Background:
Men who have sex with men (MSM) have a high prevalence of anal and penile human papillomavirus (HPV) infections with MSM living with HIV (MSMLH) bearing the highest rates. Data on anogenital high-risk HPV (hrHPV) among MSM in Rwanda and the associated risk factors are scant.
Methods:
We recruited 350 self-identified MSM aged 18 years living in Kigali, Rwanda, with 300 recruited from the community and 50 from partner clinics. Anal and penile specimens from all participants were analyzed for hrHPV using the AmpFire platform. Logistic regression was used to calculate crude odds ratios (ORs) and adjusted ORs (aORs) with 95% confidence intervals (95% CIs) as a measure of association between various factors and anal and penile hrHPV infection prevalence.
Results:
Anal hrHPV prevalence was 20.1%, was positively associated with having receptive anal sex with more partners (aOR: 9.21, 95% CI: 3.66 to 23.14), and was negatively associated with having insertive anal sex with more partners (aOR: 0.28, 95% CI: 0.12 to 0.66). Penile hrHPV prevalence was 35.0%, was negatively associated with having receptive anal sex with more partners (aOR: 0.29, 95% CI: 0.13 to 0.66), and differed significantly by HIV status, with 55.2% and 29.7% for MSMLH and HIV-negative MSM, respectively (P < 0.01).
Conclusion:
Penile hrHPV prevalence was higher than that of anal hrHPV and it was significantly higher in Rwandan MSMLH than in HIV-negative MSM. The prevalence of anal and penile HPV infections is likely variable at different locations in Africa, according to a number of factors including HIV status and sexual practices.
Key Words: MSM, anal HPV, penile HPV, HIV
INTRODUCTION
Anogenital human papillomavirus (HPV) infection is very common among men who have sex with men (MSM) and even higher among MSM living with HIV (MSMLH).1–7 High-risk HPV (hrHPV), with HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68, is the cause for virtually all cases of anal precancer and cancer. There is a paucity of data on anal and penile HPV infections in MSM living in Rwanda.
There is a growing body of evidence about anal HPV infection among MSM in sub-Saharan Africa (SSA), and multiple studies have found that anal HPV infection is significantly higher among MSM with a greater burden among MSMLH.8–10 The prevalence of anal HPV infection ranges from 40% among HIV-negative MSM to as high as 91% among MSMLH, as shown by a study performed in Nigeria.10 Anal HPV infection is also high among heterosexual individuals living with HIV, with 1 study performed in Zimbabwe showing a prevalence of anal HPV infection of 44% among a heterosexual population, including only 1 self-identified MSM.11 Data on penile HPV infection in SSA are still scanty, but some data among heterosexual men indicate a high prevalence of penile HPV infection, which is even higher among men living with HIV.12
Infection with HIV is particularly high in MSM compared with the general population, and trends indicate that HIV infection may continue to increase among MSM.13–17 There are limited data on HIV infection among MSM in SSA due to stigma and lack of access to health care services,18 as well as limited data on anal and penile HPV infections. It is therefore important to study HIV infection among MSM in SSA and its association with anogenital HPV infection.
The few studies performed among Rwandan MSM have shown evidence that this population is at particularly high risk of HIV infection and other sexually transmitted infections due to risky sexual behavior.19,20 Ntale et al found the prevalence of HIV infection among Rwandan MSM to be 4.8%,19 which is slightly higher than that among the general population, estimated at 3.0%.21 Risky sexual behavior among Rwandan MSM includes high numbers of male and female sexual partners, providing commercial sex with both men and women, low condom use during anal and vaginal sex, and high mobility.22 This is further complicated by intense societal stigma, social isolation, and discrimination, which play a significant role in individual susceptibility to engage in risky sexual behavior.20 A recent study performed in Togo found the prevalence of HIV and anal hrHPV infections among MSM to be 26.1% and 44.9%, respectively, with anal hrHPV infection being higher among MSMLH than HIV-negative MSM,23 indicating a high burden of HIV and anogenital HPV among this key population.
