Supplemental Digital Content is available in the text.
Keywords: exosome, hypoxia, miR-210, neural progenitor cells
Abstract
Hypoxia as a microenvironment is essential for the development of neural stem/progenitor cells (NSCs/NPCs). Our previous studies showed that mild hypoxia can promote proliferation of NPCs. However, the underlying mechanisms are remaining unknown. In the present study, we explored the impact of hypoxia on miR-210 secretion and its effect on cell viability. We found that short time or long time of hypoxia treatment could increase the expression of miR-210, but also promoted its secretion into the medium. The results of exosomes isolation and quantitative real-time PCR showed that hypoxia increased the levels of miR-210 in the exosome enriched from the medium. In addition, the secreted miR-210 can be absorbed by recipient NPCs. The resutls of cell viability assay showed that low levels of secreted miR-210 slightly increased cell viability of NPCs. In contrast, high levels of secreted miR-210 exhibited an inhibitory effect on cell viability. These effects were blocked by an miR-210-specific inhibitor. Taken together, hypoxia increased secretion of miR-210 in exosomes and exhibited a differential effect on cell viability of recipient NPCs.
Video abstract: http:/links.lww.com/WNR/A588.
Introduction
Hypoxia as a microenvironment is essential for the development of neural stem/progenitor cells (NSCs/NPCs). Increasing studies have showed that the proliferation of NPCs is enhanced when exposed to low, but physiologic O2 concentrations of about1.5–3% [1]. For example, our previous report had found that mild hypoxia is more beneficial to NPCs proliferation. Exposure to 3–10% O2 for 3 days promoted the proliferation of NPCs and favored their depaminergic differentiation [2,3]. Neurogenesis in the adult brain occurs mainly within two neurogenic regions, the dentate gyrus of the hippocampus and the subventricular zone (SVZ) of the forebrain. Zhang et al. [4] found that an external hypoxia environment could reduce the intrinsic O2 content in both the dentate gyrus and SVZ region. In addition, in-vivo studies also have showed that hypoxia increased the number of endogenous NPCs in both the dentate gyrus and SVZ region [5]. In contrast to the extensive studies of the impact of hypoxia on NPCs characteristics, the underlying mechanisms are rarely known. Exploring the underlying molecular mechanisms is helpful to elucidate neural development and provide basis for its clinical application on the therapeutics of neurological diseases.
Noncoding microRNAs (miRNAs) are a class of single-strand, endogenous, noncoding RNAs that are ~22 nucleotides in length. In recent years, miRNAs have been demonstrated to be involved in numerous biological processes, including cell proliferation, differentiation and apoptosis, and the incidence and progress of diseases. MicroRNA-210 (miR-210), the key hypoxia-related miRNA, was found to be upregulated in many different types of cells under hypoxia conditions. It has been revealed that miR-210 is involved in angiogenesis, cell cycle, differentiation and apoptosis, etc. [6]. Previously, we have found that the expression of miR-210 was highly induced in NPCs under hypoxia conditions [7]. The upregulation of miR-210 can directly suppress BNIP3 expression to maintain the survival of NPCs under hypoxia [8]. Recently, it is reported that miR-210 is required for normal mouse NPC-cycle proliferation and cell-cycle progress [9]. These reports suggest that miRNA-210 is essential for cell survival and proliferation in NPCs. Moreover, miRNA-210 is also enriched in exosomes derived from many types of cells, like cardiac progenitor cells [10], mesenchymal stem cells [11], hepatocellular carcinoma cells [12], etc. Therefore, it is very interesting to investigate whether hypoxia promote secretion of miR-210 in NPCs and its potential roles in cell responding to hypoxia
In the present study, based on cultured NPCs isolated from the mesencephalon of pregnant embryonic day 13.5 (E13.5) Sprague–Dawley rats, the impact of hypoxia on miR-210 secretion and its effect on cell viability were investigated.
