Abstract
The recent scientific statement of the transnational alliance for regenerative therapies in cardiovascular syndromes (TACTICS) provided an overview of the many challenges associated with pre-clinical and clinical studies of stem cell therapy for HF1 and are providing a series of guidelines and recommendations for moving this field forward1, 2. As part of this series, here we discuss the role of pre-clinical studies designed to advance stem cell therapies for CVD. The quality of this research has improved over the last 10–15 years and overall indicates that cell therapy promotes cardiac repair. However, many issues remain, including inability to provide complete cardiac recovery. Recent studies question the need for intact cells suggesting that harnessing what the cells release is the solution. Here we describe important breakthroughs and current directions in a cell-based approach to alleviating CVD.
Keywords: Stem cells, Regenerative medicine, Preclinical, Cell and tissue-based therapy
I. Introduction to stem cell therapy – landmark preclinical studies/appropriate animal models
Cardiovascular disease (CVD) is the leading cause of mortality worldwide. However, despite improvements in pharmacologic and interventional treatments, 1 in 3 men and 1 in 4 women die within a year of their first myocardial infarction (MI)3. The prevalence of heart failure (HF) and MI require new therapeutic approaches, which must be first tested in animal models to establish safety and therapeutic efficacy, prior to use in humans. Unlike pharmacologic treatments, which primarily manage the disease, stem cell administration promotes the restoration of lost functionality. However, negative outcome trials and the recent debate on the efficacy of the human clinical cell-based therapy in patients with acute MI (AMI)4 means that we must continue to find better approaches that will ensure success in human trials.
While myocyte necrosis leads to remodeling post-MI, this effect is secondary to a cascade of cellular changes that appear to be the primary cause of ventricular dilation, hypertrophy and scar formation5. In contrast to the age-old paradigm that cardiac myocytes are terminally differentiated, the current consensus is that ~0.5–2% of cardiomyocytes undergo mitosis annually6. In infarcted human hearts, myocyte growth becomes enhanced at the border zone after an ischemic event with up to 3–4-fold more dividing myocytes one week post- infarction than in end-stage heart failure7. Understanding and enhancing cardiomyocyte proliferation post-MI is a major focus of regenerative medicine.
In early murine studies mobilization of myeloid clonogenic cells from spleen and bone marrow (BM) was observed during wound healing8. Later discoveries noted the effects of neovasculogenesis after endothelial progenitor cells (EPCs) mobilized secondary to hind limb ischemia. Rabbits mobilize EPCs specifically from the BM after hind limb ischemia; which was enhanced following GM-CSF administration9. These findings paved the way for use of progenitor cell to treat disease. During these early studies, there was no notion of intrinsic self-renewing cardiac cells. In 2003 this paradigm changed; cardiac stem cells that are self-renewing, clonogenic, and multipotent were observed in adult rat hearts 10. Thus, began the concept that, with some help, the heart could heal itself. The controversy concerned the nature of that help. For many, the answer was which type of stem cell should be used to treat heart disease. The safety, efficacy and fate of each cell line needed further research in animal models to determine not only which model was best to simulate human cardiac response but which of these various cell types should be studied further.
a. Small animal studies
For preclinical development, an appropriate animal model that accurately reflects human pathological conditions is essential. Cell and molecular studies provide important mechanistic data and toxicity studies evaluate candidate drugs11, but a working heart is needed to evaluate and optimize treatments.
New therapies for CVD are usually first evaluated in small animal models (rodents), a model that provides relatively rapid and economical testing and adequate group sizes to ensure sufficient statistical power. Recent technological advances in PET-MRI imaging and echocardiography have improved the assessment of cardiovascular outcomes in rodents12. Mouse models do have inherent advantages but also some limitations. They can respond very differently than humans to treatment13, their hearts beat at 400–600 beats/min and they have a variety of anatomic differences with human hearts (reviewed by Santos et al.12). Transgenic and knock-out mice are widely available, making them particularly useful for assessing genetic factors and inducers of cardiovascular diseases. However, genetic changes can alter cardiac morphology, which can limit the advantages of these models12. Discrepancies between human and mouse embryonic stem cells, including the expression of genes regulating apoptosis, cytokine expression and cell cycle regulation can further limit the relevance of mouse models14.
Rat heart mass is roughly ten-fold greater than mice, and surgical expertise is less demanding. The rat coronary ligation model was first described in 197915, and ligation of the left anterior descending (LAD) coronary artery is the most widely used model for MI. A rat model of MI was instrumental in the evaluation and development of angiotensin-converting enzyme inhibitors16, 17 as prelude to clinical trials that resulted in the approval of captopril as a therapeutic intervention for heart failure after MI18. However, positive rat pre-clinical studies do not necessarily translate to successful clinical trials. Endothelin receptor antagonists such as bosentan, improved survival and hemodynamic characteristics in the rat after MI19, but failed to show a benefit in humans suffering from heart failure20.
b. Large animal studies
Cardiac repair studies show larger effects in rodents, increased left ventricular ejection fraction (LVEF) up to 20% and normalization of LV function, in contrast to large animal studies (mean LVEF improvement ~5–7%)21. This moderate benefit corresponds better to the results of clinical trials, giving realistic insight into the expected benefit of human cell-based cardiac therapies. The presence or absence of collateral coronary circulation is an important factor for choosing an adequate animal model for a particular study. Large animals such as pigs, dogs, or sheep satisfy many of these criteria. Dogs have an extensive collateral coronary circulation, while pigs and sheep have no functionally relevant vascular adaptation system, similar to humans22. Thus, a dog model is suitable for studying vascular adaptation to myocardial ischemia, while pigs and sheep are generally regarded as appropriate to assess the direct myocardial effects of hypoxic injuries.
Initial studies in canine hearts in the late 1970s paved the way to understand myocardial ischemia and the development of HF23. In a dog MI/reperfusion model, the angiotensin receptor blocker valsartan produced decreased infarct size and increased ejection fraction and improved diastolic function24. Ischemic cardiomyopathies can be simulated in canines through microembolization25, resulting in reduction of LVEF to less than 35%, a model that has been used for evaluation of several drugs for treating heart failure26. The drawback of these studies is that canines have a significantly more complex coronary circulation than humans, such that their maximal oxygen consumption is greater and their degree of reproducible infarct size more variable27. Therefore, despite the advantages of the canine, a better model was still needed. One such model is the sheep, where coronary anatomy is consistent with humans; and there is a lack of significant collateral circulation, allowing for reproducible infarcts. However, sheep carry zoonotic disease, a problem not associated with other large animals and ovine thoracic and gastrointestinal anatomy complicate detailed imaging, so is not ideal for typical transthoracic imaging studies28.
Swine cardiac anatomy compares favorably to that of humans; the large LAD and in most cases, the right coronary artery is dominant, supplying the posterior interventricular septum and the atrio-ventricular node in the vast majority of cases; and there is little collateral flow, all similarities to humans29. Furthermore, adult Göttingen and Yucatan minipigs possess cardiac structure and function comparable to humans, including high mortality associated with large infarcts30. For chronic studies, they remain within a manageable weight range as compared to Yorkshire pigs30. By ligation of the LAD, MI can be induced by either open- or closed-chest methods. Open-chest surgery provides easy access to coronary arteries, visual control of contractility, and facilitates the generation of defined infarction sites and sizes. However, closed-chest procedures avoid the thoracotomy-associated trauma of open-chest surgery31 as in humans. Large animal studies are very expensive (10–100x higher than similar small animal studies), limiting the number of animals in a study. A major advantage of large animal studies is the ability to use imaging modalities identical to those for humans, resulting in similar measures and outcome parameters, increasing human relevance but also costs.
This overview of pre-clinical models for regenerative medicine demonstrates that each has inherent advantages and disadvantages. Small animal studies provide an initial indication of the potential of the intervention, and must be further evaluated in large animals whose CV physiology more closely resembles humans. Only when a treatment provides sufficient therapeutic efficacy in large animals should it be moved to clinical trials.
II. Mesenchymal stem cells (MSCs)
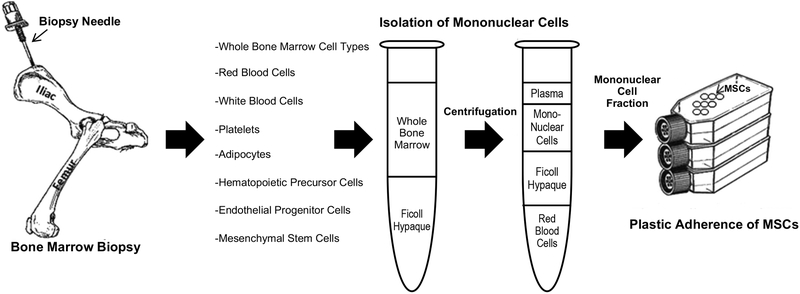
Mesenchymal stem cells (MSCs; also called mesenchymal stromal cells), have been the most commonly used stem cell for treating cardiac dysfunction. MSCs are relatively easy to isolate and can be expanded significantly ex vivo. Their immunosuppressive properties and their lack of immunogenicity make them excellent candidates for cell therapy. However, the various methods of cell expansion, isolation and characterization, necessitated a consistent definition of MSCs (Figure 1). The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy proposed distinct criteria: plastic adherence under standard culture conditions, positive for CD105, CD73 and CD90, and negative for CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR and the ability to differentiate into osteoblasts, adipocytes and chondroblasts in vitro32. MSCs have an additional therapeutic advantage; they lack MHC class II antigens and not only fail to elicit an immune response (at least initially), but also down regulate host natural killer cells and T-lympocytes33. Similarly, xenotransplantation of human bone marrow-derived MSCs (BM-MSCs) into murine34 and later porcine35 hearts revealed the ability of (a low percentage of) MSCs to integrate into the myocardium and differentiate into cardiomyocytes.
Figure 1. Procurement and isolation of MSCs.
