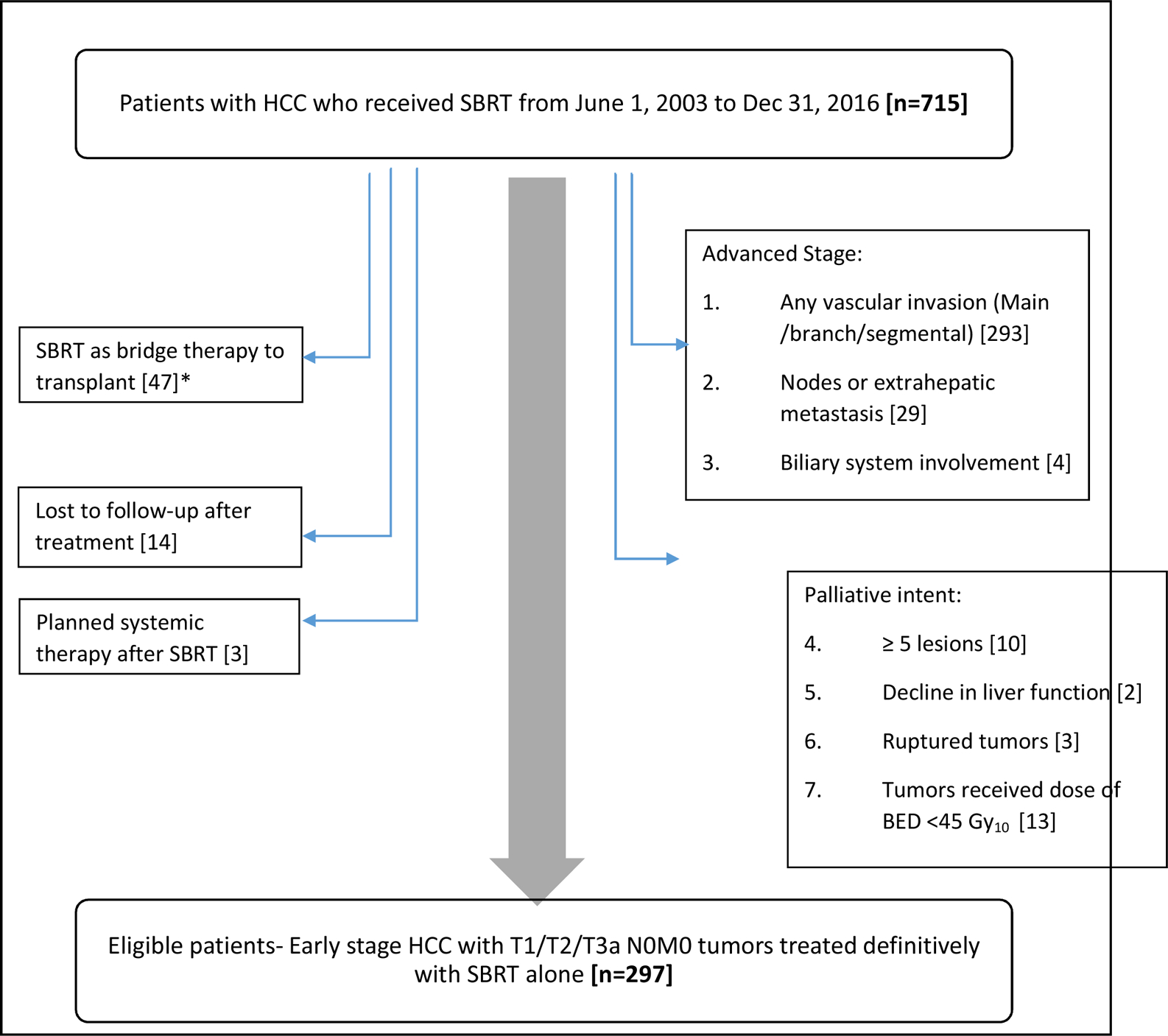
HCC- Hepatocellular carcinoma; SBRT- Stereotactic Body Radiotherapy; *At Princess Margaret Cancer Centre, patients who underwent planned SBRT as a bridge to transplant received SBRT to reduce the tumor burden to within transplant criteria, rather than for definitive treatment (and often all HCCs were not always targeted and SBRT doses were often reduced); hence they were excluded from this analysis. At University of Michigan, no patients were identified as eligible for transplant upfront. However, a subset of patients subsequently became eligible for transplant, and are included in this analysis.
Patient demographics, tumor characteristics and treatment details were retrieved from electronic medical records up to May 10, 2018. HCCs were labelled ‘recurrent’ if, at the time of irradiation, the target HCC had already recurred despite prior local-regional therapy of that lesion. Each session of prior liver-directed therapy was counted as one line of therapy.
Treatment
Treatment techniques mirrored the evolution of SBRT technologies from 2003 to 2016. Multiphasic contrast enhanced CT scans and MRI scans (when eligible), abdominal compression or breath hold {using “Active Breathing Coordinator” device (ABC™, Elekta, Stockholm, Sweden) or SDX® (Dyn’R, Toulouse, France)} motion management were used in majority of patients. Individualised dose allocation and daily image guidance were used for all patients. Details of SBRT were described in prior publications.9,12,13
Follow-Up
Follow-up was every three months for the first year, and every three/six months subsequently. Tumor response was assessed with multi-phasic CT or MRI hepatic imaging using RECIST 1·1 criteria14. Local recurrence was defined as progressive HCC (as per RECIST 1.1 criteria) within the irradiated volume. Laboratory parameters were collected at baseline and each follow-up visit. Toxicity was defined as per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) V4.03;15 with acute toxicity occurring < 90 days after SBRT. Radiation Induced Liver Disease (RILD) (both classic and non-classic) and change in ALBI score at three months after SBRT were also captured.
