Abstract
Aims
Here, we aimed to determine the therapeutic effect of longevity-associated variant (LAV)-BPIFB4 gene therapy on atherosclerosis.
Methods and results
ApoE knockout mice (ApoE−/−) fed a high-fat diet were randomly allocated to receive LAV-BPIFB4, wild-type (WT)-BPIFB4, or empty vector via adeno-associated viral vector injection. The primary endpoints of the study were to assess (i) vascular reactivity and (ii) atherosclerotic disease severity, by Echo-Doppler imaging, histology and ultrastructural analysis. Moreover, we assessed the capacity of the LAV-BPIFB4 protein to shift monocyte-derived macrophages of atherosclerotic mice and patients towards an anti-inflammatory phenotype. LAV-BPIFB4 gene therapy rescued endothelial function of mesenteric and femoral arteries from ApoE−/− mice; this effect was blunted by AMD3100, a CXC chemokine receptor type 4 (CXCR4) inhibitor. LAV-BPIFB4-treated mice showed a CXCR4-mediated shift in the balance between Ly6Chigh/Ly6Clow monocytes and M2/M1 macrophages, along with decreased T cell proliferation and elevated circulating levels of interleukins IL-23 and IL-27. In vitro conditioning with LAV-BPIFB4 protein of macrophages from atherosclerotic patients resulted in a CXCR4-dependent M2 polarization phenotype. Furthermore, LAV-BPIFB4 treatment of arteries explanted from atherosclerotic patients increased the release of atheroprotective IL-33, while inhibiting the release of pro-inflammatory IL-1β, inducing endothelial nitric oxide synthase phosphorylation and restoring endothelial function. Finally, significantly lower plasma BPIFB4 was detected in patients with pathological carotid stenosis (>25%) and intima media thickness >2 mm.
Conclusion
Transfer of the LAV of BPIFB4 reduces the atherogenic process and skews macrophages towards an M2-resolving phenotype through modulation of CXCR4, thus opening up novel therapeutic possibilities in cardiovascular disease.
Keywords: Atherosclerosis, Low-density lipoprotein, Vascular function, Immune system
See page 2498 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa522)
Translational perspective
As the main risk factor for cardiovascular diseases (CVDs) is progressive ageing of the population, unravelling the secrets of healthy ageing may be the only way to limit CVD disabilities. Here, we show that the favourable phenotype of long-living individuals can be transferred by gene therapy with the longevity-associated variant (LAV) of BPIFB4 to animal models. Indeed, the ability of LAV-BPIFB4 to finely tune endothelial function and pro/anti-inflammatory balance, as well as the fine correlation between a high BPIFB4 plasma level and a blunted atherogenic process in the clinical setting, makes LAV-BPIFB4 a suitable candidate tool for the treatment of atherosclerosis and its related CVD complications.
Introduction
Atherosclerosis, a multi-factorial disease influenced by genetic and environmental factors, represents one of the leading causes of death in industrialized society. As it is a slowly progressing disease, it is vital to find new treatments able to halt plaque progression and the underlying inflammatory substrate.1 In this regard, chemokines and small chemotactic peptides represent a potential therapeutic target.1
Recent evidence indicates that the chemokine stromal-cell derived factor-1 (SDF-1, also known as CXCL12) plays an important role in reparative processes by binding to the C-X-C chemokine receptor type 4 (CXCR4) on proangiogenic cells and leading them to sites of tissue damage.2,3 Among myeloid cells, the high expression of CXCR4 identifies transitional bone marrow precursors that replenish the mature monocyte pool for peripheral responses.4 Here, the recruitment of Ly6Chigh monocytes has resulted essential in plaque regression through their proper differentiation into M2 macrophages.5 However, the precise role of CXCR4/CXCL12 in atherosclerosis remains controversial. Pharmacological disruption of CXCR4 significantly aggravates diet-induced atherosclerotic lesion development in ApoE−/− mice.2 In addition, a common allele variant within the CXCR4 locus has been associated with atherosclerotic coronary heart disease.3 However, it has been also reported that high expression of CXCR4 in mouse heart increases infarct size, reduces cardiac function and leads to recruitment of inflammatory cells.6 Furthermore, expression of CXCR4 and CXCL12 is reportedly increased in Ly6C monocytes and macrophages within both stable and unstable carotid atherosclerotic plaques compared with healthy vessels, both at mRNA and protein levels.7
Long-living individuals (LLIs) delay or escape atherosclerosis-related cardiovascular disease (CVD).8 We have previously found that LLIs are enriched for a longevity-associated variant (LAV) in BPI fold containing family B, member 4 (BPIFB4) and that LAV-BPIFB4, when compared with wild-type (WT)-BPIFB4, is more phosphorylated at serine 75 and more cytoplasmic (due to its gained capacity to bind 14-3-3), determining endothelial nitric oxide synthase (eNOS) activation through a calcium/PKCα-mediated mechanism.9,10 Notably, systemic delivery of BPIFB4 to rodents with arterial hypertension or limb ischaemia improved endothelial function, normalized blood pressure, and accelerated revascularization, thus supporting the novel concept of utilitarian transferability of the successful genetic programme of centenarians to the treatment of CVD.10 We also gathered initial evidence that LAV-BPIFB4 exerts an immunomodulatory activity, reducing T cell activation possibly through interference with antigen presenting cells and the pro-inflammatory cytokines TNF-α and IL-1β. Furthermore, our previous study showed that mononuclear cells (MNCs) from LLIs are characterized by higher levels of BPIFB4 and reduced CXCR4 expression than non-healthy (frail) LLIs.11 Interestingly, lower CXCR4 expression in MNCs from healthy LLIs is paired with greater membrane localization of CXCR4,12 a hallmark of chemokine activation. In line with this finding, BPIFB4 knockout by siRNA blunted the migratory response of circulating pro-angiogenic cells to CXCL12.10 These seminal findings led us to hypothesize that the transfer of LAV-BPIFB4 could be a viable means to interfere with the immune-inflammatory features of vascular atherosclerosis.
To test this novel hypothesis, we performed LAV-BPIFB4 gene therapy in ApoE−/− mice fed a high-fat diet, and evaluated the impact on endothelial dysfunction and progression of the atherosclerotic disease, focusing on CXCR4-dependent monocyte polarization as a possible mechanistic mediator. Moreover, to assess the possible translational aspects of BPIFB4 in humans, we investigated the correlation between the plasma level of the protein and either carotid stenosis or intima media thickness (IMT) in two independent patient cohorts.
Methods
Animal models
All animal studies were performed in accordance with approved protocols by the IRCCS Neuromed Animal Care Review Board and by the Istituto Superiore di Sanità, Rome (number: 1163/2015-PR) and were conducted according to EU Directive 2010/63/EU for animal experiments. Animal models’ details and treatment are reported in Supplementary material online.
Cloning, vector production and in vivo gene therapy
Ten-week-old ApoE−/− male mice were treated with 1 × 1013 GC/kg of AAV-GFP, AAV-WT-BPIFB4, or AAV-LAV-BPIFB4, as described in the Supplementary material online.
Echo-Doppler analysis
Detailed procedure is available in the Supplementary material online.
Histology and lesion analysis
Detailed procedure is available in the Supplementary material online.
Transmission electron microscopy and post-embedding immune-cytochemistry
Transmission electron microscopy method is available in the Supplementary material online.