Therefore, understanding HIV infection and anogenital HPV infection in this population is important to optimize strategies to prevent both HIV infection and HPV-related anogenital cancer. Here, we report results from a cross-sectional baseline analysis of anal and penile HPV infections in a group of HIV-positive and HIV-negative Rwandan MSM.
METHODS
Study Design, Population, and Setting
This study is a prospective study of MSM living in Kigali, Rwanda. We recruited 300 MSM through community recruitment, which involved reaching out to the MSM community through their organizations as well as other individuals known to the community but who are not members of any MSM organization. We also recruited 50 MSM known to be living with HIV from partner clinics, some of which have worked with our research team previously and are known to be friendly to MSM as well as other clinics providing HIV care, all located in the city of Kigali. Recruitment also involved a snowball approach where 1 participant led us to another to reach the required number of participants. Participant recruitment occurred between March 30, 2016, and October 25, 2017, at Rwanda Military Hospital, Kigali, Rwanda. Eligibility criteria included at least 18 years of age, living in the city of Kigali, reporting any type of sex with another man in the 6 months before enrollment in the study, willingness to have HIV and CD4 cell count testing, and willingness to be contacted for follow-up visits.
Data Collection
During the appointment, an audio computer-assisted self-interview was used to complete a questionnaire in Kinyarwanda, the only local language in Rwanda. Questions included basic demographic and clinical information, including history of HIV testing and STIs, sexual behavior, and behavioral factors, including tobacco, alcohol, and nonprescription drug use.
After the audio computer-assisted self-interview, 5 mL of blood was collected for HIV testing. A penile specimen was collected for HPV testing by retracting the foreskin, if present, and rubbing the entire penile skin including the root, shaft, glans, and coronal sulcus with a 600-grit emery paper (sandpaper) followed by swabbing the penile skin with a moist Dacron swab, which was then placed in PreservCyt (Hologic, Bedford, MA) followed by vigorous shaking. An anal specimen was then collected for HPV testing by inserting a moist Dacron swab into the anal canal, which was then placed in PreservCyt. All specimens collected were transported to the Rwanda Military Hospital research laboratory for testing.
Laboratory Testing: HIV and HPV
HIV Testing
Participants received standard HIV pretest counseling followed by HIV testing according to Rwandan guidelines (rapid test with second and third tier verification depending on the initial result). Post-test counseling was provided, and the result was communicated to the participant. If the HIV test result was positive, an HIV viral load test and a CD4 cell count were performed.
HPV Testing
Anal and penile specimens were tested using the AmpFire HPV Genotyping Assay (Atila Biosystems, Inc., Mountain View, CA). The AmpFire HPV Genotyping Assay is an isothermal nucleic acid amplification-based, real-time fluorescence detection of 15 HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68) in 4 reaction tubes (the AmpFire Genotyping Assay). Testing was performed according to the manufacturer's protocol. In brief, an aliquot of the stored specimen in PreservCyt solution was pelleted by centrifugation, the supernatant was decanted, and pelleted cells were suspended in lysis buffer. The cell suspension incubated for 10 minutes at 95°C to lyse the cells. For each reaction, 2 μL of lysate was mixed with 12 μL of the reaction mix and 11 μL of one of the 4 reaction mixes. The resulting 4 reaction tubes for every sample were incubated in a Powergene 9600 fluorescence real-time polymerase chain reaction system at 60°C with fluorescence from FAM/HEX/ROX/CY5 channels measured every minute.