Statistics
Primary outcome, OS, was calculated using the Kaplan-Meier product-limit method, from the date of first fraction of SBRT to time to death or most recent follow up. Secondary outcomes included cumulative incidence of local recurrence, intrahepatic recurrence outside the irradiated volume, extrahepatic recurrence and progression-free survival (PFS). Cumulative incidence of local failure was calculated per lesion using Fine and Grey’s competing event analysis method, considering death as competing event.16 Univariate analysis of factors affecting OS and local recurrence was completed. Multivariable analysis was done using Cox proportional hazards model, including factors either potential p-value < 0.20 in the univariate analysis or deemed clinically important. Location of treatment (PM versus UM) was accounted for in the model. All P-values were two-sided, and P < 0·05 was considered significant. Missing data was excluded and statistical analyses were performed using version 9.4 of the SAS system for Windows (2002–2012 SAS Institute, Inc., Cary, NC).
RESULTS
Patient and Tumor Characteristics
One hundred and ninety-nine patients were identified from UM, and 98 patients from PM, for a total of 297 HCC patients with 436 tumors (Table 1). Previous publications from UM and PMH describe less than half of the present cohort.11–13. With a median follow up of 19·9 months, 57.5% of patients expired at last follow up.
Table 1.
Patient Demographics and Treatment Characteristics
| Factor | Description | Princess Margaret Cancer Centre (%) N=98 | University of Michigan (%) N=199 | Combined Cohort (%) N=297 |
|---|---|---|---|---|
| Age at treatment | Median (Range) | 76 (39–94) | 64.5 (22–90) | 69.3 (22–94) |
| Sex | Male/ Female (%) | 70/28 (71/29) | 151/48(76/24) | 221/76 (74/26) |
| Ethnicity (%) | Caucasian | 52 (53) | 154 (77) | 206 (69) |
| Asian | 39 (40) | 8 (4) | 47 (16) | |
| African-American | 1 (1) | 19 (10) | 20 (7) | |
| Other | 6 (6) | 18 (9) | 24 (8) | |
| ECOG Score | 0 (%) | 48 (49) | 88 (44) | 136 (46) |
| ≥1 (%) | 50 (51) | 111 (56) | 161 (54) | |
| Cirrhosis | Yes | 80 (82) | 176 (88) | 256 (86) |
| Aetiology of Cirrhosis | Hepatitis C | 20 (20) | 100 (50) | 120 (40) |
| Hepatitis B | 23 (23) | 14 (7) | 37 (12) | |
| Non-viral | 37 (38) | 63 (32) | 100 (34) | |
| None | 18 (18) | 22 (11) | 40 (14) | |
| Child-Pugh Class | A 5 | 67 (68) | 86 (43) | 153 (52) |
| A 6 | 20 (20) | 52 (26) | 72 (24) | |
| B 7 | 7 (7) | 17 (9) | 24 (8) | |
| B 8 | 1 (1) | 19 (10) | 20 (7) | |
| B 9 | 0 (0) | 15 (8) | 15 (5) | |
| C ≥ 10 | 0 (0) | 6 (3) | 6 (2) | |
| Missing | 3 (3) | 4 (2) | 7 (2) | |
| ALBI Score | Median (Range) | −2.43 (−3.27 to −0.92) | −2.26 (−3.33 to −0.49) | −2.33 (−3.33 to −0.49) |
| IQR | −2.71 to −2.13 | −2.68 to −1.72 | −2.69 to −1.91 | |
| ALBI Gradea | Grade 1 | 36 (37) | 57 (29) | 93 (31.3) |
| Grade 2 | 58 (59) | 118 (59) | 176 (59.3) | |
| Grade 3 | 4 (4) | 23 (11.6) | 27 (9) | |
| Missing | 0 | 1 (0.5) | 1 (0.3) | |
| Pre-treatment Platelets (X 109/L) | Median (Range) | 135 (34–366) | 100 (17–476) | 111 (17–476) |
| Time since original diagnosis of HCC (months) | Median (range) | 9.4 (1–209) | 8.6 (1–116) | 9 (1–209) |
| No of Liver Occurrences prior to SBRT | Median (range) | 0 (0–10) | 0 (0–6) | 0 (0–10) |
| Liver directed Therapies prior to SBRT | Yes (%) | 48 (50) | 130 (65) | 178 (60) |
| Median no. of lines (range) | 0 (0–18) | 1 (0–7) | 1 (0–18) | |
| Types of therapies | ||||
| TACE | 20 (15) | 178 (60) | 198 (46) | |
| RFA/MWA | 82 (60) | 79 (26) | 161 (37) | |
| Resection | 9 (7) | 26 (9) | 35 (8) | |
| Miscellaneousb | 25 (18) | 16 (5) | 41 (9) | |
| T stage as per AJCC/UICCc | T1 | 52 (53) | 149 (75) | 201 (68) |
| T2 | 30 (31) | 48 (24) | 78 (26) | |
| T3a | 16 (16) | 2 (1) | 18 (6) | |
| BCLC Classification | Class 0/A (Early) | 16 (16) | 64 (32) | 80 (27) |
| Class B (Intermediate) | 31 (32) | 21 (11) | 52 (18) | |
| Class Cd /D (Advanced) | 48 (49) | 110 (55) | 158 (53) | |
| Not Available | 3 (3) | 4 (2) | 7 (2) | |
| Number of lesions per patient (%) | 1 | 60 (61) | 149 (75) | 209 (70.4) |
| 2–3 | 33 (34) | 48 (24) | 81 (27.3) | |
| 4 | 5 (5) | 2 (1) | 7 (2.4) | |
| Tumor size (cm) | Median (range) | 4.4 (1.2–18.1) | 2.3 (0.5–13.4) | 2.7 (0.5–18.1) |
| Tumor size category (%) | ≤ 2 cm | 14 (14) | 87 (44) | 101 (34) |
| 2 to ≤ 3cm | 18 (18) | 54 (27) | 72 (24) | |
| 3 to ≤ 5 cm | 26 (27) | 43 (22) | 69 (23) | |
| > 5 cm | 40 (41) | 15 (8) | 55 (19) | |
| Pre-treatment AFP (ng/mL) | Median (range) | 9.5 (0–14472) | 9.7 (0–10926) | 9.7 (0–14472) |
| GTV volume (cc) | Median (range) | 53.2 (1.3–2519.1) | 10.3 (0.8–1108.6) | 15.6 (0.8–2519.1) |
| PTV volume (cc) | Median (range) | 141.8 (10–3091.6) | 42.7 (1.6–1607.4) | 56 (1.6–3091.6) |
| Prescribed Dose (Gy) | Median (range) | 39 (30–54) | 42 (27–60) | 40 (27–60) |
| Prescribed number of fractions | Median (range) | 6 (5–6) | 5 (3–5) | 5 (3–6) |
| BED (Gy)e | Median (range) | 64.4 (45–102.6) | 85.5 (51.3–180.0) | 79.2 (45–180) |
| Respiratory Motion management per lesion (N=436) (%) | Breath holdf | 41 (28) | 169 (58.3) | 210 (48) |
| Abdominal Compression | 94 (64) | 0 (0) | 94 (22) | |
| Free-breathing | 11 (8) | 108 (37.2) | 119 (27) | |
| Not available | 0 (0) | 13 (4.5) | 13 (3) | |
| Fiducials (N=436) (%) | No | 146 (100) | 255 (88) | 426 (92) |
| Yes | 0 (0) | 35 (12) | 37 (8) |
NAFLD- Non-Alcoholic Fatty Liver Disease; ECOG score- Eastern Co-operative Oncology Group performance status score; ALBI score-
Albumin Bilirubin score, RFA- radio frequency ablation; MWA- microwave ablation; TACE-trans arterial chemoembolization; BCLC- Barcelona Clinic Liver Cancer Classification, AFP- alpha-fetoprotein, GTV- gross tumor volume; PTV- planning target volume.