Materials and methods
Cell culture and hypoxia treatment
NPCs were isolated and cultured as previously described [8]. Briefly, mesencephalic cells from E13.5 Sprague–Dawley rats were mechanically dissociated and maintained in DMEM/F12 medium with 2 mM L-glutamine, 5 IU of penicillin, 5 μg/ml streptomycin, 1% N2, 1% B27 (Life Technologies, Carlsbad, California, USA), 20 ng/ml epidermal growth factor, 20 ng/ml basic fibroblast growth factor (R&D Systems, Minnesota, USA). The newly isolated cells were seeded into Ultra-low attachment T-25 flasks. After 3–5 days of culture, the cells grew up to sphere. The primary neural spheres were defined as passage zero (P0) NPCs, and be subcultured into 2–5 generations, which were applied in the subsequent experiments. The NPCs were identified as Nestin positive, as described in our previous reports [13,14]. All animal studies were approved by the Institutional Animal Care and Use Committee of Institute of Military Cognition and Brain Sciences. For hypoxia treatment, cells were put in a O2 Control Glove Cabinet (COYLAB, Grass Lake, Michigan, USA) in which the O2 concentration is adjustable. The incubator chamber was flushed with air containing 5% CO2 and balanced with N2.
RNA isolation and quantitative real-time PCR
Total RNA was extracted by TRIzol (Invitrogen, Carlsbad, California, USA). Ambion’s miRNA Isolation Kit (Ambion, Austin, Texas, USA) was used to isolate miRNAs according to the protocol. The stem-loop reverse transcription PCR technique was used to examine mature miR-210. The stem-loop RT primer for miR-210 was designed as follows: 5′-CTCGTATGGAGTGCAGGGTCCGA GGTATTCGCACT CCATACGAGTCAGCCGC-3′. The qRT-PCR was performed using the QuantiTect SYBR Green PCR Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The StepOne Plus Real-Time PCR System (Applied Biosystems, Frederick, Maryland, USA) and the supplied analytical software were used. The PCR primers for mature miR-210 were designed as follows: Forward: 5′-CTGTGCGTGTGACAGC-3′; Reverse: 5′-GTGCAGGG TCCGAGGT-3′. U6 snRNA levels were used for normalization. The PCR primers for U6 were designed as follows: Forward: 5′-AACGCTTCACGAATTTGCG T-3′; Reverse: 5′-CTC GCTTCGGCAGCACA-3′.
miRNA transfection
miR-210 mimic, miR-210 inhibitor or negative control were synthesized by GenePharma Co. (Shanghai, China). Their sequences are as following:
miR-210 mimics sense: 5′-CUGUGCGUGUGACAGCGGCUGA-3′
miR-210 mimics antisense: 5′-AGCCGCUGUCACACGCACAGUU-3′
miR-210 mimics NC sense: 5′-UUCUCCGAACGUGUCACGUTT-3′
miR-210 mimics NC antisense: 5′-ACGUGACACGUUCGGAGAATT-3′
miR-210 inhibitor: 5′-UCAGCCGCUGUCACACGCACAG-3′
miR-210 inhibitor NC: 5′-CAGUACUUUUGUGUAGUACAA-3′.
In accordance with the manufacturer’s instructions, cell transfection was performed using Lipofectamin 3000 (Invitrogen). Briefly, synthesized nucleic acids diluted in opti-MEM were incubated with Lipofectamine 3000 for 20 min before addition to cultures and transfection. After 6 h culturing, the medium was replaced using a fresh complete medium. All related experiments were performed within 48 h after transfection
Exosomes isolation
Exosomes were isolated from a conditioned culture medium using the ultracentrifugation method. Briefly, the media collected from NPCs were centrifuged at 2000×g, at 4°C for 10 min to remove cells and debris. The resulting supernatant was transferred to new tubes and centrifuged at 10000×g, at 4°C for 10 min. The supernatant was transferred to Beckman 60 Ti ultracentrifuge tubes and centrifuged at 126 000×g for 60 min at 4°C. The resulting pellet was resuspended in 1 ml PBS containing a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), and centrifuged at 135 000×g for 1 h at 4°C in a Beckman TLA110 centrfuge. The resulting pellet (containing exosomes) was resuspended in PBS containing protease inhibitors.
Exosome identification by transmission electron microscopy
Transmission electron microscopy (TEM) was used to capture the morphological features of exosome. The isolated exosomes were resuspended in PBS, and 10 μl of the diluted mixture was adsorbed on a copper mesh for 10 min. Subsequently, the copper mesh was transferred to a 3% glutaraldehyde droplet and suspended for 5 min. The copper mesh was then transferred to a drop of deionized water for cleaning, 10 cycles for 2 min each time. Finally, staining with uranyl acetate was done for 1 min. After drying naturally, a TEM (Thermo scientific, New York, USA) was used to take a picture.