MSCs isolated from bone marrow and the mononuclear cells isolated by Ficoll density centrifugation. MSCs can be separated from other mononuclear cells by their plastic adherence in culture. From Williams and Hare41
Cells with similar characteristics to BM-MSCs are found in virtually every tissue36; however, most cardiac studies have used MSCs from BM or a few other tissues (although this trend may be changing) including adipose (Ad)- and umbilical cord blood (UCB)/Wharton’s jelly-derived MSCs. BM and Ad-derived MSCs most consistently differentiate into bone, fat and cartilage, and have the highest capacity for self-renewal37. While circulating MSCs, which are thought to arise from BM, have long been debated as a source for future clinical use, they have produced contradictory results, reducing confidence in them as a source38.
a. Bone marrow-derived MSCs (BM-MSCs)
BM is of particular interest for stem cell therapy because it is a source of a variety of multipotent precursors (MSCs, mononuclear cells, and endothelial progenitor cells; see below). However, stem cells comprise only 1–3% of BM, the majority being lymphocytes; but there are other undesirable cells for cardiac repair: monocytes, pre-adipocytes and osteoblasts39. An important property of MSCs is their ability to home to sites of injury, a property first demonstrated in a baboon lethal radiation model40. Murine studies demonstrated that MSCs migrate to ischemic cardiac tissue following intravenous infusion41. Factors thought to play a role in these migratory and stimulatory properties, include the Stem cell factor/CKit (CD 117) ligand-receptor complex42, 43. Once the ability of MSCs to home was recognized, studies focused on improving cardiac function, which were encouraged by the ability of murine BM-MSCs to become functional cardiomyocytes when treated with 5-azacytidine (5-aza), a demethylase and cytosine analog. MSCs begin beating after 2-weeks of 5-aza exposure, and after three weeks, the beating becomes synchronized44.
Overall, most, but not all, studies in small and large animals, for both acute and chronic MI, show improved cardiac structure and function following BM-MSCs administration (see Narita and Suzuki for review45). Determining the precise mechanism(s) is difficult, since few cells remain in the myocardium over time. The consensus is that beneficial MSC effects are primarily paracrine, involving not only secretion of growth factors (see Table 1) but also microvesicles and exosomes46, 47. The efficacy of these cells also appears to be influenced by the route of injection48.
Table 1.
Paracrine factors secreted by MSCs
| Secreted Factor | Function |
|---|---|
| Pro-angiogenesis | |
| Fibroblast growth factor-2 (FGF-2) | Induces endothelial and smooth muscle proliferation |
| Fibroblast growth factor-7 (FGF-7) | Induces endothelial cell proliferation |
| Monocyte chemoattractant protein-1 (MCP-1) | Induces angiogenesis; recruits monocytes |
| Platelet derived growth factor (PDGF) | Smooth muscle proliferation |
| Placental growth factor (PlGF) | Promotes angiogenesis |
| Transforming growth factor-β (TGF-β) | Vessel maturation |
| Vascular endothelial growth factor (VEGF) | Endothelial cell proliferation, migration, tube formation |
| Remodeling of extracellular matrix | |
| Metalloproteinase-1 (MMP1) | Loosens matrix; tubule formation |
| Metalloproteinase-2 (MMP2) | Loosens matrix; tubule formation |
| Metalloproteinase-9 (MMP9) | Loosens matrix |
| Plasminogen activator (PA) | Degrades matrix |
| Tumor necrosis factor -α (TNF-α) | Degrades matrix; cell proliferation |
| Stem cell proliferation, recruitment, & survival | |
| Basic fibroblast growth factor (bFGF) | Enhance proliferation of endothelial and smooth muscle cells |
| Granulocyte colony stimulating factor (G-CSF) | Increases proliferation and differentiation of neutrophils |
| Insulin –like growth factor-1 (IGF-1) | Regulates cell growth and proliferation; inhibits apoptosis |
| Macrophage colony stimulating factor (M-CSF) | Increases proliferation and differentiation of monocytes |
| Thymosin-β4 (Tβ4) | Promotes cell migration |
| Stem cell-derived factor (SDF) | Progenitor cell homing |
| Secreted frizzled-related protein-1 (SFRP1) | Enhances cell m |
| Secreted frizzled-related protein-2 (SFRP2) | Inhibit apoptosis; enhance cell development |
| Immunomodulatory | |
| Heme oxygenase-1(HO1) | Inhibits T cell proliferation |
| Hepatocyte Growth Factor (HGF) | Inhibits CD4+ T cell proliferation |
| Indoleamine 2,3-dioxygenase (IDO) | Inhibits innate and adaptive immune cell proliferation |
| Inducible nitric-oxide synthase (iNOS) | Inhibits inflammation |
| Interleukin-6 (IL-6)94 | Regulates inflammation; VEGF induction |
| Prostaglandin E2 (PGE2) | Inhibits inflammation |
From Williams and Hare41
1. Small animal studies
Orlic et al. were among the first to show an improvement in cardiac function following intramyocardial injection of (enhanced green fluorescent protein (eGFP)-labeled) lineage (Lin)−/c-kit+ BM-derived stem cells into female mice in an AMI model. They observed eGFP+ cardiomyocytes, endothelial cells, smooth muscle cells and vascular structures in the infarcted region of the heart, leading to the conclusion that BM-derived cells can engraft and repair myocardium in vivo49. Since then small animal studies have reproducibly shown favorable outcomes following AMI. Reduction in infarct size and fibrosis, with enhancement of LVEF and vasculogenesis are among the common findings in the animal trials45. Additional benefits include decreased apoptosis, decreased fibrosis, increased vascular endothelial growth factor (VEGF) expression and/or increased regional blood flow in the infarct zone48, 50.
An emerging use for stem cells, particularly BM-MSCs, is in the treatment of idiopathic dilated cardiomyopathy (DCM). Given the lack of distinct areas of hypoperfusion and systolic dysfunction in the absence of coronary disease, it is a challenge to reverse this effect51. One group established a DCM model in rabbits. BM-MSCs were pre-incubated with 5-aza to induce differentiation into cardiomyocyte-like cells and injected directly into the myocardium resulting in an increase in left ventricular end-systolic pressure (LVESP) and a decrease in LV end-diastolic pressure (LVEDP). There was also an attenuation of myocardial fibrosis with an increase in VEGF52. Similarly, in a rat DCM model there was a noticeable decrease in LVEDP and an increase in LVESP53.
Intramyocardial injection of BM-MSCs after AMI produced a significant reduction in fibrosis and a noticeable engraftment of MSCs which differentiated into cardiomyocytes. Infarct size and the number of vascular cells in the myocardial structure were markedly improved54. While this study showed high engraftment of MSCs, most studies do not; with engraftment rates ranging from 6–12% of injected cells55. In an effort to improve engraftment, Simpson et al. developed an epicardial patch composed of human BM-MSCs and secured it to the acutely infarcted region. One week later, 23% of the cells engrafted into the myocardium. At four weeks, there was less LV dilation and better preservation of wall thickness, but without improvement in ventricular function when compared to controls. The authors attribute the latter finding to the small number of MSCs (1×106) that were initially embedded in the patch56.
2. Large animal studies
Both autologous and allogeneic BM-MSC produce beneficial effects in swine acute31, 35, 57, 58 and chronic30, 59-62 MI models. Early studies highlighted the engraftment and tri-lineage differentiation (CM, endothelium, vascular smooth muscle) of MSCs58, 60. In a porcine model, allogeneic BM- Di-I- and DAPI-labeled MSCs injected into the myocardium after AMI were found in the infarct region 8 weeks later. These cells expressed VEGF and specific cardiomyocyte proteins that suggested an upregulation of vasculogenesis and myocyte differentiation. Imaging and gross inspection showed an increase in LVEF and in subendocardial thickness, and a decrease in scar size in the cell treated group57, 63. While some engraftment occurs, at least in the short term, the consensus is that MSC therapy provides therapeutic efficacy through secretion of growth factors, microvessicles and exosomes (see below). Of particular importance is the observation that intramyocardial injection of BM-MSCs post-MI in Yorkshire pigs increased proliferation of endogenous cardiac stem cells (CSCs)58.
In chronic ischemic cardiomyopathy settings, BM-MSC therapy has focused on the ability of MSCs to reduce fibrosis and reverse-remodeling. Reduced myocardial thinning in the infarcted zone was reported in the treatment group following gross inspection. Histologic analysis revealed MSC engraftment up to 6-months post implantation64. Schuleri et al., assessed if cell dose is an important parameter. They injected either 20×106 vs. 200×106 autologous cells via an intramyocardial route 12-weeks post-MI. Delayed enhancement showed a decrease in infarct size in the low dose group and a decrease in infarct volume in the high dose group. Myocardial wall thickening was noted in both groups. Contractility was increased in the non-infarcted region in both MSC groups61; however, the contractility in the infarcted region was increased in only the high dose group. Quevedo et al. similarly tested the efficacy and safety of allogeneic MSCs in a swine model of chronic MI. Animals were injected via a transendocardial route. Twelve weeks post injection, the MSCs had engrafted, vasculogenesis flourished, and myocardial blood flow, LVEF and regional contractility had improved when compared to placebo60.
b. Stro-1+ BM-MSCs
A subset of BM-MSCs, BM progenitor cells (MPCs) has gained some interest over the past several years. These Stro-1+/CD34– cells are an immature subfraction of BM-MSCs65. In addition to their ability to self-renew, they can differentiate into chondrocytes, adipocytes, chondroblasts, and smooth muscle cells. Proponents of this subtype argue that MSCs in general are hard to define completely and this cell subtype can be isolated by immunoselection and has an enhanced ability to replicate and differentiate compared to traditional MSCs66. Animal studies with MPCs have produced promising results.