After running for approximately 1 hour, the amplification results were interpreted according to the exponential curves developed during the process. If the negative control showed no exponential curves and the positive control showed exponential curves, this experiment run was valid. The next step was to examine the set of 4 tubes corresponding to a specimen. Multiplex HPV infections could result in multiple exponential curves for a specimen. If no exponential curve other than the internal control (Hex channel in PM-3 tube) was present for a sample, this sample was negative. If there was no exponential amplification curve in any of the 4 tubes/any fluorescence channels, the sample failed the test. A failed sample usually indicated not enough DNA in the sample, and it was reprocessed.24 The AmpFire HPV Genotyping Assay has been compared with other assays, and the available literature was on formalin-fixed, paraffin-embedded tissues.25
Statistical Analysis
Baseline characteristics and penile and anal HPV prevalence by HIV status were compared using the Fisher exact test. The McNemar test was used to examine concordance between penile and anal HPV tests. Logistic regression was used to calculate crude odds ratios (ORs) and adjusted OR (aOR) with 95% confidence intervals (95% CIs) as a measure of association between various factors and anal and penile HPV infection prevalence. All analyses were performed with SAS statistical software (9.4; SAS Institute, Cary, NC). P values <0.05 were considered to be statistically significant.
Ethical Consideration
The study protocol was reviewed and approved by the Rwanda National Ethics Committee, and the Albert Einstein College of Medicine and the University of California San Francisco institutional review boards. All study participants provided written informed consent before enrollment to the study.
RESULTS
Baseline Characteristics
Data from 345 of 350 Rwandan MSM (295 community recruits and 50 specifically recruited MSMLH) with valid anal and penile HPV results and who reported sexual relations with another man were included in this analysis.
The prevalence of HIV infection in our study population was 19.4%, with an HIV prevalence of 5.6% in the community-based recruitment population whose HIV status at study entry was unknown. Among MSMLH, the median CD4 cell count was 537 cells/mm3, with an interquartile range of 416–622, and the median viral load was 1220 viral copies/mL, with an interquartile range of 128–20,700.
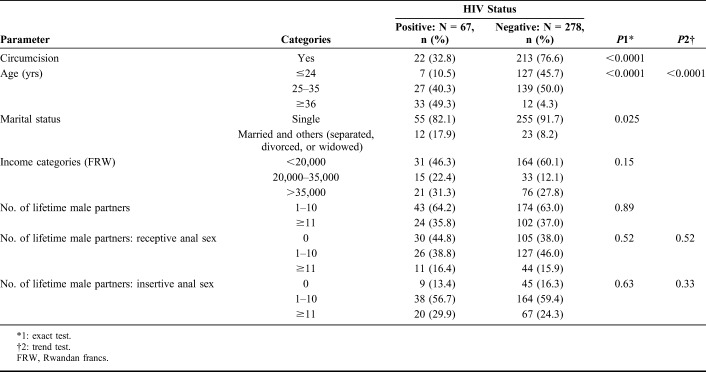
Almost half (48.1%) of our study participants were 25–35 years of age and 90% were single. Over half (56.5%) of our study participants were of very low socioeconomic status, with an average monthly income of <20,000 Rwandan francs (<22 US dollars), and most (68.1%) of our study participants were circumcised.
MSMLH were older than HIV-negative men (P < 0.001). Circumcision was less common among MSMLH than among HIV-negative men (32.8% vs. 76.6%, respectively, P < 0.001). Other factors did not differ by HIV status (Table 1).
TABLE 1.
Baseline Characteristics by HIV
Anal and Penile hrHPV Prevalence
The overall prevalence of anal hrHPV was 20.1% and penile hrHPV was 35.0% (P < 0.001). The prevalence of HPV16 (P = 0.64) and HPV18 (P = 0.49) did not differ significantly in anal vs. penile sites, but for the other hrHPV types, prevalence was significantly (P < 0.001) lower in the anus than on the penis (Fig. 1).
FIGURE 1.