Immunofluoresence
The cells were washed with PBS two times, and fixed with 4% paraformaldehyde for 30 min at room temperature. Then, the cells were washed three times and permeabilized with 0.25% Triton X-100 for 10 min. After washing three times with PBS, the cells were immersed in 1% BSA for 30 min. After three times of washing, the cells were incubated with DAPI (working concentration is 100 μg/ml) for 10 min at room temperature. The cells were examined by fluorescent microscopy at total 1000× magnification under immersion oil using an LSM 510 META ZEISS fluorescent microscope.
Cell viability assay
Cell viability was determined by CCK-8 kit (Dojindo Laboratories, Kumamoto, Japan).The NPCs were cultivated with 100 μl medium in poly-D-lysine-coated 96-well plates in 21, 3, and 0.3% O2 for the indicated times. We then added 10 μl of CCK-8 into each well and settled the culture cluster in a 37°C incubation chamber for 2 h. The optical density at 450 nm (OD450) was detected by a microplate reader (Thermo Scientific Multiskan GO, Waltham, Massachusetts, USA).
Statistical analysis
All data were expressed as the mean ± SEM, and the statistical analysis was carried out using GraphPad Prism (version 6.0, Graphpad Software Inc., California, USA). The data were assessed using the unpaired two-tailed Student’s t-test for comparisons between two groups, or one-way analysis of variance followed by Dunn’s multiple comparison post-hoc test for comparisons of more than two groups. Values less than 0.05 were considered statistically significant.
Results
Hypoxia increased expression and secretion of miR-210 in neural progenitor cells
We first detected the expression of miR-210 in NPCs under hypoxia conditions. NPCs isolated from the mesencephalon of E13.5 Sprague–Dawley rats were treated with different concentrations of O2 (21, 3, and 0.3% O2) for 0.5, 1, 2, 6, and 12 h, respectively. The results of qRT-PCR showed that the expression levels of miR-210 were kept constantly when exposed to 21% O2. However, hypoxia (3 and 0.3% O2) could evidently induce the expression of miR-210 in a time-dependent manner (Fig. 1a). Subsequently, we detected the levels of secreted miR-210 in a cell medium. The results of qRT-PCR showed that similar to the trend of miR-210 expression, the levels of secreted miR-210 did not change under 21% O2. In contrast, it significantly increased in 3 and 0.3% O2 groups (Fig. 1b). These results demonstrated that hypoxia not only increased miR-210 expression but also promoted its secretion into the cell medium.
Fig. 1.
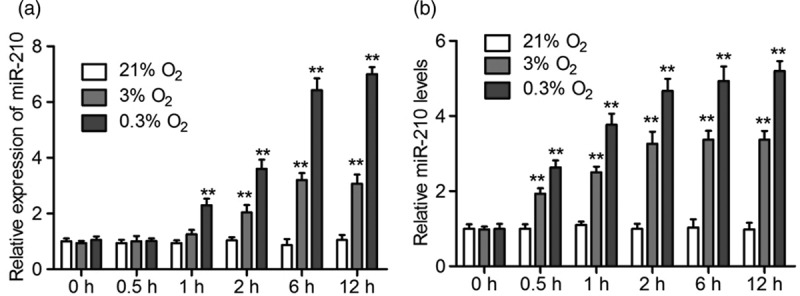
Short-time treatment of hypoxia induce miR-210 expression and its secretion in NPCs. (a and b) NPCs were exposed to 21, 3, and 0.3% O2, respectively, for 0.5, 1, 2, 6, and 12 h. The expression of miR-210 (a) and its content in the culturing medium (b) were analyzed by qRT-PCR. All experiments were performed in triplicate, and values are presented as the mean ± SEM (**P < 0.01). NPCs, neural stem/progenitor cells.
In order to further confirm the stimulation of hypoxia on miR-210 secretion, NPCs were treated with hypoxia for a longer time. The NPCs were treated with different concentrations of O2 (21, 3, and 0.3% O2) for 1, 2, and 3 days, respectively. The results of qRT-PCR showed that the expression of mir-210 was kept constantly under normoxia conditions, and hypoxia increased its expression, especially under 0.3% O2 conditions (Fig. 2a). The detection of miR-210 in the cell medium showed that the levels of miR-210 were increased when the cells were treated with hypoxia (Fig. 2b). These results further confirmed that hypoxia could promote the secretion of miR-210.
Fig. 2.