ALBI Grade 1= ≤ −2.6; Grade 2= >−2.6 to ≤−1.39; Grade 3=>−1.39
Miscellaneous liver directed therapies included- Percutaneous ethanol ablation, Irreversible electroporation, Y90 Radioembolization or any combination of these. Each session of ablation/TACE was counted separately.
UICC/ AJCC 7th Edition, 2010
BCLC C- on basis of performance score 1 or 2
BED- Biologically Effective Dose= nd {1+ d/(α/β)} where n=number of fractions, d= dose per fraction in Gy and α/β=10)
Breath hold with active breath control (ABC) or spirometric motion management system, SDX® (Dyn’R, Toulouse, France)
Patients were presented at a multidisciplinary HCC tumor board, where standard local and regional therapies were prioritized above SBRT. The cohort was heterogeneous in terms of tumor burden, liver function and performance status, as well as previous lines of treatment received (Table 1). Higher doses were used at UM compared to PM [median biologically effective dose (BED) = 85·5 Gy versus 64·4 Gy]. Twenty-three percent of patients (exclusively from UM) had a pre-planned break of a month after the third fraction of SBRT, as part of a clinical protocol,12 and 8% had fiducials implanted to aid in image guidance. Although only six percent patients had T3aN0M0 disease, 53% patients were classified as BCLC Class C/D on the basis of ECOG PS > 0 and CP C. Twenty-five patients (8·4%) ultimately underwent a liver transplant following SBRT. At PM, no patients who underwent SBRT as a bridge to transplant or who were planned to receive a liver transplant were included a priori. Despite this exclusion, five patients unexpectedly became eligible for transplant due to substantial reduction in HCC volume post SBRT. At UM, patients were not identified as eligible for transplant upfront; 20 patients subsequently went on to have a liver transplant following downsizing post SBRT.
Overall Survival
Median OS of this heterogeneous cohort, mostly ineligible for other liver-directed therapies was 25·6 months (95% CI=22·3–31·7) with one, three and five- year survival rates of 77·4%, 39·0% and 24·1% respectively. Patients with better performance status and liver function (ECOG PS 0 and CP A) had a median OS of 37 months (95% CI=25–51), compared to 23 months (95% CI= 19–24) for those with ECOG PS ≥1 and/or CP B/C. On multivariable analysis, liver transplant post SBRT, CP A liver function, AFP ≤10, and ECOG PS 0 were significantly associated with improved OS (Table 2). Median OS of those who underwent liver transplant after SBRT has not been reached, and it was 24 months (95% CI=20·5–27·2) in patients who did not undergo transplant (Figure 1). Patients with CP A had a median OS of 31·7 months (95% CI=24·0–38·5) compared to a median of 23·2 months (95% CI=18·8–28·0) for CP B or C patients. Those with AFP ≤10 had a median OS of 32·9 months (95% CI=24·9–47·2) vs 21·1 months (95% CI=17·0–25·7) for those with AFP >10. Median survival of patients with tumors larger than five cm was 28 months (95% CI=20.0–38·0), not significantly different from those with smaller tumors (median OS= 26·0 months; 95% CI=22·0–32·0). In addition, in analysis of subgroups, BCLC B patients (more than three tumors or at least 1 tumor>3cm), with ECOG PS 0 and Child Pugh A liver function had a 3 year OS of 54.6%. Calendar period was not found to be significant on univariate analysis. On multivariable analysis, there was no significant effect of treating center on OS.
Table 2.