Ex vivo transfection of mouse vessels
Detailed procedure is available in the Supplementary material online.
In vitro studies on human monocyte-derived macrophages
Detailed procedure is available in the Supplementary material online.
Cytokine detection
Cytokines were analysed by beads-based multiplex ELISA (LEGENDplex, Biolegend, USA) as described in the Supplementary material online.
Patient cohorts
The patient enrolled for the evaluation of carotid stenosis, intima-media-thickness, BPIFB4 plasma levels, and genotype characterization are described in the Supplementary material online.
Ex vivo studies of human vessels
The study protocol was approved by the local ethics committees of IRCCS Neuromed and done in accordance with the Declaration of Helsinki. All participants gave written informed consent. Institutional review board approval was obtained from IRCCS Neuromed (No. 20160106-1006). A detailed procedure is reported in the Supplementary material online.
Western blotting
Western blotting was performed on pooled protein extracts from mice vessels or on total segments of human vessels, as reported in the Supplementary material online.
Statistical analysis
Statistical analyses are available in the Supplementary material online.
Results
LAV-BPIFB4 reduces endothelial dysfunction
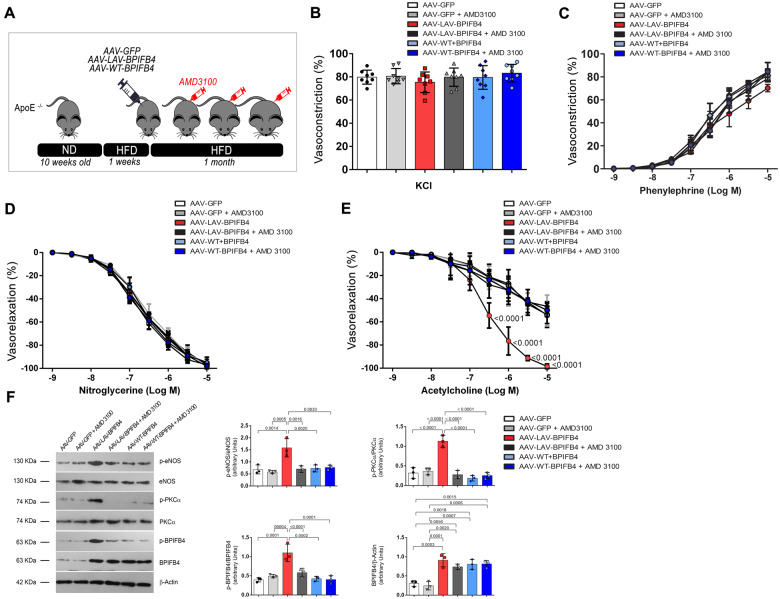
We examined the effects of LAV-BPIFB4 gene therapy in ApoE knockout mice, using GFP- and WT-BPIFB4-treated mice for comparison. Genes were delivered using adeno-associated viral (AAV) vectors injected into femoral arteries, with efficiency properly monitored in liver, myeloid cells, plasma, and vascular cells (Supplementary material online, Figures S1–S5 and Methods). In addition, each group was randomized to receive a CXCR4 antagonist, AMD3100, or vehicle to verify the effect of the receptor on the primary endpoints of the treatment (Figure 1A). The primary endpoint indicates no difference among groups with regard to KCl- or phenylephrine-induced vasoconstriction and endothelium-independent relaxation to nitroglycerine. Likewise, no difference was observed in the presence or absence of the CXCR4 inhibitor (Figure 1B–D and Supplementary material online, Figure S6A–D). In contrast, vessels obtained from ApoE knockout mice treated with AAV-LAV-BPIFB4 showed a complete rescue of acetylcholine-mediated endothelial vasorelaxation both in mesenteric and femoral arteries (Figure 1E and Supplementary material online, Figure S6D). Interestingly, AMD3100, a non-peptide antagonist of CXCR4, abolished the beneficial effect of AAV-LAV-BPIFB4 gene therapy (Figure 1E and Supplementary material online, Figure S6D).
Figure 1.
Overexpression of LAV-BPIFB4 improves the vascular reactivity of ApoE null mice fed, a high-fat diet, and a CXCR4 inhibitor abolishes this protective effect. (A) Experimental protocol; (B) vascular response of ex vivo mesenteric arteries from ApoE knockout mice to potassium (80 mmol/L KCl) and (C) the dose–responses to phenylephrine, (D) acetylcholine, and (E) nitroglycerine after 1 month of AAV-LAV-BPIFB4 treatment. AAV-GFP was used as a control. Values are mean ± standard deviation of eight independent experiments. B, C, D, E two-way ANOVA followed Tukey’s multiple comparisons test. Numbers next to the curve show adjusted P-values. (F) Representative western blot (left) and densitometric analysis (right) conducted on mesenteric artery lysates. Values are mean ± standard deviation (N = 3). One-way ANOVA followed Tukey’s multiple comparisons test. Numbers above square brackets show adjusted P-values.
Western blot analyses of mesenteric arteries explanted from AAV-LAV-BPIFB4-treated mice showed an increase of total and phosphorylated (serine 75) BPIFB4 protein (Figure 1F).9 Likewise, AAV-LAV-BPIFB4 increased the phosphorylation of eNOS at serine 1177 and of PKCα at threonine 497 (Figure 1F), in accordance with our previous published results.9 eNOS is required for CCL12/CXCR4-mediated endothelial actions and different PKC isoforms mediate CXCR4 phosphorylation/activation.13 These phosphorylation changes were blunted by AMD3100 treatment, thus confirming the importance of CXCR4 and upstream activators in LAV-BPIFB4-induced vasorelaxation (Figure 1F). Of note, the action of AAV-LAV-BPIFB4 was not dependent on total cholesterol or LDL circulating levels, which remained similarly elevated in all after AAV-LAV-BPIFB4 treatment (Supplementary material online, Table S1). Moreover, LAV-BPIFB4 exerted a protective effect on oxLDL-induced endothelial dysfunction (Supplementary material online, Figure S7).
LAV-BPIFB4 halts vascular plaque formation
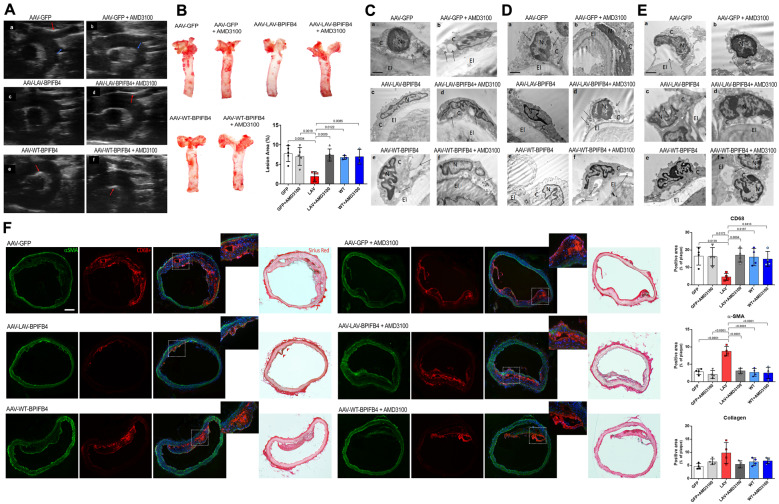
We next considered the other primary endpoint of the in vivo study, namely the progression of atherosclerosis as assessed by Echo-Doppler and histology. Ultrasound scanning of aortic arch with epi-aortic vessels revealed the presence lipid plaques in ApoE knockout mice treated with AAV-GFP and AAV-WT-BPIFB4, whereas AAV-LAV-BPIFB4 gene therapy reduced the formation of vascular plaques (Figure 2A). The quantification of the abundance of lipid streaks in aorta, which represent initial structural changes detectable in atherosclerosis, confirmed that LAV-BPIFB4 reduced vascular damage as compared to all other groups (Figure 2B). Moreover, the effect of LAV-BPIFB4 was lost in AMD3100-treated mice (Figure 2A and B), thus corroborating the CXCR4-mediated protective role of LAV-BPIFB4 on the onset of the atherogenic process.