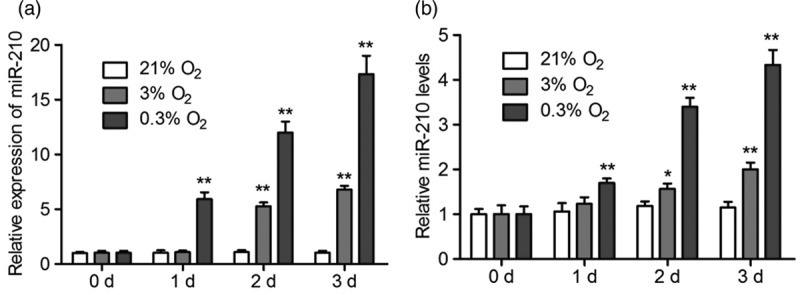
Long-time treatment of hypoxia induce miR-210 expression and secretion in NPCs. (a and b) NPCs were exposed to 21, 3 and 0.3% O2, respectively, for 1, 2, and 3 days. The expression of miR-210 (a) and its content in the culturing medium (b) were analyzed by qRT-PCR. All experiments were performed in triplicate, and values are presented as the mean ± SEM (*P < 0.05, **P < 0.01). NPCs, neural stem/progenitor cells.
MiR-210 can be secreted and absorbed by neural stem cells
It has been reported that circulating miR-210 is secreted via exosome as described above. Subsequently, we isolated the exosome by the ultracentrifugation method from the cell culturing medium and identified by TEM. As shown in Fig. 3a, the exosomes isolated from the medium had a distinct bilayer membrane and a typical cup-shaped structure. We then detected the levels of miR-210 in the exosomes and U6 were served as inner control. Our results showed that the levels of miR-210 in the exosomes enriched from 3% O2 group are much higher than that from the 21% O2 group. In contrast, the levels of U6 remained unchanged (Fig. 3b). These results demonstrated that hypoxia increased the levels of miR-210 in the exosome enriched from NPCs culturing medium.
Fig. 3.
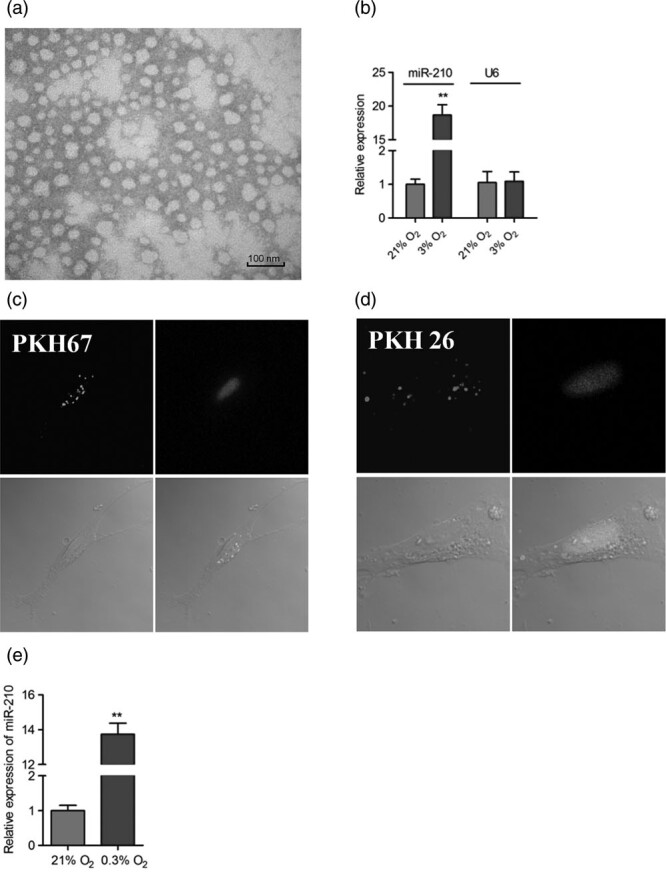
Secreted miR-210 is located in exosomes in the medium of NPCs. (a) Exosomes were collected by ultracentrifugation method and observed with TEM. Scale bar:100 nm. (b) NPCs were cultured under 21 and 3% O2 for 2 days, and then the exosomes in the medium were enriched. The expression of miR-210 in the exosomes were detected by qRT-PCR. (**P < 0.01). The experiment was performed in triplicate, and values are presented as the mean ± SEM (**P < 0.01). (c and d) Exosome collected from 0.3% O2-treated medium was stained with PKH67. (c) and PKH26 (d), respectively, and then were added into the medium of normoxia-cultured NPCs. One day later, the recipient NPCs were stained with DAPI and observed under fluorescent microscopy. (e) Exosome enriched from 0.3% O2-treated medium were added into the medium of normoxia-cultured NPCs. One day later, the expression of miR-210 in the NPCs was examined by qRT-PCR. (**P < 0.01). The experiment was performed in triplicate, and values are presented as the mean ± SEM (**P < 0.01). NPCs, neural stem/progenitor cells.