1. Large animal studies
Acutely infarcted sheep received intracoronary injection of allogeneic MPCs. There was a 40% decrease in scar size and a 50% increase in vascular density67. Another group used echocardiography to guide the injection of intramyocardial allogeneic MPCs into sheep 4-weeks post-MI. An increase in LVEF, wall thickness and vascular density was reported68. In a non-ischemic model, transendocardial administration of ovine allogenic cells produced decreased LVESV, stabilization of LVEF and decreased fibrosis69.
c. Adipose-derived stem cells
Adipose-derived stem cells (AdSC) are generated from enzymatic degradation of adipose tissue, which yields the stromal vascular fraction (SVF). The SVF then undergoes adherent culture purification to CD105+/CD34− AdSCs. Isolation of adipose tissue (liposuction) is relatively inexpensive and is much less invasive compared to BM aspiration, and yields a large number of cells. AdSCs secrete a similar variety of growth factors as BM-MSC70 and ex vivo can be differentiated into cardiomyocytes, endothelial cells, vascular smooth muscle, and evenpacemaker cells70. AdSCs, like BM-MSCs are immunoprivileged due to lack of MHC class II and they can improve the function and minimize the immune rejection of endothelial progenitor cells71. Given those similarities, their ease of isolation and the success of BM-MSCs, there is considerable interest in the potential of AdSCs to improve the function of a failing heart.
1. Small animal studies
While AdSCs and BM-MSCs overall behave similarly, an interesting study was carried out by Rasmussen et al. who isolated AdSC and BM-MSCs from an elderly patient with ischemia. These cells were injected intramyocardially into rats 1 week post-MI. Neither cell type promoted angiogenesis or reduced infarct size, but the AdSCs improved LVEF72. This result suggests differences in efficacy as the cells age.
Although not currently being used in human trials, brown adipose tissue may show promise in animal studies. Studies in rats demonstrated that CD29+ and CD133+ subpopulations of brown adipose tissue reduce infarct size and improve LV function through the induction of cardiomyocyte proliferation73, 74. However more preclinical trials are needed since most of the preclinical studies were conducted on white adipose tissue in mice or rats in an acute setting.
The therapeutic effects of adipose derived-cardiomyogenic cells (Ad-CMG), cells isolated from AdSCs that are CD90−, and retain the ability to express cardiac and skeletal muscle proteins, were studied. Three days following ligation of the LAD, mice were injected with Ad-CMG into the coronary artery. Four weeks later, the mice exhibited a reduction in remodeling, increased vasculogenesis, and stabilization of EF75.
2. Large animal studies
A porcine model introduced AdSC via coronary artery infusion following AMI. Twenty-eight days later, analysis showed a noticeable decrease in the perfusion defect, an increase in EF, vascular density and wall thickness76. Most studies with AdSCs involve AMI models, however one group utilized rabbits to study chronic ischemia. Three weeks post MI the rabbits were injected with AdSCs directly into the infarcted myocardium. AdSCs were associated with a greater vascular density, LVEF and improved EDV 5-weeks post injection compared to controls. However, not all studies demonstrate that AdSCs are very beneficial. Intracoronary administration of allogeneic AdSCs improved perfusion but not LVEF following AMI in a porcine model77 but the route of delivery may have affected the efficacy of the cells (see below). Improved perfusion and reduced infarct size was also seen upon administration of AdSCs into humanized pigs 4-weeks post-MI, but only with the highest concentration of AdSCs (4×106 cells/Kg), lower concentrations had no effect on either parameter78.
d. Umbilical cord MSCs
Fibroblast-like cells were isolated from the connective tissue of the umbilical cord (Wharton’s Jelly (WJ)) in the early 1990s79, and became a source of MSCs. These cells have gained interest for their non-invasive ease of extraction, high MSC yield and short doubling time80. There is limited preclinical data on umbilical cord blood and umbilical cord matrix MSCs (UC-MSC/UCM-MSC); however, clinical trials are currently underway. Transcriptional signature comparisons between BM- and UC-MSCs indicates distinct gene expression profiles. For example, UC-MSCs exhibit higher expression of genes associated with cell adhesion, proliferation, immune system functioning neurotrophic support, suggesting that these cells would be better than BM-MSCs for neurodegenerative diseases81
1. Small animal studies
One group tested a murine model in an acute ischemic setting. After direct intramyocardial injection of hUCM-MSCs, the systolic function was preserved 2 weeks post MI, but there were no differences in infarct size compared to controls, and both groups experienced ventricular wall thinning and dilation. Histologically, there was a lack of cell engraftment into the myocardium, which may indicate that the mechanism of ventricular preservation was due to a paracrine effect82. A transgenic mouse model with dilated cardiomyopathy received intramyocardial injections of hUC-MSCs. One month later, the LVEF was increased, the heart weight to body weight ratio decreased by 10%, and chamber dilation reduced in the cell treated group compared to placebo control mice. Histologically, VEGF, IGF-1 were upregulated and vasculogenesis increased, while apoptosis, fibrosis and vacuolization all decreased83. Rat dilated cardiomyopathy was treated with hUCM-MSCs, resulting in reduced fibrosis and cardiac dysfunction. The authors concluded that these cells worked, at least in part, by inhibiting TNF-α and TGF-ß1/Erk1/2 signaling84. Roura et al. created a fibrin patch to promote the efficacy of UC-MSC in acutely infarcted mice. The cells were retained within the patch for 4-weeks post implantation. The patch treated group yielded a smaller infarct scar (16 vs. 49%) and increased subjacent myocardial angiogenesis compared to controls85. Latifpour et al. injected hUCM-MSCs into the cyanotic region in a rabbit model of AMI. Thirty days later LVEF was significantly improved with evidence of cell engraftment, decreased scar size and chronic inflammatory markers86.
2. Large animal studies
Zhang et al. were the first to use hWJ-MSCs in swine after AMI. Six weeks after intramyocardial injection of cells, they observed improved perfusion in the cell treated group, engraftment of injected cells, some of which appeared to have differentiated into cardiomyocytes and vascular endothelial cells. Furthermore, there was an increased proliferation of immature cardiomyocytes expressing c-kit+, reduced apoptosis and increased LVEF in the treated group compared to placebo87.
Overall, MSCs obtained from a variety of tissues have demonstrated therapeutic efficacy. They tend to work similarly, reducing scar size and immune response, increasing perfusion and improving cardiac function. While MSCs have been studied for a majority of stem cell studies, many other stem cells have promoted cardiac repair.
III. Cardiac stem/progenitor cells (CSCs/CPCs)
A microenvironment or niche exists within the heart that is thought to play a critical role in maintaining stem cells in an undifferentiated state, but releasing them from this “hold” when necessary. Within the niche, stem cells give rise to cardiac progenitor cells, which migrate to sites of myocardial injury in an effort to repair the damage. Unfortunately, these endogenous stem cells quickly become depleted following large infarctions88, resulting in incomplete healing and subsequent heart failure. Although the idea of self-renewing cells in the heart was once considered unlikely, this concept is now well accepted and includes myocytes89-91 as well as stem cells within the myocardium.
a. Small animal studies
The landmark study where c-kit+ CSCs were initially identified was conducted in mice. These cells were Lin−/c-kit+ (CD117; the receptor for stem cell factor (SCF)), previously found in neonatal myocardium92, were isolated from the heart, and clonally expanded in culture10. These Lin−/c-kit+ cells were injected into the peri-infarct region following MI in rats. The resultant immunohistochemical staining and histological examination showed c-kit+ cells self-renew, and act in a clonogenic and multipotent manner to produce cardiomyocytes, smooth muscle cells, and endothelial cells10. Oskouei et al. directly compared, c-kit+ human (h) CSCs hMSCs for cardiac repair in an AMI model in immunodefficient mice. The CSCs produced greater improvement in hemodynamic parameters and were equally able to reduce scar size compared to 30-fold more hMSCs93. Overexpressing Pim1 kinase in c-kit+ hCSCs augmented their retention within the myocardium and their therapeutic efficacy in both a mouse94 and swine95 model of acute MI.
Other notable cardiac stem cells include: Cells expressing stem cell antigen-1 (not found in humans), side population cells (low in c-kit+), and islet-1 transcription factor cells (only found during the neonatal period), none of which to date have been used in clinical trials96. Epicardium-derived stem cells, while similarly not yet tested in clinical trials, may be a candidate cell type, but require more preclinical testing. During murine cardiac development, these Wt1+ cells develop into functional cardiomyocytes97. They are multipotent; resemble MSCs, participate in cardiac development, and likely have the potential to promote myocardial repair98. Determining the importance of c-kit+ CSCs for cardiac development and as a cell therapy for heart disease has been fraught with controversy. While some investigators minimize the role of CSCs99 recent studies have clarified their origin and differentiation capabilities100.
b. Large animal studies
In a chronic ischemic swine model, intracoronary administration of c-kit+ CSCs into pigs 3-months post MI demonstrated the therapeutic efficacy of these cells. Beginning one month post injection, the LVEF rose in the cell treated group and there was a regional increase in cardiac function. CSCs engrafted and some differentiated into CMs, and vascular structures101.
IV. Cardiospheres and cardiosphere-derived cells (CDCs)
Cardiospheres are a heterogeneous group of stem cells isolated from myocardial biopsies, that form clusters. The cells, initially isolated from human myocardial biopsies, express stem cell-related antigens and some cells spontaneously undergo cardiac differentiation. Upon expansion ex-vivo, these cells are called cardiosphere-derived cells (CDCs)102. CDCs have been studied as an autologous and allogeneic therapy for MI. Recently it was determined that the CD105+/CD90−/c-kit− population of CDCs represent the therapeutically active cell fraction103.
a. Small animal studies
Coronary infusion of allogeneic CDCs into rats post-MI reduced scar size, and increased cardiac function, myocyte cycling and angiogenesis104, 105 Allogeneic CDCs have also proved effective in revitalizing senescent rats106.
b. Large animal studies
CDC treatment of acute107 and chronic108 MI in the pig produces beneficial results. Recently, Gallet et al. demonstrated that CDC therapeutic effects are likely mediated via CDC-derived exosomes109.
