To the editor
From the early beginning of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, clinicians have faced not only the respiratory complications of coronavirus diseases 2019 (COVID-19) but also a series of vascular phenomena, which worsen the patient prognosis.1 Coronavirus-associated coagulopathy and thromboembolism represent one of the leading causes of death in these patients, through endothelial dysfunction and a hypercoagulable state.2–5
Notwithstanding data on vascular diseases during COVID-19 are scarce, alterations of parameters implicated in the coagulative process, such as fibrinogen, prothrombin time (PT), factor VIII and D-dimer, have been described.5 Major societies recommend routine use of thromboembolism prophylaxis with low molecular weight heparin (LMWH) or fondaparinux (if thrombocytopenia is present).6,7 Chinese data suggest that prescribing anticoagulant treatment according to high D-dimer levels and sepsis-induced coagulopathy (SIC) score equal to or higher than 4 is associated with a favourable outcome.8 Also, some data showed that heparin exerts anti-inflammatory effects, which can help reduce tissue damages caused by cytokine storm.9 However, to date, no randomised clinical trials are available to indicate type, dosage and duration of anticoagulation treatment, specifically. Moreover, risk of bleeding and prevalence of haemorrhages (possibly due to preventative strategies for thromboembolic events) were not yet precisely determined in COVID- 19, even if HAS-BLED score is useful to estimate the risk of bleeding in other conditions.10
We herein report three cases of major bleeding, occurred in three patients with COVID-19 who were prescribed anticoagulant treatment for paroxysmal atrial fibrillation. For all our patients, indication for anticoagulant was present according to Padua, and CHA2DS2-VASC score scores.11,12
Case #1
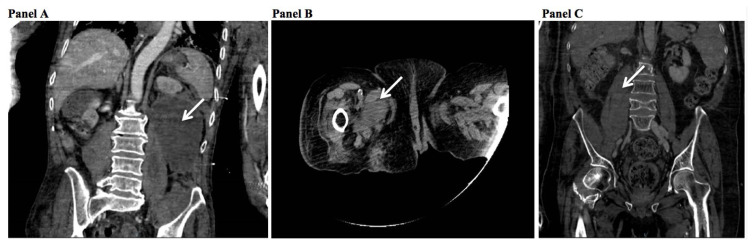
74-year-old male suffering from Parkinson disease, lung fibrosis, and hypertension was admitted to the emergency department for acute respiratory failure on April 17th, 2020, and tested positive for SARS-CoV-2. He was diagnosed with paroxysmal atrial fibrillation (CHA2DS2-VASC score=3) and was prescribed LMWH (4000 UI, subcutaneously twice daily). Padua score was 5. HAS-BLED score was 2. He was transferred of care to our unit on April 18th, 2020, where oxygen support was prescribed with high flow nasal cannula (HFNC) 40% FiO2 40 liters/minute. The patient reported an accidental fall, without apparent consequences on April 18th, with CT scan results not showing anything significant, but for lung disease. Coagulation parameters at the admission were: D-dimer 0.30 mg/l (normal values 0–0.55 mg/l), fibrinogen 216 mg/dl (normal values 200–400 mg/dL), PT 64% (normal values 70–120%), PTT 33 seconds (normal values 22–36.6 seconds), and INR 1.40. SIC score was 4. After one week he rapidly developed hypotension (blood pressure 85/45 mmHg) and severe anaemia (haemoglobin levels decreased from 13.1 to 6.9 g/dl) over three days. Coagulation parameters were: D-dimer 0.39 mg/l (normal values 0–0.55 mg/l), fibrinogen 135 mg/dl (normal values 200–400 mg/dL), PT 49% (normal values 70–120%), PTT 43 seconds (normal values 22–36.6 seconds), and INR 1.62. SIC score remained 4. He underwent a CT scan, which showed massive serum-blood collection (10 cm diameter) displacing the left kidney and hematoma with ongoing bleeding in the ipsilateral psoas muscle (see Figure 1, panel A). He received several blood and plasma transfusions and supportive care immediately, but unfortunately, he died after two days (April 28th).
Figure 1.