Exosome can be specifically stained with two lipophilic fluorochromes PKH67 (green) and PKH26 (red) [15]. We incubated the enriched exosomes with NPCs under normoxia condition. We found that the cells could be stained with green and red fluorescent probes (Fig. 3c and d), indicating that the extrinsic exosome transferred into the recipient cells. In addition, the exosome enriched from the hypoxia-treated NPCs medium could increased the levels of miR-210 in the recipient cells (Fig. 3e). These results further verified that hypoxia increased the levels of miR-210 in the exosome, which could be absorbed by recipient NPCs.
Effect of secreted miR-210 on the neural progenitor cells cell viability
To specify the potential roles of secreted miR-210 induced by hypoxia, we detected its effect on cell viability of NPCs using CCK-8 assay. First, three groups of NPCs were cultured under 21, 3, and 0.3% O2 for 2 days, respectively, and the medium containing secreted miR-210 was collected. As shown in Fig. 4a, the medium collected from 3% O2-treated group could increased cell viability of NPCs which maintained under normoxia, and the miR-21 specific inhibitor could reverse the increase of cell viability. In contrast, the medium collected from the group of 0.3% O2 exposure decreased cell viability of NPCs which maintained under normoxia condition, and an miR-21-specific inhibitor could reverse the decline of cell viability (Fig. 4b). These results suggested that different levels of secreted miR-210 exerted a differential effect on cell viability of NPCs.
Fig. 4.
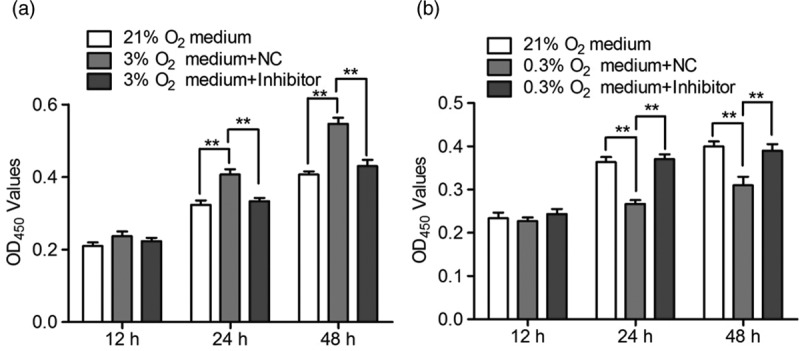
The effect of secreted mir-210 on cell viability of normoxia-cultured NPCs. (a) Control NPCs and NPCs transfected with negative control (NC) or specific inhibitor were treated with 21 and 3% O2, respectively, for 2 days. Then, the medium in each group was collected and applied to incubate the normoxia-cultured NPCs for 12, 24, and 48 h, respectively. The cell viability was measured by CCK-8 assay. (b) The same procedures were performed as described above, but the initiating control and tranfected NPCs were treated with 0.3% O2. All experiments were performed in triplicate, and values are presented as the mean ± SEM (**P < 0.01). NPCs, neural stem/progenitor cells.
The effect of different concentrations of secreted miR-210 on the neural progenitor cells cell viability
Next, we studied the effect of various doses of miR-210 on NPCs cell viability using miR-210 mimic. First, serious doses of miR-210 mimic were transfected into NPCs and their media were collected. Then, these media was applied to incubate the NPCs which maintained under normoxia conditions for 2 days. The results of CCK-8 assay found that the medium from NPCs transfected with low doses of miR-210 mimic could increase cell viability compared to the control group, but the medium from NPCs transfected with high doses of miR-210 exhibited an inhibitory effect on cell viability. As expected, miR-210 inhibition by specific inhibitor blocked these effects (Fig. 5). These resutls indicated that secreted miR-210 exerts a dual role in the regulation of cell viability.
Fig. 5.