V. Bone marrow mononuclear cells (BM-MNCs)
BM mononuclear cells (BM-MNCs) are a heterogeneous cell population which includes, MSCs, hematopoietic cells, EPCs and others. BM-MNCs are easier to isolate than the component population and have been tested in numerous studies110, but in clinical trials appear to be less effective than MSCs111.
a. Small animal studies
Early studies of MI in rats showed that intramyocardial injection BM-MNCs promoted significant vasculogenesis without an associated increase in VEGF or FGF at 2-weeks post injection. However, there was a subsequent decrease of vascularity in the 4-week group, which may have been secondary to the maturation of the scar112. A cryoablation rat model tested mixing BM-MNCs into a fibrin matrix. Eight weeks later, there was a greater enhancement of neovascularization in the cell+matrix group compared to cells alone113.
b. Large animal studies
Promising results were seen following direct BM-MNCs injection into the peri-infarct zone following LAD ligation. LVEF increased, the perfusion defect markedly decreased and the LVEDV to body weight ratio decreased in the treated group114. Alestalo et al. showed a direct correlation between improvement in LVEF following AMI and the number of retained cells seen on post mortem histologic examination115. Therefore, direct contact and retention of BMCs in ischemic tissue may be critical. Fuchs et al. were the first to establish the safety of transendocardial cell injection in a swine chronic ischemia model. They reported improved perfusion of the ischemic zone and enhancement of wall thickening 4-weeks post MI116, as well as an increase in vascularization of the myocardium and a decrease in scar size in the treated group. However, cardiac function did not change in either group117. A canine model showed the most favorable effects with regard to ventricular function118 and in sheep no difference in function reported in cell-treated animals compared to controls119. Nonetheless, in the preclinical setting, BM-MNCs have produced enhancements to neovascularization and reduced scar size; however, large animal models have shown variable effects in ventricular function.
VI. Hematopoietic and endothelial progenitor cells (EPCs)
The transplantation of BM-derived cells into mice led to the discovery of a hematopoietic source of regenerative cells. Hematopoietic stem cells (HSCs) and EPCs both are CD34+/CD133+ cells and can transform into myeloid and lymphoid cells or once mobilized from the BM into the blood after tissue injury can subsequently become endothelial cells, which can promote neovascularization120.
a. Small animal studies
Murine AMI studies have demonstrated enhanced neovascularization, EF, decreased scar size and differentiation of HSCs into endothelial cells in myocardial tissue121–123. Manipulating the EPCs provides improved repair potential. Tal et al. compared the ability of EPCs or epigenetically reprogrammed EPCs to differentiate into CMs and promote cardiac repair post-AMI124. The unmanipulated EPCs reduced infarct size, reduced LV volume and increased capillary density, but the manipulated (5-aza, valproic acid) EPCs did all three significantly better. Furthermore, the manipulated EPCs engrafted and differentiated into CMs.
b. Large animal studies
EPC conditioned media is cardioprotective following AMI in a swine model. Neutralizing insulin-like growth factor-1 (IGF-1) activity in the media abrogated these effects125
VII. Pluripotent stem cells
Transducing mouse fibroblasts with a set of transcription factors (Oct4, Sox2, Klf4, c-MYC) now known as the ‘Yamanaka factors’, produced novel pluripotent cells, induced pluripotent stem cells (iPSCs). iPSCs have surface markers and functional properties similar to embryonic stem cells (ESCs)126 and can be differentiated into hormone-responsive beating cardiac cells that mimic ESC-derived cardiomyocytes (ESC-CMs)127. Produced from autologous tissue, immune rejection and ethical concerns are no longer an issue. Using iPSC- and ESC-CMs reduces the tumor threat, but the cells remain immature and have limited ability to restore cardiac function128.
a. Small animal studies
Although initial animal studies utilized direct injection of iPSCs into the myocardium of mice, resulting in engraftment, improved cardiac function, increased wall thickness and reduced fibrosis129. iPSC-CMs engrafted long-term in a rat model of MI but failed to produce beneficial effects130
b. Large animal studies
The first studies in a large animal model examined co-injected hiPSCs and hMSCs in acutely infarcted swine. The hiPSCs enhanced vasculogenesis, but the combination of cells increased capillary density to a greater extent, likely secondary to the decreased rates of apoptosis131. Kawamura et al. placed a sheet of dermal fibroblast-derived hiPSC-CMs over the infarcted area in an ischemic swine model, which produced improved cardiac performance, angiogenesis and an attenuated LV remodeling 8-weeks post implantation132. Neither of these two studies reported tumor formation. Both ESC- and iPSC-CMs have been studied in non-human primates. Macaques received 1×109 hESC-CMs 2-weeks post-MI via intramyocardial delivery133. After 3-months, the cells continued to mature (albeit incompletely). Importantly, the cells engrafted, promoted extensive re-muscularization of the infarcted tissue, exhibited regular calcium transients and no evidence of tumors or of cells outside the heart. All of these monkeys exhibited arrhythmias133. Zhu et al. administered hPSC derived cardiovascular progenitors (hPSC-CVPs) into the myocardium of male cynomolgus monkeys 30 minutes post-MI. Cells were present 3 days later but not after 140 days, despite a modified immunosuppressive regimen. Apoptosis was reduced and cardiac function improved by the cells. However, no remuscularization was seen134.
VIII. Combination stem cell therapy:
To improve therapeutic efficacy, a new approach is to combine cells. Small and large animal studies have combined progenitor cells. Cells combines with angiogenic/growth factors increased vasculogenesis, cell survival, reduced apoptosis and enhanced cardiac function33.
a. Small animal studies
Ott et al. were the first to co-inject skeletal myoblasts and BM-MNCs into the myocardium of rats seven days post-infarction. Eight weeks later the combination group demonstrated improved EF/LVEDD/LVEDV, myotube formation and retention of BM-MNCs135. Intramyocardial injection of the same combination of cells into canines two weeks post infarction, yielded similar results compared to either cell group individually136. Quijada et al. created a fusion between murine MSCs and cardiac progenitor cells (CPCs), termed cardiac-chimeras (CC). They tested the efficacy of CCs in a mouse AMI model compared to the combination of CSC/MSCs or each cell type alone. Four weeks post injection, CC-treated animals showed enhancement of wall thickness. Cardiac function was improved in the CC group at 6 weeks, and in the MSC/CSC group at 18 weeks. Infarct size, engraftment and persistent engraftment were noted in the CC group as compared to MSC/CSC137.
b. Large animal studies
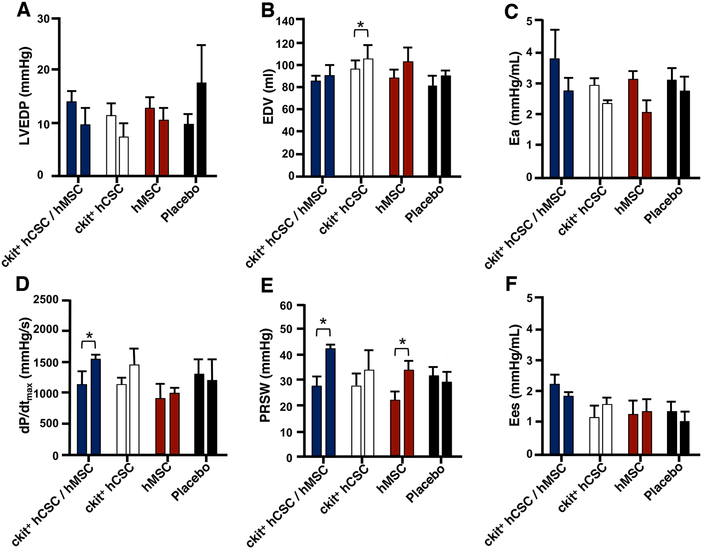
The combination of MSCs and CSCs has been studied in swine. Intramyocardial injection of hMSC and/or hCSCs were administered to immunosuppressed swine 14-days post-MI. This combination produced a 2-fold reduction in scar size, 7-fold enhanced engraftment, improved LV compliance and contractility as compared to individual cell types 4 weeks later (Fig. 2). The individual cell types produced significant improvements compared to placebo-treated animals35. In a chronic ischemic, non-immunosuppressed swine model, autologous MSCs±CSCs were administered 3 months post-MI. EF, stroke volume, cardiac output and diastolic strain were all improved in the combination group when compared to MSCs alone. Both cell treated groups significantly improved scar size, wall motion and viable tissue as compared to placebo59. A similar study using allogeneic MSCs and/or CSCs again showed that the cell combination produced greater improvements in cardiac structure and function138 at least in part by increasing cell proliferation within the myocardium59, 138.
Figure 2. Combination CSC/MSC.
Preload, afterload, and contractility changes after human cardiac stem cells (hCSC) and human mesenchymal stem cell (hMSCs). A. Left ventricular end-diastolic pressure (LVEDP) and (B) end-diastolic volume (EDV) and afterload measured by (C) arterial elastance (Ea). Combination hCSC/hMSC therapy improved contractility as measured by the (D) maximal rate of pressure change during systole (dP/dtmax) and (E) preload recruitable stroke work (PRSW), a preload-independent measure of stroke work. There was no change in (F) systolic elastance (Ees), in any of the groups. All graphs show pre-injection (2 weeks post-MI) vs 4-week post-injection values. Graphs represent mean±SEM. *P<0.05. From Williams et al.35.