Figure 2.
LAV-BPIFB4 gene therapy hinders plaque formation, preserves vascular endothelium integrity, and reduces monocyte/macrophages infiltration. (A) Ultrasound scanning of aortic arch with epi-aortic vessels in AAV-treated ApoE−/− mice fed a high-fat diet. LAV-BPIFB4-treated mice did not have any plaques, whereas all those on the other treatments had calcified plaques (red arrows) and lipidic plaques (blue arrows). (B) Oil Red O staining and quantitative analysis of atherosclerotic lesion size in the aorta. Oil Red O staining was quantified using ImageJ software. One-way ANOVA followed by Tukey’s multiple comparisons test. Numbers above square brackets show adjusted P-values. (C–E) Representative micrographs of endothelial cells from aorta (C), mesenteric (D), and femoral (E) arteries: (a) Vascular endothelium of the AAV-GFP group showed cytosolic derangement (arrows) and broken plasma membranes (arrowheads); (b) these alterations were also evident in the group treated with the CXCR4 inhibitor. Diluted cytoplasm (arrow) and markedly condensed chromatin in the nucleus (N) was observed. (c) The endothelium was preserved by gene therapy with LAV-BPIFB4. (d) AMD3100 contrasted the benefit of LAV-BPIFB4, with the endothelial cells showing a diluted cytoplasm (arrows) and disruption of the plasma membrane (arrowheads); (e) The AAV-WT-BPIFB4-treated group presented with severely altered ultrastructure of the endothelium; (f) AMD3100 administration conferred additional ultrastructural damage, with endothelial cell detachment from the elastic membrane lamina (arrows). (F) Immunofluorescence staining of aortic arches from treated ApoE knockout mice, using the monocyte/macrophage marker CD68+, the smooth muscle cell marker αSMA, and Sirius Red staining to evaluate collagen (N = 4 per group). One-way ANOVA followed by Tukey’s multiple comparisons test. Numbers above square brackets show adjusted P-values.
Ultrastructural evaluation of the aorta, femoral, and mesenteric arteries from ApoE knockout mice showed alterations in endothelial cells, such as cytosolic derangement, diluted cytoplasm, detachment of the endothelial membrane, and broken plasma membrane. Gene therapy with AAV-LAV-BPIFB4 preserved the regular architecture of the vascular endothelium, again through a CXCR4-dependent mechanism (Figure 2C–E).
An imbalance of the mononuclear phagocytic system within the vascular wall is pivotal in influencing plaque initiation and progression.14 Hence, we next verified the ability of LAV-BPIFB4 to interfere with the above mechanism by staining harvested aortic arch with CD68+, a marker highly expressed by monocytes and lesional macrophages, and with α-smooth muscle actin and collagen, which represent important compositional features of the atherosclerotic lesions. Results indicated that LAV-BPIFB4 reduces macrophages infiltration without loss of smooth muscle cells, a finding suggesting not only a slowing of the atherogenic process but also plaque stabilization.15,16 The collagen composition in the aortic arch of LAV-treated mice, evaluated by Sirius red positive area, showed a thicker fibrous cap than in vessels from other treatments, indicating a role for LAV-BPIFB4 in containing the onset and progression of the atherogenic processes (Figure 2F). In agreement with these findings, femoral arteries stained with the mono-macrophage marker MOMA-2 showed a marked reduction of positive cells in vessels from AAV-LAV-BPIFB- infected mice when compared with controls, an effect partially abolished by co-treatment with AMD3100 (Supplementary material online, Figure S5).
LAV-BPIFB4 regulates the peripheral pool of monocytes in a CXCR4-dependent manner
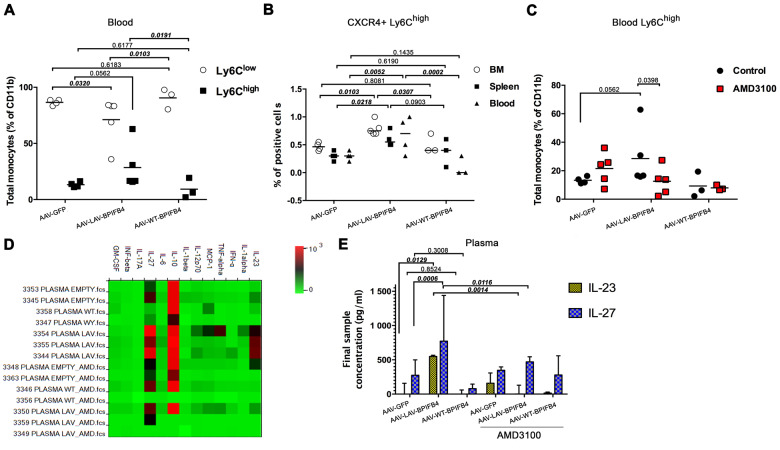
The observed reductions of CD68+ cells at the aortic arch level and of MOMA-2 positive cells in femoral arteries may reflect a redistribution of circulating monocytes in response to the treatment. Indeed, as shown by western blot analysis, peripheral monocytes and progenitor bone marrow myeloid cells expressed an enhanced level of BPIFB4 after gene therapy (Supplementary material online, Figure S5B). To test this, we next examined monocyte frequency in peripheral blood from mice treated with GFP, WT-BPIFB4, or LAV-BPIFB4. In particular, we focused on the two major subsets of murine monocytes: ‘classical’ Ly6Chigh and ‘non-classical’ Ly6Clow cells. Flow cytometry analyses demonstrated LAV-BPIFB4 gene therapy causes a reduction of Ly6Clow monocytes and an increase of Ly6Chigh monocytes when compared with controls (Figure 3A).
Figure 3.
LAV-BPIFB4 modulates monocyte dynamics in ApoE knockout mice. (A) Relative abundance of Ly6Chigh and Ly6Clow subsets in the peripheral blood of treated mice (AAV-GFP, N = 5; AAV-LAV-BPIFB4, N = 5; AAV-WT-BPIFB4, N = 3). (B) Percentage of CD11b+Ly6G-Ly6C+ monocytes expressing CXCR4 in bone marrow, spleen, and blood (AAV-GFP, N = 5; AAV-LAV-BPIFB4, N = 5; AAV-WT-BPIFB4, N = 3). (C) Effect of CXCR4 inhibitor on the frequency of peripheral blood Ly6Chigh monocytes (AAV-GFP, N = 5; AAV-LAV-BPIFB4, N = 5; AAV-WT-BPIFB4, N = 3; GFP-AMD3100, N = 5; LAV-BPIFB4-AMD3100, N = 5; WT-BPIFB4-AMD3100, N = 3). (D and E) Plasma collected from all the mice groups indicated above was assessed for circulating cytokine levels with bead-based multiplex ELISA. The relative concentrations of analytes are presented as a heat map (D) or expressed as the mean ± standard deviation of each sample determination conducted in duplicate (E). Statistical analysis by two-way ANOVA with post hoc Fisher’s Least Significant Difference (LSD) test was conducted. Numbers above square brackets show unadjusted LSD P-values.