Univariable and Multivariable Analysis for factors affecting Overall Survival and Local Progression
| Variable | Categorya | Overall Survival | Local Progression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable Analysis | Univariate analysis | Multivariable Analysis | ||||||
| HR (95% CI) | p-valueb | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Transplant post SBRT | Yes vs No | 0.08 (0.02–0.31) | 0.0003 | 0.06 (0.02–0.25) | <0.0001 | - | - | - | - |
| Pre-treatment CP score | A vs B,C | 0.50 (0.36–0.70) | <0.0001 | 0.42 (0.29–0.60) | <0.0001 | - | - | - | - |
| AFP | ≤10 (median)vs >10 | 0.67 (0.49–0.91) | 0.0112 | 0.61 (0.44–0.83) | 0.0020 | - | - | - | - |
| ECOG | 0 vs ≥1 | 0.66 (0.48–0.89) | 0.0070 | 0.71 (0.51–0.97) | 0.0339 | - | - | - | - |
| Cirrhosis | Yes vs No | 1.69 (1.03–2.80) | 0.0398 | 0.83 (0.40–1.74) | 0.6233 | - | - | ||
| Etiology of cirrhosis (ref=None) | HBV | 1.01 (0.53–1.93) | 0.0441 | NS | NS | 2.05 (0.93–4.48) | 0.1601 | - | - |
| HCV | 1.57 (0.93–2.63) | 1.00 (0.49–2.01) | - | - | |||||
| Non-viral | 1.80 (1.07–3.05) | 1.60 (0.69–3.71) | - | - | |||||
| Pre-treatment ALBI | Grade 1 vs Grade 2/3 | 0.75 (0.54–1.05) | 0.0897 | d | - | - | - | - | - |
| Age | >70 vs < 70 yrs. | 0.78 (0.58–1.06) | 0.1121 | - | - | - | - | - | - |
| T-stage | T1 vs T2/3a | 0.79 (0.57–1.09) | 0.1451 | - | - | 0.98 (0.57–1.67) | 0.9388 | - | - |
| Decline in CP score | ≥ 2 points vs <2 points | 1.79 (1.15–2.77) | 0.0093 | e | - | - | - | - | - |
| No of tumors | 1 (ref) 2–3 4 |
1.26 (0.91–1.75) 0.78 (0.25–2.46) |
0.3363 |
- | - | Ref | 0.7451 | - | - |
| 1.10 (0.63–1.91) | - | - | |||||||
| 0.67 (0.19–2.43) | - | - | |||||||
| Prior Liver directed therapy | Yes vs No | 1.06 (0.78–1.45) | 0.7152 | - | 2.08 (0.50–8.64) | 0.3147 | - | - | |
| Tumor size | ≤ 3 cm vs >3 cm | 0.96 (0.71–1.31) | 0.8143 | - | 0.53 (0.31–0.91) | 0.0212 | 0.53 (0.29–0.98 | 0.0423 | |
| ≤ 5 cm or >5 cm | 0.94 (0.64–1.40) | 0.7725 | - | 0.67 (0.33–1.36) | 0.2674 | - | - | ||
| Time since diagnosis of HCC | < 9 months vs>9 months | 0.98 (0.72–1.32) | 0.8669 | - | - | - | - | - | |
| Local progression | Yes vs No | 0.95 (0.63–1.42) | 0.7953 | - | - | - | - | - | |
| Fiducials | Yes vs No | - | - | - | 0.00 | <0.0001 | e | ||
| Respiratory motion management | Breath hold vs. compression/free breathing | - | - | - | 0.47 (0.26–0.84) | 0.0106 | 0.52 (0.28–0.98) | 0.0441 | |
| Total SBRT physical dose | - | - | f | 0.96 (0.93–0.99) | 0.0244 | - | - | ||
| Total GTV | - | - | - | 1 (0.999–1.001) | 0.4592 | - | - | ||
Reference category is the latter category in each variable.
Bold values indicate statistical significance.
As pre-treatment ALBI was correlated with pre-treatment CP score, hence ALBI was not included in the model
Was not included in the model as was correlated to Pre-treatment CP score
Not included in the model as there were too few events
Prescribed dose was not included in multivariable analysis for overall survival due to the inherent bias in dose allocation due to differing dose allocation strategies at both the participating institutions
Local Control and other Progression
Cumulative incidence of local HCC recurrence (i.e. RECIST progression of the irradiated HCC’s) was 6·3% (95% CI= 0.03–0.09) at one year and 13·7% (95% CI=0.04–0.23) at five years (Fig 3). On univariable analysis, HCC size < 3cm, higher dose, use of fiducial markers for image guidance and breath-hold immobilization had a lower likelihood of local recurrence (Table 2). Use of fiducial markers was excluded from multivariable analysis as number of events were too small. Tumor size and prescribed dose were significantly correlated with each other. When prescribed dose was excluded from the multivariable model, breath hold motion management and HCC size <3 cm were both significantly associated with improved local control (Table 2). This exploratory analysis needs validation due to few events. Only 3·7% of patients had isolated recurrence of the irradiated HCC as the first site of recurrence. The number of patients alive with no progression at 1 and 3 years is 131 and 28 respectively, and the number of patients alive with no local recurrence at 1 and 3 years is 261 and 72 respectively.
Figure 3.