IX. Paracrine effects
Considering the reported limited engraftment of transplanted cells, the idea that stem cells secrete factors that activate endogenous cells is particularly attractive139. This “secretome” is frequently enhanced by pre-incubation under stressful conditions, such as hypoxia further supports the “paracrine hypothesis”. Table 1 lists some of the potential molecular agents responsible for the paracrine effects. It is likely that a variety of different factors contribute to the regenerative and protective effects. The recruitment of resident stem cells from cardiac tissue or an increased homing of circulating progenitor cells derived from BM are likely enhanced by secreted factors.
a. Cell free medium
Studying stem cell-conditioned medium will help identify the secretome140, 141. For a pig study, MSCs derived from human embryonic stem cells were cultured in serum-free conditions and the cell medium was collected and applied intravenously140. Three weeks post-MI, pigs treated with conditioned medium showed reduced infract size and preserved cardiac function compared to the control group treated with non-conditioned medium. Capillary density was higher and collagen deposition in border and remote zones were lower in animals receiving the conditioned medium. As mentioned above, (some of) the cardioprotective effects of EPCs appear to be mediated by IGF-1125. Percutaneous intramyocardial injection of secretome of apoptotic peripheral blood mononuclear cells (Aposec) decreased infarct size and infarct transmurality measured by cardiac magnetic resonance imaging in a pig model of chronic LV dysfunction142.
b. Extracellular vesicles/Exosomes
Extracellular vesicles (EVs)/exosomes are membrane bound structures containing a variety of factors including short non-coding nucleic acids microRNAs and proteins. EVs/exosomes have sparked intense interest as the potential mediators of cell-based paracrine effects143–145. Exosomes purified from MSC-conditioned medium provide cardioprotection in an MI mouse model146. CSC-derived exosomes recapitulate the major effects of CSCs in both acute and chronic MI mouse models147. Adamiak et al. recently characterized murine iPSC-derived EVs (iPSC-EVs) which “were enriched in miRNAs and proteins with proangiogenic and cytoprotective properties”148. Importantly iPSC-EVs provided equal or greater therapeutic efficacy as iPSCs without the potential tumor formation148. As described above, CDC-derived exosomes produced equivalent levels of cardiac repair as did CDCs in a porcine model109. These exciting results await comprehensive clinical evaluations.
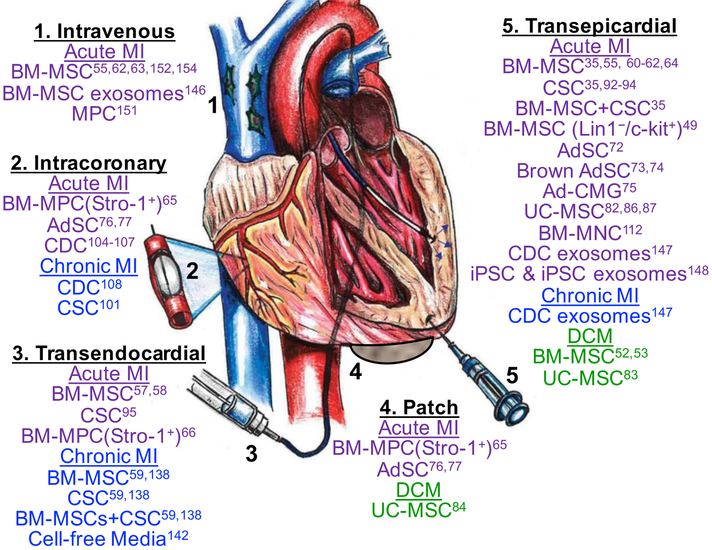
X. Delivery routes
While the search for the optimal cell type continues, so does the pursuit of the optimal delivery method. The most common routes include: intracoronary, intravenous, and intramyocardial (transendocardial/transepicardial). Despite the various delivery methods, cellular retention remains low33.
Intracoronary delivery of cells is the most utilized approach in the clinical setting149. Relatively inexpensive and well tolerated, this approach can be utilized during an acute ischemic event combined with coronary intervention150. Large animal studies on canines and swine have proven the efficacy of this method151, 152. However arterial obstruction may pose a risk with high cell doses and its application is limited in the chronic ischemia setting, secondary to the diffuse nature of the disease153. Intravenous delivery is non-invasive and well tolerated in a swine model154, yet fairly inefficient, as most of the cells are lost to other organs55.
When accompanied by cardiac mapping, the transendocardial approach, although invasive, delivers cells most accurately155, 156. Multiple swine studies have demonstrated improved cardiac function and reduced infarct size with this approach57–59, 61, 138. Caution should be taken in the elderly with thinner myocardium as there have been reports of ventricular perforation and cell clumping in areas of profound ischemia157.
The transepicardial method requires invasive thoracotomy, but is commonly utilized preclinically33. Under direct visualization, cells can be precisely injected into viable tissue surrounding the scarred area. Any perforations or hemorrhage can be controlled immediately. This delivery method produced improvements in EF, LVEDV and LVESV compared to placebo in a sheep model 8-weeks post-injection158. Other large animal studies have reported the benefits of this approach35, 64. Drawbacks include: prolonged post-op recovery, arrhythmias, embolization, and leakage of cells from the injected sites as reported in a swine study149.
XI. Patches/Biomaterials
As discussed above, generally less than 10% of injected cells are available at the site of injury within a few hours or days after delivery, and few cells actively engraft in the affected tissue. This rapid cell loss represents a major issue not only by limiting engraftment, but also for maintaining paracrine effects, many of which function only locally. Scaffolds/patches have been constructed from various biomaterials (gelatin, Matrigel, and collagen) to mimic the extracellular matrix lost secondary to MI and help retain transplanted cells. These biomaterials include: epicardial patches, self-assembling nanofibers, cell sheets, or injected gels which can be mixed with various cell types and placed on the infarcted region159, 160. Biomaterials need to fulfill a number of (sometimes contradictory) criteria to be effective including: biocompatibility, biodegradability, provide mechanical support, be an appropriate thickness and allow for precise placement161. The advent of 3D printing has expanded the availability and diversity of biomaterials allowing for cell-integration, vascularization and thicker structures162.
XII. Conclusions and Future Directions
Ranging from in vitro discoveries of the activation and differentiation of stem cells to large animal models mimicking human heart anatomy to culminating in clinical trials, stem cell therapy has been both promising and progressive33. Here, we discussed promising preclinical studies using a variety of stem cells introduced in different ways to treat a diverse assortment of animals with cardiac diseases. These pre-clinical results show that many types of cells are therapeutic but we must continue to study more cell types and use novel approaches, including combining different types of stem cells, reinjecting cells, improving retention and perhaps using the cell secretome rather than the cell itself.
As with all models, there are caveats associated with current pre-clinical studies. 1) The age of the animal is a crucial factor, with young healthy animals used for most studies. However, in humans, heart failure is primarily associated with aging3. 2) The uniformity of animals of a given strain and lack of co-morbidities, does not match human heterogeneity. 3) Lack of standardized protocols with consistent end points and outcome parameters, as well as multicenter randomized studies with centralized core lab blinded analyses as suggested by the NHLBI sponsored CAESAR consortium163 and the Working Group on Cellular Biology of the Heart of the European Society of Cardiology164. 4) The different injection routes, which appears to influence therapeutic efficacy48. Despite these caveats critical interpretation of pre-clinical models is necessary to move regenerative medicine forward.
MSCs have been the most studied cell type. They are widespread, immunomodulatory and immunoevasive and secrete exosomes and growth factors. Despite their demonstrated benefits in acute, chronic and dilated cardiomyopathy, complete recovery using MSCs alone has not occurred. Newer sources of stem cells, including umbilical cord/Wharton’s Jelly have shown potential and a different transcriptome81. Stem cells isolated from the heart, including c-kit+ CSCs and cardiospheres improve cardiac function and scar size in animal models. Pluripotent cells and their derivatives, have similarly shown some promise.
The combination of MSCs and c-kit+ CSCs has proven efficacious in large animal studies35, 59, 138 and is being translated to the clinical arena [NCT02503280]. Repeated MSC injections can be more effective than a single administration165, while Terrovitis et al. followed the transepicardial injection of CSCs with a fibrin based sealant in rats to enhance cellular retention166. Cell-free systems, particularly MVs/exosomes, with their collection of growth factors, micro RNAs, etc. may represent the next frontier either alone or in combination with cells. We anticipate, that novel pre-clinical approaches will provide more effective treatments and will pave the way for future clinical trials. The current standard of care for heart failure is to prescribe a cocktail of medications for patients to take for the remainder of their lives. The anticipated ability of stem cells to repair a compromised heart means that patients may reduce their medications and live active and healthy lives.
Figure 3. Different administration routes and cell types for treatment of heart disease.
The cell types listed under each delivery method refer only to those referenced (superscripted number) in this review. Figure adapted from Golpanian et al.167
Acknowledgments
Disclosures
This work was supported by the National Institutes of Health (NIH) grant R01HL084275 awarded to Dr. Hare. Dr. Hare is also supported by NIH grants R01HL107110, UM1HL113460, and R01HL137355; and grants from the Starr Foundation and the Soffer Family Foundation. Dr. Hare has a patent for cardiac cell-based therapy; he holds equity in Vestion Inc.; and maintains a professional relationship with Vestion as a consultant and member of the Board of Directors and Scientific Advisory Board. Vestion did not play any role in the design or conduct of the study. Dr. Joshua Hare is the Chief Scientific Officer, a compensated consultant and advisory board member for Longeveron and holds equity in Longeveron. Dr. Hare is also the co-inventor of intellectual property licensed to Longeveron. The other authors have nothing to disclose.