Monocyte polarization has been attributed to changes in the oscillation of CXCR4 expression on bone marrow precursor cells, which serve for the replenishment of the peripheral pool of Ly6Chigh monocytes.4 Additionally, a substantial portion of Ly6Chigh monocytes originates from the spleen.17 We confirmed that these central sites play a role in the observed peripheral polarization induced by gene therapy. In fact, LAV-BPIFB4, but not WT-BPIFB4 or GFP, increased the percentage of CXCR4+Ly6Chigh cells in the bone marrow and spleen of ApoE knockout mice. A similar trend was observed in peripheral blood (Figure 3B). Importantly, AMD3100 contrasted the improved effect of LAV-BPIFB4 on Ly6Chigh cell frequency, thus supporting a role for CXCR4 in these phenomena (Figure 3C). Results of multiplex bead-based immunoassay showed that LAV-BPIFB4 gene therapy increases the peripheral blood levels of IL-23 and IL-27, effects contrasted by AMD3100 (Figure 3D and E).
LAV-BPIFB4 induces an enrichment of M2 splenic macrophages and reduces the proliferative state of T cells
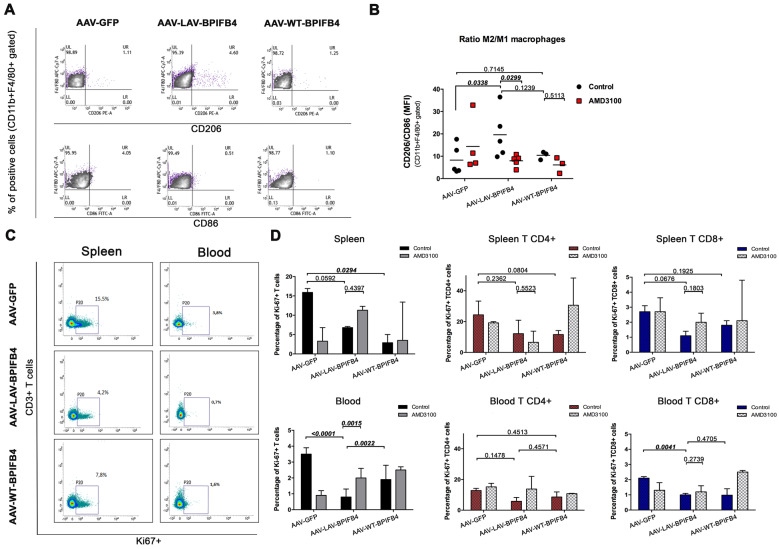
IL-23 and IL-27 are mainly secreted by monocytes and macrophages, and they have been recently implicated in suppression of atherosclerosis, partly controlling myeloid cell accumulation.5,18–20 As Ly6Chigh conversion to M2 macrophages has been seen to drive plaque regression in different models of atherosclerosis,5,17 we sought to examine whether LAV-BPIFB4 could shift the phenotype of macrophages towards the pre-resolving M2 (alternatively activated) state. Due to the limited macrophage yield from the vessel wall, we focused on readily available macrophages derived from the spleen.21 Flow cytometry analysis of CD206/CD86, a marker of the relative proportion of M2 vs. M1 cells, indicated that LAV-BPIFB4 increased M2 monocytes in the spleen of ApoE knockout mice (Figure 4A and B). LAV-BPIFB4-induced polarization of monocytes towards an M2 phenotype was prevented by concomitant CXCR4 inhibition (Figure 4B).
Figure 4.
Enhanced enrichment of M2 macrophages in the spleen of AAV-LAV-BPIFB4 ApoE knockout mice fed a high-fat diet. (A and B) CD45hi CD11b+ F4/80 + splenic macrophages were additionally stained with flow cytometric markers CD206+ or CD86+ to discern the CD11b+ F4/80 +CD206 M2 type from CD11b+ F4/80 +CD86 M1 type of splenic macrophages. A representative dot plot panel (left) is presented. The graph on the right reports the mean ± standard deviation of ratios of M2 vs. M1 splenic macrophages (CD206/CD86 ratio) from all recipient mice (N = 3–5 per group). MFI stands for mean fluorescence intensity of selected markers. (C) Analysis of the proliferation of splenic and blood CD3+ T cells in ApoE−/− mice infected with AAV-LAV-BPIFB4, AAV-WT-BPIFB4 or AAV-empty vector, treated or not with AMD3100. Representative dot plot showing changes of percentage Ki-67 expression in both spleen and blood TCD3+ cells. (D) Bar graph reporting the percentage ± standard deviation of Ki67+, CD3+ gated T cells. Percentage of Ki-67+ expression in CD4+ and CD8+ T cell subsets from the spleen and the blood of recipient mice were determined. The bar graph reports the percentage ± standard deviation. Statistical analysis by two-way ANOVA with post hoc Fisher’s Least Significant Difference (LSD) test was conducted. Numbers above square brackets show unadjusted LSD P-values.
M2 macrophages can reduce pro-inflammatory cytokine secretion and dampen inflammatory responses.22,23 Therefore, we next compared the proliferative state of CD4+ and CD8+ T cells in the spleen and peripheral blood of ApoE knockout mice treated with LAV-BPIFB4, WT-BPIFB4, or GFP (Figure 4C and D). Both LAV-BPIFB4 and WT-BPIFB4 reduced the abundance of Ki-67+ CD3 T cells, with AMD3100 inhibiting this effect only in the LAV-BPIFB4 group in peripheral blood compartment (Figure 4D). Looking at CD3 subfractions mostly affected by these changes, we found that LAV-BPIFB4 specifically reduced the abundance of proliferating cytotoxic CD8+ T cells in the spleen and in a significant manner in peripheral blood (Figure 4D).