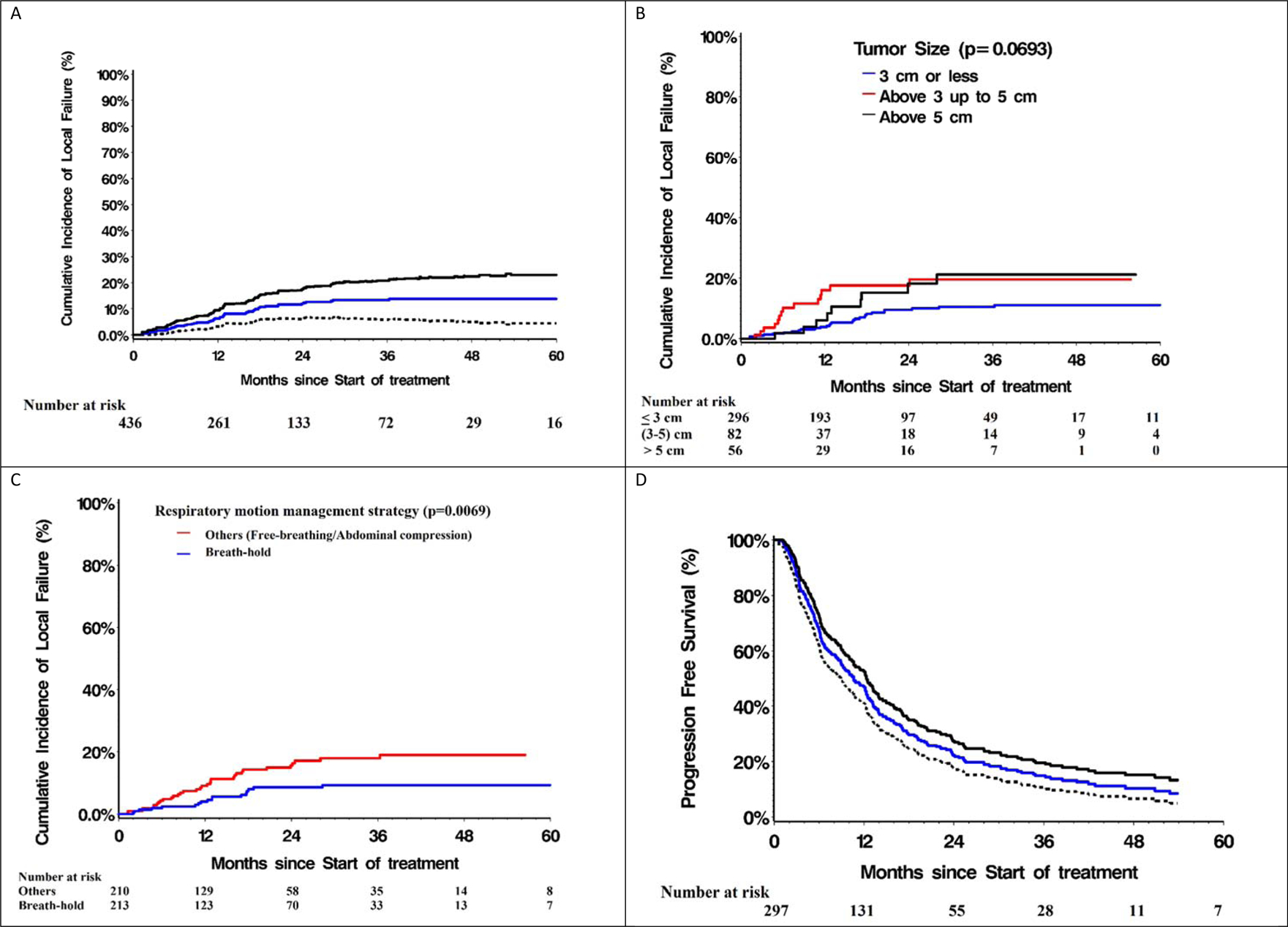
(A) Cumulative incidence of local recurrence of full cohort (with ± 95% confidence intervals) and stratified as per (B) tumor size (≤3cm, >3 cm-≤5cm and >5cm), and (C) respiratory motion management strategy (Breath hold techniques and Other techniques). (D) Progression-free survival (PFS) of full cohort (with ±95% confidence intervals).
Rates of intrahepatic recurrence/new HCC outside the irradiated HCC were higher: 31·2% and 65·7% respectively at one and five years. Cumulative rates of extrahepatic recurrence were 16·6% at 1 year and 29·3% at five years. Median PFS was 10·6 months (95% CI=8·9–12·3) with 14.9% (95% CI=10.7%−19.6%) patients alive and progression-free three years post SBRT (Fig 3).
Toxicity
Low rates of clinical toxicity were observed; 15.9% out of 214 evaluable patients experienced a worsening CP score and 21·2% of 241 evaluable patients had a worsening in ALBI grade, three months after SBRT (Table 3). No patients developed classic RILD or Grade 4 liver enzyme toxicity. Two patients who had undergone prior therapies (TACE, RFA, Y90 radioembolization and a biliary-enteric anastomosis), and a third patient with a tumor adjacent to the biliary duct, developed biliary toxicity at one, one-and-a-half and 30 months post SBRT, respectively. One patient possibly succumbed to late toxicity from a duodenal ulcer and an upper gastrointestinal bleed, 10 months post SBRT.
Table 3.
Toxicity ≥ Grade 3 (CTCAE V 4.0) in Total Cohort
| Toxicity | Grade 3 | Grade 4 | Grade 5 | Grade 3, 4 or 5 Total (N=297) |
|---|---|---|---|---|
| Biochemical | 73a (24.6%) | |||
| Bilirubin | 35 (11.8%) | 4 (1.3%) | 0 | |
| AST/ALT | 41 (13.8%) | 0 | 0 | |
| ALP | 2(0.6%) | 0 | 0 | |
| Gastrointestinal | ||||
| Biliary | 1 (0.3%) | 1 (0.3%) | 1 (0.3%) | 3 (1.0%) |
| Luminal GI toxicity | 3 (1.0%) | 0 | 1 (0.3%) | 4 (1.3%) |
| Liver | ||||
| Ascites | 23 (7.7%) | 0 | 0 | 23 (7.7%) |
| Classic RILD | 0 (0%) | |||
| CP score decline ≥ 2 at 3 months after SBRT | 34 (15.9%) | |||
| ALBI Grade increase at 3 months after SBRT | 51 (21.2%) | |||
Toxicity reported includes acute and late toxicity at least possibly attributable to SBRT.
Some patients had more than one kind of biochemical toxicity.
DISCUSSION
This pooled North American analysis of 297 HCC patients without macrovascular invasion showed that SBRT was well tolerated and demonstrates long-term tumor control in the majority of patients. Despite the inclusion of patients with large tumors and those ineligible for, or with recurrence following standard local-regional therapies, who are typically excluded from other series, the median overall survival was 31·7 and 23·2 months for CP A and CP B/C patients, respectively. In addition to baseline CP A liver function, survival was best in patients with a better performance status, lower AFP levels and in those who went on to receive a transplant. Local recurrence of the irradiated HCCs was low (3·7% as isolated first recurrence and 13% at three years), and there was no significant dose effect, emphasizing the radiation responsiveness of HCC to SBRT. Thus, SBRT is a non-invasive ablative treatment with a high chance of long-term control even in HCC patients who are ineligible for other liver-directed therapies. Most reports of HCC treated with hepatic resection (five year OS 66·5% to 79·3%) or RFA (five year OS 49·8% to 67·4%) include only patients with no or minimal medical comorbidities, with CP A liver function, and limited tumor burden.17 In contrast, 40% of our patients received SBRT for recurrent HCC after other ablative or regional therapies, 30% had multifocal lesions, 42% were > 3cm, 54% were symptomatic with a reduced performance status (ECOG ≥1) and 22% had CP B or C liver function. Patients in the present cohort are higher risk than patients in series of other ablative therapies, including radiation series from Asia which tend to include earlier stage HCC and planned TACE prior to SBRT. These series report three-year OS from 53·8% to 73·5%8,11–13, 18–20 following photon RT and five-year OS and LC rates of 25–38·7% and 81–92·8% respectively following proton therapy.21–22 In spite of poor prognostic factors in the present cohort, SBRT controlled majority of tumors and conferred long-term survival in almost a quarter.