Non-standard abbreviations
- 5-aza
5-azacytidine
- Ad-CMG
adipose derived-cardiomyogenic cells
- AdSC
Adipose-derived stem cells
- AMI
acute MI
- BM
bone marrow
- BM-MSC
bone marrow-derived mesenchymal stem cell
- BM-MNC
Bone marrow mononuclear cell
- CM
cardiomyocyte
- CDC
cardiosphere-derived cell
- CVD
Cardiovascular disease
- CC
cardiac-chimeras
- CPC
cardiac progenitor cell
- CSC
Cardiac stem cell
- ESC
embryonic stem cell
- EPC
endothelial progenitor cell
- eGFP
enhanced green fluorescent protein
- EV
Extracellular vesicle
- HF
heart failure
- HSC
Hematopoietic stem cells
- DCM
idiopathic dilated cardiomyopathy
- iPSC
induced pluripotent stem cell
- LAD
left anterior descending
- LVEF
left ventricular ejection fraction
- LVESP
left ventricular end-systolic pressure
- LVEDP
left ventricular end-diastolic pressure
- Lin
lineage
- MPC
Bone marrow-progenitor cell
- MSC
mesenchymal stem cell
- MI
myocardial infarction
- PSC-CVP
pluripotent stem cell-derived cardiovascular progenitor
- SCF
stem cell factor
- SVF
stromal vascular fraction
- TACTICS
Transnational alliance for regenerative therapies in cardiovascular syndromes
- UC
umbilical cord blood
- UCM
umbilical cord matrix
- VEGF
vascular endothelial growth factor
- WJ
Wharton’s Jelly
Literature Cited
- 1.Fernández-Avilés F, Sanz-Ruiz R, Climent AM, et al. Global position paper on cardiovascular regenerative medicine. Eur Heart J. 2017;38:2532–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-Avilés F, Sanz-Ruiz R, Climent AM, et al. Global Overview of the Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes (TACTICS) Recommendations. A Comprehensive Series of Challenges and Priorities of Cardiovascular Regenerative Medicine. Circ Res. 2017. [DOI] [PubMed] [Google Scholar]
- 3.Writing Group M, Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 4.Gyongyosi M, Wojakowski W, Lemarchand P, et al. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116:1346–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8. [DOI] [PubMed] [Google Scholar]
- 6.Eschenhagen T, Bolli R, Braun T, et al. Cardiomyocyte Regeneration: A Consensus Statement. Circulation. 2017;136:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledney GD, Stewart DA, Gruber DF, Gelston HM, Exum ED, Sheehy PA. Hematopoietic colony-forming cells from mice after wound trauma. J Surg Res. 1985;38:55–65. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. [DOI] [PubMed] [Google Scholar]
- 10.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. [DOI] [PubMed] [Google Scholar]
- 11.Vunjak Novakovic G, Eschenhagen T, Mummery C. Myocardial Tissue Engineering: In Vitro Models. Cold Spring Harbor Perspectives in Medicine. 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos A, Fernández-Friera L, Villalba M, López-Melgar B, España S, Mateo J, Mota RA, Jiménez-Borreguero J, Ruiz-Cabello J. Cardiovascular Imaging: What Have We Learned From Animal Models? Frontiers in Pharmacology. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haghighi K, Kolokathis F, Pater L, et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111:869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. Differences between human and mouse embryonic stem cells. Dev Biol. 2004;269:360–80. [DOI] [PubMed] [Google Scholar]
- 15.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circulation Research. 1979;44:503–512. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer MA, Pfeffer JM, Steinberg C, Finn P. Survival after an experimental myocardial infarction: beneficial effects of long-term therapy with captopril. Circulation. 1985;72:406–12. [DOI] [PubMed] [Google Scholar]
- 17.Schieffer B, Wirger A, Meybrunn M, Seitz S, Holtz J, Riede UN, Drexler H. Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994;89:2273–82. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer MA, Lamas GA, Vaughan DE, Parisi AF, Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988;319:80–6. [DOI] [PubMed] [Google Scholar]
- 19.Rich S, McLaughlin VV. Endothelin receptor blockers in cardiovascular disease. Circulation. 2003;108:2184–90. [DOI] [PubMed] [Google Scholar]
- 20.Rich S, McLaughlin VV. Endothelin Receptor Blockers in Cardiovascular Disease. Circulation. 2003;108:2184–2190. [DOI] [PubMed] [Google Scholar]
- 21.Zwetsloot PP, Vegh AM, Jansen of Lorkeers SJ, van Hout GP, Currie GL, Sena ES, Gremmels H, Buikema JW, Goumans MJ, Macleod MR, Doevendans PA, Chamuleau SA, Sluijter JP. Cardiac Stem Cell Treatment in Myocardial Infarction: A Systematic Review and Meta-Analysis of Preclinical Studies. Circ Res. 2016;118:1223–32. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovascular Research. 1987;21:737–746. [DOI] [PubMed] [Google Scholar]
- 23.Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633–44. [PubMed] [Google Scholar]
- 24.Jugdutt BI, Menon V. Valsartan-induced cardioprotection involves angiotensin II type 2 receptor upregulation in dog and rat models of in vivo reperfused myocardial infarction. Journal of Cardiac Failure. 2004;10:74–82. [DOI] [PubMed] [Google Scholar]
- 25.Sabbah HN, Imai M, Cowart D, Amato A, Carminati P, Gheorghiade M. Hemodynamic Properties of a New-Generation Positive Luso-Inotropic Agent for the Acute Treatment of Advanced Heart Failure. American Journal of Cardiology. 2007;99:S41–S46. [DOI] [PubMed] [Google Scholar]
- 26.Sabbah HN, Shimoyama H, Kono T, Gupta RC, Sharov VG, Scicli G, Levine TB, Goldstein S. Effects of long-term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation. 1994;89:2852–9. [DOI] [PubMed] [Google Scholar]
- 27.White FC, Roth DM, Bloor CM. The pig as a model for myocardial ischemia and exercise. Lab Anim Sci. 1986;36:351–6. [PubMed] [Google Scholar]
- 28.Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009;2:262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver ME, Pantely GA, Bristow JD, Ladley HD. A quantitative study of the anatomy and distribution of coronary arteries in swine in comparison with other animals and man. Cardiovasc Res. 1986;20:907–17. [DOI] [PubMed] [Google Scholar]
- 30.Schuleri KH, Boyle AJ, Centola M, Amado LC, Evers R, Zimmet JM, Evers KS, Ostbye KM, Scorpio DG, Hare JM, Lardo AC. The adult Gottingen minipig as a model for chronic heart failure after myocardial infarction: focus on cardiovascular imaging and regenerative therapies. Comp Med. 2008;58:568–579. [PMC free article] [PubMed] [Google Scholar]
- 31.McCall FC, Telukuntla KS, Karantalis V, Suncion VY, Heldman AW, Mushtaq M, Williams AR, Hare JM. Myocardial infarction and intramyocardial injection models in swine. Nature Protocols. 2012;7:1479–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. [DOI] [PubMed] [Google Scholar]
- 33.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8. [DOI] [PubMed] [Google Scholar]
- 35.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion VY, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. [DOI] [PubMed] [Google Scholar]
- 37.Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36:585–97. [DOI] [PubMed] [Google Scholar]
- 39.Rehman J Bone marrow tinctures for cardiovascular disease: lost in translation. Circulation. 2013;127:1935–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devine SM, Bartholomew AM, Mahmud N, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244–55. [DOI] [PubMed] [Google Scholar]
- 41.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsui Y, Zsebo KM, Hogan BL. Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature. 1990;347:667–9. [DOI] [PubMed] [Google Scholar]
- 43.Rota M, Kajstura J, Hosoda T, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narita T, Suzuki K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. Heart Fail Rev. 2015;20:53–68. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cervio E, Barile L, Moccetti T, Vassalli G. Exosomes for Intramyocardial Intercellular Communication. Stem Cells Int. 2015;2015:482171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanelidis AJ, Premer C, Lopez J, Balkan W, Hare JM. Route of Delivery Modulates the Efficacy of Mesenchymal Stem Cell Therapy for Myocardial Infarction: A Meta-Analysis of Preclinical Studies and Clinical Trials. Circ Res. 2017;120:1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. [DOI] [PubMed] [Google Scholar]
- 50.Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. [DOI] [PubMed] [Google Scholar]
- 51.Pinto YM, Elliott PM, Arbustini E, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. 2016. [DOI] [PubMed] [Google Scholar]
- 52.Mu Y, Cao G, Zeng Q, Li Y. Transplantation of induced bone marrow mesenchymal stem cells improves the cardiac function of rabbits with dilated cardiomyopathy via upregulation of vascular endothelial growth factor and its receptors. Exp Biol Med (Maywood). 2011;236:1100–7. [DOI] [PubMed] [Google Scholar]
- 53.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–35. [DOI] [PubMed] [Google Scholar]
- 54.Kudo M, Wang Y, Wani MA, Xu M, Ayub A, Ashraf M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol. 2003;35:1113–9. [DOI] [PubMed] [Google Scholar]
- 55.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–8. [DOI] [PubMed] [Google Scholar]
- 56.Simpson D, Liu H, Fan TH, Nerem R, Dudley SC, A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25:2350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karantalis V, Suncion-Loescher VY, Bagno L, et al. Synergistic Effects of Combined Cell Therapy for Chronic Ischemic Cardiomyopathy. J Am Coll Cardiol. 2015;66:1990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuleri KH, Feigenbaum GS, Centola M, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. EurHeart J. 2009;30:2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price MJ, Chou CC, Frantzen M, Miyamoto T, Kar S, Lee S, Shah PK, Martin BJ, Lill M, Forrester JS, Chen PS, Makkar RR. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol. 2006;111:231–9. [DOI] [PubMed] [Google Scholar]