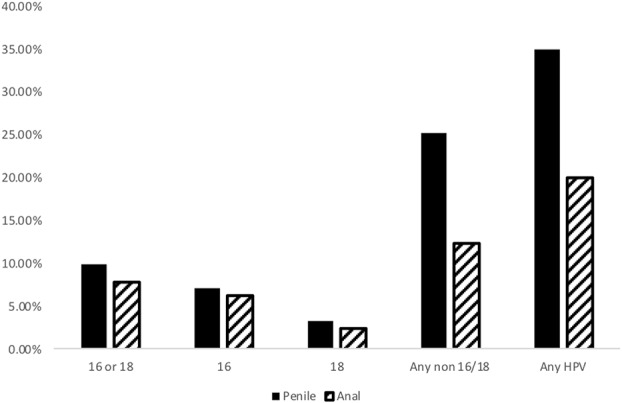
hrHPV 16, 18, and all non-16/18 hrHPV types combined. P < 0.0001 for any non-16/18 and any HPV based on the McNemar test.
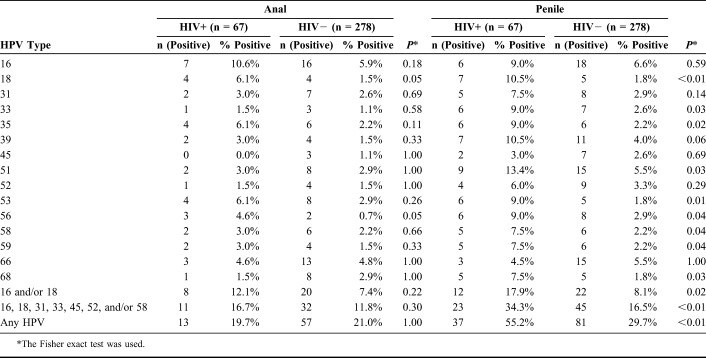
The overall prevalence of anal hrHPV infection was not significantly different by HIV status: 19.7% and 21.0% among MSMLH and HIV-negative men, respectively (P > 0.99). However, the prevalence of penile hrHPV infection was higher among MSMLH (55.2% compared with 29.7% among the HIV-negative men, P = 0.0002), Table 2.
TABLE 2.
Number of Anal and Penile High-Risk HPV Types and Overall HPV Positivity by HIV Status
There were no statistically significant differences by HIV status in the positivity of individual HPV types for anal HPV infection with each type considered separately, as shown in Table 2. For some types of penile HPV, MSMLH had significantly higher proportions of positivity: HPV 18, 33, 35, 51, 53, 56, 58, 59, and 68 (Table 2).
The prevalence of some HPV types was significantly higher on the penis than in the anus, including HPV 33, 39, 45, 51, and 56 (Fig. 2).
FIGURE 2.
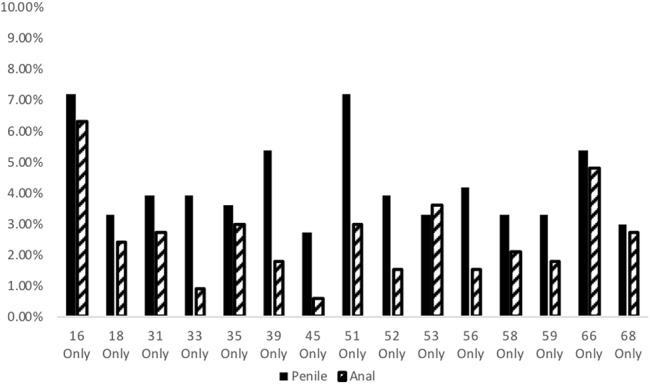
Prevalence of individual HPV genotypes by anatomical location. P < 0.05 for HPV 33, 39, 45, and 56 based on the McNemar test. P < 0.01 for HPV 51 based on the McNemar test.