At three years, our local recurrence rate of 13·7% was slightly higher than the series by Takeda et al and Sanuki et al, that reported 90%−91% three-year local control.19,20 This may in part be due to the differences in patient populations cited earlier, and since competing risks were taken into account in the present study. Use of fiducial markers to aid in image guidance and breath-hold immobilization (surrogates for higher SBRT precision and accuracy) were associated with trends for improved local control. Local control was slightly lower in tumors larger than 3 cm compared to those less than 3cm,, but there was no worsening of local control for tumors larger than 5 cm. Despite the inclusion of large tumors, durable local control was observed in the majority of the patients, with three-year local control rates of 90% (95% CI: 81%−99%) and 80% (95% CI: 67%−93%) in HCCs ≤ 3cm and > 3 cm (up to 18 cm) respectively. The study results are not easily comparable to series of RFA or other interventions, since the patients included herein were generally ineligible for or had progressive despite prior loco-regional therapies; with this caveat, our results appear to be similar to series of RFA and/or TACE used to treat similar patients.23,24 For SBRT, effectiveness and safety are related to the volume of liver that can be spared and proximity of HCC to luminal gastrointestinal tissues.
Despite the low median biologic doses used, the wide range of doses used in this cohort, and the range of tumor sizes, local control rates are high, and prescribed dose to the tumor did not significantly affect the local control rates, at least at the time points studied, i.e. two to three years. This adds to the literature supporting the view that HCC is sensitive to moderate doses of radiation. However it is possible that with longer follow up, a dose-response may be observed. Delayed local recurrence beyond 1 year was seen in a minority of patients, providing rationale for continued imaging surveillance for these patients.
SBRT was associated with low toxicity. Only 1·3% of patients experienced ≥ grade 3 gastrointestinal (GI) luminal toxicity (gastric/duodenal ulcers, gastritis or upper GI bleed). Three patients (with either prior biliary enteric anastomoses or Y90), developed biliary toxicity. SBRT should be used with caution in such patients and given the lack of dose effect on local control, lower doses are preferred when treating central HCCs adjacent to the biliary tract. Classic RILD was not observed. The dose constraints to the critical organs-at-risk (normal liver, stomach, duodenum, large and small bowel) that were adopted in this study, as reported in prior publications9 and being the basis of the ongoing trials,10 seem to show continuing safety for routine clinical use. Baseline CP score B or C was associated with an increased risk of decline in liver function post SBRT.25,26 Interestingly, El Naqa et al found that approximately 10% of HCC patients who did not receive any therapy had a worsening of CP score at 3 months.27 Novel approaches to mitigate declining liver function and to reduce the risk of toxicity post SBRT are needed, especially in patients with impaired liver function.
Patients with poor liver function are best served with a liver transplant. In such patients, there is rationale for offering SBRT as a strategy to downsize and perhaps convert previously ineligible HCC patients (due to excessive volume) to become suitable for potential liver transplant. Not surprisingly, patients who received transplant post SBRT had far better survival then those who did not, adding to the growing body of evidence that SBRT, which is also non-invasive, may be used to bridge or downsize HCC to acceptable criteria for transplant.28
The predominant pattern of intrahepatic recurrence, inherent to patients with cirrhosis and multifocal and/or recurrent HCC, provides a compelling rationale for investigating combined modality treatment in high risk patients. To this end, TACE is being investigated in clinical trials in combination with SBRT,29 as are sorafenib9 and other systemic therapies.30
Emerging paradigms for combined modality therapy include the use of immunotherapy in advanced unresectable HCC patients. PD-1 checkpoint inhibitors, and more recently the combination of the PD-L1 inhibitor atezolizumab and the VEGF-inhibitor bevacizumab have shown effectiveness in HCC patients.31 Several case series have reported the elusive abscopal effect i.e. response of unirradiated metastases when the primary HCC is irradiated,32; preclinical work suggests that hypofractionated radiation is more immunogenic than conventional radiation therapy.33The combination of immunotherapy and radiation therapy has high promise to improve the therapeutic ratio in future HCC patients, and is an area of active investigation.34
A limitation of this study is that it is retrospective, with a heterogeneous high-risk HCC population, although the majority of patients from UM were from a prospective database. Also, RECIST 1.1, which was used for response assessment, is known limited. Studies of biomarkers and more objective strategies for monitoring response are needed not only following SBRT, but also following other HCC treatments.
CONCLUSION
In summary, this large North American series demonstrated that SBRT is a safe and effective option for high risk HCC patients unsuitable for or refractory to standard local treatment options. SBRT is associated with a high likelihood of sustained local HCC control, but the majority of patients develop progression or new HCCs outside the irradiated volume, providing rationale for combined modality studies in high-risk patients. Patients with CP B & C liver function are at increased risk of developing liver toxicity, and liver transplant should be considered in these patients if downsizing occurs following SBRT.
Fig 2.