- 63.Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, Lardo AC, Heldman AW, Hare JM. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48:2116–24. [DOI] [PubMed] [Google Scholar]
- 64.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–25; discussion 1926. [DOI] [PubMed] [Google Scholar]
- 65.Gronthos S, Fitter S, Diamond P, Simmons PJ, Itescu S, Zannettino AC. A novel monoclonal antibody (STRO-3) identifies an isoform of tissue nonspecific alkaline phosphatase expressed by multipotent bone marrow stromal stem cells. Stem Cells Dev. 2007;16:953–63. [DOI] [PubMed] [Google Scholar]
- 66.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–10. [DOI] [PubMed] [Google Scholar]
- 67.Houtgraaf JH, de Jong R, Kazemi K, de Groot D, van der Spoel TI, Arslan F, Hoefer I, Pasterkamp G, Itescu S, Zijlstra F, Geleijnse ML, Serruys PW, Duckers HJ. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ Res. 2013;113:153–66. [DOI] [PubMed] [Google Scholar]
- 68.Cheng Y, Yi G, Conditt GB, et al. Catheter-based endomyocardial delivery of mesenchymal precursor cells using 3D echo guidance improves cardiac function in a chronic myocardial injury ovine model. Cell Transplant. 2013;22:2299–309. [DOI] [PubMed] [Google Scholar]
- 69.Psaltis PJ, Carbone A, Nelson AJ, Lau DH, Jantzen T, Manavis J, Williams K, Itescu S, Sanders P, Gronthos S, Zannettino AC, Worthley SG. Reparative effects of allogeneic mesenchymal precursor cells delivered transendocardially in experimental nonischemic cardiomyopathy. JACC Cardiovasc Interv. 2010;3:974–83. [DOI] [PubMed] [Google Scholar]
- 70.Ma T, Sun J, Zhao Z, Lei W, Chen Y, Wang X, Yang J, Shen Z. A brief review: adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res Ther. 2017;8:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan K, Zheng K, Li D, Lu H, Wang S, Sun X. Impact of adipose tissue or umbilical cord derived mesenchymal stem cells on the immunogenicity of human cord blood derived endothelial progenitor cells. PLoS One. 2017;12:e0178624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rasmussen JG, Frobert O, Holst-Hansen C, Kastrup J, Baandrup U, Zachar V, Fink T, Simonsen U. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transplant. 2014;23:195–206. [DOI] [PubMed] [Google Scholar]
- 73.Yamada Y, Wang XD, Yokoyama S, Fukuda N, Takakura N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem Biophys Res Commun. 2006;342:662–70. [DOI] [PubMed] [Google Scholar]
- 74.Yamada Y, Yokoyama S, Wang XD, Fukuda N, Takakura N. Cardiac stem cells in brown adipose tissue express CD133 and induce bone marrow nonhematopoietic cells to differentiate into cardiomyocytes. Stem Cells. 2007;25:1326–33. [DOI] [PubMed] [Google Scholar]
- 75.Leobon B, Roncalli J, Joffre C, Mazo M, Boisson M, Barreau C, Calise D, Arnaud E, Andre M, Puceat M, Penicaud L, Prosper F, Planat-Benard V, Casteilla L. Adipose-derived cardiomyogenic cells: in vitro expansion and functional improvement in a mouse model of myocardial infarction. Cardiovasc Res. 2009;83:757–67. [DOI] [PubMed] [Google Scholar]
- 76.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–77. [DOI] [PubMed] [Google Scholar]
- 77.Bobi J, Solanes N, Fernandez-Jimenez R, et al. Intracoronary Administration of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells Improves Myocardial Perfusion But Not Left Ventricle Function, in a Translational Model of Acute Myocardial Infarction. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dariolli R, Naghetini MV, Marques EF, Takimura CK, Jensen LS, Kiers B, Tsutsui JM, Mathias W, Lemos Neto PA, Krieger JE. Allogeneic pASC transplantation in humanized pigs attenuates cardiac remodeling post-myocardial infarction. PLoS One. 2017;12:e0176412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McElreavey KD, Irvine AI, Ennis KT, McLean WH. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem Soc Trans. 1991;19:29S. [DOI] [PubMed] [Google Scholar]
- 80.Tsagias N, Koliakos I, Karagiannis V, Eleftheriadou M, Koliakos GG. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus Med. 2011;21:253–61. [DOI] [PubMed] [Google Scholar]
- 81.Donders R, Bogie JFJ, Ravanidis S, et al. Human Wharton’s Jelly-Derived Stem Cells Display a Distinct Immunomodulatory and Proregenerative Transcriptional Signature Compared to Bone Marrow-Derived Stem Cells. Stem Cells Dev. 2018;27:65–84. [DOI] [PubMed] [Google Scholar]
- 82.Santos Nascimento D, Mosqueira D, Sousa LM, et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res Ther. 2014;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gong X, Wang P, Wu Q, Wang S, Yu L, Wang G. Human umbilical cord blood derived mesenchymal stem cells improve cardiac function in cTnT(R141W) transgenic mouse of dilated cardiomyopathy. Eur J Cell Biol. 2016;95:57–67. [DOI] [PubMed] [Google Scholar]
- 84.Zhang C, Zhou G, Chen Y, Liu S, Chen F, Xie L, Wang W, Zhang Y, Wang T, Lai X, Ma L. Human umbilical cord mesenchymal stem cells alleviate interstitial fibrosis and cardiac dysfunction in a dilated cardiomyopathy rat model by inhibiting TNFalpha and TGFbeta1/ERK1/2 signaling pathways. Mol Med Rep. 2018;17:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roura S, Bago JR, Soler-Botija C, Pujal JM, Galvez-Monton C, Prat-Vidal C, Llucia-Valldeperas A, Blanco J, Bayes-Genis A. Human umbilical cord blood-derived mesenchymal stem cells promote vascular growth in vivo. PLoS One. 2012;7:e49447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Latifpour M, Nematollahi-Mahani SN, Deilamy M, Azimzadeh BS, Eftekhar-Vaghefi SH, Nabipour F, Najafipour H, Nakhaee N, Yaghoubi M, Eftekhar-Vaghefi R, Salehinejad P, Azizi H. Improvement in cardiac function following transplantation of human umbilical cord matrix-derived mesenchymal cells. Cardiology. 2011;120:9–18. [DOI] [PubMed] [Google Scholar]
- 87.Zhang W, Liu XC, Yang L, Zhu DL, Zhang YD, Chen Y, Zhang HY. Wharton’s jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction. Coron Artery Dis. 2013;24:549–58. [DOI] [PubMed] [Google Scholar]
- 88.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941–61. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Rumyantsev PP, Borisov A. DNA synthesis in myocytes from different myocardial compartments of young rats in norm, after experimental infarction and in vitro. Biomed Biochim Acta. 1987;46:S610–5. [PubMed] [Google Scholar]
- 90.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A. 1998;95:8801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–7. [DOI] [PubMed] [Google Scholar]
- 92.Kunisada T, Yoshida H, Yamazaki H, Miyamoto A, Hemmi H, Nishimura E, Shultz LD, Nishikawa S, Hayashi S. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915–23. [DOI] [PubMed] [Google Scholar]
- 93.Oskouei BN, Lamirault G, Joseph C, Treuer AV, Landa S, Da Silva J, Hatzistergos K, Dauer M, Balkan W, McNiece I, Hare JM. Increased Potency of Cardiac Stem Cells Compared with Bone Marrow Mesenchymal Stem Cells in Cardiac Repair. Stem Cells Translational Medicine. 2012;1:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mohsin S, Khan M, Toko H, et al. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kulandavelu S, Karantalis V, Fritsch J, et al. Pim1 Kinase Overexpression Enhances ckit+ Cardiac Stem Cell Cardiac Repair Following Myocardial Infarction in Swine. J Am Coll Cardiol. 2016;68:2454–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dinsmore JH, Dib N. Stem cells and cardiac repair: a critical analysis. J Cardiovasc Transl Res. 2008;1:41–54. [DOI] [PubMed] [Google Scholar]
- 97.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chong JJ, Chandrakanthan V, Xaymardan M, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. c-kit cells minimally contribute cardiomyocytes to the heart. Nature. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hatzistergos KE, Takeuchi LM, Saur D, Seidler B, Dymecki SM, Mai JJ, White IA, Balkan W, Kanashiro-Takeuchi RM, Schally AV, Hare JM. cKit+ cardiac progenitors of neural crest origin. Proc Natl Acad Sci U S A. 2015;112:13051–13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;20:115:896–908. [DOI] [PubMed] [Google Scholar]
- 103.Cheng K, Ibrahim A, Hensley MT, Shen D, Sun B, Middleton R, Liu W, Smith RR, Marban E. Relative roles of CD90 and c-kit to the regenerative efficacy of cardiosphere-derived cells in humans and in a mouse model of myocardial infarction. J Am Heart Assoc. 2014;3:e001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, Marban L, Luthringer D, Marban E. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J Am Coll Cardiol. 2013;61:1108–19. [DOI] [PubMed] [Google Scholar]
- 105.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grigorian-Shamagian L, Liu W, Fereydooni S, Middleton RC, Valle J, Cho JH, Marban E. Cardiac and systemic rejuvenation after cardiosphere-derived cell therapy in senescent rats. Eur Heart J. 2017;38:2957–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kanazawa H, Tseliou E, Malliaras K, et al. Cellular postconditioning: allogeneic cardiosphere-derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ Heart Fail. 2015;8:322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yee K, Malliaras K, Kanazawa H, et al. Allogeneic cardiospheres delivered via percutaneous transendocardial injection increase viable myocardium, decrease scar size, and attenuate cardiac dilatation in porcine ischemic cardiomyopathy. PLoS One. 2014;9:e113805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marban L, Ghaleh B, Marban E. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lambers E, Kume T. Navigating the labyrinth of cardiac regeneration. Dev Dyn. 2016;245:751–61. [DOI] [PubMed] [Google Scholar]
- 111.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kobayashi T, Hamano K, Li TS, Katoh T, Kobayashi S, Matsuzaki M, Esato K. Enhancement of angiogenesis by the implantation of self bone marrow cells in a rat ischemic heart model. J Surg Res. 2000;89:189–95. [DOI] [PubMed] [Google Scholar]