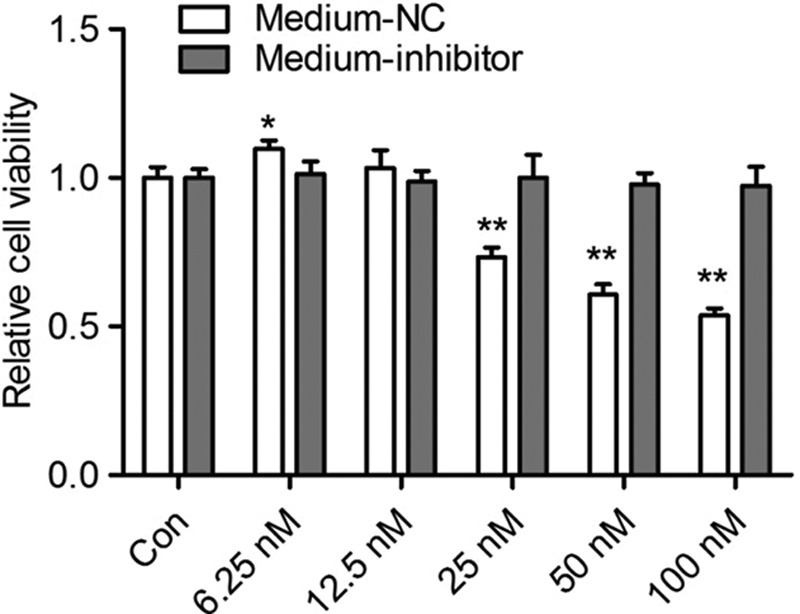
The effect of different concentrations of secreted mir-210 on cell viability of NPCs. NPCs were transfect with different doses (6.25–100 nmol) of miR-210 mimic. After 6 h of transfection, the medium were replaced with fresh complete medium and continued to culture for 2 days. Then, the medium was collected and applied to incubate the normoxia-cultured NPCs which was pretransfected with negative control (NC) or miR-210 specific inhibitor. After 2 days of culture, the cell viability was measured by CCK-8 assay. All experiments were performed in triplicate, and values are presented as the mean ± SEM (*P < 0.05, **P < 0.01). NPCs, neural stem/progenitor cells.
Discussion
Hypoxia has been shown to stimulate proliferation of endogenous NSCs in the brain. However, the mechanism of NSCs response to hypoxia has not been well defined yet. miRNA-210 has been identified as a major microRNA induced by hypoxia, and is associated with cell survival under hypoxia. For example, miR-210 expression is correlated closely with hypoxia and angiogenesis in breast cancer patients [16]. miRNA-210 inhibition was reported to significantly attenuate NSC proliferation at the early stages of differentiation [17]. In the current study, we found that the expression of miRNA-210 was sensitive to hypoxia conditions. The expression levels of miR-210 in NSCs was much higher under hypoxia conditions compared to that under normoxia conditions, and hypoxia increased its expression as well as its secretion in a time-dependent manner. More interestingly, we also found that secreted miR-210 located in the exosome in the medium of NPCs after hypoxia exposure.
Recent studies have reported that hypoxia induces the release of exosomes. King et al. [18] found that exposure of three different breast cancer cell lines to moderate (1% O2) and severe (0.1% O2) hypoxia resulted in significant increases in the number of exosomes present. Targeting HIF-1 pathway with RNA interfering prevented the enhancement of exosome release by hypoxia. In addition, the hypoxia-induced miR-210 was identified to be present at elevated levels in hypoxic exosome fractions [18]. It is also reported that hypoxia stimulates exosome production and secretion in renal tubular cells. The exosomes from hypoxic cells are protective against renal tubular cell injury [19]. In addition to the quantitative impact of exosome secretion, hypoxia stress also causes significant changes in the content and function of exosomes. An proteomic analysis of the cardiac fibroblasts exosome revealed that hypoxia conditions selectively increase the expression of proteins with extracellular matrix and signaling annotations [20]. Therefore, it is reasonable that hypoxia could regulate some cell characteristics by exosome.