In vitro exposure of PB-MNCs to LAV-BPIFB4 protein induces M2 macrophage differentiation
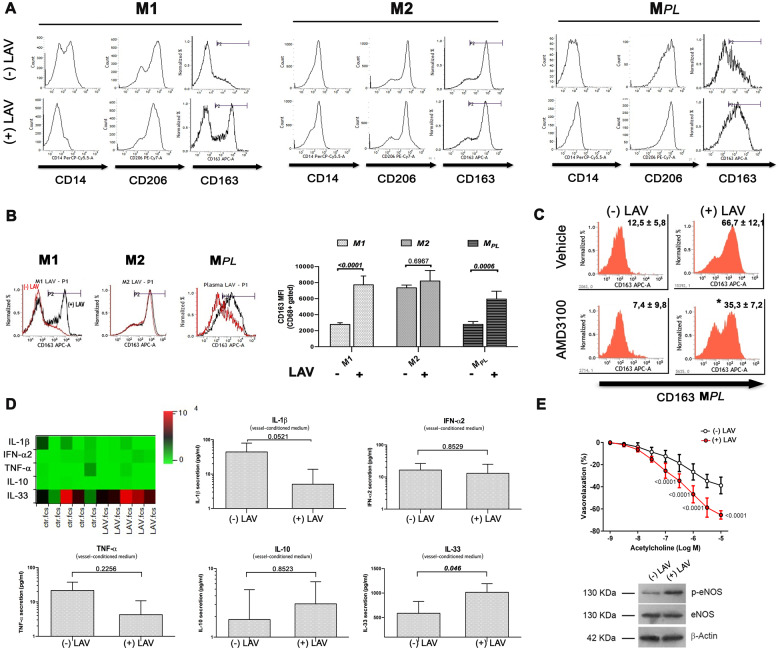
In order to confirm a direct effect of LAV-BPIFB4 on macrophage polarization, we tested the effect of human recombinant LAV-BPIFB4 protein24 on CD14+ PB-MNCs collected from atherosclerotic patients, which were induced to differentiate ex vivo into M1 or M2 macrophages, using a CellXVivo™ Kit, or into MPL macrophages, after exposure to autologous plasma. After 72 h of cytokine priming, cells were maintained in culture for an additional 72 h in the presence of LAV-BPIFB4 recombinant protein or vehicle. As shown in Figure 5A, cells harvested at the end of the conditioning included M1 macrophages displaying the canonical CD14+/CD206+/CD163− phenotype, and M2 macrophages characterized by the CD14+/CD206+/CD163+ phenotype. Flow cytometry analyses confirmed the ability of MPL to induce an M1 phenotype, whereas LAV-BPIFB4 polarized macrophages away from the M1 phenotype and towards a classical M2 anti-inflammatory state (Figure 5A and B). Of note, co-treatment with AMD3100 partially blunted the acquisition of the M2 phenotype induced by LAV-BPIFB4. This confirms the direct involvement of CXCR4 in LAV-BPIFB4’s polarizing effects (Figure 5C).
Figure 5.
In vitro conditioning with recombinant LAV-BPIFB4 protein leads to polarization of patient macrophages towards M2 phenotypes and reduces the inflammatory milieu in the vessel wall. (A) Macrophages generated from peripheral blood monocytes from atherosclerotic patients (N = 5) were cultured with or without recombinant LAV-BPIFB4 (18 ng/mL) for the last 72 h. Representative cytofluorimetric histogram profiles of CD14, CD206, and CD163 protein levels at the cell surface of macrophages (viable gated CD68+ cells) polarized towards M1, M2, or MPL (autologous plasma) phenotypes. (B) (left panel) Histogram overlay for CD163 M2 markers in M1, M2, or MPL macrophages from control, untreated- (red curve), and LAV-BPIFB4-treated cells (black curve) from a representative atherosclerotic patient. (right panel) The bar graph reports the mean fluorescence intensity (MFI) values ± standard deviation of CD163 on viable CD68+ gated cells. Two-way ANOVA followed by Tukey's multiple comparisons test. Numbers above square brackets show adjusted P-values. (C) Representative histogram profiles of CD163 protein level on MPL cell surface in controls and in cells treated with LAV-BPIFB4 (18 ng/mL) in the presence or absence of AMD3100 (20 μM) for 72 h. Percentages of positive cells are indicated in the upper right corner and are representative of four independent experiments. (D) Vessels from atherosclerotic patients (N = 5) were incubated for 24 h at 37°C in the presence or absence of 18 ng/mL LAV-BPIFB4. Conditioned medium was then assessed for cytokine secretion with bead-based multiplex ELISA. The relative concentrations of analytes detected is presented as a heat map. Statistical comparisons were performed with two-tailed Student’s t-test for simple comparisons. Numbers above square brackets show unadjusted P-values. (E) Vascular response of ex vivo human atherosclerotic femoral arteries (upper) to increasing doses of acetylcholine, before and after treatment for 1 h with LAV-BPIFB4 recombinant protein (18 ng/mL) (N = 5); two-way ANOVA followed Tukey's multiple comparisons test. Numbers reported on the curves show adjusted P-values. (Bottom) representative western blots conducted on the studied human vessels.
Finally, in order to strengthen the concept that LAV-BPIFB4 treatment can modify the inflammatory milieu within the vasculature, we measured the levels of pro- and anti-inflammatory mediators secreted by organotypic cultures of human atherosclerotic vessels. As shown in Figure 5D, stimulation with LAV-BPIFB4 protein reduced the levels of pro-inflammatory IL-1β and TNF-α, while increasing atheroprotective IL-33.25,26
Moreover, LAV-BPIFB4 improved endothelial-mediated vasorelaxation and eNOS phosphorylation of human atherosclerotic vessels (Figure 5E).
High plasma BPIFB4 associates with a reduction of atherosclerotic risk
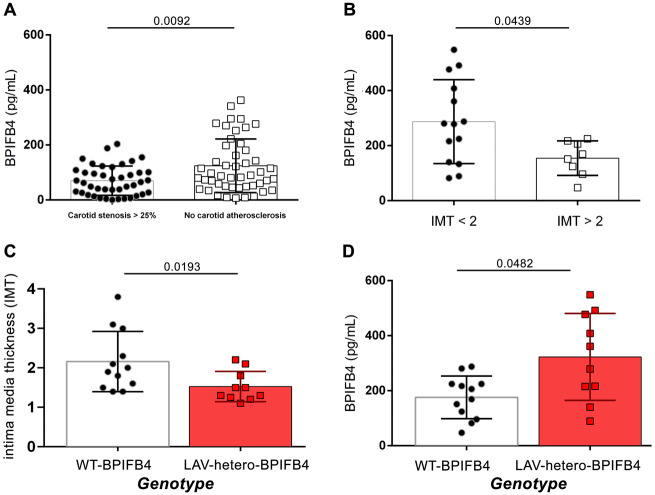
Starting form a population of 2606 individuals fully characterized in the ‘Progressione della Lesione Intimale Carotidea’ (PLIC) study, we focused on two groups of subjects stratified for the presence of subclinical carotid atherosclerosis (respectively, lower or higher than 25% of carotid stenosis; N = 90) (Table 1), and evaluated plasma levels of BPIFB4. Interestingly, our data revealed that in patients with no subclinical carotid atherosclerosis, the protein’s concentration was significantly higher as compared to patients with carotid stenosis (Figure 6A). The functional role of the circulating BPIFB4 was magnified when examining its association with IMT stratification of a cohort of 22 consecutively enrolled non-smokers, non-diabetics without previous CV events and not on statin therapy, belonging to the Campania Salute Network Registry, with hypertension as the singular cardiovascular risk factor and an absence of other comorbidities (Table 2). As shown in Figure 6B, BPIFB4 was significantly higher in plasma from patients with IMT <2 mm,27 confirming a protective role of high protein levels, a finding in line with what was previously observed in the PLIC study. Moreover, genotype stratification analysis revealed that LAV carriers significantly more frequently had IMT <2 mm cohort, and that in this cohort, LAV-BPIFB4 carriers had a higher level of circulating BPIFB4 when compared with no-carriers (Figure 6C and D).
Table 1.