- 113.Ryu JH, Kim IK, Cho SW, Cho MC, Hwang KK, Piao H, Piao S, Lim SH, Hong YS, Choi CY, Yoo KJ, Kim BS. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2005;26:319–26. [DOI] [PubMed] [Google Scholar]
- 114.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–52. [DOI] [PubMed] [Google Scholar]
- 115.Alestalo K, Korpi R, Makela J, Lehtonen S, Makela T, Yannopoulos F, Ylitalo K, Haapea M, Juvonen T, Anttila V, Lappi-Blanco E, Blanco Sequeiros R, Lehenkari P. High number of transplanted stem cells improves myocardial recovery after AMI in a porcine model. Scand Cardiovasc J. 2015;49:82–94. [DOI] [PubMed] [Google Scholar]
- 116.Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, Weissman NJ, Leon MB, Epstein SE, Kornowski R. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–32. [DOI] [PubMed] [Google Scholar]
- 117.Waksman R, Fournadjiev J, Baffour R, Pakala R, Hellinga D, Leborgne L, Yazdi H, Cheneau E, Wolfram R, Seabron R, Horton K, Kolodgie F, Virmani R, Rivera E. Transepicardial autologous bone marrow-derived mononuclear cell therapy in a porcine model of chronically infarcted myocardium. Cardiovasc Radiat Med. 2004;5:125–31. [DOI] [PubMed] [Google Scholar]
- 118.Mathieu M, Bartunek J, El Oumeiri B, et al. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. J Thorac Cardiovasc Surg. 2009;138:646–53. [DOI] [PubMed] [Google Scholar]
- 119.Bel A, Messas E, Agbulut O, Richard P, Samuel JL, Bruneval P, Hagege AA, Menasche P. Transplantation of autologous fresh bone marrow into infarcted myocardium: a word of caution. Circulation. 2003;108 Suppl 1:II247–52. [DOI] [PubMed] [Google Scholar]
- 120.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. [DOI] [PubMed] [Google Scholar]
- 122.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–7. [DOI] [PubMed] [Google Scholar]
- 123.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. [DOI] [PubMed] [Google Scholar]
- 124.Thal MA, Krishnamurthy P, Mackie AR, Hoxha E, Lambers E, Verma S, Ramirez V, Qin G, Losordo DW, Kishore R. Enhanced angiogenic and cardiomyocyte differentiation capacity of epigenetically reprogrammed mouse and human endothelial progenitor cells augments their efficacy for ischemic myocardial repair. Circ Res. 2012;111:180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hynes B, Kumar AH, O’Sullivan J, Klein Buneker C, Leblond AL, Weiss S, Schmeckpeper J, Martin K, Caplice NM. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur Heart J. 2013;34:782–9. [DOI] [PubMed] [Google Scholar]
- 126.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- 127.Pfannkuche K, Liang H, Hannes T, et al. Cardiac myocytes derived from murine reprogrammed fibroblasts: intact hormonal regulation, cardiac ion channel expression and development of contractility. Cell Physiol Biochem. 2009;24:73–86. [DOI] [PubMed] [Google Scholar]
- 128.Riggs JW, Barrilleaux BL, Varlakhanova N, Bush KM, Chan V, Knoepfler PS. Induced pluripotency and oncogenic transformation are related processes. Stem Cells Dev. 2013;22:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Riegler J, Tiburcy M, Ebert A, et al. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ Res. 2015;117:720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Templin C, Zweigerdt R, Schwanke K, et al. Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation. 2012;126:430–9. [DOI] [PubMed] [Google Scholar]
- 132.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–37. [DOI] [PubMed] [Google Scholar]
- 133.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu K, Wu Q, Ni C, et al. Lack of Remuscularization Following Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells in Infarcted Nonhuman Primates. Circ Res. 2018. [DOI] [PubMed] [Google Scholar]
- 135.Ott HC, Bonaros N, Marksteiner R, Wolf D, Margreiter E, Schachner T, Laufer G, Hering S. Combined transplantation of skeletal myoblasts and bone marrow stem cells for myocardial repair in rats. Eur J Cardiothorac Surg. 2004;25:627–34. [DOI] [PubMed] [Google Scholar]
- 136.Memon IA, Sawa Y, Miyagawa S, Taketani S, Matsuda H. Combined autologous cellular cardiomyoplasty with skeletal myoblasts and bone marrow cells in canine hearts for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2005;130:646–53. [DOI] [PubMed] [Google Scholar]
- 137.Quijada P, Salunga HT, Hariharan N, Cubillo JD, El-Sayed FG, Moshref M, Bala KM, Emathinger JM, De La Torre A, Ormachea L, Alvarez R, Gude NA, Sussman MA. Cardiac Stem Cell Hybrids Enhance Myocardial Repair. Circ Res. 2015;117:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Natsumeda M, Florea V, Rieger AC, et al. A Combination of Allogeneic Stem Cells Promotes Cardiac Regeneration. J Am Coll Cardiol. 2017;70:2504–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ Res. 2008;103:1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AAM, Goumans MJ, Strijder C, Sze SK, Choo A, Piek JJ, Doevendans PA, Pasterkamp G, de Kleijn DPV. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Research. 2011;6:206–214. [DOI] [PubMed] [Google Scholar]
- 141.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. [DOI] [PubMed] [Google Scholar]
- 142.Pavo N, Zimmermann M, Pils D, Mildner M, Petrasi Z, Petnehazy O, Fuzik J, Jakab A, Gabriel C, Sipos W, Maurer G, Gyongyosi M, Ankersmit HJ. Long-acting beneficial effect of percutaneously intramyocardially delivered secretome of apoptotic peripheral blood cells on porcine chronic ischemic left ventricular dysfunction. Biomaterials. 2014;35:3541–50. [DOI] [PubMed] [Google Scholar]
- 143.Sahoo S, Losordo DW. Exosomes and Cardiac Repair After Myocardial Infarction. Circulation Research. 2014;114:333–344. [DOI] [PubMed] [Google Scholar]
- 144.Yu B, Zhang X, Li X. Exosomes Derived from Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2014;15:4142–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lai RC, Yeo RWY, Lim SK. Mesenchymal stem cell exosomes. Seminars in Cell & Developmental Biology. 2015;40:82–88. [DOI] [PubMed] [Google Scholar]
- 146.Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DPV, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research. 2010;4:214–222. [DOI] [PubMed] [Google Scholar]
- 147.Ibrahim Ahmed G-E, Cheng K, Marbán E. Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy. Stem Cell Reports. 2014;2:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Adamiak M, Cheng G, Bobis-Wozowicz S, et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ Res. 2018;122:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sheng CC, Zhou L, Hao J. Current stem cell delivery methods for myocardial repair. Biomed Res Int. 2013;2013:547902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Pract Cardiovasc Med. 2006;3 Suppl 1:S57–64. [DOI] [PubMed] [Google Scholar]
- 151.Vela DC, Silva GV, Assad JA, Sousa AL, Coulter S, Fernandes MR, Perin EC, Willerson JT, Buja LM. Histopathological study of healing after allogenic mesenchymal stem cell delivery in myocardial infarction in dogs. JHistochemCytochem. 2009;57:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang X, Jameel MN, Li Q, Mansoor A, Qiang X, Swingen C, Panetta C, Zhang J. Stem cells for myocardial repair with use of a transarterial catheter. Circulation. 2009;120:S238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suzuki K, Brand NJ, Smolenski RT, Jayakumar J, Murtuza B, Yacoub MH. Development of a novel method for cell transplantation through the coronary artery. Circulation. 2000;102:III359–64. [DOI] [PubMed] [Google Scholar]
- 154.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103:525–36. [DOI] [PubMed] [Google Scholar]
- 155.Poglajen G, Vrtovec B. Stem cell therapy for chronic heart failure. Curr Opin Cardiol. 2015;30:301–10. [DOI] [PubMed] [Google Scholar]
- 156.Gyongyosi M, Dib N. Diagnostic and prognostic value of 3D NOGA mapping in ischemic heart disease. Nat Rev Cardiol. 2011;8:393–404. [DOI] [PubMed] [Google Scholar]
- 157.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hamamoto H, Gorman JH 3rd, Ryan LP, Hinmon R, Martens TP, Schuster MD, Plappert T, Kiupel M, St John-Sutton MG, Itescu S, Gorman RC. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: the effect of cell dosage. Ann Thorac Surg. 2009;87:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wei H, Ooi TH, Tan G, Lim SY, Qian L, Wong P, Shim W. Cell delivery and tracking in post-myocardial infarction cardiac stem cell therapy: an introduction for clinical researchers. Heart Fail Rev. 2010;15:1–14. [DOI] [PubMed] [Google Scholar]
- 160.Sui R, Liao X, Zhou X, Tan Q. The current status of engineering myocardial tissue. Stem Cell Rev. 2011;7:172–80. [DOI] [PubMed] [Google Scholar]
- 161.Reis LA, Chiu LL, Feric N, Fu L, Radisic M. Biomaterials in myocardial tissue engineering. J Tissue Eng Regen Med. 2016;10:11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mohanty S, Larsen LB, Trifol J, Szabo P, Burri HV, Canali C, Dufva M, Emneus J, Wolff A. Fabrication of scalable and structured tissue engineering scaffolds using water dissolvable sacrificial 3D printed moulds. Mater Sci Eng C Mater Biol Appl. 2015;55:569–78. [DOI] [PubMed] [Google Scholar]
- 163.Jones SP, Tang XL, Guo Y, et al. The NHLBI-sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res. 2015;116:572–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hausenloy DJ, Garcia-Dorado D, Botker HE, et al. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res. 2017;113:564–585. [DOI] [PubMed] [Google Scholar]
- 165.Guo Y, Wysoczynski M, Nong Y, Tomlin A, Zhu X, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li Q, Bolli R. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol. 2017;112:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Terrovitis J, Lautamaki R, Bonios M, Fox J, Engles JM, Yu J, Leppo MK, Pomper MG, Wahl RL, Seidel J, Tsui BM, Bengel FM, Abraham MR, Marban E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol Rev. 2016;96:1127–68. [DOI] [PMC free article] [PubMed] [Google Scholar]