The unique character of miR-210 is that it is absolutely regulated by hypoxia; however, its role under hypoxia conditions is controversial. For example, the rise of miRNA-210 level increases the apoptosis of neuronal cells through the activation of HIF-1α-VEGF signaling pathway [21]. Consistently, inhibition of microRNA-210 provides neuroprotective in hypoxic-ischemic brain injury [22]. Intracerebroventricular administration of an miR-210 inhibitor (miR-210-LNA) significantly decreased HI-induced brain infarct size and reversed the nicotine-increased vulnerability to brain HI injury in the neonate [23]. In contrast, there are also some reports found that miR-210 has a beneficial effect to any extent. It is reported that miRNA-210 also has a neuroprotective role on hypoxia-ischemic encephalopathy [24]. These differential effects of miR-210 maybe related with the disease models, extent of hypoxia injury, and the induced concentration of miR-210. Because miRNAs can simultaneously regulate many target genes, the downstream pathway was activated which is also critical for the effect of miR-210.
miRNAs not only exist intracellularly but can be released into almost all body fluids. More importantly, extracellular miRNAs or circulating miRNAs are sensitive, easily detectable, and highly stable [25,26]. Secreted miR-210 has great potential as biomarkers for the diagnosis and prognosis. This conclusion has testified by more increasing reports. It is newly reported that miR-210 might contribute to osteoarthritis development through promoting VEGF, which is an classical HIF-1 target gene. They found that upregulation of miR-210 in the synovial fluid occurs in the early stage of osteoarthritis and can be a useful biomarker for early diagnosis [27]. In patients with heart failure, mir-210 expression levels in the mononuclear cells were significantly higher than control. Plasma mir-210 levels may reflect a mismatch between the pump function of the heart and O2 demand in the peripheral tissues, and be a new biomarker for chronic heart failure [28]. In a mouse model with atherosclerosis, Li et al. found that miR-210 was upregulated and is an effective therapeutic target for atherosclerosis [29]. Another key point is that secreted miR-210 produced any biological effect on diseases. For example, it is reported that enriched levels of miR-210 caused by hypoxia resutls in exosomes with marked improved biological properties and therapeutic potential during mesenchymal stem cells therapy for cardiovascular diseases. Therefore, it is of significance to explore the role of miR-210 in different diseases, in that provide effective biological targets for clinical diagnosis and treatment.
Conclusion
In the present study, we found that short-time or long-time hypoxia treatment increased expression of miR-210, but also promoted its secretion into the medium. The levels of miR-210 in the exosome enriched from the medium were increased under hypoxia conditions. Moreover, the secreted miR-210 can be absorbed by NPCs and affects cell viability. These findings provided a reasonable molecular mechanism for the mild hypoxia-promoted proliferation of NPCs.
Acknowledgements
This research was supported by a key grant from National Natural and Science Foundation of China (No. 81430044) and a grant from Beijing Municipal Science and Technology Commission (No. Z161100000216134).
M.F. and L.Z. conceived and designed the experiments. M.Z., Y.G., F.W., X.C., and T.Z. performed experiments. M.Z. and Y.G. were involved in analysis and interpretation of data. M.Z., Y.G., and Y.Z. were involved in writing and review of the manuscript. M.F. and L.Z. were involved in study supervision.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Ming Zhao and Yan Gao contributed equally to the writing of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.neuroreport.com.
References
- 1.Csete M. Oxygen in the cultivation of stem cells. Ann N Y Acad Sci. 2005; 1049:1–8 [DOI] [PubMed] [Google Scholar]
- 2.Zhang CP, Zhu LL, Zhao T, Zhao H, Huang X, Ma X, et al. Characteristics of neural stem cells expanded in lowered oxygen and the potential role of hypoxia-inducible factor-1Alpha. Neurosignals. 2006; 15:259–265 [DOI] [PubMed] [Google Scholar]
- 3.Zhao T, Zhang CP, Liu ZH, Wu LY, Huang X, Wu HT, et al. Hypoxia-driven proliferation of embryonic neural stem/progenitor cells–role of hypoxia-inducible transcription factor-1alpha. FEBS J. 2008; 275:1824–1834 [DOI] [PubMed] [Google Scholar]
- 4.Zhang K, Zhou Y, Zhao T, Wu L, Huang X, Wu K, et al. Reduced cerebral oxygen content in the DG and SVZ in situ promotes neurogenesis in the adult rat brain in vivo. PLoS One. 2015; 10:e0140035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu LL, Wu LY, Yew DT, Fan M. Effects of hypoxia on the proliferation and differentiation of NSCs. Mol Neurobiol. 2005; 31:231–242 [DOI] [PubMed] [Google Scholar]