Clinical characteristics of patients from the PLIC study
| Parameters | PLIC population (N = 90) |
|---|---|
| Age (years) | 73 (70–77) |
| Gender (n, women) | 50 |
| Smoking habit (n, active) | 18 |
| BMI (kg/m2) | 26.70 (23.90–29.60) |
| Waist (cm) | 93.5 (87.0–99.2) |
| SBP (mmHg) | 130 (120–140) |
| DBP (mmHg) | 80 (70–85) |
| Anti-hypertensives (n, yes) | 46 |
| Fasting glucose (mg/dL) | 93.00 (86.00–104.25) |
| Oral lowering therapies (n, yes) | 5 |
| Total cholesterol (mg/dL) | 202.06 (30.91) |
| HDL (mg/dL) | 59.04 (12.42) |
| Triglycerides (mg/dL) | 88.00 (70.75–116.25) |
| LDL (mg/dL) | 123.40 (29.30) |
| ApoA-I (mg/dL) | 151.64 (18.78) |
| ApoB (mg/dL) | 105.93 (19.91) |
| Statins (n, yes) | 0 |
| ALT (U/I) | 16 (13–21) |
| AST (U/I) | 18 (16–22) |
| GGT (U/I) | 20 (14–27) |
| Creatinine (mg/dL) | 0.85 (0.76–0.95) |
| GFR (Cockcroft–Gault formula, mL/min/1.73 m2) | 69.35 (17.65) |
| Previous CVD (n, yes) | 19 |
Data are reported as mean ± standard deviation for normally distributed variables (Shapiro–Wilk test) or median (interquartile range) for non-normally distributed variables (see Supplementary material online, Statistical Analysis section).
ALT, alanine aminotransferase; ApoA-I, apolipoprotein A1; ApoB, apolipoprotein B; BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; GGT, gamma-glutamyltransferase; HDL, high-density lipoproteins; LDL, low-density lipoproteins; SBP, systolic blood pressure.
Figure 6.
High plasma BPIFB4 detected in LAV-carrier patients is associated with reduced carotid stenosis and IMT < 2mm. Graphs showing correlation between: (A) BPIFB4 plasma level in patients with carotid stenosis >25% (N = 48) and in patients with no carotid atherosclerosis (N = 42). (B) BPIFB4 level in patients stratified for IMT <2 mm (N = 14) vs. IMT >2 mm (N = 8). (C and D) Correlation between BPIFB4 protein levels with intima media thickness (C) and with plasma concentration (D) after genotype stratification of WT- or LAV-carrier patients. Results are presented as mean ± standard deviation and were analysed by two-tailed non-parametric Mann–Whitney test. Numbers above lines show unadjusted P-values.
Table 2.
Characteristics of hypertensive patients from the Campania Salute Study
| Parameters | CS population (N = 22) |
|---|---|
| Clinical characteristics | |
| Age (years) | 69 ± 7 |
| Sex (male/female) | 11/14 |
| SBP (mmHg) | 143.6 ± 15.7 |
| DBP (mmHg) | 89.56 ± 12.5 |
| Weight (kg) | 76.36 ± 11.9 |
| Height (cm) | 163.1 ± 9.5 |
| Cholesterol (mg/dL) | 212.8 ± 47.4 |
| HDL (mg/dL) | 55.25 ± 10.9 |
| TG (mg/dL) | 130.5 ± 60.4 |
| Glycaemia (mg/dL) | 96.46 ± 12.1 |
| Creatinine (mg/dL) | 0.905 ± 0.19 |
| Medication (%) | |
| β-blocker | 5 (20) |
| Ang-II receptor antagonist | 12 (48) |
| ACE-inhibitor | 8 (32) |
| Diuretics | 11 (44) |
| CCB | 9 (36) |
Data are reported as mean ± standard deviation.
CCB, amlodipine/olmesartan medoxomil; DBP, diastolic blood pressure; HDL, high-density lipoproteins; SBP, diastolic blood pressure; TG, triglycerides.
Discussion
In recent years, different approaches have been developed to counteract the progression of vascular atherosclerosis, including cholesterol-level lowering and inflammation modulation. Owing to the large numbers of inflammatory molecular and cellular mediators, it is unlikely that blockade of a single cytokine will be therapeutically effective. We report here new exciting results on the pleiotropic activity of LAV-BPIFB4 on different mechanisms of the atherogenic process. In particular, we demonstrate the efficacy of LAV-BPIFB4 in contrasting endothelial dysfunction, plaque formation/progression, inflammatory cytokine release, macrophage polarization, and T cell activation, in a murine model of atherosclerosis. These benefits were not associated with changes in the lipid profile. In addition, we provide ex vivo and in vitro evidence that these beneficial actions may extend to human vasculature until to be inversely associated to subclinical index of atherosclerosis in selected patients. Mechanistically, the effects of LAV-BPIFB4 seem to be attributable to a CXCR4-dependent mechanism.
Investigation of CXCR4 and CXCL12 expression revealed that the ligand and receptor are both up-regulated in stable and unstable carotid atherosclerotic plaques, especially in macrophages, compared with healthy vessels, both at the mRNA and protein level.7 It is, however, unclear if these expressional changes are pathogenic or compensatory. Results from a recent study using a genetic approach argue for the second possibility; in fact, in ApoE knockout mice fed a high-fat diet, vascular-specific deletion of CXCR4 resulted in larger atherosclerotic lesions compared with their relative control.28 In line with this finding, reconstitution of LDLr(−/−) mice with autologous bone marrow infected with lentivirus encoding SDF-1α antagonist or CXCR4 degrakine, which affect proteasomal degradation of CXCR4, led to progressive plaque development, intraplaque haemorrhage and disease progression. Moreover, CXCR4 knockdown augmented the adhesive capacity of neutrophils and the activation state of circulating neutrophils.29,30 An epidemiological study followed by functional validation with the CRISPR/Cas9 system confirmed that genetic variation of CXCR4 can confer different susceptibility to coronary artery disease.31 Altogether, these findings open new therapeutic perspectives for the treatment of atherosclerosis through the targeting of the CXCL12/CXCR4 signalling pathway. Our study provides new evidence that this could be indeed a valuable option. In a previous investigation, we showed that BPIFB4 and CXCR4 expression concordantly distinguished healthy LLIs and correlated with maintained MNC migration towards CXCL12.12 Here, we demonstrate that LAV-BPIFB4 gene therapy protects from atherosclerosis through a mechanism involving the CXCR4 receptor in vascular cells and MNCs.