CT scan results showing major bleedings.
Case #2
88-year-old female suffering from Parkinson disease, hypertension, and hypothyroidism tested positive for SARS-CoV-2 on March 26th, 2020.
For worsening of clinical conditions, the patient was admitted to our unit for COVID-19 pneumonia on April 2nd, 2020. Venturi mask (SpO2 60% 15 l/min) was applied. Soon after admission, she was diagnosed with paroxysmal atrial fibrillation (CHA2DS2-VASC score=5), so LMWH (4000 UI, subcutaneously twice daily) was prescribed. Coagulation parameters at the admission were: D-dimer 1.74 mg/l (normal values 0–0.55 mg/l), fibrinogen 391 mg/dl (normal values 200– 400 mg/dL), PT 108% (normal values 70–120%), PTT 26 seconds (normal values 22–36.6 seconds), and INR 1.04. Padua score was 9. SIC score was 1. HAS-BLED score was 2. She suddenly developed anaemia (haemoglobin levels decreased from 10.7 to 7.5 g/dl), with the following coagulation parameters: D-dimer 0.76 mg/l (normal values 0–0.55 mg/l), fibrinogen 91 mg/dl (normal values 200–400 mg/dL), PT 50% (normal values 70–120%), PTT 44 seconds (normal values 22–36.6 seconds), and INR 1.58. At the same time, SIC score was 1. CT scan showed a large hematoma with imbued and non-homogeneous appearance of the adductor muscles of the right thigh (see Figure 1, panel B). The patient received blood transfusions immediately. Anticoagulation was stopped for one day; then she was switched to fondaparinux 2.5 mg/daily (due to the thrombocytopenia).
Case #3
A 56-year-old male with sequelae of stroke and meningoencephalitis, diabetes, hyperthyroidism, chronic kidney disease, non-alcoholic fatty liver disease, and hypertension was admitted on April, 1rst with persistent fever and shortness of breath, requiring Venturi mask (SpO2 60% 15 l/min). He was diagnosed with paroxysmal atrial fibrillation (CHADS2- VASC2=5) and was prescribed LMWH (4000 UI, subcutaneously twice daily). Coagulation parameters at the admission were: D-dimer 1.45 mg/l (normal values 0–0.55 mg/l), fibrinogen 333 mg/dl (normal values 200–400 mg/dL), PT 94% (normal values 70–120%), PTT 33 seconds (normal values 22–36.6 seconds), and INR 1.01. SIC score was 2. His Padua score was 8. HAS-BLED score was 2. On April 28th, he suddenly developed anaemia (haemoglobin levels decreased from 10.7 to 7.1 g/dl) and hypotension. Coagulation parameters were: D-dimer 1.66 mg/l (normal values 0–0.55 mg/l), fibrinogen 359 mg/dl (normal values 200–400 mg/dL), PT 112% (normal values 70–120%), PTT 29 seconds (normal values 22–36.6 seconds), and INR 1.66. SIC score increased to 3. CT scan showed hematoma in the right psoas muscle with non-homogeneity of adjacent tissues and small liquid effusion among the intestinal loops (see Figure 1, panel C). He received several blood and plasma transfusions and supportive care immediately, with a good clinical outcome. Anticoagulation was stopped for two days, and then fondaparinux was started, due to occurring thrombocytopenia.
Our work highlights haemorrhage as an overlooked possible risk in patients with COVID-19 on anticoagulant treatment. In the study patients, we prescribed LMWH for three reasons: i) several guidelines suggest prescription of LMWH for any patients with COVID-19 to prevent thromboembolic events;6,7 ii) SIC score was above the cut-off suggested for the prescription of anticoagulant therapy in our patients,13 and iii) patients suffered from paroxysmal atrial fibrillation.12 The last reason led us to prescribe LMWH at therapeutical dosage. So, we could not exclude that, in patients with COVID-19, such a high dosage could have contributed to increasing the risk of bleeding.