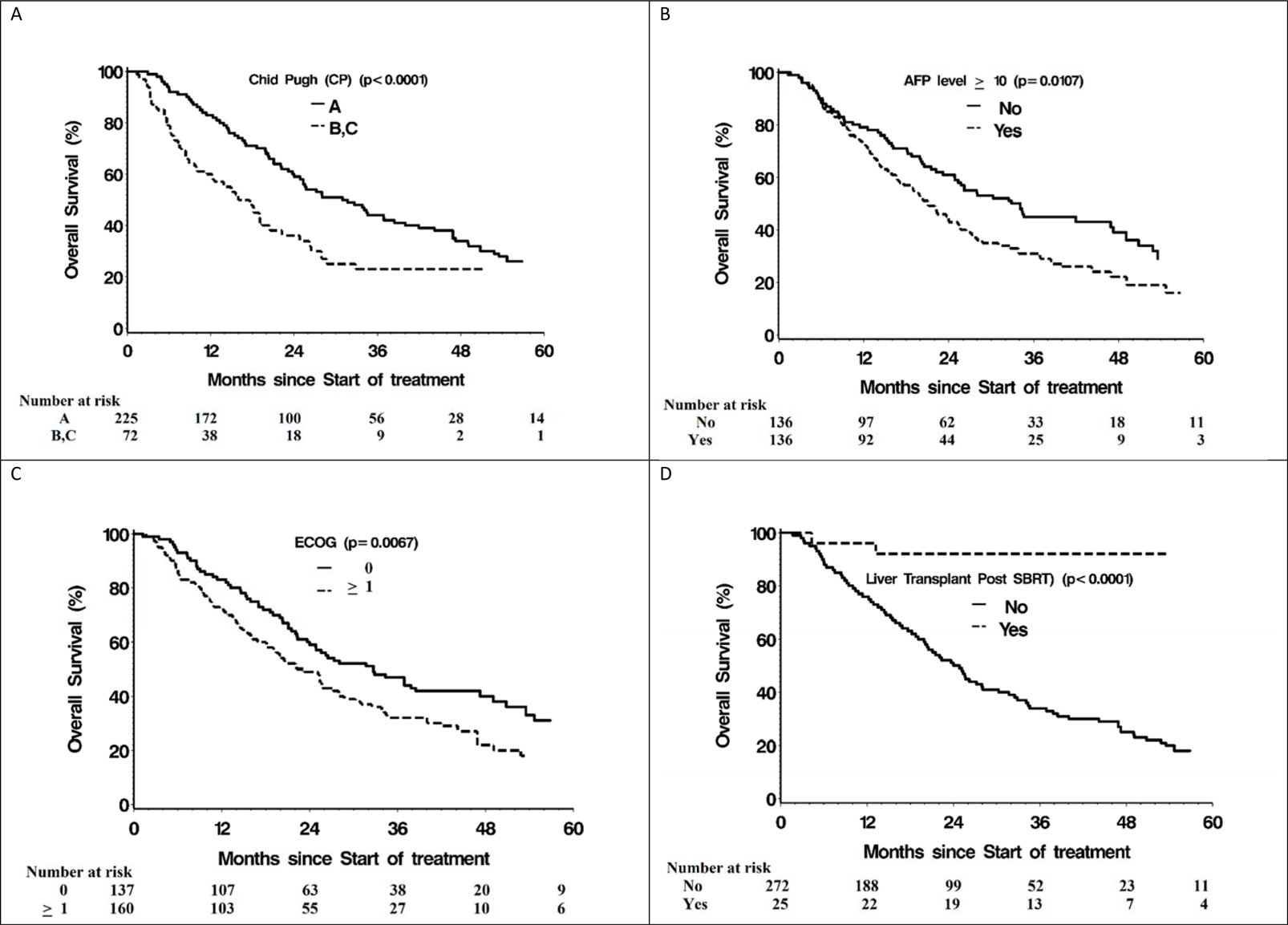
Overall Survival stratified by (A)Chid Pugh (CP) score, (B) Alpha-fetoprotein (AFP) level, (C) Eastern Co-operative Group (ECOG) Performance Score and (D) liver transplant post SBRT or not. CP- Child Pugh classification; ECOG- Eastern Co-operative Oncology Group; AFP- alpha-fetoprotein
HIGHLIGHTS.
Stereotactic radiation provides durable tumor control in early hepatocellular cancer
Patients unsuitable or refractory to other local therapies too had good outcomes
Good liver function and performance status at baseline predicted for better survival
Down staged tumors may be considered for liver transplant which improves survival
ROLE OF THE FUNDING SOURCE
This study was funded by grants from the National Institute of Health (NIH P01 Grant CA59827, NIH P30 CA46592, UL1TR000433), the A. Alfred Taubman Medical Research Institute, the National Cancer Institute of Canada (Grant 18207), and the Canadian Institutes of Health Research (CIHR Grant 20247). The sponsors had no role in any aspect of study design, data interpretation or manuscript preparation or review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
LAD has a licensing agreement for Raysearch image registration software (unrelated). AB has a consulting/advisory role with Astra Zeneca. KC received research funding from BTG and Varian Medical Systems. MF has a consulting/advisory role in GenomeDx, Myriad Pharmaceuticals, NanoString Technologies and Varian Medical Systems. She receives honoraria from Medivation/Astellas (also in Speaker’s bureau); Myriad Pharmaceuticals; Reflexion Medical and research funding from Celgene (I); Varian Medical Systems (Inst),She also received travel expenses from GenomeDx and has a pending patent for RadioType Dx, a biomarker test. ASM, EA, DO, RD, JK, JR, RW, CM, JB, CC and TSL had no conflicts of interest to declare.