Long-living individuals have the unique ability to age without experiencing major CVD. The present study strengthens the novel concept that the healthy phenotype of LLIs can be efficiently transferred to murine models and cultured human tissues by the delivery of either LAV-BPIFB4 or a recombinant protein. LAV-BPIFB4 gene therapy succeeded in the two primary endpoints, namely improving endothelial dysfunction and reducing adverse vascular effects in ApoE knockout mice fed a high-lipid diet. The benefit of LAV-BPIFB4 was also evidenced at the ultrastructural level in vessels: it prevented the cytosolic derangement, cytoplasmic dilution, and irregular plasma membrane observed in different vascular districts of control mice. Interestingly, LAV-BPIFB4 gene therapy did not affect the serum cholesterol profile, but it did contrast the ability of oxidized cholesterol to induce endothelial dysfunction by positively modulating the inflammatory/immune background of atherosclerosis.32
In line with this, LAV-BPIFB4 redistributed the pool of monocyte subpopulations, redirecting them towards a pro-resolving phenotype. This was reflected by the increased abundance of CXCR4+Ly6Chigh monocytes in bone marrow and spleen, the two major tissue reservoirs of monocytes available to mobilize towards injured tissues. In the margination process, CXCR4 is considered the retention force in the vasculature. Therefore, we speculate that the higher level of CXCR4 in blood Ly6Chigh monocytes after LAV-BPIFB4 treatment in mice may finely tune the transit time into the circulation, completing a protective intravascular differentiation process. In this context, recent studies have provided new interesting insight into the regulatory mechanisms of monocytosis relevant to atherosclerosis. Consistent with their functionally distinct immunological roles, newly recruited Ly6Chigh but not Ly6Clow monocytes uniquely differentiate into pro-resolving M2 macrophages, driving murine atherosclerotic regression at the early stages of the disease.5,32 Accordingly, we documented an enrichment of M2 splenic macrophages, which can contribute to dampen T cell activation and proliferation in a CXCR4-dependent manner, as AMD3100 counteracted most of the LAV-BPIFB4-mediated actions on the mono-macrophage compartment. This latter result is in keeping with the reported ability of CXCR4 to promote the acquisition of the M2 phenotype in healthy monocyte-derived macrophages.33 Furthermore, the described influence of LAV-BPIFB4 on the myeloid compartment is in agreement with the recorded increase in IL-23 and IL-27 in peripheral blood in the mouse model. Indeed, both cytokines modulate endothelial cell activation and myeloid cell recruitment at early and advanced stages of atherosclerosis, limiting the deleterious effects of the pathological events.34,35
Our study also provides compelling in vitro evidence that the LAV-BPIFB4 protein can exert similar beneficial effects in redirecting human monocyte-derived macrophages from an M1 pro-inflammatory to an M2 anti-inflammatory phenotype, as well as correcting the balance of cytokines, increasing eNOS phosphorylation, and improving endothelial function in human atherosclerotic vessels. All these beneficial events observed in the different anatomical districts might be finely orchestrated by the circulating levels of BPIFB4, which was found significantly increased in plasma of AAV-LAV-BPIFB4-treated mice after 1 month of gene therapy. Indeed, the undetectable level of GFP protein both in myeloid cells and aorta led us to speculate a bystander action of circulating BPIFB4 on the immune compartment and in vascular cells after its proper production and rapid secretion by transduced liver cells (Take home figure). Accordingly, elevated plasma concentration of the protein associated with protection against the onset of the atherosclerotic disease, regardless of risk factors, in two independent patient cohorts, a finding corroborating the protective role of the LAV-BPIFB4 isoform and strengthening its translational relevance.
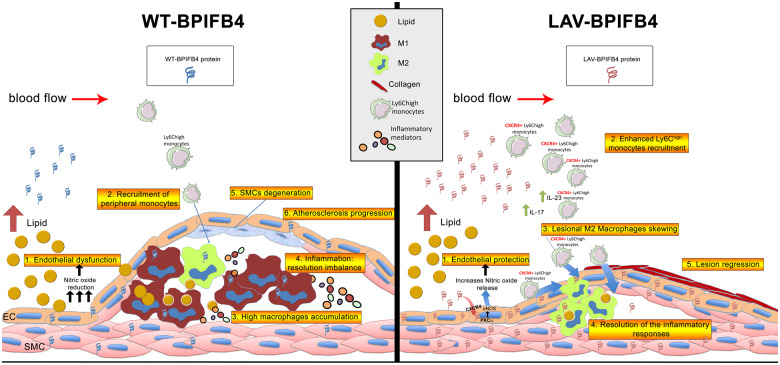
Take home figure.
LAV-BPIFB4 exerts protective effects against the onset and progression of the atherogenic process. LAV-BPIFB4 modulates both endothelial function and the immune compartment through a CXCR4-dependent mechanism. In endothelial cells, LAV-BPIFB4 promotes nitric oxide production, ensuring endothelial protection. At the same time, it is able to enhance Ly6Chigh monocyte recruitment, directing them towards the pro-resolving M2 macrophage phenotype, driving atherosclerotic regression at the early stage of the disease and favouring resolution of the inflammatory responses.
Conclusion
In conclusion, the novel findings from this study highlight the potential of LAV-BPIFB4 in contrasting vascular and immune features of atherosclerosis. This supports an alternative therapeutic vision of the management of CVDs. Hence, we foresee that LAV-BPIFB4 therapy could be a viable option to delay the occurrence of age-related cardiovascular disease, replicating successful, healthy longevity in populations at risk.
Funding
This work was supported by: research funding from Cariplo Foundation (n.2016-0874) to AAP and CV; PRIN-20157ATSLF_009 to AAP and CV; Ministry of Health (RF-2016-02364864) to AAP and CV; E. Ciaglia was supported by a fellowship from Fondazione Umberto Veronesi (FUV 2017cod.1072, FUV 2018cod.2153, FUV 2019cod.2198).
Conflict of interest: A.A.P. and C.V. own shares of LGV1 Inc. and have filed a patent. All other authors declare no financial or competing interests.
Supplementary Material
References
- 1. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT.. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 2. Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S.. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med 2006;12:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shantsila E, Tapp LD, Wrigley BJ, Montoro-García S, Lip GYH.. CXCR4 positive and angiogenic monocytes in myocardial infarction. Thromb Haemost 2013;109:255–262. [DOI] [PubMed] [Google Scholar]
- 4. Chong SZ, Evrard M, Devi S, Chen J, Lim JY, See P, Zhang Y, Adrover JM, Lee B, Tan L, Li JL, Liong KH, Phua C, Balachander A, Boey A, Liebl D, Tan SM, Chan JK, Balabanian K, Harris JE, Bianchini M, Weber C, Duchene J, Lum J, Poidinger M, Chen Q, Renia L, Wang CI, Larbi A, Randolph GJ, Weninger W, Looney MR, Krummel MF, Biswas SK, Ginhoux F, Hidalgo A, Bachelerie F, Ng LG.. CXCR4 identifies transitional bone marrow premonocytes that replenish the mature monocyte pool for peripheral responses. J Exp Med 2016;213:2293–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J, Gusarova V, Gromada J, Weinstock A, Moore KJ, Loke P, Fisher EA.. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest 2017;127:2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J, Chemaly E, Liang L, Kho C, Lee A, Park J, Altman P, Schecter AD, Hajjar RJ, Tarzami ST.. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol 2010;176:1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Merckelbach S, van der Vorst EPC, Kallmayer M, Rischpler C, Burgkart R, Doring Y, de Borst GJ, Schwaiger M, Eckstein HH, Weber C, Pelisek J.. Expression and cellular localization of CXCR4 and CXCL12 in human carotid atherosclerotic plaques. Thromb Haemost 2018;118:195–206. [DOI] [PubMed] [Google Scholar]