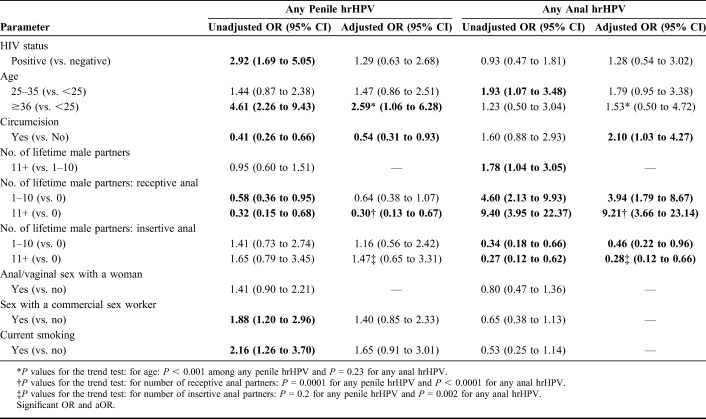
The factors associated with prevalent anal and penile hrHPV infections are shown in Table 3. Age 36 years and older compared with age younger than 25 years was positively associated (aOR = 2.76, 95% CI: 1.15 to 6.63), with prevalent penile hrHPV. Negative associations for penile hrHPV infection included being circumcised (aOR 0.52, 95% CI: 0.3 to 0.9) and more lifetime receptive anal sex (aOR = 0.29, 95% CI: 0.13 to 0.66). Positive associations with anal hrHPV infection included being circumcised (aOR = 2.1, 95% CI: 1.03 to 4.27) and receptive anal sex with many lifetime partners (aOR = 9.21, 95% CI: 3.66 to 23.14). The only negative association for anal hrHPV infection was having insertive sex with many lifetime partners: (aOR 0.28, 95% CI: 0.12 to 0.66).
TABLE 3.
Logistic Regression Model for the Association of Predictors and Any hrHPV Type for Both Penile and Anal hrHPV Infections (all Variables Were Categorical)
DISCUSSION
Our findings showed the prevalence of HIV infection among the community-based recruited group to be 5.6%. These findings are consistent with other estimates of HIV infection among Rwandan MSM where 1 study found an HIV prevalence of 4.8%.19
Although this prevalence is higher than that reported in the general Rwandan population of men, which is 2.2%, it is similar to the prevalence of HIV infection in men in urban settings, which is 5%.26
However, the prevalence of HIV infection in our study population is much lower than that reported among other MSM populations, such as those in North America and Asia which range from 40% to 50% or more1,4,15 as well as a recent study performed in Togo, which showed an HIV prevalence among MSM of 26.1%.23
A recent systematic review and meta-analysis comparing the prevalence of HIV infection between MSM and men in the general population found that MSM were almost 5 times more likely to be infected with HIV than men in the general population, with average prevalence rates of 17.8% and 6.2%, respectively.27 Our finding of an overall HIV prevalence of 19.4% is consistent with that found in a study in Monrovia, Liberia, where they found a self-reported prevalence of 19.6% among MSM.28 However, 1 study in Taiwan found 4% HIV prevalence in a community-based population of MSM, consistent with our finding.7
There is compelling evidence that the prevalence of anal HPV infection is higher among MSM and even higher among MSMLH.10,29,30 Our finding on the prevalence of anal HPV infection in Rwandan MSM differs from that reported in most other MSM populations. First, the overall prevalence of anal hrHPV infection (20.1%) was lower than that found in other MSM populations. Second, the prevalence of anal hrHPV infection was similar among MSMLH and HIV-negative men, whereas in most studies, MSMLH have a higher prevalence of anal hrHPV infection than HIV-negative MSM.1,4,5 Third, the prevalence of penile hrHPV infection was higher than that of anal hrHPV infection.