- 6.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010; 9:1072–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong L, Wang F, Huang X, Liu ZH, Zhao T, Wu LY, et al. DNA demethylation regulates the expression of miR-210 in neural progenitor cells subjected to hypoxia. FEBS J. 2012; 279:4318–4326 [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Xiong L, Huang X, Zhao T, Wu LY, Liu ZH, et al. miR-210 suppresses BNIP3 to protect against the apoptosis of neural progenitor cells. Stem Cell Res. 2013; 11:657–667 [DOI] [PubMed] [Google Scholar]
- 9.Abdullah AI, Zhang H, Nie Y, Tang W, Sun T. CDK7 and miR-210 co-regulate cell-cycle progression of neural progenitors in the developing neocortex. Stem Cell Reports. 2016; 7:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Jia Q, Xinnong C, Xie Y, Yang Y, Zhang A, et al. Role of cardiac progenitor cell-derived exosome-mediated microRNA-210 in cardiovascular disease. J Cell Mol Med. 2019; 23:7124–7131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J, Lu K, Zhang N, Zhao Y, Ma Q, Shen J, et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells Nanomed Biotechnol. 2018; 46:1659–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L, et al. Hepatocellular carcinoma cell-secreted exosomal microRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids. 2018; 11:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Zhu LL, Zhao T, Wu LY, Wu KW, Schachner M, et al. CHL1 negatively regulates the proliferation and neuronal differentiation of neural progenitor cells through activation of the ERK1/2 MAPK pathway. Mol Cell Neurosci. 2011; 46:296–307 [DOI] [PubMed] [Google Scholar]
- 14.Zhu LL, Zhao T, huang X, Liu ZH, Wu LY, Wu KW, Fan M. Gene expression profiles and metabolic changes in embryonic neural progenitor cells under low oxygen. Cell Reprogram. 2011; 13:113–120 [DOI] [PubMed] [Google Scholar]
- 15.Gangadaran P, Hong CM, Ahn BC. Current perspectives on w noninvasive tracking of extracellular vesicles with molecular imaging. Biomed Res Int. 2017; 2017:9158319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Zuo J. Emerging roles of miR-210 and other non-coding RNAs in the hypoxic response. Acta Biochim Biophys Sin (Shanghai). 2014; 46:220–232 [DOI] [PubMed] [Google Scholar]
- 17.Voloboueva LA, Sun X, Xu L, Ouyang YB, Giffard RG. Distinct effects of miR-210 reduction on neurogenesis: increased neuronal survival of inflammation but reduced proliferation associated with mitochondrial enhancement. J Neurosci. 2017; 37:3072–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012; 12:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Zhou X, Yao Q, Liu Y, Zhang H, Dong Z. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am J Physiol Renal Physiol. 2017; 313:F906–F913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosme J, Guo H, Hadipour-Lakmehsari S, Emili A, Gramolini AO. Hypoxia-induced changes in the fibroblast secretome, exosome, and whole-cell proteome using cultured, cardiac-derived cells isolated from neonatal mice. J Proteome Res. 2017; 16:2836–2847 [DOI] [PubMed] [Google Scholar]
- 21.Sun JJ, Zhang XY, Qin XD, Zhang J, Wang MX, Yang JB. MiRNA-210 induces the apoptosis of neuronal cells of rats with cerebral ischemia through activating HIF-1α-VEGF pathway. Eur Rev Med Pharmacol Sci. 2019; 23:2548–2554 [DOI] [PubMed] [Google Scholar]
- 22.Ma Q, Dasgupta C, Li Y, Bajwa NM, Xiong F, Harding B, et al. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol Dis. 2016; 89:202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Ke J, Li Y, Ma Q, Dasgupta C, Huang X, et al. Inhibition of miRNA-210 reverses nicotine-induced brain hypoxic-ischemic injury in neonatal rats. Int J Biol Sci. 2017; 13:76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu J, Zhou XY, Zhou XG, Cheng R, Liu HY, Li Y. Neuroprotective effects of microRNA-210 on hypoxic-ischemic encephalopathy. Biomed Res Int. 2013; 2013:350419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010; 56:1733–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008; 105:10513–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie W, Su W, Xia H, Wang Z, Su C, Su B. Synovial fluid microRNA-210 as a potential biomarker for early prediction of osteoarthritis. Biomed Res Int. 2019; 2019:7165406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endo K, Naito Y, Ji X, Nakanishi M, Noguchi T, Goto Y, et al. MicroRNA 210 as a biomarker for congestive heart failure. Biol Pharm Bull. 2013; 36:48–54 [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Yang C, Zhang L, Yang P. MicroRNA-210 induces endothelial cell apoptosis by directly targeting PDK1 in the setting of atherosclerosis. Cell Mol Biol Lett. 2017; 22:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