REFERENCES
- [1].European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer: EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–943 [DOI] [PubMed] [Google Scholar]
- [2].Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999. December;30(6):1434ª–40. [DOI] [PubMed] [Google Scholar]
- [3].Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2011;107(4):569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Llovet JM, Real MI, Montana X, et al. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet 2002; 359: 1734–1739. [DOI] [PubMed] [Google Scholar]
- [5].Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019; pii: S1470–2045(18)30896–9. [DOI] [PubMed] [Google Scholar]
- [6].Palma DA, Olson R, Harrow S et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. [Published online April 10, 2019]. Lancet. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- [7].National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Hepatobiliary Cancers, Version 4.2018, October 22, 2018. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf [Google Scholar]
- [8].Murray LJ, Dawson LA. Advances in Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Semin Radiat Oncol. 2017;27(3):247–255. [DOI] [PubMed] [Google Scholar]
- [9].Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631–9. [DOI] [PubMed] [Google Scholar]
- [10].ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2012 Nov 21- . Identifier NCT01730937. Randomized Phase III Study of Sorafenib Versus Stereotactic Body Radiation Therapy Followed by Sorafenib in Hepatocellular Carcinoma; 2012 Nov 21 [cited 2018 Nov 19]; [about 4 screens]. Available from: https://clinicaltrials.gov/ct2/show/NCT01730937?term=RTOG+1112&cond=HCC&rank=2 [Google Scholar]
- [11].ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2009 May 4- . Identifier NCT00892658, A Phase I Study of Sorafenib and Radiation Therapy in Patients with Hepatocellular Carcinoma; 2009 May 4 [cited 2018 Nov 19]; [about 4 screens]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT00892658 [Google Scholar]
- [12].Feng M, Suresh K, Schipper MJ, et al. Individualised adaptive stereotactic body radiotherapy for liver tumors in patients a high risk for liver damage, a phase II trial. JAMA Oncology 2018;4(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sapir E, Tao Y, Schipper MJ, et al. Stereotactic body radiation therapy as an alternative to transarterial chemoembolization for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2018; 100: 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schwartz LH, Litière S, de Vries E et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016; 62: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].National Cancer Institute, CTEP. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03, June 14, 2010 Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. [Accessed on 10th May, 2018]. [Google Scholar]
- [16].Fine J, Gray R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association, 1999;94 (446), 496–509. [Google Scholar]
- [17].Duan C, Liu M, Zhang Z, Ma K, Bie P. Radiofrequency ablation versus hepatic resection for the treatment of early-stage hepatocellular carcinoma meeting Milan criteria: a systematic review and meta-analysis. World J Surg Oncol 2013; 11:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yoon SM, Lim Y-S, Park MJ et al. Stereotactic Body Radiation Therapy as an Alternative Treatment for Small Hepatocellular Carcinoma. PLoS ONE 2013; 8(11): e79854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Takeda A, Sanuki N, Tsurugai Y et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016. ;122(13):2041–2049. [DOI] [PubMed] [Google Scholar]
- [20].Sanuki N, Takeda A, Oku Y et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: A retrospective outcome analysis in 185 patients, Acta Oncologica, 2014; 53:(3), 399–404. [DOI] [PubMed] [Google Scholar]
- [21].Qi WX, Fu S, Zhang Q, Guo XM. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: A systematic review and meta-analysis. Radiother Oncol, 2015;114; (3), 289 – 295 [DOI] [PubMed] [Google Scholar]
- [22].Hong TS, Wo JY, Yeap BY et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients with Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol. 2016;34(5):460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Choi D, Lim HK, Rhim H et al. Percutaneous Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma After Hepatectomy: Long-term Results and Prognostic Factors. Ann Surg Oncol. 2007;14,2319–2329. [DOI] [PubMed] [Google Scholar]
- [24].Peng ZW, Zhang YJ, Liang HH,et al. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262(2):689–700. [DOI] [PubMed] [Google Scholar]
- [25].Culleton S, Jiang H, Haddad CR, et al. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014. ;111(3):412–7. [DOI] [PubMed] [Google Scholar]
- [26].Velec M, Haddad CR, Craig T, et al. Predictors of liver toxicity following stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2017;97:939–946. [DOI] [PubMed] [Google Scholar]
- [27].El Naqa I, Johannsson A, Owen D et al. Modeling of Normal Tissue Complications using Imaging and Biomarkers after Radiation Therapy for Hepatocellular Carcinoma. Int J Radiation Oncol Biol Phys. 2018; 100 (2):pp 335e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sapisochin G, Barry A, Doherty M et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67(1):92–99. [DOI] [PubMed] [Google Scholar]
- [29].ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2016 Jun 9- . Identifier NCT02794337, Integrated Phase II/III Randomized Control Trial of Transarterial Chemoembolization Alone or in Combination with Stereotactic Body Radiation in Patients With Unresectable Hepatocellular Cancer; 2016 Jun 9 [cited 2019 Sept 11];[about 4 screens]. Available from: https://clinicaltrials.gov/ct2/show/NCT02794337?term=NCT02794337&rank=1 [Google Scholar]
- [30].ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2017 Oct 20. Identifier NCT03316872, Pembrolizumab and Stereotactic Radiotherapy Combined in Subjects with Advanced Hepatocellular Carcinoma - A Phase II Study; 2017 Oct 20 [cited 2019 Apr 17]; [about 4 screens]. Available from: https://clinicaltrials.gov/ct2/show/NCT03316872?term=PEMRAD&rank=1. [Google Scholar]
- [31].Finn RS, Ducreux M, Qin S, et al. aIMbrave150: A randomized phase III study of 1L atezolizumab plus bevacizumab vs sorafenib in locally advanced or metastatic hepatocellular carcinoma. DOI: 10.1200/JCO.2018.36.15_suppl.TPS4141. J Clin Oncol. 2018;36: no. 15_suppl. [Google Scholar]
- [32].Chiang C-L, Chan ACY, Chiu KWH and Kong F-M. Combined Stereotactic Body Radiotherapy and Checkpoint Inhibition in Unresectable Hepatocellular Carcinoma: A Potential Synergistic Treatment Strategy. Front. Oncol 2019, 9:1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tang C, Welsh JW, De Groot P, et al. Ipilimumab with stereotactic ablative radiation therapy: Phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res. 2017;23:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yarchoan M, Agarwal P, Villanueva A et al. Recent Developments and Therapeutic Strategies against Hepatocellular Carcinoma. Cancer Res. 2019. 79;(17) 4326–4330. [DOI] [PMC free article] [PubMed] [Google Scholar]