- 8. Terry DF, Wilcox MA, McCormick MA, Perls TT.. Cardiovascular disease delay in centenarian offspring. J Gerontol A Biol Sci Med Sci 2004;59:385–389. [DOI] [PubMed] [Google Scholar]
- 9. Spinelli CC, Carrizzo A, Ferrario A, Villa F, Damato A, Ambrosio M, Madonna M, Frati G, Fucile S, Sciaccaluga M, Capunzo M, Cali G, Milanesi L, Maciag A, Puca AA, Vecchione C.. LAV-BPIFB4 isoform modulates eNOS signalling through Ca2+/PKC-alpha-dependent mechanism. Cardiovasc Res 2017;113:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villa F, Carrizzo A, Spinelli CC, Ferrario A, Malovini A, Maciąg A, Damato A, Auricchio A, Spinetti G, Sangalli E, Dang Z, Madonna M, Ambrosio M, Sitia L, Bigini P, Calì G, Schreiber S, Perls T, Fucile S, Mulas F, Nebel A, Bellazzi R, Madeddu P, Vecchione C, Puca AA.. Genetic analysis reveals a longevity-associated protein modulating endothelial function and angiogenesis. Circ Res 2015;117:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villa F, Malovini A, Carrizzo A, Spinelli CC, Ferrario A, Maciag A, Madonna M, Bellazzi R, Milanesi L, Vecchione C, Puca AA.. Serum BPIFB4 levels classify health status in long-living individuals. Immun Ageing 2015;12:27.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spinetti G, Sangalli E, Specchia C, Villa F, Spinelli C, Pipolo R, Carrizzo A, Greco S, Voellenkle C, Vecchione C, Madeddu P, Martelli F, Puca AA.. The expression of the BPIFB4 and CXCR4 associates with sustained health in long-living individuals from Cilento-Italy. Aging (Albany NY) 2017;9:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Busillo JM, Armando S, Sengupta R, Meucci O, Bouvier M, Benovic JL.. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J Biol Chem 2010;285:7805–7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabas I, Lichtman AH.. Monocyte-macrophages and T cells in atherosclerosis. Immunity 2017;47:621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Z, Qin JJ, Zhang Y, Cheng WL, Ji YX, Gong FH, Zhu XY, Zhang Y, She ZG, Huang Z, Li H.. LILRB4 deficiency aggravates the development of atherosclerosis and plaque instability by increasing the macrophage inflammatory response via NF-kappaB signaling. Clin Sci (Lond) 2017;131:2275–2288. [DOI] [PubMed] [Google Scholar]
- 16. Molica F, Meens MJ, Dubrot J, Ehrlich A, Roth CL, Morel S, Pelli G, Vinet L, Braunersreuther V, Ratib O, Chanson M, Hugues S, Scemes E, Kwak BR.. Pannexin1 links lymphatic function to lipid metabolism and atherosclerosis. Sci Rep 2017;7:13706.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK.. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 2012;125:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fatkhullina AR, Peshkova IO, Koltsova EK.. The role of cytokines in the development of atherosclerosis. Biochemistry (Mosc) 2016;81:1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peshkova IO, Fatkhullina AR, Mikulski Z, Ley K, Koltsova EK.. IL-27R signaling controls myeloid cells accumulation and antigen-presentation in atherosclerosis. Sci Rep 2017;7:2255.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kave M, Shadman M, Alizadeh A, Samadi M.. Analysis of the association between IL-23R rs11209026 polymorphism and incidence of atherosclerosis. Int J Immunogenet 2015;42:341–345. [DOI] [PubMed] [Google Scholar]
- 21. Dutta P, Hoyer FF, Grigoryeva LS, Sager HB, Leuschner F, Courties G, Borodovsky A, Novobrantseva T, Ruda VM, Fitzgerald K, Iwamoto Y, Wojtkiewicz G, Sun Y, Da Silva N, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M.. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med 2015;212:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arango Duque G, Descoteaux A.. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 2014;5:491.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grabowska J, Lopez-Venegas MA, Affandi AJ, den Haan JMM.. CD169(+) macrophages capture and dendritic cells instruct: the interplay of the gatekeeper and the general of the immune system. Front Immunol 2018;9:2472.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vecchione C, Villa F, Carrizzo A, Spinelli CC, Damato A, Ambrosio M, Ferrario A, Madonna M, Uccellatore A, Lupini S, Maciag A, Ryskalin L, Milanesi L, Frati G, Sciarretta S, Bellazzi R, Genovese S, Ceriello A, Auricchio A, Malovini A, Puca AA.. A rare genetic variant of BPIFB4 predisposes to high blood pressure via impairment of nitric oxide signaling. Sci Rep 2017;7:9706.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aimo A, Migliorini P, Vergaro G, Franzini M, Passino C, Maisel A, Emdin M.. The IL-33/ST2 pathway, inflammation and atherosclerosis: trigger and target? Int J Cardiol 2018;267:188–192. [DOI] [PubMed] [Google Scholar]
- 26. Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY.. IL-33 reduces the development of atherosclerosis. J Exp Med 2008;205:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glaudemans AW, Slart RH, Bozzao A, Bonanno E, Arca M, Dierckx RA, Signore A.. Molecular imaging in atherosclerosis. Eur J Nucl Med Mol Imaging 2010;37:2381–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doring Y, Noels H, van der Vorst EPC, Neideck C, Egea V, Drechsler M, Mandl M, Pawig L, Jansen Y, Schroder K, Bidzhekov K, Megens RTA, Theelen W, Klinkhammer BM, Boor P, Schurgers L, van Gorp R, Ries C, Kusters PJH, van der Wal A, Hackeng TM, Gabel G, Brandes RP, Soehnlein O, Lutgens E, Vestweber D, Teupser D, Holdt LM, Rader DJ, Saleheen D, Weber C.. Vascular CXCR4 limits atherosclerosis by maintaining arterial integrity: evidence from mouse and human studies. Circulation 2017;136:388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bot I, Daissormont IT, Zernecke A, van Puijvelde GH, Kramp B, de Jager SC, Sluimer JC, Manca M, Herias V, Westra MM, Bot M, van Santbrink PJ, van Berkel TJ, Su L, Skjelland M, Gullestad L, Kuiper J, Halvorsen B, Aukrust P, Koenen RR, Weber C, Biessen EA.. CXCR4 blockade induces atherosclerosis by affecting neutrophil function. J Mol Cell Cardiol 2014;74:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zernecke A, Bot I, Djalali-Talab Y, Shagdarsuren E, Bidzhekov K, Meiler S, Krohn R, Schober A, Sperandio M, Soehnlein O, Bornemann J, Tacke F, Biessen EA, Weber C.. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res 2008;102:209–217. [DOI] [PubMed] [Google Scholar]
- 31. Runmin G, Jiamei J, Zhiliang J, Yonghua C, Zhizhou S, Guizhou T, Shuguang L.. Genetic variation of CXCR4 and risk of coronary artery disease: epidemiological study and functional validation of CRISPR/Cas9 system. Oncotarget 2018;9:14077–14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Witztum JL, Lichtman AH.. The influence of innate and adaptive immune responses on atherosclerosis. Annu Rev Pathol 2014;9:73–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cassol E, Cassetta L, Rizzi C, Alfano M, Poli G.. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J Immunol 2009;182:6237–6246. [DOI] [PubMed] [Google Scholar]
- 34. Hirase T, Hara H, Miyazaki Y, Ide N, Nishimoto-Hazuku A, Fujimoto H, Saris CJ, Yoshida H, Node K.. Interleukin 27 inhibits atherosclerosis via immunoregulation of macrophages in mice. Am J Physiol Heart Circ Physiol 2013;305:H420–9. [DOI] [PubMed] [Google Scholar]
- 35. Koltsova EK, Kim G, Lloyd KM, Saris CJ, von Vietinghoff S, Kronenberg M, Ley K.. Interleukin-27 receptor limits atherosclerosis in LDLR-/- mice. Circ Res 2012;111:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.