Our finding of low anal hrHPV infection may be explained by the fact that most individuals from our population practice insertive anal sex and that few Rwandan MSM have receptive anal sex especially for transactional purposes with provision of commercial sex being predominant among few MSM. This difference in sexual practices in this understudied population of Rwandan MSM needs further exploration, but some reports on this population agree that transactional sex is common, with 46% reporting receiving payment for sex with another man.19
The prevalence of penile hrHPV infection (35.0%) in our study was higher than that found in the previously reported study performed in Taiwan, which showed a penile hrHPV infection rate of 11%,7 but most of the other studies indicate a higher prevalence of penile hrHPV infection than our finding.31,32 However, our finding that the prevalence of penile hrHPV infection was higher among MSMLH than HIV-negative men is consistent with some other studies, such as those performed in the Netherlands33 and in Peru where external genital sites including the penis were combined34 and also consistent with a study among heterosexual men in Uganda.12
Risk factors for penile hrHPV infection included absence of circumcision and more partners for insertive anal sex, whereas anal hrHPV infection was associated with being circumcised and having more partners for receptive anal sex. Circumcision being protective for penile hrHPV infection is consistent with a study performed in India32 as well as studies performed on heterosexual men in Uganda.35–37 It is noteworthy that the 2014–2015 Rwanda Demographic and Health Survey showed that 30% of men aged 15–49 years were circumcised and that circumcision was highest (44%) among those aged 20–24 years and lowest (18%) among those aged 40–45 years.26
Our study was limited by having fewer individuals practicing receptive anal sex with many individuals practicing insertive anal sex, although this explains why we have low anal hrHPV prevalence and high penile hrHPV prevalence. In addition, we think that there may be a selection bias especially among the 50 MSMLH recruited from partner clinics who tend to be older and very few reported having receptive anal sex. Overall, having sex with another man being self-reported might also have contributed to misclassification of the MSM status and, hence, the unusual findings. These unusual findings may not reflect the actual situation in the entire Rwandan MSM community because we have information that we have a significant number of MSM living outside the city of Kigali.38
The current policy on HPV vaccination in Rwanda is to immunize all girls aged 12 years, and the coverage has been very good (≥90%) over the past 9 years, with the first coverage rounds in 2011 achieving coverage ranging between 93% and 95%.39 With more evidence on HPV infection among MSM being presented, we hope that HPV vaccination will be introduced soon for boys and for adolescents and young adults, including MSM, in the near future.
CONCLUSIONS
In this study of urban Rwandan MSM, an unusual pattern of anal HPV infection was observed. Penile hrHPV prevalence was higher than anal hrHPV in both MSMLH and HIV-negative MSM, and it was significantly higher in MSMLH than in HIV-negative men. The prevalence of anal and penile hrHPV infections is likely variable at different locations in Africa according to a number of factors including HIV status and sexual practices.
Understanding the prevalence of anogenital HPV as well as sexual practices in this key population is paramount to dealing with HPV-related anogenital cancers (anal and penile) and their relationship with HIV infection. This study, as the first to report on anal and penile HPV infections, is valuable to the scientific community in Rwanda where health issues for key populations, including MSM, are of growing public health concern, and MSM are currently being given access to essential services such as HIV pre-exposure prophylaxis and perhaps for the future, HPV vaccination.
ACKNOWLEDGMENTS
The authors acknowledge the great contribution of the participants to this study as well as all the study team including nurses, laboratory technicians, the data manager, and the administration and finance team.
Footnotes
Supported by an NCI/NIH Grant (5U54CA19016304).
The authors have no conflicts of interest to disclose.
G.M. wrote up the initial article draft. P.E.C., K.A., and J.M.P. conceived the study design and performed the initial in-depth editing and review of the article. H.-Y.K. performed the analysis. A.M., P.T., T.M.Z., A.M.B., A.A., and L.M. reviewed the article and contributed in various aspects of realizing the study.