Unfortunately, although validated scores are available to weight possible contraindications of anticoagulants (i.e., risk of bleedings)10 against the indication for it, such scores are not validated explicitly in COVID-19 patients. This validation process would be necessary because SARS-CoV-2 is not only able to interfere with the coagulation cascade in a prothrombotic way14, but it can also induce a sepsis-like syndrome, including disseminated intravascular coagulation, of which haemorrhage can be an outcome.2 So, a careful evaluation of the relative risk of thrombosis and bleeding should be required in these patients.
Importantly, it seems that existing scores10–12 were not conclusive in balancing the indication to use anticoagulants with the risk of bleeding in our patients. In fact, while according to Padua, CHA2DS2-VASC, and SIC scores anticoagulants were recommended, HAS-BLED indicated a moderate risk of bleeding in all cases presented in this paper.
Given these considerations, clinicians could decide to reduce dosage of heparin; however, no data are supporting this choice. By contrast, several data have shown that heparin should be prescribed in order to prevent COVID-19 associated coagulopathy,6 suggesting that the risk of bleeding is less hazardous.
In conclusion, our experience showed that life-threatening major bleedings can occur during anticoagulation treatment and that a careful clinical and laboratory monitoring of such patients with COVID-19 is required. This proactive approach is essential especially in people who have an increased risk of bleedings, i.e., elderly, although validated scores may indicate that this risk is only moderate. In other terms, scores to evaluate the risk of bleeding may need to be validated explicitly in patients with COVID-19.
Acknowledgements
We want to thank all our patients and our nurses. We also thank the Infectious Diseases and Tropical Medicine (IDTM) of the University “Magna Graecia” (UMG) COVID-19 Group, which is composed, besides the main authors, by the following: Eugenio Arrighi, Giorgio Settimo Barreca, Flavia Biamonte, Bernardo Bertucci, Maria Teresa Busceti, Anna Cancelliere, Francesco Saverio Costanzo, Chiara Davoli, Adele Emanuela De Francesco, Paolo Fusco, Luigia Gallo, Aida Giancotti, Amerigo Giudice, Giuseppe Greco, Valentina La Gamba, Angelo Lamberti, Maria Carla Liberto, Elena Lio, Rosaria Lionello, Nadia Marascio, Giovanni Matera, Maria Chiara Pelle, Maria Petullà, Graziella Perri, Giada Procopio, Angela Quirino, Marco Ricchio, Vincenzo Scaglione.
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.14768. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannegieter SC, Klok FA. COVID–19 associated coagulopathy and thromboembolic disease: Commentary on an interim expert guidance. Res Pract Thromb Haemost. 2020 doi: 10.1002/rth2.12350. doi: 10.1002/rth2.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.14830. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020 doi: 10.1016/j.jcv.2020.104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14810. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lombardy Section of the Italian Society of Infectious and Tropical Diseases. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2) https://www.infezmed.it/media/journal/Vol_28_2_2020_4.pdf. [PubMed] [Google Scholar]
- 8.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 doi: 10.1111/jth.14817. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi C, Wang C, Wang H, et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe covid-19 patients: a retrospective clinical study. medRxiv. 2020 doi: 10.1101/2020.03.28.20046144. doi: 10.1101/2020.03.28.20046144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The euro heart survey. Chest. 2010 doi: 10.1378/chest.10-0134. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 11.Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalised medical patients at risk for venous thromboembolism: The Padua Prediction Score. J Thromb Haemost. 2010 doi: 10.1111/j.1538-7836.2010.04044.x. doi: 10.1111/j.1538-7836.2010.04044.x. [DOI] [PubMed] [Google Scholar]
- 12.Mason PK, Lake DE, Dimarco JP, et al. Impact of the CHA 2DS 2-VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med. 2012 doi: 10.1016/j.amjmed.2011.09.030. doi: 10.1016/j.amjmed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iba T, Di Nisio M, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: A retrospective analysis of a nationwide survey. BMJ Open. 2017 doi: 10.1136/bmjopen-2017-017046. doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong M, Liang X, Wei Y. Changes in Blood Coagulation in Patients with Severe Coronavirus Disease 2019 (COVID–19): a Meta–Analysis. Br J Haematol. 2020 doi: 10.1111/bjh.16725. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]