REFERENCES
- 1.Palefsky JM, Holly EA, Ralston ML, et al. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)– positive and HIV-negative homosexual men. J Infect Dis. 1994:361–367. [DOI] [PubMed] [Google Scholar]
- 2.Critchlow CW, Hawes SE, Kuypers JM, et al. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS. 1998;12:1177–1184. [DOI] [PubMed] [Google Scholar]
- 3.van der Snoek EM, Niesters HGM, Mulder PGH, et al. Human papillomavirus infection in men who have sex with men participating in a Dutch gay-cohort study. Sex Transm Dis. 2003;30:639–644. [DOI] [PubMed] [Google Scholar]
- 4.Lin C, Hsieh MC, Hung HC, et al. Human papillomavirus prevalence and behavioral risk factors among HIV-infected and HIV- uninfected men who have sex with men in Taiwan. Medicine (Baltimore). 2018;97:e13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somia IKA, Teeratakulpisarn N, Jeo WS, et al. Prevalence of and risk factors for anal high-risk HPV among HIV-negative and HIV-positive MSM and transgender women in three countries at South-East Asia. Medicine (Baltimore). 2018;97:e9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ucciferri C, Tamburro M, Falasca K, et al. Prevalence of anal, oral, penile and urethral Human Papillomavirus in HIV infected and HIV uninfected men who have sex with men. J Med Virol. 2018;90:358–366. [DOI] [PubMed] [Google Scholar]
- 7.Strong C, Zou H, Ko NY, et al. Prevalence and risk factors of anogenital human papillomavirus infection in a community sample of men who have sex with men in Taiwan: baseline findings from a cohort study. Sex Transm Infect. 2019;96:62–68. [DOI] [PubMed] [Google Scholar]
- 8.Bouassa RM, Mbeko Simaleko M, Camengo SP, et al. Unusual and unique distribution of anal high- risk human papillomavirus (HR-HPV) among men who have sex with men living in the Central African Republic. PLoS One. 2018;13:e0197845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller EE, Rebe K, Chirwa TF, et al. The prevalence of human papillomavirus infections and associated risk factors in men-who-have-sex-with-men in Cape Town, South Africa. BMC Infect Dis. 2016;16:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowak RG, Gravitt PE, He X. Prevalence of anal high-risk human papillomavirus infections among HIV-positive and HIV-negative men who have sex with men in Nigeria. Sex Transim Dis. 2016;43:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinyowa S, Palefsky JM, Chirenje ZM, et al. Anal human papillomavirus infection in HIV-positive men and women at two opportunistic infections clinics in Harare, Zimbabwe. BMC Public Health. 2018;18:1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobian AA, Grabowski MK, Kigozi G, et al. High-risk human papillomavirus prevalence is associated with HIV infection among heterosexual men in Rakai, Uganda Aaron. Sex Transm Infect. 2014;89:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Hao C, Huan X, et al. HIV incidence and associated factors in a cohort of men who have sex with men in Nanjing, China. Sex Transm Dis. 2010;37:208–213. [DOI] [PubMed] [Google Scholar]
- 14.Scheer S, Kellogg T, Klausner JD, et al. HIV is hyperendemic among men who have sex with men in San Francisco: 10-year trends in HIV incidence, HIV prevalence, sexually transmitted infections and sexual risk behaviour. Sex Transm Infect. 2008;84:493–498. [DOI] [PubMed] [Google Scholar]
- 15.Stall R, Wisniewski SR, Friedman MS, et al. Running in place: implications of HIV incidence estimates among urban men who have sex with men in the United States and other industrialized countries. AIDS Behav. 2009;13:615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. Subpopulation estimates from the HIV incidence surveillance system—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:985–989. [PubMed] [Google Scholar]
- 17.van Griensven F, Varangrat A, Wimonsate W, et al. Trends in HIV prevalence, estimated HIV incidence, and risk behavior among men who have sex with men in Bangkok, Thailand, 2003-2007. J Acquir Immune Defic Syndr. 2010;53:234–239. [DOI] [PubMed] [Google Scholar]
- 18.Smith AD, Tapsoba P, Peshu N, et al. Men who have sex with men and HIV/AIDS in sub-Saharan Africa. Lancet. 2009;374:416–422. [DOI] [PubMed] [Google Scholar]
- 19.Ntale RS, Rutayisire G, Mujyarugamba P, et al. HIV seroprevalence, self-reported STIs and associated risk factors among men who have sex with men: a cross-sectional study in Rwanda, 2015. Sex Transm Infect. 2019;95:71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adedimeji A, Sinayobye JD, Asiimwe-Kateera B, et al. Social contexts as mediator of risk behaviors in Rwandan men who have sex with men (MSM): implications for HIV and STI transmission. PLoS One. 2019;14:e0211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.RBC. October 2019 Rwanda population-based HIV impact assessment summary results. Prelim Find Dissem. 2019:2–7. [Google Scholar]
- 22.Chapman J, Koleros A, Delmont Y, et al. High HIV risk behavior among men who have sex with men in Kigali, Rwanda : making the case for supportive prevention policy. AIDS Care. 2011;23:449–455. [DOI] [PubMed] [Google Scholar]
- 23.Ferré VM, Gbeasor-Komlanvi FA, Collin G, et al. Prevalence of human papillomavirus, human immunodeficiency virus, and other sexually transmitted infections among men who have sex with men in Togo: a national cross-sectional Survey. Clin Infect Dis. 2019;69:1019–1026. [DOI] [PubMed] [Google Scholar]
- 24.Atila Biosystems. Genotype 15 High Risk HPV by Fluorescent Detection, 2019. [Online]. Available at: https://atilabiosystems.com/our-products/genotype-15-high-risk-hpv-by-fluorescent-detection/. [Google Scholar]
- 25.Tang Y, Lozano L, Chen X, et al. An isothermal, multiplex amplification assay for detection and genotyping of human papillomaviruses in formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2019;22:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institute of Statistics of Rwanda (NISR) [Rwanda], Ministry of Health (MOH) [Rwanda]. Rwanda Demographic and Health Survey 2014–2015. Rockville, MD: NISR, MOH, and ICF International, 2015. [Google Scholar]
- 27.Hessou PHS, Glele-Ahanhanzo Y, Adekpedjou R, et al. Comparison of the prevalence rates of HIV infection between men who have sex with men (MSM) and men in the general population in sub-Saharan Africa : a systematic review and meta-analysis. BMC Public Health. 2019;19:1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieber M, Reynolds CW, Lieb W, et al. Human papillomavirus knowledge, attitudes, practices, and prevalence among men who have sex with men in Monrovia. Liberia. 2018;22:326–332. [DOI] [PubMed] [Google Scholar]
- 29.Lee CH, Lee SH, Lee S, et al. Anal human papillomavirus infection among HIV-infected men in Korea. PLoS One. 2016;11:e0161460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn JA, Belzer M, Chi X, et al. Pre-vaccination prevalence of anogenital and oral human papillomavirus in young HIV-infected men who have sex with men. Papillomavirus Res. 2019;7:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Bilsen WPH, Kovaleva A, Bleeker MCG, et al. HPV infections and flat penile lesions of the penis in men who have sex with men. Papillomavirus Res. 2019;8:100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghavendran A, Hernandez AL, Lensing S, et al. Genital human papillomavirus infection in Indian HIV-seropositive men who have sex with men. Sex Transm Dis. 2018;44:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Aar F, Mooij SH, van der Sande MA, et al. Anal and penile high-risk human papillomavirus prevalence in HIV-negative and HIV-infected MSM. AIDS. 2013;27:2921–2931. [DOI] [PubMed] [Google Scholar]
- 34.Blas MM, Brown B, Menacho L, et al. HPV prevalence in multiple anatomical sites among men who have sex with men in Peru. PLoS One. 2015;10:e0139524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson LE, Gravitt P, Tobian AA, et al. Male circumcision reduces penile high-risk human papillomavirus viral load in a randomised clinical trial in Rakai, Uganda. Sex Transm Infect. 2013;89:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tobian AA, Kong X, Gravitt PE, et al. Male circumcision and anatomic sites of penile high-risk human papillomavirus in Rakai, Uganda Aaron. Int J Canc. 2012;129:2970–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serwadda D, Wawer MJ, Makumbi J, et al. Circumcision of HIV-infected men: effects on high risk human papillomavirus infections in a Randomized trial in Rakai, Uganda. Int J Canc. 2011;201:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murenzi G. Oral Communication from a community advisory board set up as part of the U54 MSM studies. 2019. [Google Scholar]
- 39.Binagwaho A, Wagner CM, Gatera M, et al. Achieving high coverage in Rwanda's national human papillomavirus vaccination programme. Bull World Health Organ. 2012;90:623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]